Abstract
An exacerbated type 1 response to leishmanial antigens is the basis of tissue destruction observed in mucosal leishmaniasis (ML). After therapy, a persistent production of high levels of inflammatory cytokines can confer a poor prognosis. Herein we investigated whether the clinical conditions defined during the active phase of ML affect the magnitude of long-term anti-Leishmania immune response. Twenty clinically cured ML cases were studied. Peripheral blood mononuclear cells (PBMC) were cultured with L. braziliensis antigens (Lb-Ag), Toxoplasma gondii antigens (Tg-Ag), concanavalin-A (Con-A) or medium alone, and the lymphocyte proliferative response and cytokine secretion were quantified. Medical records were reviewed for Montenegro skin test (MST) during diagnosis, duration of ML disease or time elapsed after clinical cure. The duration of disease was correlated positively with MST (r = 0·61). Lb-Ag induced interferon (IFN)-γ was correlated positively with duration of illness (r = 0·69) as well as the frequency of secreting cells [enzyme-linked immunospot (ELISPOT)] assay. No association was observed for Tg-Ag or Con-A. Disease duration was correlated negatively with interleukin (IL)-10 production (r = −0·76). Moreover, a negative correlation between length of time after clinical cure and TNF levels (r = −0·94) or the IFN-γ : IL-10 ratio (r = −0·89) were also seen. We suggest that the magnitude of the IFN-γ inflammatory response triggered by ML can be driven by the time of leishmanial antigens exposition during the active phase of the disease. This pattern could persist even long-term after cure. However, despite IFN-γ levels, the decrease of the TNF and IFN-γ : IL-10 ratio reflects the control of proinflammatory responses achieved by cure of ML, possibly preventing disease relapses.
Keywords: clinical cure, duration of illness, interferon-γ, interleukin-10, mucosal leishmaniasis
Introduction
Several reports have shown unequivocally that an exacerbated effector response directed to leishmanial antigens is the basis of severe tissue destruction observed in mucosal leishmaniasis (ML) patients. The magnitude of T cell responses to Leishmania tends to be higher in ML than in cutaneous leishmaniasis (CL) in terms of the ability of lymphocytes to proliferate [1], the frequency of leishmanial-reactive lymphocytes in tissues [2], the levels of interferon (IFN)-γ and tumour necrosis factor (TNF) [1,3–5], parasite-specific cytotoxicity [6,7] and the activation status of circulating T lymphocytes [8,9]. Besides the higher inflammatory type 1 effector responses, type 2 immune responses also increase upon infection with Leishmania, which probably contributes to parasite replication [9–11]. A deficient regulatory function related to the low production of IL-10 [10] and a deficit in the expression of interleukin (IL)-10 receptor [7] may be key factors accounting for deregulated types 1 and 2 immune responses in ML.
Despite recent advances in our understanding of ML, the mechanisms triggering exacerbated immune responses that lead to mucosal lesions remain unknown. Studies have shown that genetic factors driving high TNF and IL-6 cytokine expression are potentially associated with mucosal involvement [12,13]. However, the IFNG+874T/A polymorphism was not connected with either susceptibility or severity to leishmaniasis [14]. The IL-17 is probably another important cytokine that can contribute to chronic inflammatory disease detected in ML [15].
Given the characteristics of ML immunopathogenesis, patients usually respond poorly to anti-leishmanial treatments. In addition, relapse may occur in up to 50% of treated ML patients [16–18]. Until now, mucosal relapse is still unpredictable based on clinical observations [19,20], but patients presenting low in-situ expression of IL-10 and higher cytotoxic cells [TCD8+ and natural killer (NK)] in active lesions have an increased risk for recurrence of mucosal lesions [17]. High and low levels of IFN-γ have been observed in long-term cured ML patients, without correlation to higher or lower IFNG+874T/A genotype patterns. This indicates that a higher sustained IFN-γ production of this cytokine can be dependent upon other factors which potentially drive the IFN-γ secretion upon leishmanial stimuli [14], then influencing the prognostic of ML post-therapy. The hypothesis to be considered is that the period of disease reflects the time of exposition to parasite antigens, with consequent immune system stimuli. Conversely, as the disease is controlled, a decrease in leishmanial stimuli leads to a progressive reduction of lymphocyte activation.
In attempts to identify factors driving the production of high levels of IFN-γ by long-term patients after ML cure, we evaluated whether the magnitude of T lymphocyte reactivity to Leishmania-antigens is affected by clinical parameters such as the time–course of active ML or the length of time after clinical cure.
Material and methods
Patients, ethics statement and isolation of peripheral blood mononuclear cells (PBMC)
Twenty subjects with a past history of mucosal leishmaniasis followed regularly at the outpatient care unit of Hospital das Clínicas/USP or IPEC/FIOCRUZ were enrolled into this study. Medical records of the patients were reviewed for clinical and laboratorial parameters, such as delayed-type hypersensitivity response (leishmanin skin test/Montenegro skin test: MST). Patients were treated with antimonial therapy according to the guidelines of Brazilian Ministry of Health [19] and were considered clinically cured 1 year after the end of therapy. Mucosal healing was defined as macroscopic absence of hyperaemia, oedema, ulceration and granulomatous or vegetative lesion, determined by clinical and endoscopic rhinolaryngological examination [17,20]. As relapses of ML usually occur up to 5 years after clinical cure post-therapy [21–23], this time-point was used to classify patients into two groups: group 1, less than 5 years of the end of treatment (3·2 ± 1·2 years, median 3·4 years, n = 10); and group 2, more than or equal to 5 years of the end of treatment (8·2 ± 3·7 years, median 7·0 years, n = 10). None of these subjects had episodes of recurrence after being considered clinically cured. No difference regarding age was seen between these groups, and the gender distribution was similar (Table 1).
Table 1.
Lymphocyte proliferation and interferon (IFN)-γ production in response to Leishmania (Viannia) braziliensis antigens in clinically cured mucosal leishmaniasis patients evaluated in different periods after the end of therapy (less than and more than 5 years)
| Mononuclear cells responses/profile upon stimulation with Leishmania braziliensis antigens | |||||
|---|---|---|---|---|---|
| Period of clinical cure | Age (years)/gender | LPR (SI) | T CD4+ (%) | T CD8+ (%) | IFN-γ (pg/ml) |
| < 5 years (n = 10) | 56·4 ± 15·8 | 26·8 ± 24·8 | 36·3 ± 16·4 | 20·5 ± 9·1 | 5,770 ± 6,284 |
| 4M/6F | |||||
| ≥ 5 years (n = 10) | 58·5 ± 11·6 | 13·2 ± 21·0 | 24·3 ± 10·2 | 16·8 ± 9·2 | 4,992 ± 8,707 |
| 6M/4F | |||||
Results expressed as means ± standard deviation. No statistical differences were observed between the groups. F = female; LPR = lymphocyte proliferative responses; M = male; SI = stimulation index.
Written informed consent was obtained from all participants. This protocol was approved by the Ethical Committee for Human Research from Fundação Oswaldo Cruz, Ministério da Saúde, Brazil, as well as abiding by the Helsinki Declaration on human subject research (CEP FIOCRUZ P007/2011, protocol 0048·0.009·000-10).
Heparinized venous blood (maximum volume of 20 ml) was withdrawn from subjects and PBMC were purified by a Ficoll-Hypaque gradient (Sigma Chemical Co., St Louis, MO, USA). Cells were analysed in terms of lymphocyte proliferative response (LPR), T cell phenotypic characterization and cytokine profile.
Lymphocyte proliferative response to leishmanial antigens
For LPR, PBMC (3 × 105/well, final volume of 200 μl) were cultured for 5 days in the presence of disrupted promastigotes of L. (V.) braziliensis (MHOM/BR/75/M2903, Lb-Ag; 10 μg/well, an equivalent of 106 parasites), disrupted tachyzoites of Toxoplasma gondii (Tg-Ag, 2 × 106 parasites/well), mitogen (concanavalin A: ConA, 4 μg/well; Sigma) or medium alone, as described previously [11]. [3H]-thymidine (1 μCi/well; Amersham International, Amersham, UK) was added to the wells and radioactivity uptake was measured in a scintillation beta counter (1600CA; Packard Instrumental Company, Downers Grove, IL, USA). Results were expressed as the stimulation index (SI), defined as the mean counts per minute (cpm) in wells containing antigen or mitogen divided by the background (mean counts in non-stimulated wells). Indices equal to or higher than 2·5 were considered positive. The background ranged from 92 to 508 cpm throughout the study. Culture supernatants were collected at different times and stored at −20°C until measurement of cytokine levels.
Phenotypical analysis of Leishmania braziliensis-reactive cells
PBMC (3 × 106 cells) were cultured in the presence of 50 μg of Lb-Ag under the conditions described elsewhere [11]. After 5 days in culture, blast cells were separated by a discontinuous Percoll (Sigma) gradient, washed twice and adjusted to 106 cells/200 μl in cold phosphate-buffered saline (PBS) containing 0·01% of sodium azide and 10% of fetal calf serum. Cells were labelled with 5 μl of monoclonal antibodies for CD3+ (T3-RD1), CD4+ [T4-fluorescein isothiocyanate (FITC)] and CD8+ [T8-phycoerythrin-cyanin 5 (PE-Cy5)] (Coulter Diagnosis, Hialeah, FL, USA). Cells were defined by forward- and side-scatter gating. Thirty thousand events were acquired in each sample run and the data were analysed with EXPO32™ software in an EPICS ALTRA flow cytometer (Beckman-Coulter, Miami, FL, USA).
Cytokine measurement
Cytokines in culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA). PBMC were stimulated in vitro for 1 day (TNF, IL-18), 3 days (IL-10, IL-5) and 5 days (IFN-γ), with Leishmania antigens. The monoclonal antibodies were purchased from Pharmingen (San Diego, CA, USA; IFN-γ, TNF, IL-10, IL-5) or R&D Systems (Minneapolis, MN, USA; IL-18). The procedures were performed according to the manufacturer's instructions. Samples were tested in duplicate and cytokine concentrations were determined using the softmax®pro version 4·0 program (Life Sciences Edition, Molecular Devices Corporation, USA). The minimum cytokine levels detected were 62·5 pg/ml for IFN-γ, 31·2 pg/ml for TNF or IL-10 and 15·6 pg/ml for IL-5 and IL-18. For each patient, not all cytokines were measured due to the variable amount of collected peripheral blood and consequently the yield of purified mononuclear cells.
Quantitation of IFN-γ-producing lymphocytes by enzyme-linked immunospot (ELISPOT) assay
For quantitative determination of the frequency of cells releasing IFN-γ, a commercial kit (ELISPOT human IFN-γ; R&D Systems) was utilized. Patients with duration of illness varying from 8 to 36 months were selected (Table 2). In brief, PBMC in complete RPMI medium were plated onto a polyvinylidene difluoride microplate coated with IFN-γ-specific capture monoclonal antibody. Four duplicated dilutions were used for each in-vitro stimulus or medium alone (2 × 105, 1 × 105, 2 × 104 and 1 × 104 cells per well). Parasite antigens and mitogen were used at the same final concentrations used in the LPR assays. Tg-Ag and Con-A were used as control. Cells were incubated for 72 h at 37°C in a humidified atmosphere of 5% CO2. After washing, biotinylated polyclonal antibodies specific for human IFN-γ were added to the wells. Alkaline phosphatase-conjugated streptavidin and a substrate solution (BCIP/NBT) were used to reveal the reaction. Blue-black-coloured precipitate spots appeared at the sites of cytokine localization, with each individual spot representing an individual IFN-γ-secreting cell. The spots were counted in an automated ELISPOT reader system (ImmunoSpot® image analyser; Cellular Technology Ltd, Cleveland, OH, USA). The results (total number of spots and the spot-size) were calculated using immunospot software (ImmunoSpot version 3·2) and expressed as the number of spot-forming cells (SFC) per number of cells (we have chosen 2 × 105 cell/ml dilution) and mean size of spots in mm2.
Table 2.
Frequencies of interferon (IFN)-γ-producing cells in response to Leishmania (Viannia) braziliensis antigens and other unrelated stimuli maintained in the blood of cured mucosal leishmaniasis long-term after therapy
| IFN-γ-producing cells by ELISPOT assays | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean number of spots‡ per 2 × 105 cells | Mean size of spots (mm2)‡ | |||||||||||
| Case number | Period of illness | Control§ | Con-A§ | Lb-Ag§ | Tg-Ag§ | Con-A | Lb-Ag | Tg-Ag | ||||
| Pt 20 | 8 m | 0 |  |
162 |  |
2 |  |
64 |  |
0·18 | 0·20 | 0·09 |
| Pt 17 | 12 m | 1 |  |
317 |  |
3 |  |
10·5 |  |
0·17 | 0·10 | 0·06 |
| Pt 21 | 24 m | 162 |  |
328 |  |
68 |  |
381 |  |
0·23 | 0·26 | 0·32 |
| Pt 14 | 36 m | 317 |  |
401 |  |
85 |  |
7·5 |  |
0·13 | 0·19 | 0·11 |
†,‡ The spots of IFN-γ-producing cells were quantified by enzyme-linked immunospot (ELISPOT) assays in peripheral blood mononuclear cells (PBMC) cultures in the absence (control) or presence of exogenous stimuli. §The pictures are representative of the duplicate of the wells obtained from each patient after the cells were stimulated with such antigen. Con-A = convanavalin A; Lb-Ag = Leishmania braziliensis antigens; m = months; Pt = patient; Tg-Ag = Toxoplasma gondii antigens.
Statistical analysis
The comparisons between groups of < 5 years and ≥ 5 years of clinical cure were performed using the Mann–Whitney U-test for non-parametric values. Spearman's test was used for correlation of non-parametric values. The data were analysed by using GraphPad Prism version 5·01 for Windows (GraphPad Software, San Diego, CA, USA), and the results were considered statistically different when P-value was ≤ 0·05. Results are expressed as mean ± standard deviation and/or median.
Results
The immune response primed during the active mucosal disease can affect the long-lasting production of cytokines
The MST intensity performed at the diagnosis time was correlated positively with the duration of the active disease (r = 0·61, P = 0·004, n = 20; Fig. 1), suggesting that leishmanial-antigen-triggered T lymphocyte responses could be shaped during the active phase of the parasitic infection. Then, we further evaluated whether the magnitude of the recall response to leishmanial antigens in long-term cured ML patients was also influenced by the time of ML evolution.
Figure 1.
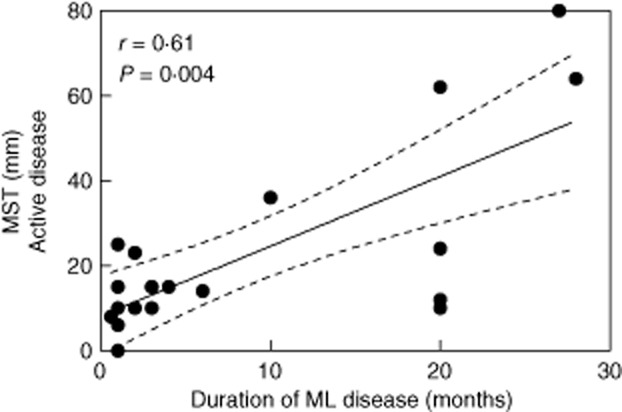
Delayed-type hypersensitivity to leishmanial antigens (Montenegro skin test: MST) is associated with the duration of illness in active mucosal leishmaniasis (ML) patients. The graph shows the best-fitted lines with 95% confidence intervals. R = correlation coefficient; P = significance level. Each point represents one subject (n = 20).
The IFN-γ production in response to stimulation with Lb-Ag was correlated positively with the duration of the ML disease (r = 0·69; P = 0·0008; n = 20; Fig. 2a). In contrast, no association was observed when PBMC of the same patients were stimulated with Tg-Ag (Fig. 2b) or mitogen (data not shown).
Figure 2.
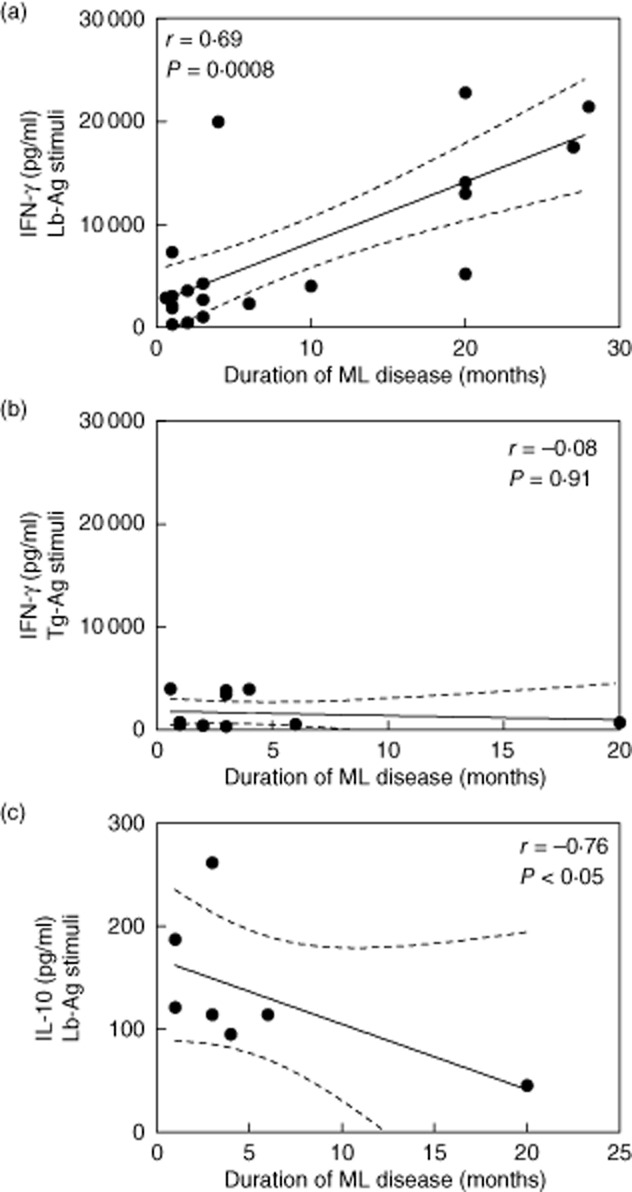
Levels of cytokines produced by long-term cured mucosal leishmaniasis (ML) subjects in correlation with the duration of illness of these active ML patients. Peripheral blood mononuclear cells were stimulated in-vitro with Leishmania braziliensis antigens (Lb-Ag) or Toxoplasma gondii antigens (Tg-Ag). The production of interferon (IFN)-γ (a, n = 20; b, n = 12) or interleukin (IL)-10 (c, n = 7) were quantified in the cell supernatants after 5 and 3 days culture, respectively. The graphs show the best-fitted lines with 95% confidence intervals. R = correlation coefficient; P = significance level. Each point represents one subject.
The frequency of IFN-γ secreting cells (SFC) from four patients is shown in Table 2. Patients whose disease lasted longer than 24 months had higher numbers of SFC in response to Lb-Ag stimuli than patients who had a shorter disease duration (Table 2). Consistently, such an association was not observed when cells were stimulated by Tg-Ag or Con-A. In all patients, the mean size of the spots was not related to the number of spots (Table 2).
We also observed a negative correlation between disease duration and IL-10 production (r = −0·76, P < 0·05, n = 7; Fig. 2c).
Taken together, these results indicate that the intensity of IFN-γ production by ML patients long-term after their clinical cure is primed during the active phase of the disease and that this response is specific for leishmanial antigens.
Influence of the length of time after clinical cure on the intensity of T cell responses against leishmanial antigens
To evaluate the influence of the length of time after clinical cure on T cell responses, lymphocyte proliferation and production of IFN-γ in response to stimulation by Lb-Ag were analysed. As shown in Table 1, no significant differences were observed between the groups of patients evaluated up to or more than 5 years after completion of treatment.
Analysis of cytokine production by PBMC from cured ML patients revealed that low and high IFN-γ producers (> 10 000 pg/ml) were found in both groups (data not shown). Regarding other proinflammatory cytokines produced under Lb-Ag stimuli, eight of nine patients analysed produced IL-18 (311·1 ± 234·7 pg/ml; median = 320 pg/ml; n = 8), but no correlation was observed between this cytokine and IFN-γ levels (n = 9). Conversely, a negative correlation between the levels of TNF and the time after clinical cure was seen (r = −0·94, P = 0·01, n = 5; Fig. 3a).
Figure 3.
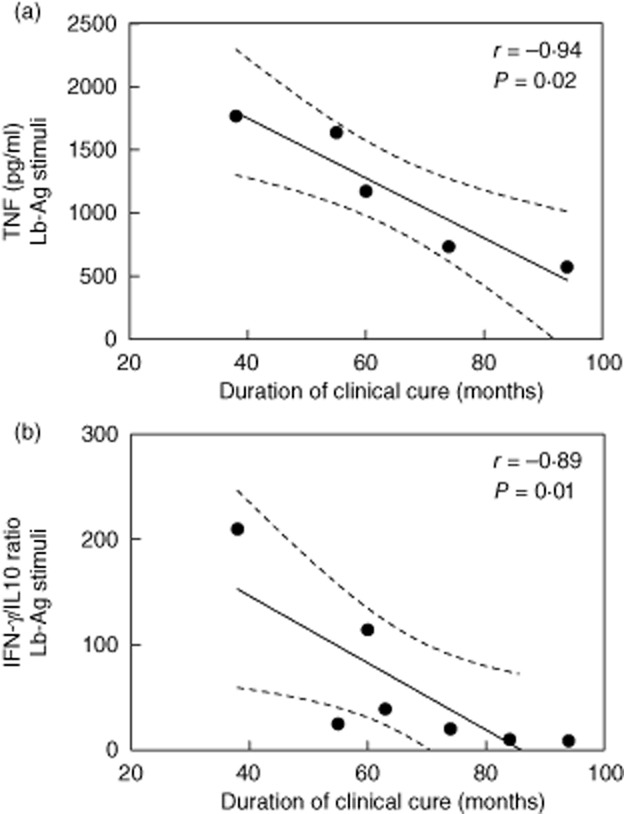
The production of tumour necrosis factor (TNF) levels and interferon (IFN)-γ : interleukin (IL)-10 ratio by long-term cured mucosal leishmaniasis (ML) subjects were correlated inversely with duration of clinical cure. Peripheral blood mononuclear cells were stimulated in-vitro with Leishmania (Viannia) braziliensis antigens (Lb-Ag). The production of IFN-γ, TNF or IL-10 was quantified in the cell supernatants after 5 and 3 days in culture, respectively. (a) (TNF) and (b) (IFN-γ : IL-10 ratio) show the best-fitted lines with 95% confidence intervals. R = correlation coefficient; P = significance level. Each point represents one subject.
A counter-regulatory IL-10 production was also detected (79·7 ± 48·5 pg/ml; median = 79·7 pg/ml; n = 7) in both groups. Interestingly, the IFN-γ : IL-10 ratio correlated negatively with time after clinical cure (r = −0·89; P = 0·01; n = 7; Fig. 3b). Eight of 10 patients produced IL-5 (105·3 ± 106·5 pg/ml; median = 70 pg/ml; n = 8). Again, except for TNF, no significant association was observed between these cytokines with the time after clinical cure.
Discussion
In this study, we have demonstrated that the intensity of IFN-γ and IL-10 production by cured ML patients was associated with disease duration. Moreover, the time of clinical cure did not affect Leishmania-induced IFN-γ levels, but was correlated inversely with the IFN-γ : IL-10 ratio and TNF production. These results indicate that the profile of these cytokines produced after cure is shaped during the active phase of the disease and that proinflammatory responses were progressively down-modulated after therapy.
The recurrence of ML is estimated to occur within the first 5 years after clinical cure in approximately 15–50% of cases, even after successful antimonial therapy [17,18]. The exacerbated immune response developed by ML patients may account for this unfavourable prognosis [1,8–11]. After clinical cure, ML patients take longer to achieve the equilibrium of Leishmania-reactive TCD4+ : CD8+ lymphocyte ratio than CL patients [11]. Indeed, it has been shown that lymphocytes from some ML patients continued to produce high levels of IFN-γ upon leishmanial stimulus even long-term after healing [11].
Regardless of the time of clinical cure after therapy (less or more than 5 years), ML patients exhibited similar LPR indices, IFN-γ production and TCD4+ : CD8+ lymphocyte ratio in response to Leishmania antigens. Nevertheless, all of them remained cured. These results suggest that an effective modulation of effector immune response to leishmanial antigens is difficult to be achieved by ML patients. An ineffective modulation of the immune responses is also observed during active ML [7,8,10] and can persist along the clinical cure, contributing to the recurrence of mucosal leishmaniasis [5].
IFN-γ and TNF are the main proinflammatory cytokines presumably associated with hyperresponsiveness in active ML [1,3,4,8,10]. Herein we showed that cells from cured ML patients maintain the ability to produce IFN-γ and TNF in response to leishmanial antigens. However, the levels of IL-18, a known inducer of T helper type 1 (Th1) immune responses, were similar in these patients, in agreement with data reported for tuberculosis patients [24]. Given that there was no correlation between IL-18 and IFN-γ levels, IL-18 could not be regarded as the regulator, or at least as the only regulator of IFN-γ production in cured ML patients. It is important to consider that IL-18 function also depends upon a complex network of signals, such as a secondary stimulus by IL-12 [24,25].
The immune response recall with preferential production of type 1 cytokines may be beneficial to the host, due to their importance in effector responses against possible reactivated parasites after clinical cure. However, high levels of these cytokines, along with a lack in their regulatory mechanisms, can lead to tissue damage during an eventual parasite reactivation. The IFN-γ : IL-10 ratio has been considered as an important parameter to predict the clinical evolution or the responses to therapy [26]. IL-10 can counterbalance the proinflammatory effect of IFN-γ, leading to an adequate regulation of immune responses [26], thus preventing recurrence of leishmaniasis. Indeed, a higher IFN-γ : IL-10 ratio has been observed in the mucosal lesions of patients who relapsed after treatment compared to those who presented a stable clinical cure [17]. These data support the idea that the immune profile of patients with more severe mucosal leishmaniasis is prone to be more inflammatory throughout the course of the disease.
In the current investigation, over time we observed a gradual modulation of inflammatory cytokine production after clinical cure. Clinically cured ML patients maintained the production of IL-10, which was influenced by the disease duration. TNF production, as well as the IFN-γ : IL-10 ratio, were correlated inversely with the time elapsed after clinical cure. These results constitute important evidence for the regulation of immune responses in these patients, which was associated potentially with sustained ML cure.
It is known that an intrinsic characteristic such as genetic background can also influence the production of cytokines, as demonstrated for TNF and IL-6, whose exacerbated production favoured tissue damage in ML [12,13]. Decreased IL-10 production [10], low expression of the IL-10 receptor [7] or enhanced expression of the IFN-γ receptor [27] have also been associated with mucosal disease. A study has shown that, under similar in-vitro priming experimental conditions, cells from some healthy individuals produced high levels of IFN-γ, while cells from other individuals produced low levels of the cytokine upon stimulation with Leishmania [28].
Herein, we observed a positive correlation between the duration of ML disease and IFN-γ production. This response was specific for leishmanial antigens, as no correlation was found when Toxoplasma antigens were used as stimuli. A positive association between time–course of the disease and the MST results at diagnosis was also observed, highlighting the importance of time of exposure to Leishmania antigens during active disease. Reinforcing these data, the frequency of IFN-γ-producing cells was related directly to the duration of illness. These results indicate that the magnitude of IFN-γ production is triggered by leishmanial stimuli during the active disease. In an observational study [29], in which 80% of patients with tegumentary leishmaniasis that progressed to ML had a cutaneous lesion for more than 1 year [30], the authors hypothesized that prolonged stimulation of the immune system during the primary disease, due probably to a high antigenic load rather than the absence of therapeutic intervention, may have contributed to a strong adaptive response. Studies performed in the in the 1980s indicated the duration of illness as a determinant factor for the magnitude of lymphocyte responsiveness to parasite antigens [1]. The long-term persistence of the parasite stimulus could then predispose to hyperactivated immune responses against Leishmania.
In conclusion, the results obtained in this study favour the hypothesis that the pattern of immune response developed during the active ML disease is determinant for its clinical prognosis. In cured ML patients, the amount of inflammatory (IFN-γ) and regulatory (IL-10) cytokines produced in response to Leishmania is influenced by clinical variables, such as the time–course, during active disease. Also, the time elapsed after clinical cure may contribute, in direct proportion, to an appropriate modulation of the immune response, essential to the control of parasite replication without triggering tissue damage.
Acknowledgments
The authors thank Dr A. Bertho for flow cytometric analysis; Dr J. C. Lima Jr for ELISPOT reading and image capture and Dr David William Provance Junior for the English critical review. We are also grateful to Ms R. Pellegrino for secretarial assistance and Ms A. Saavedra for their valuable suggestions. This work was funded by IOC/FIOCRUZ internal funds, PAPESIV/VPPDT/FIOCRUZ and by FAPERJ APQ-1. A. G.-S. is a PhD sponsored by CNPq/FIOCRUZ research visitor programme. A. M. D.-C. is a CNPq and FAPERJ (CNE) research fellow.
Disclosures
The authors declare no financial or commercial conflict of interests.
Author contributions
R. S. N., R. C. B. and D. S. M. carried out the immunological studies and participated in the data acquisition. R. S. N. and A. G.-S. participated in the analysis and interpretation of data, drafting the manuscript or revising it critically for important intellectual content. V. S. A., M. S. M. and M. P. O.-N. collected the clinical data and accompanied the patients. S. G. C. and A. M. D.-C. conceived the study and participated in its design and co-ordination and helped to draft the manuscript. All authors read and approved the final manuscript.
References
- 1.Carvalho EM, Johnson WD, Barreto E, et al. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J Immunol. 1985;135:4144–4148. [PubMed] [Google Scholar]
- 2.Conceição-Silva F, Dórea RC, Pirmez C, Schubach A, Coutinho SG. Quantitative study of Leishmania braziliensis braziliensis reactive T cells in peripheral blood and in the lesions of patients with American mucocutaneous leishmaniasis. Clin Exp Immunol. 1990;79:221–226. doi: 10.1111/j.1365-2249.1990.tb05182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Da-Cruz AM, Oliveira MP, De Luca PM, Mendonça SC, Coutinho SG. Tumor necrosis factor-alpha in human American tegumentary leishmaniasis. Mem Inst Oswaldo Cruz. 1996;91:225–229. doi: 10.1590/s0074-02761996000200019. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro-de-Jesus A, Almeida RP, Lessa H, Bacellar O, Carvalho EM. Cytokine profile and pathology in human leishmaniasis. Braz J Med Biol Res. 1998;31:143–148. doi: 10.1590/s0100-879x1998000100020. [DOI] [PubMed] [Google Scholar]
- 5.Amato VS, Andrade HF, Duarte MI. Mucosal leishmaniasis: in situ characterization of the host inflammatory response, before and after treatment. Acta Trop. 2003;85:39–49. doi: 10.1016/s0001-706x(02)00260-7. [DOI] [PubMed] [Google Scholar]
- 6.Brodskyn CI, Barral A, Boaventura V, Carvalho E, Barral-Netto M. Parasite-driven in vitro human lymphocyte cytotoxicity against autologous infected macrophages from mucosal leishmaniasis. J Immunol. 1997;159:4467–4473. [PubMed] [Google Scholar]
- 7.Faria DR, Gollob KJ, Barbosa J, Jr, et al. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect Immun. 2005;73:7853–7859. doi: 10.1128/IAI.73.12.7853-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaze ST, Dutra WO, Lessa M, et al. Mucosal leishmaniasis patients display an activated inflammatory T-cell phenotype associated with a nonbalanced monocyte population. Scand J Immunol. 2006;63:70–78. doi: 10.1111/j.1365-3083.2005.01707.x. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho LP, Passos S, Bacellar O, et al. Differential immune regulation of activated T cells between cutaneous and mucosal leishmaniasis as a model for pathogenesis. Parasite Immunol. 2007;29:251–258. doi: 10.1111/j.1365-3024.2007.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacellar O, Lessa H, Schriefer A, et al. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70:6734–6740. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Da-Cruz AM, Bittar R, Mattos M, et al. T-cell-mediated immune responses in patients with cutaneous or mucosal leishmaniasis: long-term evaluation after therapy. Clin Diagn Lab Immunol. 2002;9:251–256. doi: 10.1128/CDLI.9.2.251-256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabrera M, Shaw MA, Sharples C, et al. Polymorphism in tumor necrosis factor genes associated with mucocutaneous leishmaniasis. J Exp Med. 1995;182:1259–1264. doi: 10.1084/jem.182.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castellucci L, Menezes E, Oliveira J, et al. IL6 -174 G/C promoter polymorphism influences susceptibility to mucosal but not localized cutaneous leishmaniasis in Brazil. J Infect Dis. 2006;194:519–527. doi: 10.1086/505504. [DOI] [PubMed] [Google Scholar]
- 14.Matos GI, Covas CJ, Bittar RC, et al. IFNG +874T/A polymorphism is not associated with American tegumentary leishmaniasis susceptibility but can influence Leishmania induced IFN-gamma production. BMC Infect Dis. 2007;247:33–38. doi: 10.1186/1471-2334-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacellar O, Faria D, Nascimento M, et al. Interleukin 17 production among patients with American cutaneous leishmaniasis. J Infect Dis. 2009;200:75–78. doi: 10.1086/599380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franke ED, Wignall FS, Cruz ME, et al. Efficacy and toxicity of sodium stibogluconate for mucosal leishmaniasis. Ann Intern Med. 1990;113:934–940. doi: 10.7326/0003-4819-113-12-934. [DOI] [PubMed] [Google Scholar]
- 17.Tuon FF, Gomes-Silva A, Da-Cruz AM, Duarte MI, Neto VA, Amato VS. Local immunological factors associated with recurrence of mucosal leishmaniasis. Clin Immunol. 2008;128:442–446. doi: 10.1016/j.clim.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Netto EM, Marsden PD, Llanos-Cuentas EA, et al. Long-term follow-up of patients with Leishmania (Viannia) braziliensis infection and treated with glucantime. Trans R Soc Trop Med Hyg. 1990;84:367–370. doi: 10.1016/0035-9203(90)90321-5. [DOI] [PubMed] [Google Scholar]
- 19.Brazil: Ministério da Saúde, Secretaria de Vigilância em Saúde. 2010. Manual for Surveillance of American Tegumentary Leishmaniasis/Ministério da Saúde, Secretaria de Vigilância em Saúde. 180 p.: il. (Série A. Normas e Manuais Técnicos) 2. ed. atual. Brasília: Editora do Ministério da Saúde. [PubMed]
- 20.Mattos MS. O desafio para a realização de ensaios clínicos em leishmaniose tegumentar [The challenge for clinical trials in cutaneous leishmaniasis] Rev Soc Bras Med Trop. 2006;39(Suppl. III):94–95. [Google Scholar]
- 21.Jones TC, Johnson WD, Jr, Barretto AC, et al. Epidemiology of American cutaneous leishmaniasis due to Leishmania braziliensis braziliensis. J Infect Dis. 1987;156:73–83. doi: 10.1093/infdis/156.1.73. [DOI] [PubMed] [Google Scholar]
- 22.Zajtchuk JT, Casler JD, Netto EM, et al. Mucosal leishmaniasis in Brazil. Laryngoscope. 1989;99:925–939. doi: 10.1288/00005537-198909000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira MR, Macêdo VO, Carvalho EM, et al. An evolutionary study of mucosal leishmaniasis (a 7- to 17-year follow-up) due to Leishmania (Viannia) braziliensis in Três Braços, Bahia. Rev Soc Bras Med Trop. 1995;28:325–332. doi: 10.1590/s0037-86821995000400004. [DOI] [PubMed] [Google Scholar]
- 24.Lee JS, Song CH, Kim CH, et al. Profiles of IFN-gamma and its regulatory cytokines (IL-12, IL-18 and IL-10) in peripheral blood mononuclear cells from patients with multidrug-resistant tuberculosis. Clin Exp Immunol. 2002;128:516–524. doi: 10.1046/j.1365-2249.2002.01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshimoto T, Takeda K, Tanaka T, et al. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J Immunol. 1998;161:3400–3407. [PubMed] [Google Scholar]
- 26.Gomes-Silva A, Bittar RC, Nogueira RS, et al. Can interferon-gamma and interleukin-10 balance be associated with severity of human Leishmania (Viannia) braziliensis infection? Clin Exp Immunol. 2007;149:440–444. doi: 10.1111/j.1365-2249.2007.03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Da-Cruz AM, Bertho AL, Oliveira-Neto MP, Coutinho SG. Flow cytometry analysis of cellular infiltrate from American tegumentary leishmaniasis lesions. Br J Dermatol. 2005;153:537–543. doi: 10.1111/j.1365-2133.2005.06647.x. [DOI] [PubMed] [Google Scholar]
- 28.Pompeu MML, Brodskyn C, Teixeira MJ, et al. Differences in gamma interferon production in vitro predict the pace of the in vivo response to Leishmania amazonensis in healthy volunteers. Infect Immun. 2001;69:7453–7460. doi: 10.1128/IAI.69.12.7453-7460.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pessôa SB, Barreto MP. 1948. p. 527. Leishmaniose Tegumentar Americana [American Tegumentar Leishmaniasis]. Rio de Janeiro: Serviço de documentação, Imprensa Nacional, Ministério da Educação e Saúde.
- 30.Marsden PD. Mucosal leishmaniasis (‘espundia’ Escomel, 1911) Trans R Soc Trop Med Hyg. 1986;80:859–876. doi: 10.1016/0035-9203(86)90243-9. [DOI] [PubMed] [Google Scholar]


