Abstract
Sarcoidosis is a systemic, inflammatory disorder, which in a proportion of patients runs a chronic progressive course despite immunosuppressive treatment. Therapeutic granulocyte and monocyte apheresis (GMA) has been shown to be an effective treatment option for other systemic inflammatory disorders, but has not yet been investigated in sarcoidosis. The aim of this study was to evaluate the response to GMA in sarcoidosis. Seven patients with sarcoidosis refractory to standard immunosuppressive therapy received 10 GMA sessions. All patients underwent chest X-ray, spirometry, a Chronic Respiratory Disease Questionnaire (CRQ-SAS), blood tests and bronchoscopy with bronchoalveolar lavage (BAL) before treatment and at 2–4 weeks and 3 months (except bronchoscopy) after the last treatment session. Bronchoalveolar lavage fluid (BALF) cell differential counts were recorded and T cells from blood and BALF were analysed for markers of activity, differentiation and T regulatory function. Compared to baseline, five of seven patients reported an improvement in dyspnoea score. In BALF there was an increase in the percentage of macrophages and a decrease in the percentage of lymphocytes and CD4+/FoxP3+ T cells. Furthermore, the decrease in BALF CD4+/FoxP3+ T cells correlated significantly with an improvement in dyspnoea score. In peripheral blood there was a statistically significant increase in the percentage of CD4+/CD27− T cells and a trend towards an initial increase in the percentage of CD4+/FoxP3+ T cells, followed by a statistically significant decrease. The effects of GMA on regulatory T cells are consistent with those observed in other inflammatory disorders and could potentially translate into a clinical benefit.
Keywords: bronchoscopy, investigational, therapies, leukapheresis, sarcoidosis, T-lymphocytes
Introduction
Sarcoidosis is a systemic, inflammatory disorder of unknown aetiology, characterized by the formation of non-caseating granulomas in affected organs. The disease is considered T helper type 1 (Th1)-mediated with the accumulation of CD4+ T lymphocytes and an increased CD4/CD8 ratio in bronchoalveolar lavage fluid (BALF) [1]. However, dysregulated neutrophils and monocytes may also have a role. In particular, there have been reports indicating a changed phagocytic activity and an altered expression of certain adhesion molecules of these cells [2–4]. Also, pulmonary neutrophils in sarcoidosis display an activated phenotype, and an increased percentage of BALF neutrophils has been associated with a worse prognosis [4,5]. Although the outcome is often good, a proportion of patients develop a chronic progressive disease [6]. For these patients, the therapeutic options are limited to immunosuppressive agents, commonly associated with significant adverse events [1]. Thus, there is a need for new treatment modalities with a more favourable benefit : risk ratio. Therapeutic granulocyte and monocyte apheresis (GMA) involves the extracorporeal removal of activated granulocytes and monocytes, and represents an interesting alternative to conventional pharmacological therapy. To date, GMA has been employed mainly in the treatment of ulcerative colitis (UC) [7–15]. There is also emerging evidence of its efficacy in other inflammatory conditions [16–18]; however, there are no previous reports of GMA treatment for sarcoidosis. The mechanism of action of GMA appears to extend beyond the absorption of granulocytes and monocytes, suggesting that it disrupts the immunological cascade to such an extent that a clinical response is induced [19,20]. More recently, alterations in T cell subsets has been associated with the beneficial effect of GMA. In particular, in UC a decrease in regulatory CD4+ T cells (Treg) in the intestinal mucosa and an increase in peripheral blood CD4+ Treg has been reported by several authors to correlate with a favourable clinical response [21–23]. Treg cells suppress proliferation and cytokine production of activated T cells and are known to play an important role in maintaining peripheral tolerance [24,25]. They can be identified through their co-expression of CD4 and CD25bright or by forkhead box protein 3 (FoxP3 transcription factor). The FoxP3 Treg marker may be more reliable, as CD 25 is also expressed by activated T cells [26]. In sarcoidosis it has been hypothesized that the observed Th1 bias may be due to a dysfunction of regulatory T cells, and our group has previously described a decreased level of CD4+/FoxP3+ cells in the BALF and blood of subjects with sarcoidosis compared to healthy controls [27].
To determine if GMA could potentially be a therapeutic option in sarcoidosis, we conducted a pilot study evaluating the T cell response in blood and BALF of patients with symptomatic sarcoidosis, refractory to standard immunosuppressive treatment. We compared pre- and post-treatment values for BALF and blood CD4/CD8 ratio, percentages of memory T cells (CD45RO), B cells (CD19), natural killer (NK) cells (CD56(+)CD16(+), CD3(−)), regulatory T cells (FoxP3) and cells expressing markers of early (CD27) and very early (CD69) T cell activation. We also analysed soluble CD27 (s-CD27, T cell activation marker) in blood and BALF and tumour necrosis factor (TNF)-α in BALF and compared BALF cell differential counts before and 2–4 weeks after the last treatment session.
Materials and methods
Study subjects
Seven patients with treatment refractory sarcoidosis, followed at the Respiratory Medicine Unit (Karolinska University Hospital, Stockholm, Sweden), were included. Diagnosis was in accordance with the criteria of the World Association of Sarcoidosis and other Granulomatous Disorders [1]. Disease duration ranged from 2 to 11 years. Treatment refractory disease was defined as the presence of markers of disease activity despite recent or ongoing immunosuppressive therapy, of which the dose was kept stable throughout the duration of the study. All patients underwent pulmonary chest X-ray, dynamic spirometry, blood tests and bronchoscopy with bronchoalveolar lavage (BAL). In addition, all patients completed the McMaster Chronic Respiratory Disease Questionnaire, Self-Administered Standardized (CRQ-SAS, Swedish version). The investigations were performed before, within 2–4 weeks and at 3 months after the last GMA treatment session. Bronchoscopy, s-CD27 and TNF-α were performed only pretreatment and at the 2–4-week follow-up. Written informed consent was obtained from all subjects, and approval was granted from the regional ethical review board. Patient characteristics are summarized in Table 1.
Table 1.
Characteristics of participants
| Characteristics of participants | |
|---|---|
| Number | 7 |
| Gender (male/female) | 6/1 |
| Age median (range) | 47 (35–59) |
| Radiographic stage (0/I/II/III/IV) | (0/0/3/0/4) |
| VC % predicted, median (range) | 98 (72–124) |
| FEV1 % predicted, median (range) | 79 (62–136) |
| Dyspnoea score (1–7) at baseline, median (range) | 5·4 (4·2–7) |
| Fatigue score (1–7) at baseline, median (range) | 4 (1–6·2) |
| Immunosupressive treatment (Predn/Predn+MTX/MTX) | 5/1/1 |
Predn = prednisolone, MTX = methotrexate. Prednisolone dose ranged from 5 to 15 mg daily. Methotrexate dose ranged from 10 to 15 mg/week. VC = vital capacity; FEV1 = forced expiratory volume in 1 s.
Adsorptive granulocyte and monocyte apheresis (GMA)
Leucocyte apheresis was conducted in a set of 10 outpatient sessions over 7 consecutive weeks, using the Adacolumn apheresis device (Japan Immunoresearch Laboratories Company Limited (JIMRO), Otsuka, Japan). Blood inlet and outlet were established via suitable veins in the left and right arm. Anti-coagulation was delivered as an infusion of 1500 U of heparin (Pharmacia, Stockholm, Sweden). Each apheresis session had a duration of 60 min and was performed with a perfusion rate of 30 ml/min.
BAL procedure and handling of BAL fluid and whole blood
All BAL samples were collected and analysed as described previously [28]. Briefly, under local anaesthesia a flexible fiberoptic bronchoscope (Olympus Optical Co. Ltd, Tokyo, Japan) was wedged in a subsegmental bronchus in the middle lobe. Five aliquots of 50 ml of sterile, phosphate-buffered saline (PBS) solution (37°C) were instilled and subsequently aspirated and collected in a siliconized plastic bottle kept on ice. The BAL fluid was strained through a Dacron net (Millipore, Cork, Ireland) and centrifuged at 400 g for 10 min at 4°C. The cell pellet was resuspended in RPMI-1640 medium (Sigma Aldrich, Irvine, UK) and the viability was determined by blue cell exclusion. Smears for differential counts were determined by May–Grünwald–Giemsa staining of cytospin slides. The BAL fluid CD4/CD8 T cell ratio was determined by flow cytometric analysis (FACSCanto; BD Biosciences, Mountain View, CA, USA) using monoclonal antibodies against CD4 [phycoerythrin–cyanin 7 (PE-Cy7)] and CD8 [peridinin chlorophyll (PerCP)-Cy5·5] (Dako Cytomation Norden AB, Solna, Sweden). Whole blood was drawn from a suitable antecubital vein and collected in heparinized tubes.
Enzyme-linked immunosorbent assay (ELISA), immunofluorescent staining and flow cytometry
All reagents and antibodies were purchased from BD Bioscience (Mountain View, CA, USA), unless otherwise stated. The expression of cell surface and intracellular molecules was examined using a fluorescence activated cell sorter (FACS)Canto II flow cytometer. Data were processed using FACSDiva version 6·1.2 (BD Bioscience) and Infinicyt (Cytognos, Salamanca, Spain) software.
Aliquots of fresh BAL cells (106 cells resuspended in 100 μl cell wash) or whole blood (100 μl) were surface-stained for either: (i) CD3 (FITC), CD4 (PE-Cy7), CD8 [allophycocyanin (APC)-Cy7], CD19 (APC), CD45 (PerCP-Cy5·5) and CD56/CD16 (PE) in combination, (ii) CD3 (Pacific Blue), CD4 (APC-H7), CD8 (AmCyan), CD25 (PE-Cy5), CD27 (APC), CD45RO (PE-Cy7) and CD69 (FITC) or matched isotype controls. The cell suspensions were incubated for 20 min in the dark [BAL cells at 4°C and blood cells at room temperature (RT)]. BAL cells were thereafter washed twice in cold cell wash, centrifuged for 5 min at 400 g at 4°C and resuspended in 500 ml cell wash (i) or kept on ice (ii). For whole blood, the erythrocytes were removed through incubation with FACS lysing solution for 8 min at RT, and the remaining white blood cells were washed twice with RT cell wash and thereafter resuspended in 500 ml cell wash (i) or kept on ice (ii).
For (ii), BAL and blood cells were further fixed and permeabilized for 45 min at 4°C in the dark, using a FoxP3 intracellular staining kit (eBioscience, San Diego, CA, USA). The cells were washed twice with 2 ml 1 × permeabilization buffer, centrifuged for 5 min at 400 g at 4°C and incubated with 2% normal rat serum for 15 min at 4°C. BAL and blood cells were then stained intracellularly with anti-human FoxP3 (PE, clone PCH101) or anti-immunoglobulin (Ig)G2a antibodies (PE) (both purchased from eBioscience) for 30 min at 4°C, then washed twice with permeabilization buffer and resuspended in 500 ml cell wash.
S-CD27 α in blood and BALF and TNF-α in BALF were analysed by ELISA kits following the manufacturer's instructions (human TNF-α total platinum ELISA and human sCD27 instant ELISA; eBioscience, produced by Bender MedSystems GmbH, Campus Vienna Biocenter 2, 1030 Vienna, Austria).
Statistical analyses
Statistical analyses were performed using GraphPad Prism version 6 (GraphPad Software Inc., San Diego, CA, USA). The Wilcoxon signed-rank test was used for comparisons between pre- and post treatment values. Spearman's rank correlation was used to calculate pairwise correlations. P < 0·05 was considered significant.
Results
Patient questionnaires, chest X-ray and spirometry
Five of seven patients reported an improvement in dyspnoea scores at 3 months after the last treatment session (Fig. 1). Furthermore, there was a significant correlation between an improvement in dyspnoea score at 3 months post-treatment and a decrease in the percentage of BALF CD4+/FoxP3+ T cells (r = −0·96, P = 0·0016, Fig. 1). There was no difference in the fatigue scores before and after treatment. At baseline, three of seven patients were in chest X-ray stadium 2 and the remaining four patients were in radiological stadium 4. No post-treatment change in radiological stadium was observed in any of the patients. There was no correlation between radiographic stage and the improvement in dyspnoea score (three of four patients in radiographic stage 4 and two of three patients in radiographic stage 2 reported an improvement). Furthermore, there were no statistically significant or clinically relevant changes from baseline in the spirometric parameters.
Figure 1.
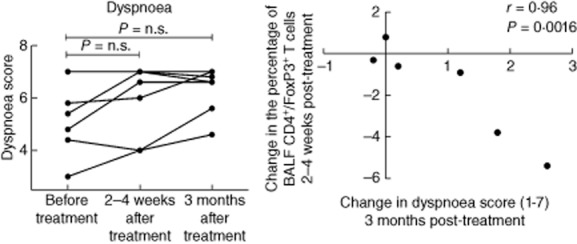
(a) Dyspnoea score [Swedish version of the Chronic Respiratory Disease Questionnaire, Self-Administered Standardized CRQ-SAS)], before treatment and within 2–4 weeks and 3 months after the last treatment session. Score 7 = no dyspnoea, score 1 = severe dyspnoea. (b) Spearman's rank correlation between change from baseline in dyspnoea score and in the percentage of bronchoalveolar fluid (BALF) CD4+/forkhead box protein 3 (FoxP3)+ T cells.
BAL fluid cell differential counts and major lymphocyte populations
Bronchoalveolar lavage was performed immediately before and within 2–4 weeks of the last GMA treatment. The results for the cell differential counts and the CD4/CD8 ratio are presented in Table 2. Six of seven patients had a tendency to an increase in the percentage of macrophages and a decrease in the percentage of lymphocytes (not significant, Fig. 2). The percentage of CD45RO+ memory T cells, CD19+ B cells, CD56+/CD16+/CD3− NK cells and activated CD4+ T cells (CD27− and/or CD69+ cells) in the BALF did not change significantly from baseline. Absolute numbers of the above cell populations were also calculated, and did not change significantly compared to baseline. Furthermore, there was no change in the BALF levels of s-CD27 and TNF-α (data not presented).
Table 2.
Bronchoalveolar fluid (BALF) cell concentration and differential cell count
| BALF cell concentration and differential cell count | ||
|---|---|---|
| Before treatment | After treatment | |
| Total number of cells | 17·3 (7·6–44·7) | 17·6 (10·5–39·5) |
| Cellconcentration (×106/l) | 136·6 (83·1–262·8) | 147·8 (88·9–213·8) |
| Macrophages (%) | 76·6 (58·0–93·6) | 79·4 (61·8–95·0) |
| Lymphocytes (%) | 20·0 (4·2–40·0) | 15·6 (2·6–37·6) |
| Neutrophils (%) | 2·2 (1·2–9·6) | 2·4 (0·2–13·2) |
| Eosinophils (%) | 0·4 (0·0–2·2) | 0·0 (0·0–3·4) |
| Basophils (%) | 0·0 (0·0–0·2) | 0·0 (0·0–0·4) |
| Mastcells | 4·0 (0·0–36·0) | 9·0 (0·0–39·0) |
| CD4/CD8 ratio | 3·9 (2·6–8·5) | 4·0 (3·0–11·6) |
(a) Data are shown as median (min–max). (b) Mast cells are shown as the number of cells observed in 10 visual fields (×16 magnification).
Figure 2.
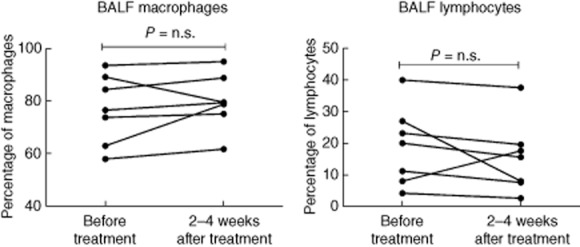
Percentages of macrophages and lymphocytes in bronchoalveolar fluid (BALF) measured before and within 2–4 weeks after the last treatment session.
Peripheral blood analysis
Blood samples were taken immediately before treatment and at 2–4 weeks and 3 months after the last treatment. Compared to baseline, there was a statistically significant increase in the percentage of CD4+/CD27− T cells at 2–4 weeks and 3 months (Figs 3 and 4). Similarly, an increase in the percentage of CD8/CD 27– T cells was seen at 3 months (Fig. 3). The percentages of CD4+ and CD8+ T cells expressing CD69+ were low and did not change significantly after treatment. Serum levels of s-CD27 also did not change compared to baseline (data not presented). TNF-α was not measured in peripheral blood. There were no significant changes in the levels of s-Ca and s-ACE following GMA.
Figure 3.
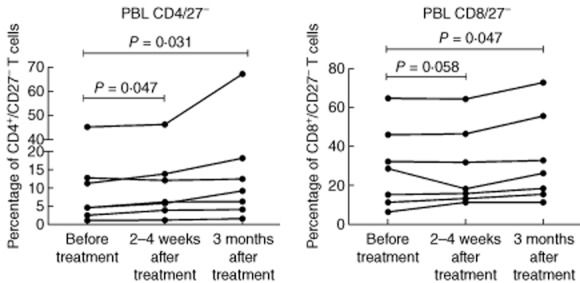
Percentages of peripheral blood CD4+/CD27− and CD8+/CD27− T cells before treatment, within 2–4 weeks and at 3 months after the last treatment session.
Figure 4.
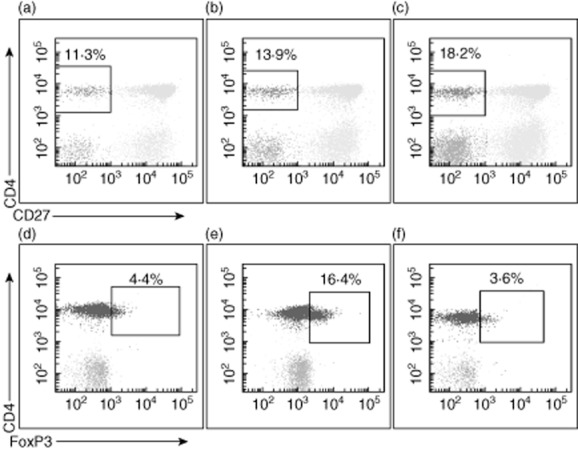
Examples of representative dot-plots for peripheral blood CD4+/CD27− T cells and CD4+ T cells expressing forkhead box protein 3 (FoxP3). Data are shown before treatment (a,d), within 2–4 weeks after treatment (b,e) and at 3 months after the last treatment session (c,f).
Analysis of regulatory Treg
In BALF there was a trend towards a post-treatment decrease in the percentage of CD4+ Treg, with six of seven patients experiencing a decrease in the percentage of CD4+/FoxP3+ cells (Figs 4 and 5) and five of seven patients experiencing a decrease in the percentage of CD4+/CD25+ T cells. Conversely, in peripheral blood, there was a trend towards an increase in the percentage of CD4+/FoxP3+ T cells at the time of the post-treatment bronchoscopy (five of seven patients), followed by a statistically significant decrease compared to baseline at 3 months (P = 0·047, Figs 4 and 5). Changes in the percentage of peripheral blood CD4+/CD25+ cells were only partially consistent with the observed changes in CD4+/FoxP3+ cells.
Figure 5.
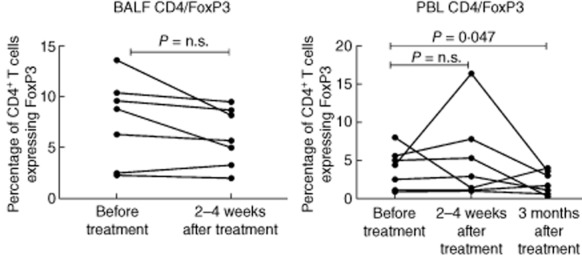
Percentages of bronchoalveolar fluid (BALF) and peripheral blood CD4+ T cells expressing forkhead box protein 3 (FoxP3) before treatment, within 2–4 weeks and at 3 months (peripheral blood only) after the last treatment session.
Discussion
This study is, to our knowledge, the first study to evaluate GMA treatment for chronic progressive sarcoidosis. In particular, we were interested to determine if the treatment would influence the T cell profiles in BALF and blood, and whether a beneficial effect on Treg would occur similar to that described in other systemic inflammatory conditions.
We included seven patients with active disease, refractory to standard immunosuppressive treatment. Of these, five patients reported a decrease in dyspnoea at 3 months after the last treatment session. Furthermore, there was a significant correlation between an improvement in dyspnoea score at 3 months and a decrease in the percentage of BALF CD4+/FoxP3+ T cells. These findings are encouraging.
In BALF, compared to baseline, there was a trend towards an increase in the percentage of macrophages and a decrease in the percentage of lymphocytes. A larger number of subjects could have strengthened the observation. Sarcoidosis is a T cell-mediated disease where the accumulation of lymphocytes is limited mainly to the lungs, suggesting that the lungs are the primary target for the immune response [29,30]. Thus, a decrease in pulmonary lymphocytes could indicate a reduced disease activity.
This may be substantiated further by the statistically significant post-treatment increase in the percentage of peripheral blood CD4+ and CD8+/CD27− T cells. These fully differentiated effector T cells are produced in response to prolonged T cell activation, which occurs following an initial up-regulation of CD27 and the release of a soluble form of the molecule (sCD27) [31–33]. In sarcoidosis, a raised concentration of sCD27 has been observed in the lung epithelial lining fluid, together with a high number of CD27– BALF T cells [34]. This indicates that, in the lung, persistent antigen stimulation causes shedding of CD27 and the expansion of CD27− effector T cells. The pulmonary compartmentalization of these fully differentiated effector T cells probably augments the inflammatory process in situ. Thus, an increase of CD27− T cells in peripheral blood suggests that GMA treatment may cause a migration of these effector T cells from the pulmonary compartment and into the blood. Eventually this would cause a reduced number of proinflammatory CD27− T cells in the lung and a subsequent dampening of the localized immune response. It is not clear what initiates this migration of cells, but it may be that GMA treatment induces a change in the T cell homeostasis. We were unable to demonstrate an accompanying decrease in BALF CD27− T cells. The subgroup of highly activated BALF CD4+ CD27−CD69+ T cells was evaluated separately, and did also not change. Furthermore, there was no change in the serum and BALF levels of s-CD27 when pre- and post-treatment values were compared. However, this could be due simply to the relatively short follow-up period.
With regard to the treatment effect on Treg at 2–4 weeks after the last treatment session, we found a trend towards a decrease in the percentage of CD4+/FoxP3+ T cells in BALF accompanied by an increase in peripheral blood. This suggests that, following GMA treatment, there is an outflow of these cells from the lung and into the blood. At 3 months after the last treatment session, however, there was a statistically significant decrease in the percentage of CD4+/FoxP3+ T cells in blood. Our results are largely consistent with the findings in UC, where previous studies have demonstrated a decrease in the intestinal mucosal levels of CD4+/FoxP3+ T cells together with an increase of these cells in peripheral blood in clinical responders. In UC the increase in blood CD4+/FoxP3+ cells may be persistent up to 10 weeks after treatment [21–23]. In our study we did not capture the precise duration of the increase in blood Treg.
An obvious limitation of our study is the small sample size and the lack of a randomized placebo-controlled design. Furthermore, while GMA appears to be a reasonable treatment approach in UC, where patients typically exhibit high levels of activated granulocytes and monocytes, the treatment rationale in sarcoidosis, a lymphocyte-driven disease, may be less clear. In this respect, the effects on regulatory T cells, similar to that seen in UC, is an encouraging finding. However, it may be that a more effective modulation of the immune response would occur if the apheresis treatment was tailored to specifically target inflammatory cells typically involved in sarcoidosis.
This is, to our knowledge, the first study to evaluate GMA treatment for sarcoidosis. Currently there are no pharmacological agents that have been proven to be curative for the treatment of sarcoidosis. Furthermore, the immunosuppressive agents used are associated with significant adverse events. Thus, there is a pressing need for new treatment modalities with more benign safety profiles. GMA could potentially represent such an alternative; however, larger studies are needed to further evaluate its efficacy in sarcoidosis.
Acknowledgments
The authors are grateful for the assistance of H. Blomqvist, M. Dahl, B. Dahlberg, B. Engvall and G. de Forest. This work was supported by Otsuka, Dr Raphael Gruber, Director, Adacolumn Business Univ-EU, the Swedish Research Council, the Swedish Heart-Lung Foundation, the King Oscar II Jubilee Foundation, the Torsten and Ragnar Söderbergs Foundation, the Mats Kleeberg Foundation, King Gustaf V's and Queen Victoria's Freemasons' Foundation, Karolinska Institutet and through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet. None of the funding sources had any influence on the production of this manuscript.
Disclosure
All authors completed the disclosure form and declare support with disposable devices for the study from Otsuka but no other relationships or activities that could appear to have influenced the submitted work.
Author contributions
H. O. contributed to the conception and design of the study, to the acquisition, analysis and interpretation of data and to drafting and revising the article. V. M. contributed to the conception and the design of the study, to the acquisition of data and to critically revising the article. K. C. contributed to the conception and the design of the study, to the analysis of data and to critically revising the article. J. L. contributed to the conception and the design of the study, to the acquisition of data and to critically revising the article. A. K. contributed to the conception and the design of the study, to the acquisitionn, analysis and interpretation of data and to drafting and critically revising the article. J. G. contributed to the conception and the design of the study, to the acquisition, analysis and interpretation of data and to drafting and critically revising the article. All authors have finally approved the version to be published.
References
- 1.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Schmekel B, Håkansson L, Hällgren R, Nou E, Stålenheim G, Venge P. Neutrophil phagocytosis in sarcoidosis. Reduced C3b receptor-mediated phagocytosis in active and silent sarcoidosis. Clin Exp Immunol. 1985;60:191–195. [PMC free article] [PubMed] [Google Scholar]
- 3.Dubaniewicz A, Typiak M, Wybieralska M, et al. Changed phagocytic activity and pattern of Fcγ and complement receptors on blood monocytes in sarcoidosis. Hum Immunol. 2012;73:788–794. doi: 10.1016/j.humimm.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Fortunati E, Kazemier KM, Grutters JC, Koenderman L, Van den Bosch vJ. Human neutrophils switch to an activated phenotype after homing to the lung irrespective of inflammatory disease. Clin Exp Immunol. 2009;155:559–566. doi: 10.1111/j.1365-2249.2008.03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drent M, Mansour K, Linssen C. Bronchoalveolar lavage in sarcoidosis. Semin Respir Crit Care Med. 2007;28:486–495. doi: 10.1055/s-2007-991521. [DOI] [PubMed] [Google Scholar]
- 6.Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 7.Shimoyama T, Sawada K, Hiwatashi N, et al. Safety and efficacy of granulocyte and monocyte adsorption apheresis in patients with active ulcerative colitis: a multicenter study. J Clin Apher. 2001;16:1–9. doi: 10.1002/jca.1000. [DOI] [PubMed] [Google Scholar]
- 8.Domènech E, Hinojosa J, Esteve-Comas M, et al. Granulocyte aphaeresis in steroid-dependent inflammatory bowel disease: a prospective, open, pilot study. Aliment Pharmacol Ther. 2004;20:1347–1352. doi: 10.1111/j.1365-2036.2004.02288.x. [DOI] [PubMed] [Google Scholar]
- 9.Kanke K, Nakano M, Hiraishi H, Terano A. Clinical evaluation of granulocyte/monocyte apheresis therapy for active ulcerative colitis. Dig Liver Dis. 2004;36:811–817. doi: 10.1016/j.dld.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Ljung T, Thomsen OØ, Vatn M, et al. Granulocyte, monocyte/macrophage apheresis for inflammatory bowel disease: the first 100 patients treated in Scandinavia. Scand J Gastroenterol. 2007;42:221–227. doi: 10.1080/00365520600979369. [DOI] [PubMed] [Google Scholar]
- 11.Hanai H, Watanabe F, Takeuchi K, et al. Leukocyte adsorptive apheresis for the treatment of active ulcerative colitis: a prospective, un-controlled, pilot study. Clin Gastroenterol Hepatol. 2003;1:28–35. doi: 10.1053/jcgh.2003.50005. [DOI] [PubMed] [Google Scholar]
- 12.Hanai H, Watanabe F, Yamada M, et al. Adsorptive granulocyte and monocyte apheresis versus prednisolone in patients with corticosteroid-dependent moderately severe ulcerative colitis. Digestion. 2004;70:36–44. doi: 10.1159/000080079. [DOI] [PubMed] [Google Scholar]
- 13.Habermalz B, Sauerland S. Clinical effectiveness of selective granulocyte, monocyte adsorptive apheresis with the Adacolumn device in ulcerative colitis. Dig Dis Sci. 2010;55:1421–1428. doi: 10.1007/s10620-009-0845-x. [DOI] [PubMed] [Google Scholar]
- 14.Thanaraj S, Hamlin PJ, Ford AC. Systematic review: granulocyte/monocyte adsorptive apheresis for ulcerative colitis. Aliment Pharmacol Ther. 2010;32:1297–1306. doi: 10.1111/j.1365-2036.2010.04490.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhu M, Xu X, Nie F, Tong J, Xiao S, Ran Z. The efficacy and safety of selective leukocytapheresis in the treatment of ulcerative colitis: a meta-analysis. Int J Colorectal Dis. 2011;26:999–1007. doi: 10.1007/s00384-011-1193-9. [DOI] [PubMed] [Google Scholar]
- 16.Sanmartí R, Marsal S, Valverde J, et al. Adsorptive granulocyte/monocyte apheresis for the treatment of refractory rheumatoid arthritis: an open pilot multicentre trial. Rheumatology (Oxf) 2005;44:1140–1144. doi: 10.1093/rheumatology/keh701. [DOI] [PubMed] [Google Scholar]
- 17.Sakanoue M, Takeda K, Kawai K, Kanekura T. Granulocyte and monocyte adsorption apheresis for refractory skin diseases due to activated neutrophils, psoriasis, and associated arthropathy. Ther Apher Dial. 2013;17:477–483. doi: 10.1111/1744-9987.12113. [DOI] [PubMed] [Google Scholar]
- 18.Sawada K, Kashiwamura S, Okamura H, et al. Selective granulocyte and monocyte apheresis as a new adjunct to enhance the efficacy of interferon-alpha + ribavirin in patients with high plasma hepatitis C virus. Dig Liver Dis. 2005;37:515–521. doi: 10.1016/j.dld.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Hanai H, Takeda Y, Eberhardson M, et al. The mode of actions of the Adacolumn therapeutic leucocytapheresis in patients with inflammatory bowel disease: a concise review. Clin Exp Immunol. 2011;163:50–58. doi: 10.1111/j.1365-2249.2010.04279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.C Leitner G, Worel N, Vogelsang H. Selective granulocyte and monocyte apheresis as a non-pharmacological option for patients with inflammatory bowel disease. Transfus Med Hemother. 2012;39:246–252. doi: 10.1159/000341801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamikozuru K, Fukunaga K, Hirota S, et al. The expression profile of functional regulatory T cells, CD4+CD25high+/forkhead box protein P3+, in patients with ulcerative colitis during active and quiescent disease. Clin Exp Immunol. 2009;156:320–327. doi: 10.1111/j.1365-2249.2009.03904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuadrado E, Alonso M, de Juan MD, Echaniz P, Arenas JI. Regulatory T cells in patients with inflammatory bowel diseases treated with Adacolumn granulocytapheresis. World J Gastroenterol. 2008;14:1521–1527. doi: 10.3748/wjg.14.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoyama Y, Fukunaga K, Fukuda Y, et al. Demonstration of low-regulatory CD25high+CD4+ and high-pro-inflammatory CD28-CD4+ T-cells subsets in patients with ulcerative colitis: modified by selective granulocyte and monocyte adsorption apheresis. Dig Dis Sci. 2007;52:2725–2731. doi: 10.1007/s10620-006-9560-z. [DOI] [PubMed] [Google Scholar]
- 24.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr OpinRheumatol. 2003;15:430–435. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 26.Roncador G, Brown PJ, Maestre L, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single cell level. Eur J Immunol. 2005;35:1681–1691. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 27.Idali F, Wahlström J, Müller-Suur C, Eklund A, Grunewald J. Analysis of regulatory T cell associated forkhead box P3 expression in the lungs of patients with sarcoidosis. Clin Exp Immunol. 2008;152:127–137. doi: 10.1111/j.1365-2249.2008.03609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eklund A, Blaschke E. Relationship between changed alveolar-capillary permeability and angiotensin converting enzyme activity in serum in sarcoidosis. Thorax. 1986;41:629–634. doi: 10.1136/thx.41.8.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grunewald J, Eklund A, Hed J, Takahashi H, Wigzell H. Aspects on the alveolar accumulation of T cells in sarcoidosis. Sarcoidosis. 1992;9:142–144. [PubMed] [Google Scholar]
- 30.Darlington P, Haugom-Olsen H, von Sivers K, et al. T-cell phenotypes in bronchoalveolar lavage fluid, blood and lymph nodes in pulmonary sarcoidosis – indication for an airborne antigen as the triggering factor in sarcoidosis. J Intern Med. 2012;272:465–471. doi: 10.1111/j.1365-2796.2012.02543.x. [DOI] [PubMed] [Google Scholar]
- 31.van Lier RA, Borst J, Vroom TM, et al. Tissue distribution and biochemical and functional properties of Tp55 (CD27), a novel T cell differentiation antigen. J Immunol. 1987;139:1589–1596. [PubMed] [Google Scholar]
- 32.Sugita K, Hirose T, Rothstein DM, Donahue C, Schlossman SF, Morimoto C. CD27, a member of the nerve growth factor receptor family, is preferentially expressed on CD45Ra+ CD4 T cell clones and involved in distinct immunoregulatory functions. J Immunol. 1992;149:3208–3216. [PubMed] [Google Scholar]
- 33.Hintzen RQ, de Jong R, Hack CE, et al. A soluble form of the human T-cell differentiation antigen CD 27 is released after triggering of the TCR /CD3 complex. J Immunol. 1991;147:29–35. [PubMed] [Google Scholar]
- 34.Hol BE, Hintzen RQ, van Lier RA, Alberts C, Out TA, Jansen HM. Soluble and cellular markers of T cell activation in patients with pulmonary sarcoidosis. Am Rev Respir Dis. 1993;148:643–649. doi: 10.1164/ajrccm/148.3.643. [DOI] [PubMed] [Google Scholar]


