Abstract
Sjögren's syndrome (SS) is a chronic autoimmune disease characterized by salivary and lacrimal gland dysfunction. Clinical observations and results from animal models of SS support the role of aberrant epithelial cell apoptosis and immune homeostasis loss in the glands as triggering factors for the autoimmune response. Vasoactive intestinal peptide (VIP) promotes potent anti-inflammatory effects in several inflammatory and autoimmune disease models, including the non-obese diabetic (NOD) mouse model of SS. With the knowledge that VIP modulates monocyte function through vasoactive intestinal peptide receptors (VPAC) and that immune homeostasis maintenance depends strongly upon a rapid and immunosuppressant apoptotic cell clearance by monocytes/macrophages, in this study we explored VPAC expression on monocytes from primary SS (pSS) patients and the ability of VIP to modulate apoptotic cell phagocytic function and cytokine profile. Monocytes isolated from individual pSS patients showed an increased expression of VPAC2 subtype of VIP receptors, absent in monocytes from control subjects, with no changes in VPAC1 expression. VPAC2 receptor expression could be induced further with lipopolysaccharide (LPS) in pSS monocytes and VIP inhibited the effect. Moreover, monocytes from pSS patients showed an impaired phagocytosis of apoptotic epithelial cells, as evidenced by reduced engulfment ability and the failure to promote an immunosuppressant cytokine profile. However, VIP neither modulated monocyte/macrophage phagocytic function nor did it reverse their inflammatory profile. We conclude that monocytes from pSS patients express high levels of VPAC2 and display a deficient clearance of apoptotic cells that is not modulated by VIP.
Keywords: apoptotic cell clearance, monocytes, Sjögren's syndrome patients, VPAC receptors
Introduction
Sjögren's syndrome (SS) is a chronic autoimmune disorder that affects 0·5–1% of the adult population, with a high economic impact in health care. The disease hallmark is a salivary and lacrimal gland dysfunction, although co-morbidities are found depending upon genetic background, hormones and environmental triggers [1–5]. Clinical observations and results from patient cell approaches and animal models of SS point to the loss of salivary gland homeostasis as a triggering factor for the autoimmune response which would, in turn, promote further damage to the gland [6–11]. In line with this, evidence of aberrant expression of inflammatory and apoptosis mediators in salivary gland epithelial cells from pSS patients and murine models was reported [12–17]. Immune homeostasis depends strongly upon a rapid and immunosuppressant apoptotic cell clearance by monocytes/macrophages to prevent an inflammatory response and self-tolerance breakdown [18–21]. Consistently, evidence on an impaired or delayed clearance of apoptotic cells by macrophages was reported in systemic lupus erythematosus (SLE) patients, although its direct aetiopathogenic role is still unclear [22–24]. Regarding SS, a deficient phagocytosis of apoptotic cells was described in the non-obese diabetic (NOD) mouse model of the disease [25–27]. In particular, macrophages isolated from NOD mice at the SS-like stage expressed a predominant M1 inflammatory activation profile and presented a defective engulfment of apoptotic acinar cells [26].
Vasoactive intestinal peptide is a prosecretory and vasodilating neuropeptide with potent immunomodulatory effects through the activation of vasoactive intestinal peptide receptor VPAC1 and VPAC2 receptors on monocytes, macrophages and T cells [28–32]. VIP promotes anti-inflammatory and tolerogenic effects in several inflammatory and autoimmune disease models [30,33–37]. Particularly in the NOD mouse model of SS, a local gene therapy with an adenoviral construct encoding VIP restored salivary secretion and reduced autoimmune markers [38]. Also, a predominant M2 alternative activation profile was promoted by VIP in NOD mice macrophages at the SS-like stage [26,27,39].
In this study we analysed Sjögren syndrome individual patients' monocytes to explore their VPAC receptor expression profile and function, particularly the effect of VIP on the phagocytosis of epithelial apoptotic cells. We observed that monocytes from pSS patients showed increased expression of VPAC2 that was absent in normal subjects' monocytes, and that this effect paralleled an impaired phagocytosis of apoptotic cells, with reduced engulfment and failure to express an immunosuppressant cytokine profile that was not restored by VIP.
Materials and methods
Patients
Blood samples were collected from patients that fulfilled the American–European Consensus Group Criteria for pSS [40] (n = 38), followed-up at the Rheumatology Unit, Department of Medicine of the CEMIC, Buenos Aires, Argentina, and from healthy volunteers as the control group (n = 16). All the participants were women who signed an informed consent to participate in this study, approved by the Argentine Society of Clinical Investigation Review Board and Ethical Committee. The age range, disease duration, extraglandular manifestations and systemic treatments are indicated in Table 1. All patients were positive for anti-Ro (SSA) serum antibodies, and 12 patients received only local symptomatic management.
Table 1.
Characteristics of primary Sjögren's syndrome (pSS) patients and healthy volunteers (control)
| Participants | Age (mean ± s.d.) | Extraglandular manifestations (number of patients) | Disease duration (mean ± s.d.) | Systemic treatment (number of patients) |
|---|---|---|---|---|
| pSS Patients n = 38 | 29–79 years (55·9 ± 11·9) | Autoimmune thyroid disease (7); synovitis (2); cutaneous vasculitis (4); Raynaud phenomenon (2) | 2–33 years (12·2 ± 9·8) | Hydroxicloroquine (19), levothyroxine (6), prednisone (3), pilocarpine (1) |
| Control n = 16 | 24–61 years (42·2 ± 13·2) | n.a. | n.a. | n.a. |
Patients fulfilled the American–European Consensus Group Criteria for pSS and all of them were positive for anti-Ro (SSA) serum antibodies as described in Material and methods. Drug prescription to pSS patients is indicated, except for 12 patients who only received local symptomatic management. Control subjects received no drug treatment. All the participants were women. s.d. = standard deviation; n.a. = not applicable.
Peripheral blood mononuclear cells (PBMC)
PBMC from patients and controls were isolated from heparinized peripheral blood by density gradient centrifugation on Ficoll-Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden). Monocytes were purified by attachment to a culture plate for 2 h and washed three times with warm phosphate-buffered saline (PBS) to remove non-adherent cells, and adherent monocytes were recovered with a cell scraper. Cell population purity was checked by fluorescence activated cell sorter (FACS) analysis using anti-CD14 monoclonal antibody (mAb) and was found to be > 85% for each set of experiments. In some experiments CD14+ cells were separated by performing positive selection with CD14+ micro-magnetic beads, according to the manufacturer's instructions (Miltenyi Biotec, Bergisch Gladbach, Germany), with a purity of 95%. CD14+ cells from each patient or control sample were used separately for VPAC determination, plated for phagocytosis experiments or incubated with 1 μg/ml lipopolysaccharide (LPS) in the presence or absence of 100 nM VIP for 24 h before being homogenized for reverse transcription–quantitative polymerase chain reaction (RT–qPCR) determinations.
Epithelial cell apoptosis induction
The human salivary gland (HSG) epithelial cell line was kindly provided by Dr Bruce Baum (NICDH-NIH, Bethesda, MD, USA) and cultured in 24-well flat-bottomed polystyrene plates (Becton Dickinson, Franklin Lakes, NJ, USA) in complete Dulbecco's modified Eagle's medium (DMEM) 10% fetal calf serum (FCS) (Gibco, Invitrogen, Buenos Aires, Argentina). HSG cells displayed secretory properties and markers according to their salivary gland epithelial phenotype [41]. Apoptotic HSG cell suspension was obtained by incubating HSG cells at 70% confluence during 24 h with 50 nM staurosporine (Sigma Chemical Co, St Louis, MO, USA). The frequency of apoptotic HSG cells was assessed by propidium iodide (PI) and fluorescein isothiocyanate (FITC)-annexin V staining following the manufacturer's recommendations (BD Biosciences, San José, CA, USA) and analysed by flow cytometry (FACS) using WinMDI software®.
Phagocytosis of apoptotic HSG cells by patients' monocytes
Phagocytosis of apoptotic HSG cells by monocytes was determined by FACS. HSG cells were induced to apoptosis during 24 h with 50 nM staurosporine and stained with carboxyfluorescein succinimidyl ester (CFSE; eBioscience, San Diego, CA, USA). Apoptotic HSG cell suspension was added to each well of the 24-well plate containing adherent monocyte monolayers from individual patients or control subjects for 60, 90 or 120 min at 37°C at a 1:1 or 3:1 relationship (apoptotic HSG : monocyte cells) in the presence or absence of 100 nM VIP (Polypeptide Group, Strasbourg, France). Non-ingested cells were washed out and monocytes detached by addition of Tryple® (Gibco, Grand Island, NY, USA). The monocyte population was stained with phycoerythrin (PE)-conjugated anti-CD14 mAb (BD Biosciences) and the percentage of phagocytosis was determined as CD14/CFSE double-stained cells by FACS. Ten thousand events were acquired in a FACSAria II® cytometer and results were analysed using the WinMDI software®. Samples were incubated in parallel with a non-relevant, isotype-matched CD14 antibody as a background control.
Cytokine production
To assess tumour necrosis factor (TNF)-α, interleukin (IL)-10 and IL-6 production by monocytes after phagocytosis experiments, cells were incubated further for 24 h and supernatants collected for cytokine determination by enzyme-linked immunosorbent assay (ELISA) (e-Bioscience).
RT–PCR and RT–qPCR
Total RNA isolation was performed using TRIzol (Invitrogen, Carlsbad, CA, USA) and cDNA were generated from 1 μg of RNA using a Moloney murine leukaemia virus (MMLV) reverse transcriptase, RNAsin RNAse inhibitor and oligo dT kit (Promega-Biodynamics, Buenos Aires, Argentina). Each cDNA was then amplified using specific primers for VPAC1, VPAC2, VIP and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or CD14 antigen were used as housekeeping genes (Table 2). PCR products and DNA size markers were fractionated on 2% agarose gels, visualized with ethidium bromide staining, and band density was expressed in arbitrary units (AU) normalized to GAPDH. Bands were semi-quantified with ImageJ® and intensity expressed in AU relative to GAPDH. qPCR was performed using Mezcla Real (Biodynamics, Buenos Aires, Argentina), according to the manufacturer's instructions.
Table 2.
Primers used in this study. Oligonucleotide primers were designed using the online tool Primer3® (Whitehead Institute for Biomedical Research)
| Gene | Forward | Reverse |
|---|---|---|
| Vpac1 | CCCCTGGGTCAGTCTGGTG | GAGACCTAGCATTCGCTGGTG |
| Vpac2 | CCAGATGTCGGCGGCAACG | GCTGATGGGAAACACGGCAAAC |
| Vip | CAGTAACAGCCAACCCTTAGCC | TGAGAAGAGTCAGGAGCACAAGG |
| Gapdh | TGATGACATCAAGAAGGTGGTGAAG | TCCTTGGAGGCCATGTAGGCCAT |
| Cd14 | CAAGTGTGAAGCCTGGAAGCCG | AGCAGCAACAAGCAGGACGC |
Statistical analysis
The significance of the results was analysed by Student's t-test and Mann–Whitney U-test for non-parametric samples. When multiple comparisons were necessary, the Student–Newman–Keuls test was used after analysis of variance. Differences between groups were considered significant at P < 0·05 using the GraphPad Prism4 software (GraphPad, San Diego, CA, USA).
Results
VPAC2 is highly expressed in pSS monocytes
We first analysed the expression of both subtypes of VIP receptors, VPAC1 and VPAC2, on monocytes isolated from pSS patients and control subjects. Figure 1a shows that pSS monocytes express the VPAC2 subtype of VIP receptors, which were absent in monocytes from normal subjects. The expression of VPAC2 in pSS monocytes was similarly high, regardless of whether the housekeeping gene used was GAPDH or CD14 as a monocyte specific marker (Fig. 1b). In contrast, VPAC1 subtype was expressed in both patient and control CD14+ cells at similar levels (Fig. 1c). We could not detect VIP expression in the monocyte population of any of the patients' or control subjects' blood samples tested (not shown).
Figure 1.
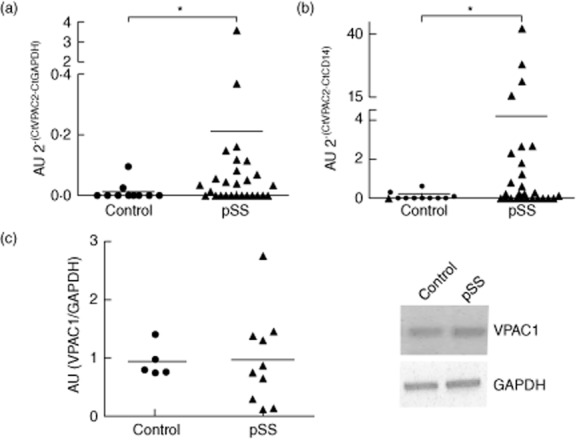
Vasoactive intestinal peptide receptor (VPAC) expression in patients' CD14+ cells. (a,b) Monocytes were isolated from primary Sjögren's syndrome (pSS) or control subjects and analysed for VPAC2 expression by quantitative reverse transcription–polymerase chain reaction (qRT–PCR) as described in Material and methods using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and CD14 to calculate relative expression. Values represent VPAC2 relative expression to GAPDH (2–(CtVPAC2-CtGAPDH)×10 exp3) or to CD14 (2–(CtVPAC2-CtGAPDH)×10 exp3) calculated from each pSS or control subject monocyte sample. Media calculated is also shown. *P < 0·05, Mann–Whitney U-test. (c) VPAC1 receptor expression was assessed in the same samples of (a) by RT–PCR, as described in Material and methods. Band intensity expressed in arbitrary units (AU) relative to GAPDH was semi-quantified with ImageJ®. Values represent VPAC1 expression in AU obtained with each different pSS or control subject monocyte sample and the media value is also depicted.
VPAC2 expression in pSS monocytes is increased by LPS and inhibited by VIP
Because the VPAC2 receptor subtype was up-regulated in mouse peritoneal macrophages as well as in a murine macrophage cell line after stimulation through Toll-like receptors (TLRs) [28,42], we explored whether this receptor could be induced through TLR-4 in monocytes from pSS patients. Thus, we incubated pSS monocytes with 1 μg/ml LPS for 24 h in vitro. A more than threefold increase in VPAC2 expression over basal levels (incubated in the absence of LPS) was observed only in pSS monocytes (Fig. 2, right panel). In contrast, VPAC2 receptors, absent in normal monocytes, could not be induced further by LPS treatment in our experimental conditions (Fig. 2, left panel). Interestingly, VIP inhibited the effect of LPS on VPAC2 expression on pSS monocytes (Fig. 2, right panel).
Figure 2.
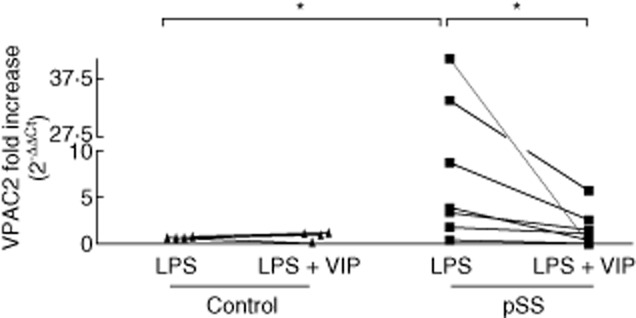
Lipopolysaccharide (LPS) induction of vasoactive intestinal peptide receptor (VPAC)2 expression in patients' CD14+ cells and vasoactive intestinal peptide (VIP) modulation. Monocytes from different primary Sjögren's syndrome (pSS) patients or control subjects (control) were isolated and incubated for 24 h with 1 μg/ml LPS in the presence or absence of 100 nM VIP as described in Material and methods before they were analysed for VPAC2 expression by quantitative reverse transcription–polymerase chain reaction (qRT–PCR). Values represent the fold increase of VPAC2 expression in each patient or control monocyte sample incubated with LPS for 24 h with or without VIP, over VPAC2 expression in the same incubation period in the absence of stimuli. *P < 0·05, Mann–Whitney U-test.
Impaired phagocytosis of apoptotic cells by pSS monocytes is not restored by VIP
Sjögren's syndrome is associated with an increased apoptosis of salivary gland epithelial cells and loss of gland immune homeostasis. Considering that apoptotic cell clearance by phagocytic cells in a suppressant manner is essential for tissue homeostasis maintenance, we next explored the phagocytosis of apoptotic epithelial HSG cells by pSS patients' and control subjects' monocytes and the effect of VIP. Figure 3a shows an impaired phagocytic function of pSS monocytes at 90-min incubation time, as determined by FACS compared with monocytes from normal subjects. Representative cytometry plots in Fig. 3b show the percentage of phagocytosis of HSG apoptotic cells by control and pSS monocytes at 90 min. A microphotograph of CFSE-stained HSG apoptotic cell body ingested by pSS monocytes is shown in Fig. 3c. The impairment in apoptotic cell phagocytosis observed in CD14+ cells from pSS patients was assessed at two different monocyte : apoptotic cell relationships (Fig. 3d). As shown in Fig. 3e, 100 nM VIP had no effect on phagocytosis of apoptotic cells by pSS monocytes at a 1:1 relationship and 90-min incubation setting. As expected for normal monocytes/macrophages, phagocytosis of apoptotic cells induced a suppressant cytokine profile with lower levels of the proinflammatory cytokine TNF-α and increased levels of IL-10 only in normal subjects' monocytes (Fig. 4a,b). In contrast, TNF-α production increased in pSS CD14+ cells, whereas IL-10 did not, supporting an impaired apoptotic cell clearance (Fig. 4a,b). IL-6 levels also increased when pSS patients' CD14+ cells engulfed apoptotic HSG cells (data not shown). We next investigated if VIP could reverse the proinflammatory profile of monocytes after apoptotic cell phagocytosis. Figure 4c,d shows that 100 nM VIP, the same concentration that limited LPS-induced VPAC2 expression (Fig. 2), could not reduce TNF-α or increase IL-10 production by pSS monocytes.
Figure 3.
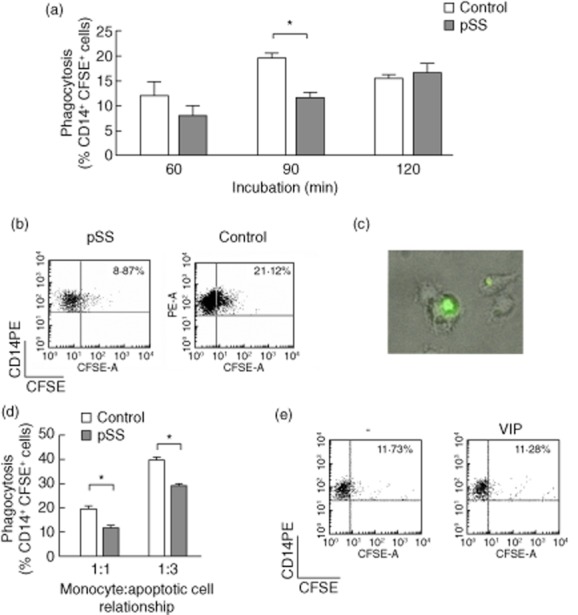
Impaired phagocytosis of apoptotic human salivary gland (HSG) cells by primary Sjögren's syndrome (pSS) monocytes. Monocytes were isolated from pSS or control subjects' peripheral blood mononuclear cells (PBMCs) and plated for 24 h for phagocytosis experiments, as indicated in Material and methods. (a) HSG cells stained with carboxyfluorescein succinimidyl ester (CFSE) and induced to apoptosis for 24 h with 50 nM staurosporine were added to monocytes from individual pSS patients or control subjects plated on 24-well plates for 60, 90 or 120 min at a 1:1 apoptotic HSG : monocyte relationship. Values are mean ± standard error of the mean (s.e.m.) frequency of CD14+CFSE+ cells of at least six pSS or control monocyte samples determined by fluorescence activated cell sorter (FACS). *P < 0·05, Mann–Whitney U-test. (b) Representative dot-plot of 1:1 apoptotic cell : pSS or control monocyte incubated for 90 min. (c) Microphotography (×400) representative of at least eight experiments with pSS monocytes. (d) Monocytes from pSS patients or control subjects plated for 90 min at a 1:1 or 1:3 monocyte : apoptotic cell. Values are mean ± s.e.m. frequency of CD14+CFSE+ cells of six pSS and three control monocyte samples for a 1:1 relationship or four pSS and three control samples for a 1:3 relationship determined by FACS. *P < 0·05. (e) Monocytes from pSS patients incubated for 90 min at a 1:1 apoptotic cell:monocyte relationship in the presence or absence of 100 nM VIP. Representative dot-plot of three similar experiments.
Figure 4.
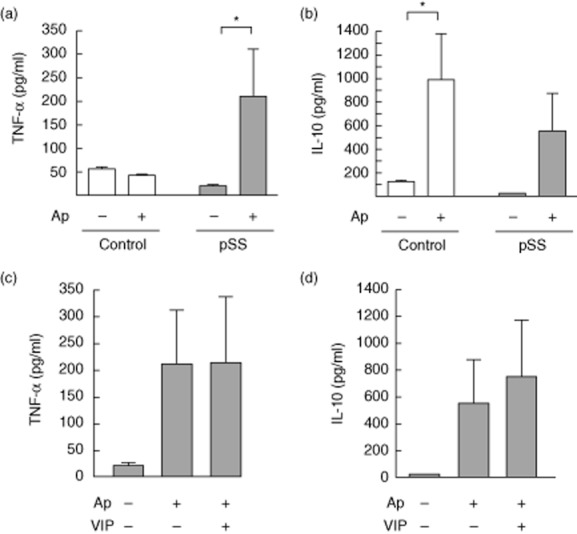
Inflammatory profile upon phagocytosis of apoptotic cells by primary Sjögren's syndrome (pSS) monocytes not modulated by vasoactive intestinal peptide (VIP). Monocytes from pSS patients or control subjects were incubated for an additional 24 h after phagocytosis of human salivary gland (HSG) apoptotic cells (Ap) or without apoptotic cells added (–) and the levels of tumour necrosis factor (TNF)-α (a) and interleukin (IL)-10 (b) were determined by enzyme-linked immunosorbent assay (ELISA). Values are mean ± s.e.m. of at least six different pSS or control monocytes. *P < 0·05. (c) Monocytes from pSS patients were incubated for an additional 24 h in the presence or absence of 100 nM VIP after phagocytosis of HSG Ap or without Ap added (–) and the levels of cytokines were determined by ELISA. Values are mean ± s.e.m. of six different pSS monocyte samples.
Discussion
In this study we present data to indicate that pSS patients' monocytes display a high expression of VPAC2 which can be modulated by LPS and VIP. Conversely, we showed that pSS patients' monocytes exhibit an impaired phagocytosis of apoptotic epithelial cells that could not be restored by VIP treatment in vitro. To our knowledge, there are no previous reports on apoptotic epithelial cell clearance defects by pSS patients' monocytes. Accordingly, our results indicate that TNF-α and IL-6 were released only by pSS monocytes when they phagocytized apoptotic HSG cells, consistent with a proinflammatory non-silent defective process. However, VIP did not improve phagocytosis of HSG apoptotic cells by pSS monocytes, nor did it contribute to a suppressant microenvironment by down-regulating TNF-α, IL-6 or enhancing IL-10.
A defect of in-vitro apoptotic cell clearance was described in systemic lupus erythematosus (SLE). Cultured macrophages differentiated from monocytes or stem cells of SLE patients displayed morphological abnormalities and showed an impaired phagocytosis of apoptotic cells [22,23]. Macrophages from SLE and rheumatoid arthritis (RA) patients were smaller, with less ability to differentiate and to adhere to apoptotic Jurkat T cells than control cells [43]. However, only SLE macrophages showed reduced engulfment of apoptotic cells, indicating that not only adherence and differentiation but multiple signals were impaired in the phagocytic process, similar to the results shown here for pSS monocytes, where phagocytosis of apoptotic HSG cells appears hampered at different levels. The observation that engulfment ability was reduced but not completely abolished in the short incubation time assayed here suggests that a basal impairment can be compensated in vitro through rapidly conveying monocyte signalling. Finally, our results on defective apoptotic epithelial cell clearance by macrophages is also consistent with previous observations in patients' glands and murine models of SS, where increased apoptosis of epithelial cells and aberrant expression of apoptosis markers and inflammatory mediators were proposed to have a role in the initiation and perpetuation of SS [14].
Previous observations in the NOD mouse model of SS indicated that VIP injected locally into NOD females at the SS-like stage reduced proinflammatory cytokines and normalized salivary secretory function [38]. Consistently, VIP promoted an alternative activation profile in NOD mice macrophages and favoured a suppressant apoptotic cell clearance by NOD macrophages [26,27]. However, which biological circuits will be mediated preferentially by VIP in vivo is not easily predictable, as was shown clearly by Abad and coworkers in VIP knock-out mice that are resistant to experimental autoimmune encephalitis and LPS-induced endotoxaemia [44,45].
Monocytes from pSS patients but not those from control subjects expressed VPAC2 receptors as revealed by qRT–PCR, and the expression of this subtype receptor was increased when the cells were stimulated in vitro with LPS. VPAC1 is the unique subtype expressed in normal human resting monocytes [46,47]. However, various stimuli are known to modulate VPAC expression in immune cells. In particular, the VPAC2 receptor subtype was up-regulated in mouse peritoneal macrophages and macrophage cell lines when they were primed in vitro with inflammatory stimuli through TLR-4 [42]. Here we showed that the effect of LPS on VPAC2 expression occurred only in pSS but not in normal monocytes, and that it averaged a threefold increase, in line with previous observations in murine macrophages. Interestingly, a higher expression of VPAC2 was observed on fibroblast-like synovial cells from rheumatoid arthritis patients compared with osteoarthritis patients [48], suggesting that receptor differential expression might regulate VIP local effects in vivo. The increased expression of VPAC2 receptors in resting pSS monocytes shown here might reflect a compensating mechanism operating in vivo.
Of note, the higher expression of VPAC2 receptors in pSS monocytes did not suffice to modulate their functional profile in the presence of VIP ex vivo. In fact, this enhanced VPAC2 expression, and even the expression of VPAC1 comparable to normal subjects' monocytes, could not favour a suppressant phagocytosis when the cells were treated with VIP in vitro. This observation strongly supports a prominent role for a deficient phagocytosis of apoptotic cells in the pathogenesis of pSS that is refractory to the anti-inflammatory effect of VIP. The observation also confirms that the anti-inflammatory efficacy of VIP is more evident when immune cells are primed in vitro with potent proinflammatory stimuli. Certainly, in rheumatoid arthritis and ostheoarthritis patients' synovial cells, VIP potently inhibited proinflammatory signals when cells were stimulated in vitro with poli I : C [49]. Similarly, in the present results, the inhibitory effect of VIP was stated only on VPAC2 induction by LPS.
Our results indicate collectively that pSS patients' monocytes highly express VPAC2 receptors and display an impaired phagocytic function of apoptotic epithelial cells that could not be modulated by VIP. The functional relevance of the higher expression of VPAC2 receptors in pSS monocytes and whether it is a compensatory mechanism or just an epiphenomenon due to damage-associated molecular pattern (DAMP) ligand stimulation of pSS monocytes in vivo is a matter of future studies. However, the consistency of this observation in pSS patients, regardless of the disease outcome and duration, and the absolute absence of VPAC2 expression in the control group monocytes with normal apoptotic cell clearance, suggest its potential as a functional biomarker in pSS.
Acknowledgments
V. H., L. F. and E. G. carried out all the experiments and statistical analysis. A. E. and O. H. followed-up pSS patients and provided blood samples. R. R., O. H. and C. P. L. designed the study, discussed the results and prepared the manuscript. All authors read and approved the final manuscript. This work was funded by the National Research Council CONICET (PIP 2012-2015); the University of Buenos Aires (UBACyT 20020100100505) and the National Agency of Sciences and Technology ANPCyT (PICT 2011-0144).
Disclosure
No conflicts of interest to declare.
References
- 1.García-Carrasco M, Fuentes-Alexandro S, Escárcega RO, Salgado G, Riebeling C, Cervera R. Pathophysiology of Sjögren's syndrome. Arch Med Res. 2006;37:921–932. doi: 10.1016/j.arcmed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Meijer JM, Meiners PM, Vissink A, et al. Effectiveness of rituximab treatment in primary Sjögren's syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:960–968. doi: 10.1002/art.27314. [DOI] [PubMed] [Google Scholar]
- 3.Konttinen YT, Fuellen G, Bing Y, et al. Sex steroids in Sjögren's syndrome. J Autoimmun. 2012;39:49–56. doi: 10.1016/j.jaut.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Tzioufas AG, Vlachoyiannopoulos PG. Sjogren's syndrome: an update on clinical, basic and diagnostic therapeutic aspects. J Autoimmun. 2012;39:1–3. doi: 10.1016/j.jaut.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Vitali C, B Bootsma H, Bowman SJ, et al. Classification criteria for Sjogren's syndrome: we actually need to definitively resolve the long debate on the issue. Ann Rheum Dis. 2013;72:476–478. doi: 10.1136/annrheumdis-2012-202565. [DOI] [PubMed] [Google Scholar]
- 6.Rosignoli F, Roca V, Meiss R, Leceta J, Gomariz RP, Pérez Leirós C. Defective signalling in salivary glands precedes the autoimmune response in the non-obese diabetic mouse model of sialadenitis. Clin Exp Immunol. 2005;142:411–418. doi: 10.1111/j.1365-2249.2005.02930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitsias DI, Kapsogeorgou EK, Moutsopoulos HM. Sjögren's syndrome: why autoimmune epithelitis? Oral Dis. 2006;12:523–532. doi: 10.1111/j.1601-0825.2006.01292.x. [DOI] [PubMed] [Google Scholar]
- 8.Manoussakis MN, Kapsogeorgou EK. The role of intrinsic epithelial activation in the pathogenesis of Sjögren's syndrome. J Autoimmun. 2010;35:219–224. doi: 10.1016/j.jaut.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Rosignoli F, Goren NB, Perez Leiros C. Alterations in nitric oxide synthase activity and expression in submandibular glands of NOD mice. Clin Immunol. 2001;101:86–93. doi: 10.1006/clim.2001.5097. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen KH, Brayer J, Cha S, et al. Evidence for anti-muscarinic acetylcholine receptor antibody-mediated secretory dysfunction in NOD mice. Arthritis Rheum. 2000;43:2297–2306. doi: 10.1002/1529-0131(200010)43:10<2297::AID-ANR18>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 11.Barrera MJ, Bahamondes V, Sepúlveda D, et al. Sjögren's syndrome and the epithelial target: a comprehensive review. J Autoimmun. 2013;42:7–18. doi: 10.1016/j.jaut.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Robinson CP, Yamachika S, Alford CE, et al. Elevated levels of cysteine protease activity in saliva and salivary glands of the nonobese diabetic (NOD) mouse model for Sjögren syndrome. Proc Natl Acad Sci USA. 1997;94:5767–5771. doi: 10.1073/pnas.94.11.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calafat M, Larocca L, Roca V, et al. Vasoactive intestinal peptide inhibits TNF-alpha-induced apoptotic events in acinar cells from nonobese diabetic mice submandibular glands. Arthritis Res Ther. 2009;11:R53. doi: 10.1186/ar2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voulgarelis M, Tzioufas AG. Pathogenetic mechanisms in the initiation and perpetuation of Sjögren's syndrome. Nat Rev Rheumatol. 2010;6:529–537. doi: 10.1038/nrrheum.2010.118. [DOI] [PubMed] [Google Scholar]
- 15.Jonsson R, Vogelsang P, Volchenkov R, Espinosa A, Wahren-Herlenius M, Appel S. The complexity of Sjögren's syndrome: novel aspects on pathogenesis. Immunol Lett. 2011;141:1–9. doi: 10.1016/j.imlet.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Kong L, Robinson CP, Peck AB, et al. Inappropriate apoptosis of salivary and lacrimal gland epithelium of immunodeficient NOD-scid mice. Clin Exp Rheumatol. 1998;16:675–681. [PubMed] [Google Scholar]
- 17.Nguyen CQ, Cornelius JG, Cooper L, et al. Identification of possible candidate genes regulating Sjögren's syndrome-associated autoimmunity: a potential role for TNFSF4 in autoimmune exocrinopathy. Arthritis Res Ther. 2008;10:R137–R137. doi: 10.1186/ar2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fadok VA, Bratton D, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 20.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlin LM, Stamatiades EG, Auffray C, et al. Nr4a1-dependent ly6c(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaipl US, Voll R, Sheriff A, Franz S, Kalden JR, Herrmann M. Impaired clearance of dying cells in systemic lupus erythematosus. Autoimmun Rev. 2005;4:189–194. doi: 10.1016/j.autrev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Shao WH, Cohen P. Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Arthritis Res Ther. 2011;13:202. doi: 10.1186/ar3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumann I, Kolowos W, Voll RE, et al. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:191–201. doi: 10.1002/1529-0131(200201)46:1<191::AID-ART10027>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 25.Stoffels K, Overbergh L, Giulietti A, et al. NOD macrophages produce high levels of inflammatory cytokines upon encounter of apoptotic or necrotic cells. J Autoimmun. 2004;23:9–15. doi: 10.1016/j.jaut.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Hauk V, Calafat M, Larocca L, et al. Vasoactive intestinal peptide/vasoactive intestinal peptide receptor relative expression in salivary glands as one endogenous modulator of acinar cell apoptosis in a murine model of Sjögren's syndrome. Clin Exp Immunol. 2011;166:309–316. doi: 10.1111/j.1365-2249.2011.04478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larocca L, Hauk V, Calafat M, et al. Modulation of macrophage inflammatory profile in pregnant nonobese diabetic (NOD) mice. Mol Cell Endocrinol. 2011;333:112–118. doi: 10.1016/j.mce.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 28.Delgado M, Munoz-Elias E, Kan Y, et al. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit tumor necrosis factor alpha transcriptional activation by regulating nuclear factor-KB and cAMP response element-binding protein/c-Jun. J Biol Chem. 1998;273:31427–31436. doi: 10.1074/jbc.273.47.31427. [DOI] [PubMed] [Google Scholar]
- 29.Dorsam G, Voice J, Kong Y, Goetzl EJ. Vasoactive intestinal peptide mediation of development and functions of T lymphocytes. Ann NY Acad Sci. 2000;921:79–91. doi: 10.1111/j.1749-6632.2000.tb06953.x. [DOI] [PubMed] [Google Scholar]
- 30.Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7:563–568. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- 31.Voice JK, Grinninger C, Kong Y, Bangale Y, Paul S, Goetzl EJ. Roles of vasoactive intestinal peptide (VIP) in the expression of different immune phenotypes by wild-type mice and T cell-targeted type II VIP receptor transgenic mice. J Immunol. 2003;170:308–314. doi: 10.4049/jimmunol.170.1.308. [DOI] [PubMed] [Google Scholar]
- 32.Gomariz RP, Arranz A, Juarranz Y, et al. Regulation of TLR expression, a new perspective for the role of VIP in immunity. Peptides. 2007;28:1825–1832. doi: 10.1016/j.peptides.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Arranz A, Abad C, Juarranz Y, Leceta J, Martinez C, Gomariz RP. Vasoactive intestinal peptide as a healing mediator in Crohn's disease. Neuroimmunomodulation. 2008;15:46–53. doi: 10.1159/000135623. [DOI] [PubMed] [Google Scholar]
- 34.Rosignoli F, Torroba M, Juarranz Y, et al. VIP and tolerance induction in autoimmunity. Ann NY Acad Sci. 2006;1070:525–530. doi: 10.1196/annals.1317.073. [DOI] [PubMed] [Google Scholar]
- 35.Tan YV, Waschek JA. Targeting VIP and PACAP receptor signalling: new therapeutic strategies in multiple sclerosis. ASN Neuro. 2011;3:pii: e00065. doi: 10.1042/AN20110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez-Rey E, Delgado M. Vasoactive intestinal peptide and regulatory T-cell induction: a new mechanism and therapeutic potential for immune homeostasis. Trends Mol Med. 2007;13:241–251. doi: 10.1016/j.molmed.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Pozo D, Gonzalez-Rey E, Chorny A, Anderson P, Varela N, Delgado M. Tuning immune tolerance with vasoactive intestinal peptide: a new therapeutic approach for immune disorders. Peptides. 2007;28:1833–1846. doi: 10.1016/j.peptides.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lodde BM, Mineshiba F, Wang J, et al. Effect of human vasoactive intestinal peptide gene transfer in a murine model of Sjogren's syndrome. Ann Rheum Dis. 2006;65:195–200. doi: 10.1136/ard.2005.038232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larocca L, Calafat M, Roca V, Franchi AM, Pérez Leiros C. VIP limits LPS-induced nitric oxide production through IL-10 in NOD mice macrophages. Int Immunopharmacol. 2007;7:1343–1349. doi: 10.1016/j.intimp.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 40.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aframian DJ, Cukierman E, Nikolovski J, Mooney DJ, Yamada KM, Baum BJ. The growth and morphological behavior of salivary epithelial cells on matrix protein-coated biodegradable substrata. Tissue Eng. 2000;6:209–216. doi: 10.1089/10763270050044380. [DOI] [PubMed] [Google Scholar]
- 42.Herrera JL, Gonzalez-Rey E, Fernandez-Montesinos R, Quintana FJ, Najmanovich R, Pozo D. Toll-like receptor stimulation differentially regulates vasoactive intestinal peptide type 2 receptor in macrophages. J Cell Mol Med. 2009;13:3209–3217. doi: 10.1111/j.1582-4934.2009.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tas SW, Quartier P, Botto M, Fossati-Jimack L. Macrophages from patients with SLE and rheumatoid arthritis have defective adhesion in vitro, while only SLE macrophages have impaired uptake of apoptotic cells. Ann Rheum Dis. 2006;65:216–221. doi: 10.1136/ard.2005.037143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abad C, Tan YV, Lopez R, et al. Vasoactive intestinal peptide loss leads to impaired CNS parenchymal T-cell infiltration and resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2010;107:19555–19560. doi: 10.1073/pnas.1007622107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abad C, Tan YV, Cheung-Lau G, Nobuta H, Waschek J. VIP deficient mice exhibit resistance to lipopolysaccharide induced endotoxemia with an intrinsic defect in proinflammatory cellular responses. PLOS ONE. 2012;7:e36922. doi: 10.1371/journal.pone.0036922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lara-Marquez M, O'Dorisio M, O'Dorisio T, Shah M, Karacay B. Selective gene expression and activation-dependent regulation of vasoactive intestinal peptide receptor type 1 and type 2 in human T cells. J Immunol. 2001;166:2522–2530. doi: 10.4049/jimmunol.166.4.2522. [DOI] [PubMed] [Google Scholar]
- 47.Couvineau A, Laburthe M. VPAC receptors: structure, molecular pharmacology and interaction with accessory proteins. Br J Pharmacol. 2012;166:42–50. doi: 10.1111/j.1476-5381.2011.01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juarranz Y, Gutiérrez-Cañas I, Santiago B, Carrión M, Pablos JL, Gomariz RP. Differential expression of vasoactive intestinal peptide and its functional receptors in human osteoarthritic and rheumatoid synovial fibroblasts. Arthritis Rheum. 2008;58:1086–1095. doi: 10.1002/art.23403. [DOI] [PubMed] [Google Scholar]
- 49.Carrión M, Juarranz Y, Pérez-García S, et al. RNA sensors in human osteoarthritis and rheumatoid arthritis synovial fibroblasts: immune regulation by vasoactive intestinal peptide. Arthritis Rheum. 2011;63:1626–1636. doi: 10.1002/art.30294. [DOI] [PubMed] [Google Scholar]


