Abstract
It has always been known that anti-tissue transglutaminase 2 (anti-TG2) antibodies are produced in the small intestine. Their serum titres correlate with mucosal damage degree and decrease on a gluten-free diet (GFD).
We aimed to correlate intestinal anti-TG2 antibodies levels with degree of mucosal damage and GFD duration.
Thirty-four active, 71 potential and 24 CD patients on GFD for at least 2 years were enrolled. Anti-TG2 deposits were detected in intestinal biopsies by double immunofluorescence. Biopsies were cultured for 24 h with medium, and with gliadin peptic tryptic digest (PTG) or A-gliadin peptide 31–43 (P31-43). Anti-TG2 antibodies secreted into supernatants were measured by enzyme-linked immunosorbent assay (ELISA). All active CD patients secreted high titres of anti-TG2 antibodies into culture medium that increased with the worsening of mucosal injury (Spearman's r = 0·71; P < 0·0001). Seventy of 71 potential CD patients and 15 of 24 treated CD patients secreted low titres of anti-TG2 antibodies into supernatants, eight of nine negative treated patients being on GFD for more than 10 years. An inverse correlation between antibody titres and duration of GFD was found, (Spearman's r = −0·52; P < 0·01). All active, 53 of 71 potential and six of 24 treated, CD patients showed anti-TG2 mucosal deposits. Five of six positive treated CD patients had been on GFD for fewer than 6 years and were also positive for secreted anti-TG2. In treated patients, PTG/P31-43 was not able to induce secretion of anti-TG2 antibodies into culture medium.
Measurement of anti-TG2 antibodies in biopsy supernatants proved to be more sensitive than detection by immunofluorescence to reveal their intestinal production. Intestinal antiTG2 antibodies titres correlated positively with the degree of mucosal damage and inversely with the duration of GFD.
Keywords: anti-tissue transglutaminase 2, coeliac disease, gluten-free diet, intestinal antibodies
Introduction
Coeliac disease (CD) is a T cell-mediated inflammatory disorder of the small intestine caused by gluten in genetically susceptible individuals [1]. CD is characterized by highly specific autoantibodies directed against transglutaminase 2 (TG2) [2]. It is now well known that serum levels of anti-TG2 correlate with intestinal damage [3,4]. This finding has been considered in the recently revised European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) criteria, and used to avoid biopsies in symptomatic patients with high titres of anti-TG2 [5]. CD-specific autoantibodies disappear from serum after the beginning of a gluten-free diet (GFD) [6].
Anti-TG2 antibodies are found both in blood and small intestine, where they are produced, and have been shown to co-localize with extracellular TG2 in the active phase of the disease [7]. In recent decades several techniques have been used to reveal intestinal production of anti-TG2 antibodies, such as measurement in faeces [8] or duodenal juice [9], or in supernatants of cultured biopsies [10–12], the detection of mucosal deposits [7] or of plasma cells secreting them [13] and their expression by phage display library of RNA coding [14]. Some assays were considered unreliable as diagnostic tests [15]; others, even if with high diagnostic sensitivity and specificity, too demanding to be performed routinely [14].
Recently we have demonstrated that the measurement of antibodies released into culture supernatants is more sensitive than detection of their deposits to assess intestinal production of anti-TG2 in patients with potential CD [16]. Picarelli et al. have shown recently that the organ culture system is a useful tool to assist the histology in diagnosing CD, mainly in cases without villous atrophy or in seronegative patients [17]. Concerning patients on a gluten-free diet (GFD), the same authors showed that anti-endomysium (EMA) was not found in supernatants of biopsy samples cultured with medium alone, but was detected when biopsy samples from the same patients were treated with gliadin peptides [18]. Furthermore, Maki et al. [19] showed that, in the organ culture system conducted with biopsies of treated CD patients, gliadin induced secretion of autoantibodies into culture supernatants, reduced epithelial cell height and increased the density of lamina propria CD25+ cells. However, these changes could be demonstrated only in biopsies from CD patients who had recently started a GFD, in when the small-intestinal mucosal TG2-specific IgA autoantibody deposits were still present.
It is well known that the disappearance of serum anti-TG2 is observed progressively after the beginning of a GFD. It has been considered a useful tool to evaluate the diet compliance, although it is not yet clear if it is helpful to assess complete histological recovery [20].
The aims of our study were to correlate, in coeliac patients, the titres of intestinal antibodies and the degree of mucosal damage and to investigate the effect of GFD on their intestinal production.
Material and methods
Patients
One hundred and twenty-nine CD patients were enrolled at the Department of Medical Translational Sciences, Section of Pediatrics, University Federico II in Naples. One hundred and five patients (range 2–16 years, mean = 6 years) underwent a small intestinal biopsy because of clinical suspicion of CD. They had serum levels of anti-TG2 over the cut-off (7 U/ml) and/or positive EMA antibodies. Thirty-four of 105 patients showed villous atrophy with a grade Marsh 3 (Marsh 3a, n = 13; 3b, n = 11; 3c, n = 10) [21]; they received a diagnosis of CD. Seventy-one of 105 patients showed an architecturally normal intestinal mucosa with a grade of 0/1 (Marsh 0, n = 34; 1, n = 37); they were coded as potential CD patients. Twenty-four of 129 patients (range 8–48 years, mean = 19 years) on a GFD for at least 2 years also underwent a small intestinal biopsy. All patients on a GFD had architecturally normal intestinal mucosa (Marsh 0, n = 10; 1, n = 14) and serum levels of anti-TG2 below the cut-off. At the time of their initial diagnosis, four of 24 patients were potential CD and when they started the GFD presented a mucosa with Marsh 0 or 1 lesion; in fact, they were put on a GFD because of clinical symptoms that disappeared after beginning the GFD. Immunoglobulin (Ig)A deficiency was excluded in all patients.
Duodenal biopsy and organ culture system
During upper gastrointestinal endoscopy, at least five duodenal biopsies were taken from all patients. Two fragments were fixed in 10% formalin, paraffin-embedded and then treated for histological and morphometric analysis. Moreover, for potential CD patients, 4-μm-thick paraffin haematoxylin-stained sections were used to evaluate villous height crypt depth ratio (Vh/CrD); Vh/CrD ≥ 2 was considered normal [22]. One of the duodenal specimens was embedded in a cryostat-embedding medium (Killik; Bio-Optica, Milan, Italy) and stored in liquid nitrogen until used. The remaining fragments were cultured for 24 h at 37°C with medium alone. Moreover, fragments from CD patients on a GFD were cultured for 24 h either in the presence or absence of peptic–tryptic digest of gliadin (PTG, 1 mg/ml) or A-gliadin peptide P31-43 (100 μg/ml). Organ culture was performed as reported previously [23]. After 24 h of culture, the tissues were embedded in optimal cutting temperature (OCT) and stored in liquid nitrogen. The culture supernatants were collected and stored at −80°C until analysed.
Measurement of anti-TG2 IgA antibodies secreted into culture supernatants
Mucosal anti-TG2 IgA antibodies secreted into culture supernatants were measured in undiluted supernatants by enzyme-linked immunosorbent assay (ELISA EU-tTG IgA kit; Eurospital, Trieste, Italy), according to the manufacturer's instructions. When the value of anti-TG2 was higher than the last point of standard curve, supernatants were diluted 1 : 10 in culture medium. The cut-off value for anti-TG2 IgA antibodies in culture supernatants was 2·8 U/ml, as calculated previously in our laboratory [16].
Detection of intestinal deposits of anti-TG2 IgA antibodies and immunohistochemistry
The presence of intestinal deposits of anti-TG2 IgA was investigated in non-cultured fragments from all CD patients. Five-μm cryostat sections were stained using a double-immunofluorescence method, as described previously [24]. The stained sections were evaluated using a confocal microscope (LSM510; Zeiss, Oberkochen, Germany). Immunohistochemical stainings for CD3+ and γδ-T cell receptor (TCR)+ intraepithelial lymphocytes and CD25+ lamina propria mononuclear cells, as well as evaluation of cell densities, were performed in potential CD patients, as reported previously [25].
Statistics
Statistical analysis was performed using GraphPad Prism 4 for Windows, version 4·03 (GraphPad Software, San Diego, CA, USA). Quantitative data were expressed as medians. The Mann–Whitney U-test was used to compare titres of anti-TG antibodies in supernatants between the groups. A paired t-test was used to compare changes of anti-TG2 titres in supernatants after culture with PTG/P3143 within the group of CD patients on a GFD. Spearman's correlation was used to compare anti-TG2 titres in supernatants and laboratory values as Vh/CrD ratios, CD3+ intraepithelial lymphocyte (IEL) density, γδ-TCR+ IELs density and CD25+ mononuclear cells in potential CD patients. Moreover, Spearman's correlation was used to compare anti-TG2 titres in supernatants and duration of the GFD in treated CD patients and to compare anti-TG2 secreted into culture medium and degree of villous atrophy (according to Marsh classification) in active CD patients. A P-value < 0·05 was considered statistically significant.
Stepwise canonical discriminant analysis was adopted to select variables that discriminated between the three groups of cases. Wilks' lambda was used to estimate the capacity of each variable to discriminate among the three groups, ranging between 0 and 1, where 1 = complete overlap and 0 = complete separation. The stepwise multivariate procedure selects the first variable that minimizes Wilks' lambda, then includes the subsequent variables progressively, according to their contribution to lowering Wilks' lambda. The variance ratio F provides an estimate of each variable's contribution to the discrimination between groups.
Ethical approval
Written informed consent was obtained from adult patients and from parents of children enrolled. The study protocol was approved by the Ethical Committee of the University ‘Federico II’ Naples, Italy (CE 230/05).
Results
IgA anti-TG2 in culture supernatants
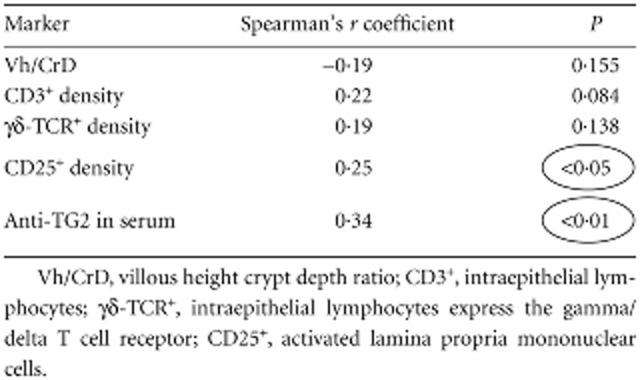
All CD patients in the active phase of disease secreted high titres of anti-TG2 antibodies into culture supernatants (range 15·3–2000 U/ml; median = 222·1 U/ml) (Fig. 1). The antibody titre increased gradually with the worsening of mucosal injury; that is, from grade Marsh 3a to 3c lesion (Fig. 2), with a Spearman's correlation coefficient = 0·71 (P < 0·0001). All potential CD patients, except one, secreted anti-TG2 antibodies into culture supernatants. The antibody titres were variable, ranging from 4·2 to 1247·0 U/ml (median = 26·4 U/ml) (Fig. 1); however, there was no statistical difference between Marsh 0 and 1 lesions (Marsh 0 median = 29·9; 1 = 23·8; P > 0·05) and between potential and Marsh 3a CD patients (Fig. 2). Moreover, titres of secreted anti-TG2 correlated with serum titres of anti-TG2 (Table 1), confirming our previous data [16]. In 62 potential CD patients an immunohistochemical analysis of intestinal biopsies was performed and the correlation between the antibody titres of anti-TG2 secreted into culture supernatants with other parameters such as villous height/crypt depth ratio, density of intraepithelial lymphocytes CD3+, γδ-TCR+ and the CD25+ mononuclear cell density in lamina propria was evaluated. Our data showed that only lamina propria CD25+ mononuclear cell density was correlated with increased levels of secreted anti-TG2 antibodies (Spearman's correlation coefficient = 0·25; P < 0·05) (Table 1).
Figure 1.
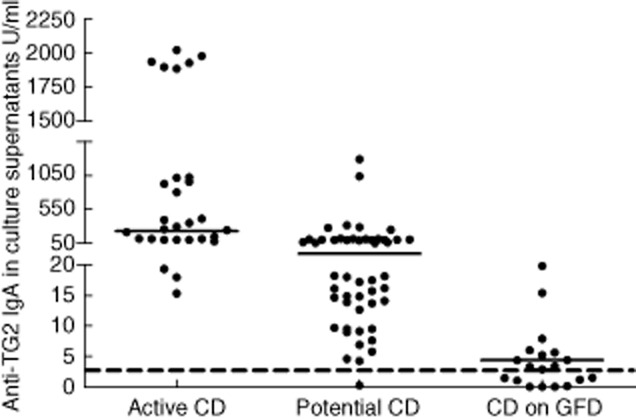
Titres of anti-transglutaminase 2 (TG2) immunoglobulin (Ig)A, expressed as U/ml, in medium culture supernatants. The dotted line represents the cut-off of 2·8 U/ml; horizontal lines represent median values. The y-axis is divided into three segments in the same graph to also show the low titres of anti-TG2 secreted into culture medium by potential and treated coeliac disease (CD) patients. GFD, gluten-free diet.
Figure 2.
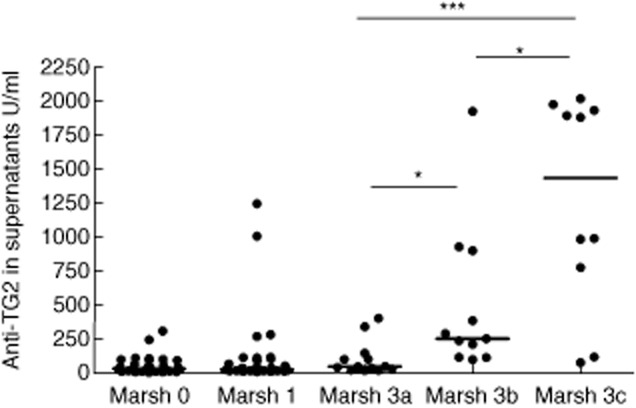
Anti-transglutaminase 2 (TG2) immunoglobulin (Ig)A, expressed as U/ml, secreted into culture medium by active (Marsh 3a, 3b, 3c) and potential (Marsh 0, 1) coeliac disease (CD) patients according to grade of mucosal lesion. *P < 0·05; ***P < 0·001; Mann–Whitney U-test.
Table 1.
Spearman's correlation between titres of anti-transglutaminase 2 (TG2) secreted into culture supernatants by potential coeliac disease (CD) patients and markers
 |
When CD patients on a GFD were considered, 15 of 24 secreted low titres of anti-TG2 antibodies (range 3·3–27·2 U/ml; median = 4·35 U/ml) (Fig. 1). Antibody titres did not correlate with the mucosal lesion degree (Spearman's r = 0·02, P = 0·9). Eight of nine negative patients had been on a GFD for more than 10 years. The ninth negative patient had been on GFD for less than 2 years, but he had a Marsh 1 lesion at diagnosis. All four patients in remission who had Marsh 0/1 mucosa when put on a GFD (potential CD patients) produced lower titres of anti-TG2 compared to patients with similar duration of the GFD but with Marsh 3 mucosal lesion at the time of diagnosis. Only one patient on GFD for 12 years continued to produce low amounts of anti-TG2 antibodies (4·34 U/ml). He also had type 1 diabetes. There was an inverse correlation between antibody titres and duration of GFD (Spearman's correlation coefficient = −0·52, P < 0·01).
Finally, CD patients on a GFD did not show a significant increase in antibody titres after in-vitro challenge with PTG or P31-43. PTG/P31-43 stimulation was not able to induce an increase of anti-TG2 antibody secretion into culture supernatants of small intestinal fragments, even in the nine patients with no basal production of intestine anti-TG2 antibodies (data not shown).
Intestinal deposits of anti-TG2 IgA
Mucosal deposits of IgA anti-TG2 were detected in duodenal mucosa of all active and 53 of 71 potential CD patients. Among the 18 potential CD patients without mucosal deposits, only one was also negative for supernatant anti-TG2. Considering CD patients on a GFD, six of 24 showed mucosal deposits. Five of six had been on a GFD for less than 6 years and secreted anti-TG2 into culture medium, with titres ranging from 22 to 27 U/ml. The last patient (also affected by type 1 diabetes) had been on a GFD for 12 years. Patients on a GFD with mucosal deposits secreted anti-TG2 into culture supernatants with levels higher than the patients without them (P < 0·01, Fig. 3). Moreover, the intensity of mucosal deposit staining of IgA anti-TG2 was correlated directly with titres of secreted anti-TG2 (Spearman's r = 0·63, P < 0·001).
Figure 3.
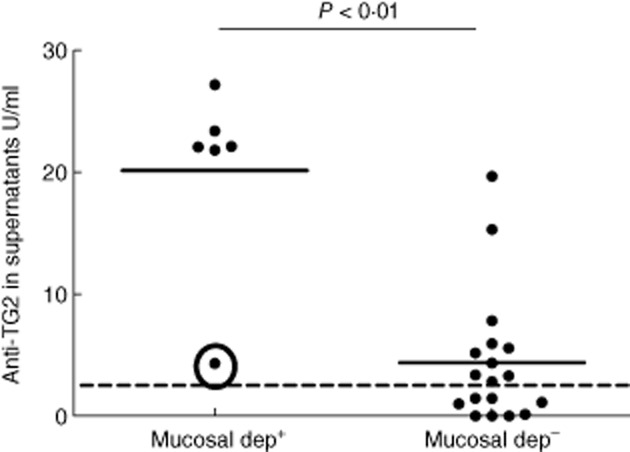
Coeliac disease (CD) patients on gluten-free diet (GFD) with or without mucosal deposits coded as mucosal dep+ and mucosal dep–, respectively. Mucosal dep+ patients secreted anti-transglutaminase 2 (TG2) immunoglobulin (Ig)A antibodies into culture medium with titres significantly higher than mucosal dep– patients. The dotted line represents the cut-off of 2·8 U/ml. The circle shows the type 1 diabetes patient on GFD for 12 years who produced low amounts of anti-TG2 antibodies; Mann–Whitney U-test.
A concordance of 76·7% was observed between the two techniques used to evaluate the mucosal production of anti-TG2 antibodies in our study population.
Multivariate discriminant analysis
As described in the Methods, we attempted a multivariate discriminant analysis in order to identify those variables that, in the multivariate model, are the most efficient to discriminate among groups. As shown in Table 2, Wilks' lambda was lowered exclusively by the two variables related to the production of anti-TG2 in the mucosa (mucosal deposits and anti-TG2 production into culture supernatants). The anti-TG2 antibody level in the serum did not add any significant improvement to the discriminating function after inclusion of the above variables.
Table 2.
Multivariate discriminant analysis
| Variables entered/removed†,‡ | ||||
|---|---|---|---|---|
| Wilks' lambda | ||||
| Exact F | ||||
| Step | Entered | Statistic | Statistic | Sig. |
| 1 | Mucosal deposits | 0·579 | 37·156 | 0·000 |
| 2 | Anti-TG2 into culture medium | 0·506 | 20·492 | 0·000 |
At each step, the variable that minimizes the overall Wilks' lambda is entered.
Minimum partial F to enter is 3·84.
Maximum partial F to remove is 2·71. TG2, transglutaminase 2.
By using the discriminant equation obtained, it is possible to allocate each individual case to the group for which he has the highest probability computed by the two selected variables. When we compared active and potential cases, 73·5% of active CD were identified correctly by the equation versus 95·8% of potential cases. The equation was obviously less efficient to discriminate potential from remissions (65 versus 79·2%).
Discussion
High titres of anti-TG2 antibodies are found in the serum of most coeliac patients. They are related to mucosal damage severity and disappear from the serum in patients on a GFD. Anti-TG2 antibodies are synthesized in the intestinal mucosa and, as described previously, several techniques have been devised to prove this. To identify the most suitable test for detection of the intestinal production of anti-TG2 antibodies, we have recently compared two of these techniques in potential CD patients: the search for anti-TG2 intestinal deposits and measurement of the same antibodies secreted into supernatants after organ culture [16]. Our data showed higher sensitivity and specificity of the anti-TG2 dosage into culture supernatant for this purpose [16]. This test is objective, not bound to operator experience, not too demanding and not expensive.
In this study, we confirmed previous results and showed for the first time that there was a correlation between levels of intestinal anti-TG2 antibodies and severity of mucosal damage. A similar correlation has already been found between serum anti-TG2 antibody levels and duodenal histology in paediatric [20,26] and adult [26,27] coeliac populations. However, our data showed that the intestinal production of these autoantibodies correlated with mucosal lesion degree only in active CD. Potential CD patients with Marsh 0 mucosa produced titres of intestinal anti-TG2 antibodies comparable to those with Marsh 1. Moreover, in this potential coeliac population intestinal anti-TG2 titres correlated with an increased number of CD25+ mononuclear cells in lamina propria, a proven marker of mucosa inflammation [28].
Furthermore, we investigated intestinal production of anti-TG2 with the same two assays in CD patients on a GFD for at least 2 years. Two-thirds (63%) of patients on a GFD secreted low amounts of these autoantibodies, while mucosal deposits were detected in fewer than one-third (25%) of patients. This means that in most cases anti-TG2 antibodies, when produced, do not accumulate as mucosal deposits; in these cases the absence of mucosal deposits could depend upon the low titres and decreasing affinity of anti-TG2 antibodies for their antigen as a consequence of a long period of GFD. In our hands, anti-TG2 detection in culture medium seems to be more sensitive in revealing their intestinal production. Studies on the follow-up of CD patients on GFD [29–31] have shown that titres of circulating anti-TG2 antibodies decreased significantly during the first 12 months of the diet and then disappeared in the next 12 months. The disappearance of serum CD-specific autoantibodies was correlated with GFD compliance, but not always associated with the total recovery of small intestinal mucosa [20]. Our data appear to show that the early disappearance of circulating specific CD autoantibodies, following a strict GFD, does not mean the end of their intestinal production. After the disappearance from serum, anti-TG2 antibodies as deposits would disappear slowly from the intestine, and only after a long period of GFD, small intestinal mucosa would cease to produce them.
Anti-TG2 antibodies in coeliac mucosa are produced by TG2-specific plasma cells that reduce their number on GFD [13]. The density of these intestinal plasma cells in treated CD patients is lower than in active CD, but higher than in non-CD subjects. Recent evidence [13,32] shows that secreting plasma cells from human intestine can live in vitro for several weeks and could potentially live in vivo for months or years, thanks to a microenvironment favourable to their long-term survival. Only after a long period of GFD do these secreting cells disappear and, as result, intestinal anti-TG2 production ends.
Finally, our data show that in vitro 24-h PTG stimulation was not able to induce a statistically significant increase of antibody secretion in short-term GFD CD patients as well as in patients on long-term GFD. The ability of gliadin peptides to induce antibody secretion (EMA and/or anti-TG2 antibodies) into supernatants of cultured biopsies from treated CD patients has been investigated widely in the last 20 years, with contradictory results [14,17–19]. Maki et al. [19] hypothesized that secretion of specific CD autoantibodies from cultured small intestinal biopsies of treated CD patients is related to the presence of anti-TG2 mucosal deposits that are usually detected in those patients who have been on a GFD for a short time-period. Our data appear to show that secretion of anti-TG2 antibodies is detected regardless of the presence of mucosal deposits. Absence or a very low density of TG2-specific plasma cells and/or the short duration of organ culture might explain the inability of gliadin peptides to stimulate antibody secretion in treated CD patients.
In conclusion, our results show that the measurement of intestinal antibodies in biopsy supernatants represents a valid, quantitative test to investigate the production of these autoantibodies at each stage of disease. In our hands, it is more sensitive than detection of mucosal deposits by immunofluorescence. The production of intestinal anti-TG2 antibodies could represent the very early stage of gluten-induced mucosal injury, when the integrity of small intestinal mucosa is still conserved and anti-TG2 are not detectable in serum. This test has a potentially great impact in clinical practice to unravel the wide spectrum of gluten sensitivity.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2:647–655. doi: 10.1038/nri885. [DOI] [PubMed] [Google Scholar]
- 2.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 3.Bazzigaluppi E, Parma B, Tronconi GM, et al. IgA anti-actin antibodies in children with celiac disease: comparison of immunofluorescence with ELISA assay in predicting severe intestinal damage. Ital J Pediatr. 2010;36:25. doi: 10.1186/1824-7288-36-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alessio MG, Tonutti E, Brusca I, et al. Correlation between IgA tissue transglutaminase antibody ratio and histological finding in celiac disease. J Pediatr Gastroenterol Nutr. 2012;55:44–49. doi: 10.1097/MPG.0b013e3182470249. [DOI] [PubMed] [Google Scholar]
- 5.Husby S, Koletzko S, Korponay-Szabó IR, et al. for the ESPGHAN Working Group on Coeliac Disease Diagnosis, on behalf of the ESPGHAN Gastroenterology Committee European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–160. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 6.Hogen Esch CE, Wolters VM, Gerritsen SA, et al. Specific celiac disease antibodies in children on a gluten-free diet. Pediatrics. 2011;128:547–552. doi: 10.1542/peds.2010-3762. [DOI] [PubMed] [Google Scholar]
- 7.Korponay-Szabo IR, Halttunen T, Szalai Z, et al. In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut. 2004;53:641–648. doi: 10.1136/gut.2003.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Picarelli A, Sabbatella L, Di Tola M, et al. Antiendomysial antibody detection in fecal supernatants: in vivo proof that small bowel mucosa is the site of antiendomysial antibody production. Am J Gastroenterol. 2002;97:95–98. doi: 10.1111/j.1572-0241.2002.05426.x. [DOI] [PubMed] [Google Scholar]
- 9.Mawhinney H, Love AH. Anti-reticulin antibody in jejunal juice in coeliac disease. Clin Exp Immunol. 1975;21:394–398. [PMC free article] [PubMed] [Google Scholar]
- 10.Picarelli A, Di Tola M, Sabbatella L, et al. Usefulness of the organ culture system in the in vitro diagnosis of coeliac disease: a multicentre study. Scand J Gastroenterol. 2006;41:186–190. doi: 10.1080/00365520510024151. [DOI] [PubMed] [Google Scholar]
- 11.Carroccio A, Di Prima L, Pirrone G, et al. Anti-transglutaminase antibody assay of the culture medium of intestinal biopsy specimens can improve the accuracy of celiac disease diagnosis. Clin Chem. 2006;52:1175–1180. doi: 10.1373/clinchem.2005.061366. [DOI] [PubMed] [Google Scholar]
- 12.Picarelli A, Libanori V, De Nitto D, et al. Organ culture system as a means to detect celiac disease. Ann Clin Lab Sci. 2010;40:85–87. [PubMed] [Google Scholar]
- 13.Di Niro R, Mesin L, Zheng NY, et al. High abundance of plasma cells secreting transglutaminase 2-specific IgA autoantibodies with limited somatic hypermutation in celiac disease intestinal lesions. Nat Med. 2012;18:441–445. doi: 10.1038/nm.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marzari R, Sblattero D, Florian F, et al. Molecular dissection of the tissue transglutaminase autoantibody response in celiac disease. J Immunol. 2001;166:4170–4176. doi: 10.4049/jimmunol.166.6.4170. [DOI] [PubMed] [Google Scholar]
- 15.Kappler M, Krauss-Etschmann S, Diehl V, et al. Detection of secretory IgA antibodies against gliadin and human tissue transglutaminase in stool to screen for coeliac disease in children: validation study. BMJ. 2006;332:213–214. doi: 10.1136/bmj.38688.654028.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tosco A, Aitoro R, Auricchio A, et al. Intestinal anti-tissue transglutaminase antibodies in potential coeliac disease. Clin Exp Immunol. 2013;171:69–75. doi: 10.1111/j.1365-2249.2012.04673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picarelli A, Di Tola M, Marino M, et al. Usefulness of the organ culture system when villous height/crypt depth ratio, intraepithelial lymphocyte count, or serum antibody tests are not diagnostic for celiac disease. Transl Res. 2013;161:172–180. doi: 10.1016/j.trsl.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Picarelli A, Maiuri L, Frate A, et al. Production of antiendomysial antibodies after in-vitro gliadin challenge of small intestine biopsy samples from patients with coeliac disease. Lancet. 1996;348:1065–1067. doi: 10.1016/S0140-6736(96)03060-7. [DOI] [PubMed] [Google Scholar]
- 19.Stenman SM, Lindfors K, Korponay-Szabo IR, et al. Secretion of celiac disease antibodies after in vitro gladin challenge is dependent on small-bowel mucosal transglutaminase2-specific IgA deposits. BMC Immunol. 2008;9:6. doi: 10.1186/1471-2172-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tursi A, Brandimarte G, Giorgetti GM. Lack of usefulness of anti-transglutaminase antibodies in assessing histologic recovery after gluten-free diet in celiac disease. J Clin Gastroenterol. 2003;37:387–391. doi: 10.1097/00004836-200311000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of celiac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–1194. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Kurppa K, Collin P, Viljamaa M, et al. Diagnosing mild enteropathy celiac disease: a randomized, controlled clinical study. Gastroenterology. 2009;136:816–823. doi: 10.1053/j.gastro.2008.11.040. [DOI] [PubMed] [Google Scholar]
- 23.Mazzarella G, Maglio M, Paparo F, et al. An immunodominant DQ8 restricted gliadin peptide activates small intestinal immune response in in vitro cultured mucosa from HLA-DQ8 positive but not HLA-DQ8 negative coeliac patients. Gut. 2003;52:57–62. doi: 10.1136/gut.52.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tosco A, Maglio M, Paparo F, et al. Immunoglobulin A anti-tissue transglutaminase antibody deposits in the small intestinal mucosa of children with no villous atrophy. J Pediatr Gastroenterol Nutr. 2008;47:293–298. doi: 10.1097/MPG.0b013e3181677067. [DOI] [PubMed] [Google Scholar]
- 25.Paparo F, Petrone E, Tosco A, et al. Clinical, HLA and small bowel immunohistochemical features of children with positive serum antiendomysium antibodies and architecturally normal small intestinal mucosa. Am J Gastroenterol. 2005;100:2294–2298. doi: 10.1111/j.1572-0241.2005.41134.x. [DOI] [PubMed] [Google Scholar]
- 26.Donaldson MR, Book LS, Leiferman KM, et al. Strongly positive tissue transglutaminase antibodies are associated with Marsh 3 histopathology in adult and pediatric celiac disease. J Clin Gastroenterol. 2008;42:256–260. doi: 10.1097/MCG.0b013e31802e70b1. [DOI] [PubMed] [Google Scholar]
- 27.Kalhan S, Joseph P, Sharma S, et al. Comparative study of histopathological Marsh grading with clinical and serological parameters in celiac iceberg of north India. Indian J Pathol Microbiol. 2011;54:279–283. doi: 10.4103/0377-4929.81593. [DOI] [PubMed] [Google Scholar]
- 28.Maiuri L, Picarelli A, Boirivant M, et al. Definition of the initial immunologic modifications upon in vitro gliadin challenge in the small intestine of celiac patients. Gastroenterology. 1996;110:1368–1378. doi: 10.1053/gast.1996.v110.pm8613040. [DOI] [PubMed] [Google Scholar]
- 29.Tommasini A, Not T, Kiren V, et al. Mass screening for coeliac disease using antihuman transglutaminase antibody assay. Arch Dis Child. 2004;89:512–515. doi: 10.1136/adc.2003.029603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vahedi K, Mascart F, Mary JY, et al. Reliability of antitransglutaminase antibodies as predictors of gluten-free diet compliance in adult celiac disease. Am J Gastroenterol. 2003;98:1079–1087. doi: 10.1111/j.1572-0241.2003.07284.x. [DOI] [PubMed] [Google Scholar]
- 31.Fabiani E, Catassi C International Working Group. The serum IgA class anti-tissue transglutaminase antibodies in the diagnosis and follow up of coeliac disease. Results of an international multi-centre study. International Working Group on Eu-tTG. Eur J Gastroenterol Hepatol. 2001;13:659–665. doi: 10.1097/00042737-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Mesin L, Sollid LM, Di Niro R. The intestinal B-cell response in celiac disease. Front Immunol. 2012;3:313. doi: 10.3389/fimmu.2012.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]


