Abstract
Numerous studies have suggested the importance of leptin against autoimmune diseases such as systemic lupus erythematosus (SLE), multiple sclerosis (MS) and psoriasis. To summarize our current understanding of the role of leptin in inflammatory responses and rheumatoid arthritis (RA), a systematic review was conducted to assess the discrepancy of leptin in RA and its effect on immunity according to different studies. Recently, emerging data have indicated that leptin is involved in the pathological function of RA, which is common in autoimmune disorders. This review discusses the possible consequences of leptin levels in RA. Blocking the key signal pathways of leptin and inhibiting the leptin activity-like leptin antagonist may be a promising way for potential therapeutic treatment of RA at risk of detrimental effects. However, leptin was increased in patients with RA and may also regulate joint damage. Thus, more understanding of the mechanism of leptin in RA would be advantageous in the future.
Keywords: autoimmune diseases, inflammation, leptin, rheumatoid arthritis
Introduction
Leptin, the ob gene product, is a 16-kDa non-glycosylated peptide hormone secreted by adipocytes, and has drawn much attention since its identification in 1995 [1]. Leptin belongs to one of the type I cytokine superfamily members, and has a long-helix structure similar to interleukin (IL)-2, IL-6 and granulocyte colony-stimulating factor (G-CSF) [2]. Rheumatoid arthritis (RA) is a systemic and chronic inflammatory autoimmune disease with unknown aetiology. Synovial inflammation and proliferation can contribute to cartilage and bone tissue invasion, ending with joint destruction and bone malformation. This disorder is usually considered to result from infection by some pathogens or carrying of the susceptibility genes, which cause dysfunction of the immune system. It is may also be associated with T cell immune responses and B cell-induced antibodies. In addition, cytokines from various immune cells play important roles in the pathogenesis of RA. In recent years, a variety of studies have been carried out to suggest that leptin has a critical role not only in the regulation of metabolic processes, but also in control of immune homeostasis, which shows diverse effects on the innate immune system. Normal leptin levels crucially maintain and regulate the body's immune function. Emerging evidence indicates that leptin acts as a proinflammatory cytokine in immune responses such as systemic lupus erythematosus (SLE) [3–6], RA [7–10], multiple sclerosis (MS) [11–13] and psoriasis [14–16]. Leptin could be a member of the cytokine network managing the inflammatory immune response and host defence mechanisms. Therefore, in this review, we review the biological characteristics, pathogenesis and therapeutic potential of leptin in RA.
Search strategy
The inclusion criteria for the studies in the present review comprised observational studies (prospective or retrospective), clinical trials or clinical trial long-term extensions which focused on the association between leptin and RA. These papers were published in the English language and used quantitative study designs for evaluation. The study characteristics of the main original papers in the final inclusion are shown in Table 1.
Table 1.
Leptin expression levels in RA
| Author | Study year | Pro-destructive and anti-destructive | Disease | Parameter | Increase/decrease compared with controls | Correlation with disease activity | Comment |
|---|---|---|---|---|---|---|---|
| Manavathongchai et al. [9] | 2013 | n.a. | Human RA | Serum leptin levels | Increase | No | Hypertension in RA patients is associated with increasing leptin levels rather than systemic inflammation |
| Park et al. [17] | 2013 | n.a. | Human RA | Serum leptin levels | n.a. | Positive correlation | Serum leptin has a significant association with LDL cholesterol levels, which can predict radiographic progression of RA |
| Muraoka et al. [18] | 2013 | Proinflammatory | Human RA | Leptin receptor mRNAs | Increase | n.a. | Leptin may up-regulate IL-6 production in rheumatoid synovial fibroblasts by activating JAK2/STAT-3 |
| Chen et al. [10] | 2013 | Anti-inflammatory | Human RA | Plasma leptin levels | No significant difference | n.a. | Leptin may be involved in keeping energy homeostasis in the anti-inflammatory responses during RA remission |
| Hayashi et al. [19] | 2012 | n.a. | Human RA | Serum leptin levels | n.a. | No | Serum leptin levels in the high disease activity group were significantly lower than in the low disease activity group. They were positively correlated with the BMI and waist circumference |
| Kopec-Medrek et al. [20] | 2012 | n.a. | Human RA | Plasma leptin levels | No significant difference | No | Physiological relationship between leptin and body mass was not changed during the treatment with infliximab |
| Hamaguchi et al. [21] | 2012 | n.a. | Murine type IIcollagen-induced arthritis (CIA) | Serum leptin levels | No significant difference | n.a. | High-fat diet may lead to functional alterations of adipose tissues during developing arthritis |
| Sugioka et al. [22] | 2012 | n.a. | Mice collagen antibody-induced arthritis (CAIA) | Serum leptin levels | Increase | n.a. | RA patients with high BMI who have leptin resistance may indicate reduced inflammation |
| Olama et al. [23] | 2012 | Anti-destructive | Human RA | Serum and synovial leptin levels | Increase | Positive correlation | The synovial/serum leptin ratio may appropriately reflect the extent of local consumption of the leptin in the joint than the synovial leptin level |
| Kontny et al. [24] | 2012 | n.a. | Human RA | Articular adiposetissue and synovial membrane | n.a. | n.a. | Articular adipose tissue could be an important contributor in the pathological processes taking place in the RA joint |
| Ferraz-Amaro et al. [25] | 2011 | n.a. | Human RA | Serum leptin levels | No significant difference | n.a. | Long-term inhibition of TNF-α does not obviously alter leptin serum levels in RA patients |
| Yoshino et al. [26] | 2011 | Proinflammatory | Human RA | Serum leptin levels | No significant difference | Positive correlation | Leptin was positively associated with CRP level in RA patients, indicating that it may act as proinflammatory cytokine in RA |
| Engvall et al. [27] | 2010 | n.a. | Human RA | Serum leptin levels | Increase | n.a. | Leptin concentrations increased with the treatment of hydroxychloroquine or infliximab |
| Xibille-Friedmann et al. [28] | 2010 | n.a. | Human RA | Serum leptin levels | Increase | Positive correlation | Change of leptin levels may relate to disease progression and IL-17 titres |
| Targońska-Stepniak et al. [29] | 2010 | Proinflammatory | Human RA | Serum leptin levels | n.a. | Positive correlation | Leptin could act as a proinflammatory cytokine |
| Rho et al. [30] | 2009 | Anti-destructive | Human RA | Serum leptin levels | Increase | Positive correlation | Leptin correlated negatively with radiographic joint damage |
| Seven et al. [31] | 2009 | n.a. | Human RA | Serum and synovial fluid leptin levels | Increase | No | Chronic inflammation may be an important determinant of plasma leptin level |
| Martin et al. [32] | 2008 | n.a. | Mice RA | Serum leptin levels | Decrease | n.a. | Leptin gene expression is susceptible to the white adipose tissue mass rather than the caloric intake |
| Targonska-Stepniak et al. [95] | 2008 | n.a. | Human RA | Serum leptin levels | Increase | Positive correlation | Some important dependence appears between aggressive process of RA and increased leptin levels |
| Hizmetli et al. [33] | 2007 | n.a. | Human RA | Plasma leptin levels | No significant difference | No | leptin levels are associated with BMI both in RA patients and healthy individuals |
| Hayward et al. [34] | 2007 | n.a. | Mice RA | Serum leptin levels | Decrease | n.a. | Down-regulation of leptin may try to decrease the metabolic rate, bone loss and depletion of adipose stores |
| Caetano-Lopes et al. [35] | 2007 | Proinflammatory | Mice RA | Serum leptin levels | n.a. | n.a. | ObR antagonists may be a promising therapeutics in leptin-sensitive early stages of RA |
| Otero et al. [36] | 2006 | n.a. | Human RA | Plasma leptin levels | Increase | n.a. | Leptin may modulate the inflammatory environment in RA patients |
| Gunaydin et al. [37] | 2006 | n.a. | Human RA | Serum leptin levels | Increase | No | Leptin probably plays an important role in the pathogenesis of RA |
| Harle et al. [38] | 2006 | n.a. | Human RA | Serum leptin levels | No significant difference | No | Leptin levels are not linked to inflammation |
| Popa et al. [39] | 2005 | n.a. | Human RA | Plasma leptin levels | No significant difference | Negative correlation | Active chronic inflammation may reduce plasma leptin production |
| Bokarewa et al. [7] | 2003 | Anti-destructive | Human RA | Plasma leptin levels | Increase | No | Local consumption of leptin in the joint cavity related to non-erosive joint disease |
| Anders et al. [40] | 1999 | n.a. | Human RA | Serum leptin levels | Increase | No | Anti-rheumatic treatment may reduce the assumed proinflammatory stimulus for abnormal leptin production by restricting the disease activity via a reduction of local cytokines |
BMI = body mass index; n.a. = not available; RA = rheumatoid arthritis; LDL = low-density lipoprotein; IL = interleukin; TNF = tumour necrosis factor; CRP = C-reactive protein; JAK2 = Janus kinase 2; STAT-3 = signal transducer and activator of transcription-3.
Relevant publications were accessed by two investigators (Guo Tian and Jia-Ning Liang) through a systematic literature search using the keywords ‘RA’, ‘Rheumatoid Arthritis’ and ‘leptin’ in PubMed, Embase and the Cochrane Library. Other literature was retrieved from cross-references in both original and review papers. If discordance appeared, it was assessed further by a third investigator (Dian Zhou), and a final decision was made by the majority of the votes.
The biological activity of leptin
Leptin has a dual role with respect to its anti- and proinflammatory response. On one hand, the increased level of leptin in infection and inflammation induces and sustains the T cell immune response and its function [41]. On the other hand, leptin levels are increased in RA patients, but they in synovial fluid are less than in plasma, indicating local consumption of leptin in the joint cavity [7], and the levels of disassociated leptin can cross-react with other cytokines. Meanwhile, it promotes the production of cytokine antagonists, as does IL-1, to regulate the body's inflammatory response [42]. Popa et al. [39] argued that leptin production can be increased when patients suffering from sepsis with raised cytokines such as IL-1β and tumour necrosis factor (TNF)-α, but can be inhibited when in a chronic inflammatory process. It can act through the central nervous system and diminish osteoblast activity, or can have an osteogenic effect by binding directly to its receptors on the surface of osteoblast cells.
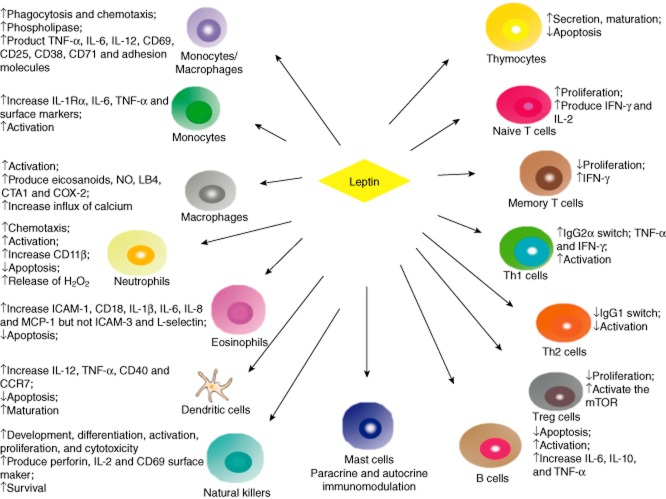
Leptin, as a potent modulator in immune responses, has direct effects on both innate and adaptive immunity (Fig. 1). Leptin activity on the immune system is basically proinflammatory. In innate immunity, it can stimulate immune cells such as activation of proliferation and phagocytosis of monocytes and macrophages, chemotaxis of neutrophils, maturation of dentritic cells (DC), cytotoxicity and survival of natural killer (NK) cells, whereas it down-regulates apoptosis of neutrophils, eosinophils and DCs. Leptin also increases the production of proinflammatory cytokines such as TNF-α, IL-6 and IL-12 [43], and induces CD69, CD25, CD38, CD71 and adhesion molecule expression via activation of monocytes or macrophages [44,45]. Gruen et al. [46] found that leptin exerts a potent chemotaxis for monocytes and macrophages, which needs the presence of full-length leptin receptors on migrating cells. Moreover, leptin administration mediates the production of proinflammatory cytokines TNF-α and IL-6 through monocytes. Leptin also increases the production of monocyte surface markers and activates human monocytes; thus, leptin may be a powerful stimulatory hormone on human peripheral blood monocytes. In neutrophils, leptin can stimulate chemotaxis through the p38 mitogen-activated protein kinase (MAPK) pathway [47], as well as the release of H2O2 [48]. Leptin has also activated impacts upon neutrophils and NK cells and incentives for their gene expression [48,49]. Regarding eosinophils, leptin can promote the cell surface release of intercellular adhesion molecule-1 (ICAM-1) and CD18, but not ICAM-3 and L-selectin. Moreover, leptin can also stimulate eosinophil chemokinesis and cause the release of inflammatory cytokines such as IL-1β, IL-6, IL-8, growth-related oncogene-alpha and monocyte chemotactic protein 1 (MCP-1) [50]. Leptin stimulating the proinflammatory cytokine production can indirectly regulate adaptive immunity. Mattioli et al. [51] argued that leptin-induced cytokines such as IL-12 and TNF-α in DC serve to licence naive CD4+ T cells for T helper type 1 (Th1) priming. In addition, leptin can induce CD40 expression by activating protein kinase B (Akt) in murine DCs [52]. Al-Hassi et al. [53] reported that leptin can stimulate DC maturation and CCR7 production in blood DC. Mast cells are immune cells secreting many mediators and cytokines that influence different inflammatory and immune processes. Taildeman et al. [54] have identified the production of leptin and leptin receptors in mast cells in the various studied tissues, indicating paracrine and/or autocrine immunomodulatory effects of leptin on mast cells.
Figure 1.
Effects of leptin on innate and adaptive immune system cells. Leptin regulates levels in some ways such as development, proliferation, apoptotic, maturation and activation both in innate immunity and adaptive immunity. In innate immunity, leptin increases phagocytosis and chemotaxis of monocytes and macrophages, regulates maturation and production of cytokines both between natural killer (NK) and dendritic cells (DC), as well as paracrine and autocrine immunomodulation in mast cells. In adaptive immunity, it promotes proliferation of naive T cells but not memory and regulatory T cells (Treg) cells. Leptin increases T helper type 1 (Th1) cell activation and immunoglobulin (Ig)G2α switching while it down-regulates IgG1 switching in Th2 cells. Meanwhile, it stimulates the release of cytokines in T and B cells.
In adaptive immunity, leptin dramatically stimulates secretion, maturation and survival of thymocytes, proliferation of naive T cells and activation of Th1 and B cells. It also reduces apoptosis of thymocytes and B cells and inhibits production of regulatory T cells (Tregs). Leptin can mediate the specific aspects of T cell function with differential effects on diverse subpopulations of lymphocytes, and can also stimulate the production of various proinflammatory cytokines such as interferon (IFN)-γ and IL-2 in T lymphocytes [55,56]. Leptin regulates immunoglobulin (Ig)G2a-switching in B cells and modulates the production of TNF-α and IFN-γ in Th1 cells, but suppresses action on Th2 cells and IgG1-switching [57]. De Rosa et al. found that freshly isolated human Treg cells secrete leptin and induce high levels of LepR. Leptin can restrain rapamycin-induced proliferation of Tregs via activation of the mammalian target of rapamycin (mTOR), a 289-kDa serine or threonine protein kinase. Oscillating mTOR activity may be necessary for Treg cell activation [58]. It might account for why Treg cells are retarded to T cell receptor stimulation in vitro, in which high levels of leptin and energy status support mTOR activation [59]. Furthermore, leptin modulates human B cells to produce cytokines such as IL-6, IL-10 and TNF-α by activating Janus kinase 2/signal transducer and activator of transcription-3 (JAK2/STAT-3) and p38MAPK/extracellular-regulated kinase 1/2 (ERK1/2) signalling pathways, which may result in its inflammatory and immunoregulatory features [60].
Leptin signalling
In recent years, studies of leptin binding signalling to its receptor have brought a greater understanding of the biochemical and molecular mechanisms of leptin function. The early finding of OB-R led to the quick recognization of the JAK/STAT pathway as one of the main leptin-activated signalling cascades [61,62]. Further studies showed that the OB-Rb included intracellular motifs required for activation of the JAK/STAT pathway [63]. As well as JAK/STAT, other pathways such as MAPK/ERK and AMP-activated protein kinase (AMPK) and PI3K/Akt are also involved in leptin signalling. Together, all these pathways comprise a network that modulates the leptin response.
JAK/STAT pathway
Leptin binding to its receptor causes dimerization, so that dimer-associated JAK2 closes and leads to phosphorylation. Activated JAK2 phosphorylation of tyrosine 1138 (Tyr1138) subsequently binds to the SH2 domain of STAT-3 [64]. Tyr985-phosphorylated STAT-3 is separated from lepRb, dimerizing and entering the nucleus. STAT-3 dimers, as a transcription factor, adjust the expression of targeting genes such as suppressor of cytokine signalling 3 (SOCS3) and neuropeptides [65–67]. There is another way to mediate JAK2 signalling: after leptin stimulation of lepRb phosphorylation on Tyr1077, a combination phospho-Tyr1077 with the SH2 domain of STAT-5 allows STAT-5 phosphorylation and activation by JAK2 [68]. In addition, STAT-5 activation is also partially susceptible to phospho-Tyr1138 [69] (Fig. 2).
Figure 2.
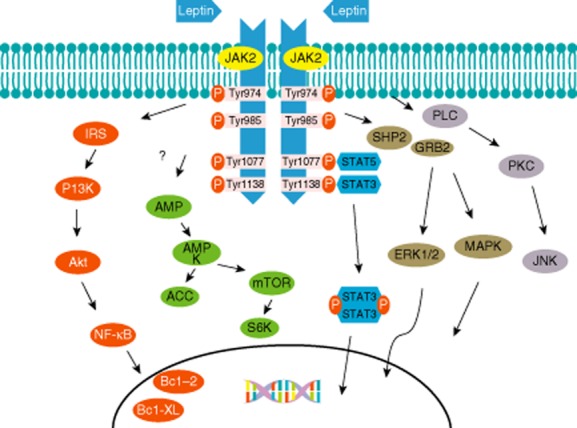
Leptin signalling pathways. After leptin binding to leptin receptor (LEPRb) and activating Janus kinase 2 (JAK2), the possible pathways are as follows: (1) activated JAK2 phosphorylation of the tyrosine residues on the leptin receptor induces signal transducer and activator of transcription (STAT)-3 and STAT-5 binding to tyrosine residues. The phosphorylated STAT-3 dimers enter the nucleus and are involved in the gene expression. (2) After the phosphorylated tyrosine (Tyr) 974 and Tyr 985 recruitment of Src homology (SH)2,SHP2 activates the mitogen-activated protein kinase (MAPK) pathways such as extracellular-regulated kinase (ERK)1/2, p38 MAPK and p42/44 MAPK pathways by interacting with growth factor receptor-bound protein 2 (GRB2), which regulate the expression of cytokine and chemokine genes. (3) PI3K, with its p85 binding to the insulin receptor substrate (IRS) proteins, and protein kinase B (Akt) regulate nuclear factor kappa B (NF)-κB, which can adjust the production of prosurvival factors such as Bcl-2 and Bcl-XL. (4) Leptin participates in the modulation of the CaMKK2/AMP-activated protein kinase (AMPK)/acetyl-CoA carboxylase (ACC) and mammalian target of rapamycin (mTOR)/ribosomal S6 kinase (S6K) pathways. (5) Leptin linking to obese receptor (OB-Rb) can stimulate phospholipase C (PLC) for activation of c-JunN-terminal kinase (JNK) by activating protein kinase C (PKC).
MAPK/ERK pathway
The phosphorylated Tyr 974 and Tyr 985 recruitment of SH2 domain-containing phosphatase 2 (SHP2) activates the MAPK pathways, such as ERK1/2, p38 MAPK and p42/44 MAPK, by interacting with the growth factor receptor-bound protein 2 (GRB2) [63,70,71], which enter the nucleus and mainly mediate cytokine and chemokine genes. SHP2 can sometimes also inhibit JAK2/STAT-3 signalling [72]. The phosphorylated Tyr985 also recruits the SH2 domain of SOCS3, but SOCS3 inversely restrains the activation of the LepRb/JAK2 pathways [73].
PI3K/Akt pathway
PI3K/Akt is an important signalling pathway. PI3K and Akt are requisite for the induction of immune and inflammatory responses [74]. The pathway regulates many effectors, including the anti-apoptotic transcription factor nuclear factor kappa B (NF-κB). Akt suppresses the function of transcription factor forkhead box protein O1 (FoxO1) or forkhead transcription factor like 1 (FKHR-L1), which can induce the production of Bim from the Bcl-2 family proteins. It is reported that FoxO1 can directly regulate the function of leptin in the hypothalamus [75]. Dimer made by leptin and OB-Rb also stimulates insulin signalling such as insulin receptor substrate 1/2 (IRS1/2) via phosphorylated JAK2 [76]. The IRS proteins binding to p85 of PI3K activate the catalytic domain. In mononuclear cells leptin can stimulate the p85 subunit in PI3K by phosphorylating Sam68 (Src-associated substrate in mitosis of 68 kDa) [77]. In thymic cells, leptin stimulates anti-apototic activation via JAK2 on the basis of the involvement in the IRS1/PI3K pathway [74]. NF-κB can adjust the production of various genes covering prosurvival factors such as Bcl-2 and Bcl-XL [56]. Additionally, in DC, leptin can activate NF-κB. However, leptin signalling deficiency results in increasing levels of inhibitor of kappa B (IκB)-α, which is the inhibitor protein of NF-κB [78].
AMPK pathway
Leptin is involved in the regulation of the CaMKK2/AMP-activated protein kinase (AMPK)/acetyl-CoA carboxylase (ACC) and the mTOR/ribosomal S6 kinase (S6K) pathways, but it is not clear how leptin influences this pathway directly or indirectly in hypothalamic neurones. Su et al. [79] suggested that glucose may improve leptin signalling through modulation of AMPK activity. It has not been shown that deletion of AMPK in either pro-opiomelanocortin (POMC) neurones or Agouti-related peptide (AgRP) neurones can change leptin sensitivity [80], which indicates that the CaMKK2/AMPK/ACC pathway can regulate leptin metabolic action [81].
Other pathways
The serine and threonine kinase family of protein kinase C (PKC) isoforms have been concerned with many cellular effects [82]. Leptin seems to have a stimulatory as well as an inhibitory role on PKC. Other pathways may also be involved in leptin signalling. For example, leptin linking to OB-Rb can stimulate phospholipase C (PLC) for activation of c-Jun N-terminal protein kinase (JNK) by activating PKC [56]. Additionally, it is reported that leptin action in pancreatic islets may constrain the PKC-modulated component of the PLC/PKC signalling system which usually induces insulin release. However, leptin-activated PKC is suggested by the ability of PKC inhibitors (GF109203X and Go6976) to inhibit leptin-induced invasiveness of colon epithelial cells [83].
Leptin receptor biological activity
Leptin receptor (ObR), a protein receptor encoded by the diabetes (db) gene, belongs to the type I cytokine superfamily receptor members, and has three different types: long (Ob-Rb), short (Ob-Ra,c,d,f) and soluble (Ob-Re). Ob-Rb can be found in many types of cells, such as immune cells, macrophages, T cells, NK cells and polymorphonuclear cells, as well as neurones and intestinal epithelial cells [55,84–86]. JAK/STAT binding is a key process in conveying information concerning cell kinin receptors. Leptin binding to receptor affects the physiological function and metabolic pathway via the JAK2/STAT signalling pathway. Short-type receptor (Ob-Ra, Ob-R c, Ob-Rd) is expressed mainly in the choroid plexus, kidneys, liver and lungs, involved in mediating leptin into the brain tissue and also the degradation and removal of leptin. It has been stated that the secretagogue and anti-secretagogue effects of leptin are usually regulated by Ob-Ra and Ob-Rb, respectively [87]. The soluble receptor (Ob-Re) in blood circulation can combine with leptin. Smith et al. [88] found that placental secretion of Ob-Ra, Ob-Rb and Ob-Re may tend to adjust leptin action and transportation in the fetus and placenta. Approximately 5–25% of the OB-R subtypes lie at the cell surface, while others are in intracellular pools [89]. With the control of amino acids 8–29 of the intracellular domain, lysosomal internalization and degradation are regulated by OB-Ra and OB-Rb [90]. Leptin shows that internalization and reduction of surface receptors are more important for OB-Rb [89]. More studies are needed to illuminate the association both between the relative levels of the intracellular or cell-surface pools of OB-R and their effects on the regulatory defects of leptin sensitivity. Otvos et al. [91] used the peptide-based ObR agonists and antagonist in-vitro and in-vivo models of the disease to identify ObR as an RA target. Leptin and Allo-aca lessened the extent of joint swelling and the amount of arthritic joints in rat models suffering from adjuvant-induced arthritis. On the basis of the experimental model, leptin shows a distinct impact upon RA. The diverse character of RA may result from various influences of leptin and exposure of ObR antagonism, meaning that targeting ObR antagonists may become useful methods in leptin-sensitive early stages of RA.
Leptin and rheumatoid arthritis
Rheumatoid arthritis is a common type of autoimmune disease in humans, characterized by synovitis and joint destruction. Although RA has been much investigated, the disease pathogenesis remains unclear. Nevertheless, cytokines play a crucial role in participation of activating the synovial cell to joint destruction. Leptin comes mainly from adipose cells. Most studies have found significantly elevated serum levels of leptin in RA patients [7,18,22,26–28,30,36,40,92,93], while others have found decreased levels [19,21,32,34]. Although the relationship is complex, leptin has been shown to be the major factor linkage of food intake with bone metabolism [94].
Leptin levels in serum, synovial fluid and synovial tissue and its influence on joint damage in RA
A significant association may exist between RA patients' risk of severity and leptin levels [17,26,28,39,95,96] (Table 1). Plasma leptin levels have been observed to be higher than in healthy controls [36]. Targonska-Stepniak et al. [95] reported that serum leptin levels were higher in patients with erosive RA. However, some studies have reported that serum leptin levels of RA patients were not different from controls [20,25,33,38], and were concerned significantly with body mass index (BMI) but not disease activity stage (DAS) [33,39]. Anders et al. observed that leptin serum levels are not correlated with disease activity in RA patients [40]. Emerging studies of leptin in synovial fluid and synovial tissue in RA have also been reported. Seven et al. [31] found that serum and synovial fluid leptin levels were obviously higher (P < 0·05) in RA patients than in their control group. Accordingly, these also appeared in moderate disease activity (DAS > 2·7) compared to low disease activity (DAS < 2·7). The amount of leptin release from articular adipose tissue (AAT) was similar (P = 0·9) to that secreted by synovial membrane (SM) [24], but AAT treated with IL-1β produced four times more leptin in contrast with SM. It seems that AAT might be an important contributor to the pathological processes taking place in the RA joint. Another study also reported that leptin had higher levels in systemic circulation than locally in synovial fluid, and was neither associated with resistin levels nor with other proinflammatory markers in body fluids from RA patients [97]. Similarly, Bokarewa et al. [7] found higher serum leptin levels than synovial fluid in RA patients, but the latter evidently dropped compared to the former in arthritis patients in the absence of joint erosion, suggesting that leptin consumption in the joints may be involved in protection against erosions, and therefore that leptin protects against bone erosion. This suggests that leptin may down-regulate the erosive course in the joints. Leptin triggered the production of the IL-1 receptor antagonist [98]. Treatment of RA patients using anakinra (IL-1 receptor antagonist) has been well tolerated, and hence prevented the joint destructive process [99].
In short, there are several potential explanations for these differing results among these studies: first, the relatively small sample size may not precisely represent the larger patients; secondly, the baseline characteristics such as disease duration, age, race and BMI can be inconsistent, which may affect the expression level of leptin; thirdly, patients with other co-existing autoimmune diseases cannot be excluded from studies, which could be potential confounding factors; and fourthly, different measurement methods of leptin levels, such as radioimmunoassay [20,37,40,100] and enzyme-linked immunosorbent assay (ELISA), may also cause deviation [8,23,30]. In addition, discrepancies in leptin levels in RA patients could be due to different treatments that intervene with the endocrine system, which usually complicates human experimental researches. Thus, more studies are still needed to illuminate the relationship between leptin levels and RA patients.
Proinflammatory and anti-inflammatory effects of leptin on RA
Besides its well-known role in the immune system, leptin performs its main actions in autoimmune diseases such as RA. Recently, increased reports have appeared to support this in in-vitro and in-vivo studies. It is unlikely that leptin alone affects cartilage to induce an inflammatory response. Leptin plays a proinflammatory and detrimental role during joint inflammation which, in synergy with IFN-γ or IL-1, triggers nitric oxide synthase type II (NOS2) release from mouse chondrocytes [101,102]. RA patients with erosive joint disease have higher leptin concentrations than those without erosions, and leptin levels may increase the risk of progressive joint destruction [95]. The leptin level was related positively to DAS28 values, which may be linked to the proinflammatory leptin role [29]. Also, no correlation has been documented previously between serum leptin levels and the presence of joint erosions [33]. As a possible proinflammatory cytokine, leptin increases IL-6 production in RA by activating JAK2/STAT-3. Targeting leptin and the JAK/STAT pathway could provide a hopeful method in the future [18]. Yoshino et al. [26] found that leptin was related significantly to CRP levels in RA patients, indicating that it may act as proinflammatory cytokine in RA. In a mouse serum transfer RA model, Allo-aca added subcutaneously partially suppresses the proinflammatory effects of leptin. ObR antagonists may be applied in the leptin-sensitive early stages of RA [91].
However, targeting leptin according to its anti-inflammatory effect in joint erosions of RA has been described. A reduced leptin level in synovial fluid is involved with non-erosive joint disease, indicating that leptin has a protective role against RA destruction [7]. Leptin may reduce radiographic joint damage [30]. It seems that enhancing the effects of leptin may benefit RA patients. Alternatively, this inverse association between leptin and radiographic damage may result from the fact that higher leptin in RA truly deteriorates joint damage, but this influence is sheltered by adiponectin, which protects against joint damage due to its anti-inflammatory properties [103]. It is also reported that recombinant leptin could relieve the severity of joint manifestations in Staphylococcus aureus-induced arthritis, as well as the inflammatory response [96]. Serum leptin, synovial leptin and the synovial/serum leptin ratio in RA patients were found to be significantly higher than in control subjects. Interestingly, serum leptin in RA patients with effusion was higher than the matched synovial leptin [23], suggesting that local consumption of leptin in the joint cavity could protect against the destructive course of RA. Figenschau et al. [104] showed increased proliferation and an enhanced synthesis of extracellular matrix by leptin-induced chondrocytes. Thus, leptin has a direct effect on cartilage generation, including skeletal growth and development. Long-term treatment with anti-TNF-α is potentially effective in ameliorating body mass decrease in RA patients. In addition, no significant alterations in plasma leptin were observed during etanercept therapy. Leptin may play an important role in maintaining energy homeostasis in the anti-inflammatory responses during RA remission [10].
Taken together, the results of different clinical studies suggest that leptin levels potentially contribute to joint damage by regulating immune responses. More studies including patients with early RA are needed to clarify the role of leptin on disease outcome, particularly the progression of joint damage.
Studies concerning the effect of leptin on radiographic joint damage in RA
Recently, a growing number of studies have shown that raised adiposity might protect against radiographic damage in RA. Although its mechanism has not been illustrated, the potential relation of serum leptin levels to the progression of radiographic joint damage in RA patients was explored. Giles et al. [105] did not observe any meaningful association of leptin with radiographic progression. Leptin also did not correlate with Sharp-van der Heijde score (SHS) in both univariate and multivariable modelling. A significant association between leptin and radiographic damage was not observed [106]. Low-density lipoprotein (LDL) cholesterol could synergistically raise the probability of radiographic progression by adjustment in patients with high serum leptin levels, and not in those without. Thus, LDL cholesterolaemia, which has an association with serum leptin levels, might be applied to predict the radiographic progression of RA [17]. Meyer et al. [107] found that there was no association between leptin level and radiographic progression by total SHS. This result was in accord with that of Rho et al. [30], who thought that there was a negative relationship between leptin and radiographic joint damage, but it was attenuated by adjustment for BMI. Therefore, targeting leptin may be helpful in individuals with RA.
Cellular studies concerning the effect of leptin on cartilage
Many researches have reported the function of leptin in cartilage metabolism. Leptin has a double role in the immune response. Leptin can act not only to down-regulate osteoblasts activity via the central nervous system, but also expresses an osteogenic role by binding directly to its receptors on the surface of osteoblast cells [35]. Nitric oxide (NO) can mediate cartilage destruction by triggering de-differentiation and chondrocyte apoptosis. Kim et al. observed that cytokine-induced NO, as a gaseous mediator, can lead to many inflammatory alterations on cartilage, such as chondrocyte phenotype loss, chondrocyte apoptosis and metalloprotease activation. Leptin may behave as a proinflammatory cytokine due to its synergistic role with IFN-γ on NO synthase type II [108]. Leptin may directly affect cartilage generation [104], suggesting a new role of leptin in skeletal growth and development. Furthermore, through injection into the joint cavity of the rat knee, leptin could strongly stimulate anabolic functions of chondrocytes by inducing insulin-like growth factor 1 (IGF-1) and transforming growth factor (TGF-β) [109]. Additionally, Busso et al. [41] observed that leptin-deficient mice (ob/ob) developed less severe arthritis than control mice. The production of IL-1β and TNF-α mRNA in the synovium of ob/ob arthritic knees was less than in +/? mice. They also showed that B lymphocytes induced leptin receptor mRNA, which indicated that leptin may have a direct influence on B cell function; therefore, leptin could involve the pathopoiesia of antigen-induced arthritis via humoral and cell-mediated immune responses. These findings showed that leptin might play a positively protective role against the destructive course of arthritis. However, Hui et al. [110] found that leptin coming from joint white adipose tissue could trigger cartilage degradation by promoting and activating the matrix metalloproteinases. Leptin served as a proinflammatory adipokine with a catabolic role on cartilage metabolism by raising proteolytic enzymes and working synergistically with other proinflammatory stimuli. Conde et al. [111] argued that leptin may induce vascular cell adhesion molecule 1 (VCAM-1) production in human and murine chondrocytes, suggesting that leptin could keep cartilage-degrading processes through the induction of factors responsible for leucocyte and monocyte permeation at inflamed joints; thus, leptin tends to a prodegenerative function in cartilage and may be of importance between obesity and cartilage metabolism. Furthermore, more studies are needed to detail the pathopoiesia of leptin in joint destruction in RA.
Genetic studies concerning leptin in RA
Although previously there have been many researches concerning leptin/lepR gene polymorphism [112–115], they have been relatively sparse in relation to RA. Kimber et al. [116] studied four single nucleotide polymorphisms (SNPs) (A409E, R612H, W664R and H684P) in the extracellular domain of LepR in order to understand more clearly their consequences on LepR signalling. In-vitro STAT-3 assays showed that three mutant LepRs have not been able to respond following leptin binding (A409E, W664R, H684P) but R612H exerted significantly reduced activity. This reduced activity may reduce levels of mutant LepR production on the cell surface in view of W664R, H684P and R612H [84], which may provide an explanation for the association between RA and lepR. In a study of the Ningxia Hui population in China, lepR SNP A668G exerted an association with susceptibility to knee osteoarthritis, meaning that the SNP may be regarded as one of the candidate genes to predict the risk of knee osteoarthritis [117], but whether lepR SNP A668G affects susceptibility to RA still needs more research. Yiannakouris et al. [118] and Koh et al. [119] found that LepR A668G related to plasma levels of leptin, as mentioned previously. This difference suggested that LepR gene SNPs may be susceptible to genetic background and environmental factors in various ethnic populations. Garcia-Bermudez et al. [120] argued that the leptin rs2167270 (G > A) polymorphism was not found to be involved with susceptibility to RA, or the risk of cardiovascular disease. Skibola et al. [121] found that there existed genetic interactions between the G2548A and LepR Q223R polymorphisms. Among the population with LepR 223RR, the risk for non-Hodgkin lymphoma (NHL) may be elevated in leptin 2548GA and leptin 2548AA relative to leptin 2548GG genotypes, showing that gene–gene interactions between leptin and its receptor may bring about immune dysfunction involved with obesity and NHL. However, in obese patients, serum leptin levels were related inversely to bone mineral density (BMD) and male sex, but positively with the existence of the leptin −2548A allele [122]. More data are needed to explore the mechanism of leptin polymorphism in RA.
It is noted that gene-expression changes may not make a difference in function. Leptin levels do not necessary reflect their effect. Further, the impact of adipokines such as leptin also relies upon the physiological context, and thus their effects can differ in normal health compared to diseased states.
Effect of traditional disease-modifying anti-rheumatic drugs (DMARDs) or biological agents on serum leptin in RA
Recently, studies have been conducted focusing on both DMARDs or biological agents and leptin in RA. A previous study suggested that circulating levels of leptin in RA patients were not affected by treatment with biologicals such as adalimumab [38]. Klaasen and colleagues observed that leptin serum levels displayed no significant changes after 16 weeks of therapy for RA with adalimumab. Additionally, leptin levels were not affected by long-term glucocorticoid (GC) treatment [123]. Long-term treatment with anti-TNF-α is potentially effective in ameliorating body mass decrease in RA patients. No significant alterations in plasma leptin were observed during etanercept therapy. Leptin may have an important role in maintaining energy homeostasis in the anti-inflammatory responses during RA remission [10]. Moreover, treatment for RA with infliximab could cause an increase in plasma leptin levels [20,27]. It has been reported that TNF-α induces the production of preformed leptin from human mature adipocytes and existing differentiated pre-adipocytes [124]. However, in another study, infliximab treatment did not change plasma levels of leptin after 6 months of treatment [125], similar to a previous study [126]. However, some caution should be noted with regard to these differences. First, these study samples were relatively small, thus potential differences might be unable to be found. Secondly, different baseline levels may result in these phenomena. Thirdly, leptin levels were estimated at various time-points in these studies after the start of treatment. Given the effects of different therapeutic interventions on leptin, it is too early to draw conclusions.
Effect of leptin on cardiovascular risk in RA
Many reports indicated that RA patients were at greater risk of developing coronary heart disease [127]. A study by Derdemezis et al. [125] found that anti-TNF treatment effects on cardiovascular disease (CVD) risk were not regulated by changes in leptin levels. Dessein et al. also argued that there was no significant relationship between leptin levels and cardiovascular risk in all patients and either female and male or black and white patients with RA. However, several leptin–cardiovascular risk relations were affected by age, suggesting that younger but not older RA patients may retain obesity-induced endothelial activation that was modulated directly by leptin [128]. Moreover, the leptin : adiponectin (L : A) ratio is concerned with the common carotid artery resistive index, and may be an marker for predicting cardiovascular risk in RA patients [129]. However, the association between leptin and cardiovascular risk in RA is complex; larger studies need to be conducted [130].
Leptin antagonism as a therapeutic target for RA
As a key mediator against infection, immune responses, and even the induction of autoimmune diseases, leptin needs more research to identify whether leptin can become the pathogenesis of targeting autoimmune diseases in humans. Reduction of leptin levels in RA patients by fasting improves the clinical symptoms of autoimmune disease [131],, thus leptin antagonism has been proposed for the prevention of developing RA in people genetically susceptible to RA and other autoimmune diseases. A number of diverse approaches can be designed for antagonists. However, blocking the key signal pathways such as JAK/STAT may cause harmful effects. Recently, the progression of leptin mutants with antagonism and proteins inhibiting leptin activity offers new hope for possible treatment [132]. Many leptin mutants may be regarded as potent leptin antagonists both in vitro and in vivo [133]. A S120A/T121A binding site III leptin mutant may become a competitive inhibitor of leptin receptor signalling due to its linkage to the receptor but not its stimulation [133]. Nevertheless, PEGylation (polyethylene glycol) of this mutant is implied, which could promote the circulation lifetime of the S120A/T121A leptin antagonist, because leptin has a short half-life in circulation [134]. Additionally, the monoclonal antibody (mAb) against the human leptin receptor could provide a useful strategy, because of being a tissue-specific leptin antagonist. The antibody-blocking peripheral immune actions of leptin and leptin-induced production of TNF-α by human monocytes and T cell production suppresses leptin proinflammatory activity [135]. Leptin may be partly responsible for low-level systemic inflammation. Targeting leptin may be regarded as a therapeutic method in some clinical conditions, such as proinflammatory status or autoimmune diseases, which can control an excess of immune response. In vitro, neutralization using leptin mAb, when stimulating anti-CD3 and anti-CD28, leads to Treg cell proliferation [136]. In leptin- and ObR-deficient mice, Matarese et al. [137] showed an enhanced proliferation of Treg cells, indicating the possibility for treatment of immune and autoimmune diseases. Leptin receptors were also identified on mast cells, which could provide new insights in several therapeutic diseases. Recently, some molecules such as mAb have been reported to run as leptin antagonists and block leptin signalling [135]. Perhaps leptin antagonists could be a tool to control many inflammatory processes in which mast cells are present.
In addition, a surprising role of leptin has been detailed in the regulation of bone formation [138,139]. The primary osteoblasts themselves can secrete leptin. In vitro, leptin can induce mineralization [139] and mediate stromal cells to differentiate into osteoblasts [140]. Glucocorticoids are effectively anti-inflammatory molecules useful for the treatment of RA, bowel disease and other inflammatory diseases. Glucocorticoids exert regulation of rat leptin production in vitro [141], and McLaughlin et al. [142] has found an evident rise in serum leptin by dexamethasone therapy, which may provide an idea for RA treatment. However, leptin antagonism could make individuals obese; glucocorticoid therapy has been applied for many years in the treatment of arthritis, but is limited due to side effects, including osteoporosis, diabetes and weight gain. Furthermore, as obese individuals have very high levels of leptin, if leptin had the effects proposed, autoimmune disease should be more common in severe obesity, but no evidence has accounted for this phenomenon.
Conclusion
In summary, RA is a multi-system disorder with joint inflammation, which causes patients chronic pain, disability and increased mortality. Prevailing studies suggest that leptin is of great importance in the pathogenesis and treatment of RA through in-vitro and in-vivo models. More studies are needed to elucidate the mechanism of leptin in RA, although numerous data from a variety of models support the key role of leptin in immunity and autoimmune diseases. Leptin also has direct effects on both innate and adaptive immunity. Sparse clinical findings have been reported for therapeutic RA by induction of possible leptin signalling on molecule and gene levels. Blocking the key signal pathways of leptin and inhibiting leptin activity, such as with leptin antagonists, may be a promising way for the therapeutic potential of RA at risk of detrimental effects. In particular, it has a dual role with respect to its anti-inflammatory and proinflammatory response. However, leptin is increased in patients with RA and may also regulate joint damage [30]. Hence, further understanding of the mechanism of leptin would be advantageous in the future in RA treatment.
Disclosures
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.MacDougald OA, Hwang CS, Fan H, Lane MD. Regulated expression of the obese gene product (leptin) in white adipose tissue and 3T3-L1 adipocytes. Proc Natl Acad Sci USA. 1995;92:9034–9037. doi: 10.1073/pnas.92.20.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang F, Basinski MB, Beals JM, et al. Crystal structure of the obese protein leptin-E100. Nature. 1997;387:206–209. doi: 10.1038/387206a0. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Gonzalez A, Gonzalez-Lopez L, Valera-Gonzalez IC, et al. Serum leptin levels in women with systemic lupus erythematosus. Rheumatol Int. 2002;22:138–141. doi: 10.1007/s00296-002-0216-9. [DOI] [PubMed] [Google Scholar]
- 4.Xu WD, Zhang M, Zhang YJ, Liu SS, Pan HF, Ye DQ. Association between leptin and systemic lupus erythematosus. Rheumatol Int. 2014;34:559–563. doi: 10.1007/s00296-013-2774-4. [DOI] [PubMed] [Google Scholar]
- 5.Wislowska M, Rok M, Stepien K, Kuklo-Kowalska A. Serum leptin in systemic lupus erythematosus. Rheumatol Int. 2008;28:467–473. doi: 10.1007/s00296-008-0526-7. [DOI] [PubMed] [Google Scholar]
- 6.Vadacca M, Zardi EM, Margiotta D, et al. Leptin, adiponectin and vascular stiffness parameters in women with systemic lupus erythematosus. Intern Emerg Med. 2013;8:705–712. doi: 10.1007/s11739-011-0726-0. [DOI] [PubMed] [Google Scholar]
- 7.Bokarewa M, Bokarew D, Hultgren O, Tarkowski A. Leptin consumption in the inflamed joints of patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:952–956. doi: 10.1136/ard.62.10.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elwakkad AS, Said RN, Muhammad SI, Saleh MT, Elhamshary A. Role for leptin and prolactin in human juvenile rheumatic diseases. Pak J Biol Sci. 2007;10:1984–1989. doi: 10.3923/pjbs.2007.1984.1989. [DOI] [PubMed] [Google Scholar]
- 9.Manavathongchai S, Bian A, Rho YH, et al. Inflammation and hypertension in rheumatoid arthritis. J Rheumatol. 2013;40:1806–1811. doi: 10.3899/jrheum.130394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CY, Tsai CY, Lee PC, Lee SD. Long-term etanercept therapy favors weight gain and ameliorates cachexia in rheumatoid arthritis patients: roles of gut hormones and leptin. Curr Pharm Des. 2013;19:1956–1964. doi: 10.2174/1381612811319100014. [DOI] [PubMed] [Google Scholar]
- 11.Matarese G, Carrieri PB, Montella S, De Rosa V, La Cava A. Leptin as a metabolic link to multiple sclerosis. Nat Rev Neurol. 2010;6:455–461. doi: 10.1038/nrneurol.2010.89. [DOI] [PubMed] [Google Scholar]
- 12.Rotondi M, Batocchi AP, Coperchini F, et al. Severe disability in patients with relapsing–remitting multiple sclerosis is associated with profound changes in the regulation of leptin secretion. Neuroimmunomodulation. 2013;20:341–347. doi: 10.1159/000353567. [DOI] [PubMed] [Google Scholar]
- 13.Rey LK, Wieczorek S, Akkad DA, Linker RA, Chan A, Hoffjan S. Polymorphisms in genes encoding leptin, ghrelin and their receptors in German multiple sclerosis patients. Mol Cell Probes. 2011;25:255–259. doi: 10.1016/j.mcp.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Kapoor S. Comorbidities associated with leptin and psoriasis. J Drugs Dermatol. 2013;12:515. [PubMed] [Google Scholar]
- 15.Zhu KJ, Zhang C, Li M, Zhu CY, Shi G, Fan YM. Leptin levels in patients with psoriasis: a meta-analysis. Clin Exp Dermatol. 2013;38:478–483. doi: 10.1111/ced.12171. [DOI] [PubMed] [Google Scholar]
- 16.Aly DG, Abdallah IY, Hanafy NS, Elsaie ML, Hafiz NA. Elevated serum leptin levels in nonobese patients with psoriasis. J Drugs Dermatol. 2013;12:e25–29. [PubMed] [Google Scholar]
- 17.Park YJ, Cho CS, Emery P, Kim WU. LDL cholesterolemia as a novel risk factor for radiographic progression of rheumatoid arthritis: a single-center prospective study. PLOS ONE. 2013;8:e68975. doi: 10.1371/journal.pone.0068975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muraoka S, Kusunoki N, Takahashi H, Tsuchiya K, Kawai S. Leptin stimulates interleukin-6 production via janus kinase 2/signal transducer and activator of transcription 3 in rheumatoid synovial fibroblasts. Clin Exp Rheumatol. 2013;31:589–595. [PubMed] [Google Scholar]
- 19.Hayashi H, Satoi K, Sato-Mito N, et al. Nutritional status in relation to adipokines and oxidative stress is associated with disease activity in patients with rheumatoid arthritis. Nutrition. 2012;28:1109–1114. doi: 10.1016/j.nut.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Kopec-Medrek M, Kotulska A, Widuchowska M, Adamczak M, Wiecek A, Kucharz EJ. Plasma leptin and neuropeptide Y concentrations in patients with rheumatoid arthritis treated with infliximab, a TNF-alpha antagonist. Rheumatol Int. 2012;32:3383–3389. doi: 10.1007/s00296-011-2182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamaguchi K, Itabashi A, Kuroe Y, et al. Analysis of adipose tissues and stromal vascular cells in a murine arthritis model. Metabolism. 2012;61:1687–1695. doi: 10.1016/j.metabol.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Sugioka Y, Tada M, Okano T, Nakamura H, Koike T. Acquired leptin resistance by high-fat feeding reduces inflammation from collagen antibody-induced arthritis in mice. Clin Exp Rheumatol. 2012;30:707–713. [PubMed] [Google Scholar]
- 23.Olama SM, Senna MK, Elarman M. Synovial/serum leptin ratio in rheumatoid arthritis: the association with activity and erosion. Rheumatol Int. 2012;32:683–690. doi: 10.1007/s00296-010-1698-5. [DOI] [PubMed] [Google Scholar]
- 24.Kontny E, Plebanczyk M, Lisowska B, Olszewska M, Maldyk P, Maslinski W. Comparison of rheumatoid articular adipose and synovial tissue reactivity to proinflammatory stimuli: contribution to adipocytokine network. Ann Rheum Dis. 2012;71:262–267. doi: 10.1136/annrheumdis-2011-200123. [DOI] [PubMed] [Google Scholar]
- 25.Ferraz-Amaro I, Arce-Franco M, Muniz J, et al. Systemic blockade of TNF-alpha does not improve insulin resistance in humans. Horm Metab Res. 2011;43:801–808. doi: 10.1055/s-0031-1287783. [DOI] [PubMed] [Google Scholar]
- 26.Yoshino T, Kusunoki N, Tanaka N, et al. Elevated serum levels of resistin, leptin, and adiponectin are associated with C-reactive protein and also other clinical conditions in rheumatoid arthritis. Intern Med. 2011;50:269–275. doi: 10.2169/internalmedicine.50.4306. [DOI] [PubMed] [Google Scholar]
- 27.Engvall IL, Tengstrand B, Brismar K, Hafstrom I. Infliximab therapy increases body fat mass in early rheumatoid arthritis independently of changes in disease activity and levels of leptin and adiponectin: a randomised study over 21 months. Arthritis Res Ther. 2010;12:R197. doi: 10.1186/ar3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xibille-Friedmann D, Bustos-Bahena C, Hernandez-Gongora S, Burgos-Vargas R, Montiel-Hernandez JL. Two-year follow-up of plasma leptin and other cytokines in patients with rheumatoid arthritis. Ann Rheum Dis. 2010;69:930–931. doi: 10.1136/ard.2009.111732. [DOI] [PubMed] [Google Scholar]
- 29.Targonska-Stepniak B, Dryglewska M, Majdan M. Adiponectin and leptin serum concentrations in patients with rheumatoid arthritis. Rheumatol Int. 2010;30:731–737. doi: 10.1007/s00296-009-1053-x. [DOI] [PubMed] [Google Scholar]
- 30.Rho YH, Solus J, Sokka T, et al. Adipocytokines are associated with radiographic joint damage in rheumatoid arthritis. Arthritis Rheum. 2009;60:1906–1914. doi: 10.1002/art.24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seven A, Guzel S, Aslan M, Hamuryudan V. Serum and synovial fluid leptin levels and markers of inflammation in rheumatoid arthritis. Rheumatol Int. 2009;29:743–747. doi: 10.1007/s00296-008-0764-8. [DOI] [PubMed] [Google Scholar]
- 32.Martin AI, Castillero E, Granado M, Lopez-Menduina M, Villanua MA, Lopez-Calderon A. Adipose tissue loss in adjuvant arthritis is associated with a decrease in lipogenesis, but not with an increase in lipolysis. J Endocrinol. 2008;197:111–119. doi: 10.1677/JOE-07-0491. [DOI] [PubMed] [Google Scholar]
- 33.Hizmetli S, Kisa M, Gokalp N, Bakici MZ. Are plasma and synovial fluid leptin levels correlated with disease activity in rheumatoid arthritis. Rheumatol Int. 2007;27:335–338. doi: 10.1007/s00296-006-0264-7. [DOI] [PubMed] [Google Scholar]
- 34.Hayward MD, Jones BK, Saparov A, et al. An extensive phenotypic characterization of the hTNFalpha transgenic mice. BMC Physiol. 2007;7:13. doi: 10.1186/1472-6793-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caetano-Lopes J, Canhao H, Fonseca JE. Osteoblasts and bone formation. Acta Reumatol Port. 2007;32:103–110. [PubMed] [Google Scholar]
- 36.Otero M, Lago R, Gomez R, et al. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1198–1201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gunaydin R, Kaya T, Atay A, Olmez N, Hur A, Koseoglu M. Serum leptin levels in rheumatoid arthritis and relationship with disease activity. South Med J. 2006;99:1078–1083. doi: 10.1097/01.smj.0000240625.27772.79. [DOI] [PubMed] [Google Scholar]
- 38.Harle P, Sarzi-Puttini P, Cutolo M, Straub RH. No change of serum levels of leptin and adiponectin during anti-tumour necrosis factor antibody treatment with adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:970–971. doi: 10.1136/ard.2005.040857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popa C, Netea MG, Radstake TR, van Riel PL, Barrera P, van der Meer JW. Markers of inflammation are negatively correlated with serum leptin in rheumatoid arthritis. Ann Rheum Dis. 2005;64:1195–1198. doi: 10.1136/ard.2004.032243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anders HJ, Rihl M, Heufelder A, Loch O, Schattenkirchner M. Leptin serum levels are not correlated with disease activity in patients with rheumatoid arthritis. Metabolism. 1999;48:745–748. doi: 10.1016/s0026-0495(99)90174-9. [DOI] [PubMed] [Google Scholar]
- 41.Busso N, So A, Chobaz-Peclat V, et al. Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol. 2002;168:875–882. doi: 10.4049/jimmunol.168.2.875. [DOI] [PubMed] [Google Scholar]
- 42.Madiehe AM, Mitchell TD, Harris RB. Hyperleptinemia and reduced TNF-alpha secretion cause resistance of db/db mice to endotoxin. Am J Physiol Regul Integr Comp Physiol. 2003;284:R763–770. doi: 10.1152/ajpregu.00610.2002. [DOI] [PubMed] [Google Scholar]
- 43.Loffreda S, Yang SQ, Lin HZ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 44.Vadacca M, Margiotta DP, Navarini L, Afeltra A. Leptin in immuno-rheumatological diseases. Cell Mol Immunol. 2011;8:203–212. doi: 10.1038/cmi.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zarkesh-Esfahani H, Pockley G, Metcalfe RA, et al. High-dose leptin activates human leukocytes via receptor expression on monocytes. J Immunol. 2001;167:4593–4599. doi: 10.4049/jimmunol.167.8.4593. [DOI] [PubMed] [Google Scholar]
- 46.Gruen ML, Hao M, Piston DW, Hasty AH. Leptin requires canonical migratory signaling pathways for induction of monocyte and macrophage chemotaxis. Am J Physiol Cell Physiol. 2007;293:C1481–1488. doi: 10.1152/ajpcell.00062.2007. [DOI] [PubMed] [Google Scholar]
- 47.Montecucco F, Bianchi G, Gnerre P, Bertolotto M, Dallegri F, Ottonello L. Induction of neutrophil chemotaxis by leptin: crucial role for p38 and Src kinases. Ann NY Acad Sci. 2006;1069:463–471. doi: 10.1196/annals.1351.045. [DOI] [PubMed] [Google Scholar]
- 48.Caldefie-Chezet F, Poulin A, Vasson MP. Leptin regulates functional capacities of polymorphonuclear neutrophils. Free Radic Res. 2003;37:809–814. doi: 10.1080/1071576031000097526. [DOI] [PubMed] [Google Scholar]
- 49.Zhao Y, Sun R, You L, Gao C, Tian Z. Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem Biophys Res Commun. 2003;300:247–252. doi: 10.1016/s0006-291x(02)02838-3. [DOI] [PubMed] [Google Scholar]
- 50.Wong CK, Cheung PF, Lam CW. Leptin-mediated cytokine release and migration of eosinophils: implications for immunopathophysiology of allergic inflammation. Eur J Immunol. 2007;37:2337–2348. doi: 10.1002/eji.200636866. [DOI] [PubMed] [Google Scholar]
- 51.Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005;174:6820–6828. doi: 10.4049/jimmunol.174.11.6820. [DOI] [PubMed] [Google Scholar]
- 52.Lam QL, Zheng BJ, Jin DY, Cao X, Lu L. Leptin induces CD40 expression through the activation of Akt in murine dendritic cells. J Biol Chem. 2007;282:27587–27597. doi: 10.1074/jbc.M704579200. [DOI] [PubMed] [Google Scholar]
- 53.Al-Hassi HO, Bernardo D, Murugananthan AU, et al. A mechanistic role for leptin in human dendritic cell migration: differences between ileum and colon in health and Crohn's disease. Mucosal Immunol. 2013;6:751–761. doi: 10.1038/mi.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taildeman J, Perez-Novo CA, Rottiers I, et al. Human mast cells express leptin and leptin receptors. Histochem Cell Biol. 2009;131:703–711. doi: 10.1007/s00418-009-0575-3. [DOI] [PubMed] [Google Scholar]
- 55.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 56.Lam QL, Lu L. Role of leptin in immunity. Cell Mol Immunol. 2007;4:1–13. [PubMed] [Google Scholar]
- 57.Siegmund B, Lehr HA, Fantuzzi G. Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology. 2002;122:2011–2025. doi: 10.1053/gast.2002.33631. [DOI] [PubMed] [Google Scholar]
- 58.Gomez R, Conde J, Scotece M, Gomez-Reino JJ, Lago F, Gualillo O. What's new in our understanding of the role of adipokines in rheumatic diseases? Nat Rev Rheumatol. 2011;7:528–536. doi: 10.1038/nrrheum.2011.107. [DOI] [PubMed] [Google Scholar]
- 59.De Rosa V, Procaccini C, Cali G, et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 60.Agrawal S, Gollapudi S, Su H, Gupta S. Leptin activates human B cells to secrete TNF-alpha, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J Clin Immunol. 2011;31:472–478. doi: 10.1007/s10875-010-9507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol. 2003;24:225–253. doi: 10.1016/j.yfrne.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 62.Hegyi K, Fulop K, Kovacs K, Toth S, Falus A. Leptin-induced signal transduction pathways. Cell Biol Int. 2004;28:159–169. doi: 10.1016/j.cellbi.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 63.Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- 64.White DW, Kuropatwinski KK, Devos R, Baumann H, Tartaglia LA. Leptin receptor (OB-R) signaling. Cytoplasmic domain mutational analysis and evidence for receptor homo-oligomerization. J Biol Chem. 1997;272:4065–4071. doi: 10.1074/jbc.272.7.4065. [DOI] [PubMed] [Google Scholar]
- 65.Soebiyanto RP, Sreenath SN, Qu CK, Loparo KA, Bunting KD. Complex systems biology approach to understanding coordination of JAK-STAT signaling. Biosystems. 2007;90:830–842. doi: 10.1016/j.biosystems.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 67.Terrell AM, Crisostomo PR, Wairiuko GM, Wang M, Morrell ED, Meldrum DR. Jak/STAT/SOCS signaling circuits and associated cytokine-mediated inflammation and hypertrophy in the heart. Shock. 2006;26:226–234. doi: 10.1097/01.shk.0000226341.32786.b9. [DOI] [PubMed] [Google Scholar]
- 68.Mutze J, Roth J, Gerstberger R, Hubschle T. Nuclear translocation of the transcription factor STAT5 in the rat brain after systemic leptin administration. Neurosci Lett. 2007;417:286–291. doi: 10.1016/j.neulet.2007.02.074. [DOI] [PubMed] [Google Scholar]
- 69.Gong Y, Ishida-Takahashi R, Villanueva EC, Fingar DC, Munzberg H, Myers MG., Jr The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem. 2007;282:31019–31027. doi: 10.1074/jbc.M702838200. [DOI] [PubMed] [Google Scholar]
- 70.van den Brink GR, O'Toole T, Hardwick JC, et al. Leptin signaling in human peripheral blood mononuclear cells, activation of p38 and p42/44 mitogen-activated protein (MAP) kinase and p70 S6 kinase. Mol Cell Biol Res Commun. 2000;4:144–150. doi: 10.1006/mcbr.2001.0270. [DOI] [PubMed] [Google Scholar]
- 71.Bjorbaek C, Buchholz RM, Davis SM, et al. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276:4747–4755. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 72.Li C, Friedman JM. Leptin receptor activation of SH2 domain containing protein tyrosine phosphatase 2 modulates Ob receptor signal transduction. Proc Natl Acad Sci USA. 1999;96:9677–9682. doi: 10.1073/pnas.96.17.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bjorbak C, Lavery HJ, Bates SH, et al. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem. 2000;275:40649–40657. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- 74.Mansour E, Pereira FG, Araujo EP, et al. Leptin inhibits apoptosis in thymus through a Janus kinase-2-independent, insulin receptor substrate-1/phosphatidylinositol-3 kinase-dependent pathway. Endocrinology. 2006;147:5470–5479. doi: 10.1210/en.2006-0223. [DOI] [PubMed] [Google Scholar]
- 75.Kitamura T, Feng Y, Kitamura YI, et al. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- 76.Elbatarny HS, Maurice DH. Leptin-mediated activation of human platelets: involvement of a leptin receptor and phosphodiesterase 3A-containing cellular signaling complex. Am J Physiol Endocrinol Metab. 2005;289:E695–702. doi: 10.1152/ajpendo.00125.2005. [DOI] [PubMed] [Google Scholar]
- 77.Sanchez-Margalet V, Martin-Romero C, Santos-Alvarez J, Goberna R, Najib S, Gonzalez-Yanes C. Role of leptin as an immunomodulator of blood mononuclear cells: mechanisms of action. Clin Exp Immunol. 2003;133:11–19. doi: 10.1046/j.1365-2249.2003.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lam QL, Liu S, Cao X, Lu L. Involvement of leptin signaling in the survival and maturation of bone marrow-derived dendritic cells. Eur J Immunol. 2006;36:3118–3130. doi: 10.1002/eji.200636602. [DOI] [PubMed] [Google Scholar]
- 79.Su H, Jiang L, Carter-Su C, Rui L. Glucose enhances leptin signaling through modulation of AMPK activity. PLOS ONE. 2012;7:e31636. doi: 10.1371/journal.pone.0031636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Claret M, Smith MA, Batterham RL, et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1247–1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dempsey EC, Newton AC, Mochly-Rosen D, et al. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol. 2000;279:L429–438. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- 83.Attoub S, Noe V, Pirola L, et al. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, rho-, and rac-dependent signaling pathways. FASEB J. 2000;14:2329–2338. doi: 10.1096/fj.00-0162. [DOI] [PubMed] [Google Scholar]
- 84.Mackey-Lawrence NM, Petri WA., Jr Leptin and mucosal immunity. Mucosal Immunol. 2012;5:472–479. doi: 10.1038/mi.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Faggioni R, Feingold KR, Grunfeld C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J. 2001;15:2565–2571. doi: 10.1096/fj.01-0431rev. [DOI] [PubMed] [Google Scholar]
- 86.Wrann CD, Laue T, Hubner L, et al. Short-term and long-term leptin exposure differentially affect human natural killer cell immune functions. Am J Physiol Endocrinol Metab. 2012;302:E108–116. doi: 10.1152/ajpendo.00057.2011. [DOI] [PubMed] [Google Scholar]
- 87.Malendowicz LK, Gorska T, Tortorella C, et al. Acute in vivo effects of leptin and leptin fragments on corticosteroid hormone secretion and entero-insular axis in the rat. Int J Mol Med. 2004;13:829–834. [PubMed] [Google Scholar]
- 88.Smith JT, Waddell BJ. Leptin receptor expression in the rat placenta: changes in ob-ra, ob-rb, and ob-re with gestational age and suppression by glucocorticoids. Biol Reprod. 2002;67:1204–1210. doi: 10.1095/biolreprod67.4.1204. [DOI] [PubMed] [Google Scholar]
- 89.Barr VA, Lane K, Taylor SI. Subcellular localization and internalization of the four human leptin receptor isoforms. J Biol Chem. 1999;274:21416–21424. doi: 10.1074/jbc.274.30.21416. [DOI] [PubMed] [Google Scholar]
- 90.Sweeney G. Leptin signalling. Cell Signal. 2002;14:655–663. doi: 10.1016/s0898-6568(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 91.Otvos L, Jr, Shao WH, Vanniasinghe AS, et al. Toward understanding the role of leptin and leptin receptor antagonism in preclinical models of rheumatoid arthritis. Peptides. 2011;32:1567–1574. doi: 10.1016/j.peptides.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 92.Roth B, Manjer J, Ohlsson B. Microscopic colitis is associated with several concomitant diseases. Drug Target Insights. 2013;7:19–25. doi: 10.4137/DTI.S12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mohammad-Shahi M, Haidari F, Rashidi B, Saei AA, Mahboob S, Rashidi MR. Comparison of the effects of genistein and daidzein with dexamethasone and soy protein on rheumatoid arthritis in rats. BioImpacts. 2011;1:161–170. doi: 10.5681/bi.2011.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sandell LJ. Obesity and osteoarthritis: is leptin the link? Arthritis Rheum. 2009;60:2858–2860. doi: 10.1002/art.24862. [DOI] [PubMed] [Google Scholar]
- 95.Targonska-Stepniak B, Majdan M, Dryglewska M. Leptin serum levels in rheumatoid arthritis patients: relation to disease duration and activity. Rheumatol Int. 2008;28:585–591. doi: 10.1007/s00296-007-0480-9. [DOI] [PubMed] [Google Scholar]
- 96.Hultgren OH, Tarkowski A. Leptin in septic arthritis: decreased levels during infection and amelioration of disease activity upon its administration. Arthritis Res. 2001;3:389–394. doi: 10.1186/ar332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Senolt L, Housa D, Vernerova Z, et al. Resistin in rheumatoid arthritis synovial tissue, synovial fluid and serum. Ann Rheum Dis. 2007;66:458–463. doi: 10.1136/ard.2006.054734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gabay C, Dreyer M, Pellegrinelli N, Chicheportiche R, Meier CA. Leptin directly induces the secretion of interleukin 1 receptor antagonist in human monocytes. J Clin Endocrinol Metab. 2001;86:783–791. doi: 10.1210/jcem.86.2.7245. [DOI] [PubMed] [Google Scholar]
- 99.Cohen S, Hurd E, Cush J, et al. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:614–624. doi: 10.1002/art.10141. [DOI] [PubMed] [Google Scholar]
- 100.Toussirot E, Nguyen NU, Dumoulin G, Aubin F, Cedoz JP, Wendling D. Relationship between growth hormone–IGF-I–IGFBP-3 axis and serum leptin levels with bone mass and body composition in patients with rheumatoid arthritis. Rheumatology. 2005;44:120–125. doi: 10.1093/rheumatology/keh421. [DOI] [PubMed] [Google Scholar]
- 101.Otero M, Lago R, Lago F, Reino JJ, Gualillo O. Signalling pathway involved in nitric oxide synthase type II activation in chondrocytes: synergistic effect of leptin with interleukin-1. Arthritis Res Ther. 2005;7:R581–591. doi: 10.1186/ar1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Otero M, Gomez Reino JJ, Gualillo O. Synergistic induction of nitric oxide synthase type II: in vitro effect of leptin and interferon-gamma in human chondrocytes and ATDC5 chondrogenic cells. Arthritis Rheum. 2003;48:404–409. doi: 10.1002/art.10811. [DOI] [PubMed] [Google Scholar]
- 103.Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Figenschau Y, Knutsen G, Shahazeydi S, Johansen O, Sveinbjornsson B. Human articular chondrocytes express functional leptin receptors. Biochem Biophys Res Commun. 2001;287:190–197. doi: 10.1006/bbrc.2001.5543. [DOI] [PubMed] [Google Scholar]
- 105.Giles JT, van der Heijde DM, Bathon JM. Association of circulating adiponectin levels with progression of radiographic joint destruction in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1562–1568. doi: 10.1136/ard.2011.150813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Giles JT, Allison M, Bingham CO, 3rd, Scott WM, Jr, Bathon JM. Adiponectin is a mediator of the inverse association of adiposity with radiographic damage in rheumatoid arthritis. Arthritis Rheum. 2009;61:1248–1256. doi: 10.1002/art.24789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Meyer M, Sellam J, Fellahi S, et al. Serum level of adiponectin is a surrogate independent biomarker of radiographic disease progression in early rheumatoid arthritis: results from the ESPOIR cohort. Arthritis Res Ther. 2013;15:R210. doi: 10.1186/ar4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Otero M, Lago R, Lago F, et al. Leptin, from fat to inflammation: old questions and new insights. FEBS Lett. 2005;579:295–301. doi: 10.1016/j.febslet.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 109.Dumond H, Presle N, Terlain B, et al. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 2003;48:3118–3129. doi: 10.1002/art.11303. [DOI] [PubMed] [Google Scholar]
- 110.Hui W, Litherland GJ, Elias MS, et al. Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Ann Rheum Dis. 2012;71:455–462. doi: 10.1136/annrheumdis-2011-200372. [DOI] [PubMed] [Google Scholar]
- 111.Conde J, Scotece M, Lopez V, et al. Adiponectin and leptin induce VCAM-1 expression in human and murine chondrocytes. PLOS ONE. 2012;7:e52533. doi: 10.1371/journal.pone.0052533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Becer E, Mehmetcik G, Bareke H, Serakinci N. Association of leptin receptor gene Q223R polymorphism on lipid profiles in comparison study between obese and non-obese subjects. Gene. 2013;529:16–20. doi: 10.1016/j.gene.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 113.Parveen F, Agrawal S. A study of forty-seven single nucleotide polymorphisms among recurrent miscarriage using classification and regression tree analysis. Am J Reprod Immunol. 2013;70:529–537. doi: 10.1111/aji.12152. [DOI] [PubMed] [Google Scholar]
- 114.Lee HJ, Kim H, Ku SY, Choi YM, Kim JH, Kim JG. Association between polymorphisms in leptin, leptin receptor, and beta-adrenergic receptor genes and bone mineral density in postmenopausal Korean women. Menopause. 2014;21:67–73. doi: 10.1097/GME.0b013e31829366ed. [DOI] [PubMed] [Google Scholar]
- 115.Fourati M, Mnif M, Kharrat N, et al. Association between Leptin gene polymorphisms and plasma leptin level in three consanguineous families with obesity. Gene. 2013;527:75–81. doi: 10.1016/j.gene.2013.05.064. [DOI] [PubMed] [Google Scholar]
- 116.Kimber W, Peelman F, Prieur X, et al. Functional characterization of naturally occurring pathogenic mutations in the human leptin receptor. Endocrinology. 2008;149:6043–6052. doi: 10.1210/en.2008-0544. [DOI] [PubMed] [Google Scholar]
- 117.Ma XJ, Guo HH, Hao SW, et al. Association of single nucleotide polymorphisms (SNPs) in leptin receptor gene with knee osteoarthritis in the Ningxia Hui population. Yi chuan. 2013;35:359–364. doi: 10.3724/sp.j.1005.2013.00359. [DOI] [PubMed] [Google Scholar]
- 118.Yiannakouris N, Yannakoulia M, Melistas L, Chan JL, Klimis-Zacas D, Mantzoros CS. The Q223R polymorphism of the leptin receptor gene is significantly associated with obesity and predicts a small percentage of body weight and body composition variability. J Clin Endocrinol Metab. 2001;86:4434–4439. doi: 10.1210/jcem.86.9.7842. [DOI] [PubMed] [Google Scholar]
- 119.Koh JM, Kim DJ, Hong JS, et al. Estrogen receptor alpha gene polymorphisms Pvu II and Xba I influence association between leptin receptor gene polymorphism (Gln223Arg) and bone mineral density in young men. Eur J Endocrinol. 2002;147:777–783. doi: 10.1530/eje.0.1470777. [DOI] [PubMed] [Google Scholar]
- 120.Garcia-Bermudez M, Gonzalez-Juanatey C, Rodriguez-Rodriguez L, et al. Lack of association between LEP rs2167270 (19 G>A) polymorphism and disease susceptibility and cardiovascular disease in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2011;29:293–298. [PubMed] [Google Scholar]
- 121.Skibola CF, Holly EA, Forrest MS, et al. Body mass index, leptin and leptin receptor polymorphisms, and non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2004;13:779–786. [PubMed] [Google Scholar]
- 122.Franek E, Nowak J, Safranow K, et al. 2548)A leptin gene polymorphism in obese subjects is associated with serum leptin concentration and bone mass. Pol Arch Med Wewn. 2010;120:175–180. [PubMed] [Google Scholar]
- 123.Klaasen R, Herenius MM, Wijbrandts CA, et al. Treatment-specific changes in circulating adipocytokines: a comparison between tumour necrosis factor blockade and glucocorticoid treatment for rheumatoid arthritis. Ann Rheum Dis. 2012;71:1510–1516. doi: 10.1136/annrheumdis-2011-200646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang HH, Kumar S, Barnett AH, Eggo MC. Tumour necrosis factor-alpha exerts dual effects on human adipose leptin synthesis and release. Mol Cell Endocrinol. 2000;159:79–88. doi: 10.1016/s0303-7207(99)00194-x. [DOI] [PubMed] [Google Scholar]
- 125.Derdemezis CS, Filippatos TD, Voulgari PV, Tselepis AD, Drosos AA, Kiortsis DN. Effects of a 6-month infliximab treatment on plasma levels of leptin and adiponectin in patients with rheumatoid arthritis. Fundam Clin Pharmacol. 2009;23:595–600. doi: 10.1111/j.1472-8206.2009.00717.x. [DOI] [PubMed] [Google Scholar]
- 126.Gonzalez-Gay MA, Garcia-Unzueta MT, Berja A, et al. Anti-TNF-alpha therapy does not modulate leptin in patients with severe rheumatoid arthritis. Clin Exp Rheumatol. 2009;27:222–228. [PubMed] [Google Scholar]
- 127.Sattar N, McCarey DW, Capell H, McInnes IB. Explaining how ‘high-grade’ systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation. 2003;108:2957–2963. doi: 10.1161/01.CIR.0000099844.31524.05. [DOI] [PubMed] [Google Scholar]
- 128.Dessein PH, Norton GR, Woodiwiss AJ, Tsang L, Solomon A. Age impacts on the independent relationships of leptin with cardiometabolic risk and surrogate markers of enhanced early atherogenesis in black and white patients with rheumatoid arthritis: a cross-sectional study. Rheumatol Int. 2014;34:329–339. doi: 10.1007/s00296-013-2933-7. [DOI] [PubMed] [Google Scholar]
- 129.Kang Y, Park HJ, Kang MI, et al. Adipokines, inflammation, insulin resistance, and carotid atherosclerosis in patients with rheumatoid arthritis. Arthritis Res Ther. 2013;15:R194. doi: 10.1186/ar4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dessein PH, Solomon A. Towards the elucidation of the true impact of adipocytokines on cardiovascular risk in rheumatoid arthritis. Arthritis Res Ther. 2013;15:127. doi: 10.1186/ar4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 132.Gertler A. Development of leptin antagonists and their potential use in experimental biology and medicine. Trends Endocrinol Metab. 2006;17:372–378. doi: 10.1016/j.tem.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 133.Peelman F, Van Beneden K, Zabeau L, et al. Mapping of the leptin binding sites and design of a leptin antagonist. J Biol Chem. 2004;279:41038–41046. doi: 10.1074/jbc.M404962200. [DOI] [PubMed] [Google Scholar]
- 134.Babaei A, Zarkesh-Esfahani SH, Bahrami E, Ross RJ. Restricted leptin antagonism as a therapeutic approach to treatment of autoimmune diseases. Hormones. 2011;10:16–26. doi: 10.14310/horm.2002.1289. [DOI] [PubMed] [Google Scholar]
- 135.Fazeli M, Zarkesh-Esfahani H, Wu Z, et al. Identification of a monoclonal antibody against the leptin receptor that acts as an antagonist and blocks human monocyte and T cell activation. J Immunol Methods. 2006;312:190–200. doi: 10.1016/j.jim.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 136.Fernandez-Riejos P, Najib S, Santos-Alvarez J, et al. Role of leptin in the activation of immune cells. Mediat Inflamm. 2010;2010:568343. doi: 10.1155/2010/568343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Matarese G, De Rosa V, La Cava A. Regulatory CD4 T cells: sensing the environment. Trends Immunol. 2008;29:12–17. doi: 10.1016/j.it.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 138.Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 139.Reseland JE, Syversen U, Bakke I, et al. Leptin is expressed in and secreted from primary cultures of human osteoblasts and promotes bone mineralization. J Bone Miner Res. 2001;16:1426–1433. doi: 10.1359/jbmr.2001.16.8.1426. [DOI] [PubMed] [Google Scholar]
- 140.Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140:1630–1638. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- 141.Fukuda H, Iritani N. Transcriptional regulation of leptin gene promoter in rat. FEBS Lett. 1999;455:165–169. doi: 10.1016/s0014-5793(99)00877-7. [DOI] [PubMed] [Google Scholar]
- 142.McLaughlin F, Mackintosh J, Hayes BP, et al. Glucocorticoid-induced osteopenia in the mouse as assessed by histomorphometry, microcomputed tomography, and biochemical markers. Bone. 2002;30:924–930. doi: 10.1016/s8756-3282(02)00737-8. [DOI] [PubMed] [Google Scholar]