Abstract
Spontaneous abortion in early pregnancy due to unknown reasons is a common problem. The excess complement activation and consequent placental inflammation and anti-angiogenic milieu is emerging as an important associated factor in many pregnancy-related complications. In the present study we sought to examine the expression of complement inhibitory proteins at the feto–maternal interface and levels of complement split products in the circulation to understand their role in spontaneous abortion. Consenting pregnant women who either underwent elective abortion due to non-clinical reasons (n = 13) or suffered miscarriage (n = 14) were recruited for the study. Systemic levels of complement factors C3a and C5a were measured by enzyme-linked immunosorbent assay (ELISA). Plasma C5 and C3 protein levels were examined by Western blot. Expressions of complement regulatory proteins such as CD46 and CD55 in the decidua were investigated by quantitative polymerase chain reaction (PCR) and Western blot. The median of plasma C3a level was 82·83 ng/ml and 66·17 ng/ml in elective and spontaneous abortion patients, respectively. Medians of plasma C5a levels in elective and spontaneous abortion patients were 0·96 ng/ml and 1·14 ng/ml, respectively. Only plasma C5a levels but not C3a levels showed significant elevation in spontaneous abortion patients compared to elective abortion patients. Further, there was a threefold decrease in the mRNA expressions of complement inhibitory proteins CD46 and CD55 in the decidua obtained from spontaneous abortion patients compared to that of elective abortion patients. These data suggested that dysregulated complement cascade may be associated with spontaneous abortion.
Keywords: C3a, C5a, CD46, membrane co-factor protein, spontaneous abortion
Introduction
Spontaneous abortion is a common problem in early pregnancy. Approximately 15% of clinically recognized first-trimester pregnancies undergo miscarriage [1,2]. The loss of subclinical pregnancy is even higher, and is reported to be approximately 60% based on measurement of human chorionic gonadotrophin levels [3]. Commonly known causes of spontaneous abortion are chromosomal abnormalities in the fetus, congenital or acquired abnormalities in the uterus, the presence of thyroid and anti-phospholipid antibodies and maternal infections [4–10]. However, the underlying cause for miscarriage in many spontaneous abortion cases is not known.
Dysregulated or over-activated complement system is emerging as an associated factor in many pregnancy complications. The complement system, an important arm of the innate immunity, is a cascade containing more than 30 proteins and is involved in protection against invading pathogens. The complement cascade, when activated by classical, mannose-binding lectin (MBL) and alternative pathways, deposits several split products on the cell membrane, ultimately creating a cytotoxic cell lysis complex. The complement split products also include free circulating anaphylotoxins such as C3a and C5a, which can initiate inflammation and tissue injury [11]. The complement cascade is controlled by several soluble and membrane-bound factors such as factor I, CD55, CD46 and CD59.
The association of uncontrolled complement with fetal death has been demonstrated in experimental animals. The murine complement cascade inhibitor protein Crry deficiency in mice is known to cause fetal demise. Fetal death in Crry knock-out mice was found to be due to the complement deposition and consequent placental inflammation [12]. In an antibody-independent mouse model of spontaneous abortion and intrauterine growth restriction (IUGR), Girardi et al. showed that significant local complement activation occurs at the feto–maternal interface and complement split product C5a induces the production of potent anti-angiogenic factor, soluble vascular endothelial growth factor (VEGF) receptor 1 (sFlt-1) [13]. Hypocomplementaemia has been shown recently to be predictive of poor pregnancy outcomes in pregnancies with anti-phospholipid syndrome [14]. Human and experimental animal studies have implicated the association of complement system with pre-eclampsia, which is a serious complication related to pregnancy. In a prospective study of pregnant women, Lynch et al. reported that elevated alternative pathway component Bb was associated strongly with pre-eclampsia and preterm birth [15,16]. Mutations of complement regulatory proteins such as membrane co-factor protein (MCP or CD46), complement factor I (CFI) and complement factor H (CFH) have been shown to be associated with autoimmune as well as non-autoimmune-mediated pre-eclampsia [17]. In an anti-phospholipid antibody (aPL) mouse model and in aPL and systemic lupus erythematosus patients, Cohen et al. demonstrated that various clinical parameters of placental injury were associated with complement activation [18]. In this study we analysed the plasma complement factors C3, C5, C3a, C5a and the expression of membrane-bound complement regulatory proteins in placental decidua of a small cohort of pregnant women that included patients who either underwent elective abortion for non-clinical reasons or suffered miscarriage. We show that the plasma C5a levels were elevated and decidual CD46 and CD55 mRNA levels were reduced in spontaneous miscarriage patients compared to elective abortion patients.
Materials and methods
Patient population
This study was approved by the Institutional Review Board at the University of Texas Medical Branch at Galveston. Abortion tissues (5–14 weeks) and blood were obtained from consenting elective abortion patients at Planned Parenthood Houston and spontaneous abortion patients at The University of Texas Medical Branch at Galveston clinics in Galveston. Spontaneous abortion tissues were collected from patients diagnosed for fetal death by ultrasound at the time when the patient arrived at the hospital. Blood was collected before conducting the elective abortion and at the time when the patient arrived at the hospital in cases of spontaneous abortion. Plasma was separated with ethylenediamine tetraacetic acid (EDTA) and stored at −80°C until used for immunoassay or Western blot. Placental tissues were dissected carefully to exclude any membrane contamination, flash-frozen and stored at −80°C until used for RNA and protein isolation. Gestational ages were calculated from the date of the last menstrual period with either ultrasound or clinical confirmation. Miscarriage patients with any kind of maternal infection, recurrent miscarriage, chromosomal abnormalities in the fetus, or any kind of physical insult to the uterus, were excluded from the study. Blood was collected from 14 (11 of gestational week < 10 and three of ≥ 10) elective abortion patients and 13 (10 of gestational week < 10 and three of ≥ 10) miscarriage patients. Placental tissues were collected from 13 (seven of gestational week < 10 and six of ≥ 10) elective abortion patients and eight (seven of gestational week <10 and one of = 10) spontaneous abortion patients.
Plasma C3a and C5a measurement
Plasma C3a and C5a concentrations were measured using enzyme-linked immunosorbent assay (ELISA) kits (HK354-01 and HK349-01, respectively) purchased from Hycult Biotech (Plymouth Meeting, PA, USA), according to the instructions provided by the manufacturer.
Isolation of total RNA and quantitative real-time polymerase chain reaction (PCR)
Total RNA was isolated from the tissues using the RNeasy mini kit (Qiagen, Valencia, CA, USA), according to the procedure recommended by the manufacturer. RNA extraction was followed by DNase I (Qiagen) treatment to remove DNA contamination. Total RNA of 1 μg was reverse-transcribed into complementary DNA using avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI, USA) and random oligonucleotide hexamers (Life Technologies, Grand Island, NY, USA). Quantitative real-time PCR was carried out using CFX96 system and SYBR green master mix (BioRad, Hercules, CA, USA). A comparative cycle of threshold fluorescence (CT) method was used with housekeeping gene as internal control to quantify the target gene expression.
Western blotting
The tissues were homogenized in radioimmunoprecipitation assay (RIPA) buffer (Cell Signaling Technology, Danvers, MA, USA) with protease inhibitor tablets (one tablet/10 ml) (Roche Life Sciences, Indianapolis, IN, USA). Protein concentrations in the homogenates and in plasma were determined by Pierce bicinchoninic acid assay (BCA) protein assay kit (ThermoScientific, Rockford, IL, USA) against bovine serim albumin (BSA) standards. Equal amounts of proteins were separated by polyacrylamide gel electrophoresis using NuPAGE 4–12% Bis-Tris mini gels (Life Technologies) with 3-(N-morpholino)propanesulphonic acid sodium dodecyl sulphide (MOPS SDS) as running buffer at 200 V for 50 min. After electrophoresis, proteins were electrotransferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked with TBST (20 mM Tris, 500 mM sodium chloride, 1% Tween 20, pH 7·5) containing 5% fat-free milk for 1 h at room temperature. Blocked membranes were incubated with primary antibodies for C3c (abcam, Cambridge, MA, USA) or C5 (abcam) or CD46 (abcam) or CD55 (clone IH4) at 4°C. After washing, the membranes were incubated overnight at 4°C with horseradish peroxidase (HRP) conjugated anti-rabbit secondary antibody (Southern Biotech, Birmingham, AL, USA). The bound antibody was visualized on a blue sensitive autoradiography film using supersignal west pico chemiluminiscence substrate (ThermoScientific) according to the instructions provided by the manufacturer. The films were scanned and the protein band densitometric analysis was performed using the AlphaEase Flurochem 8000 software (Alpha Innotech, Santa Clara, CA, USA).
Statistical analysis
The data were analysed statistically with Prism version 6·00 for Windows (GraphPad Software, San Diego, CA, USA). The within-group significance was analysed by Student's t-test and non-parametric Mann–Whitney test. P < 0·05 was considered statistically significant.
Results
Spontaneous abortion patients have elevated plasma C5a levels and reduced plasma C5 protein levels
The plasma C3a levels ranged from 62·8 to 97·8 ng/ml in the patients who underwent elective abortion (n = 14) with a median value of 82·83 ng/ml. In spontaneous abortion patients (n = 13) plasma C3a levels were in the range of 44·5–131·16 ng/ml, with a median value of 66·17 ng/ml. Although the median of plasma C3a levels showed a tendency to decrease in spontaneous abortion patients compared to that of elective abortion patients, the difference was not statistically significant (Fig. 1). Plasma C5a levels in elective and spontaneous abortion patients ranged from 0·58 to 1·4 ng/ml and 0·86 to 2·14 ng/ml, respectively. The median of plasma C5a levels in elective abortion patients was 0·96 ng/ml. The median plasma C5a levels in spontaneous abortion patients was 1·14 ng/ml, which was significantly higher compared to that of elective abortion patients (Fig. 2). We also examined the C3 and C5 protein levels in the plasma by Western blot (Fig. 3a,b, upper panels). Consistent with C3a levels, plasma C3 levels showed a trend of increase in spontaneous abortion patients, but the densitometry analysis of Western blot did not show statistically significant differences in C3 protein between spontaneous and elective abortion patients (Fig. 3a, lower panel). The plasma C5 protein levels in the spontaneous miscarriage patients were reduced compared to that of elective abortion patients, but the difference in the median of densities of protein bands was not statistically significant (Fig. 3b, lower panel). Overall, these results suggested that spontaneous abortion patients had elevated levels of circulating C5a compared to elective abortion patients.
Figure 1.
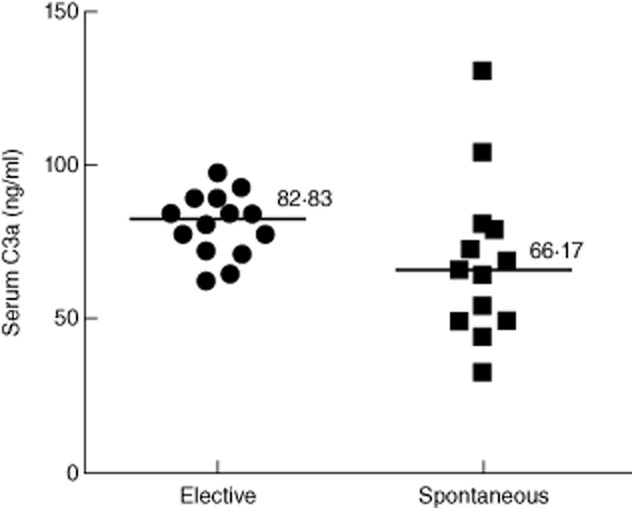
Plasma C3a levels from elective abortion (n = 14) and spontaneous abortion (n = 13) patients. Median of plasma C3a levels measured using enzyme-linked immunosorbent assay did not show significant differences between elective and spontaneous abortion cases (P = 0·06).
Figure 2.
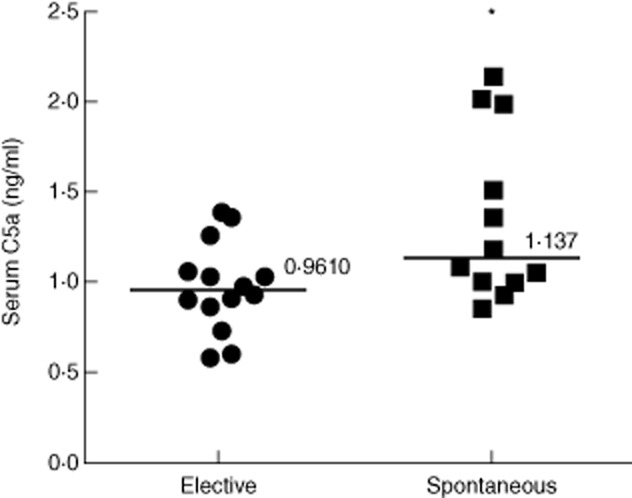
Plasma C5a levels from elective abortion (n = 14) and spontaneous abortion (n = 13) patients. The median of plasma C5a levels was elevated significantly in spontaneous abortion patients compared to that of elective abortion patients (*P = 0·025) as measured by enzyme-linked immunosorbent assay.
Figure 3.
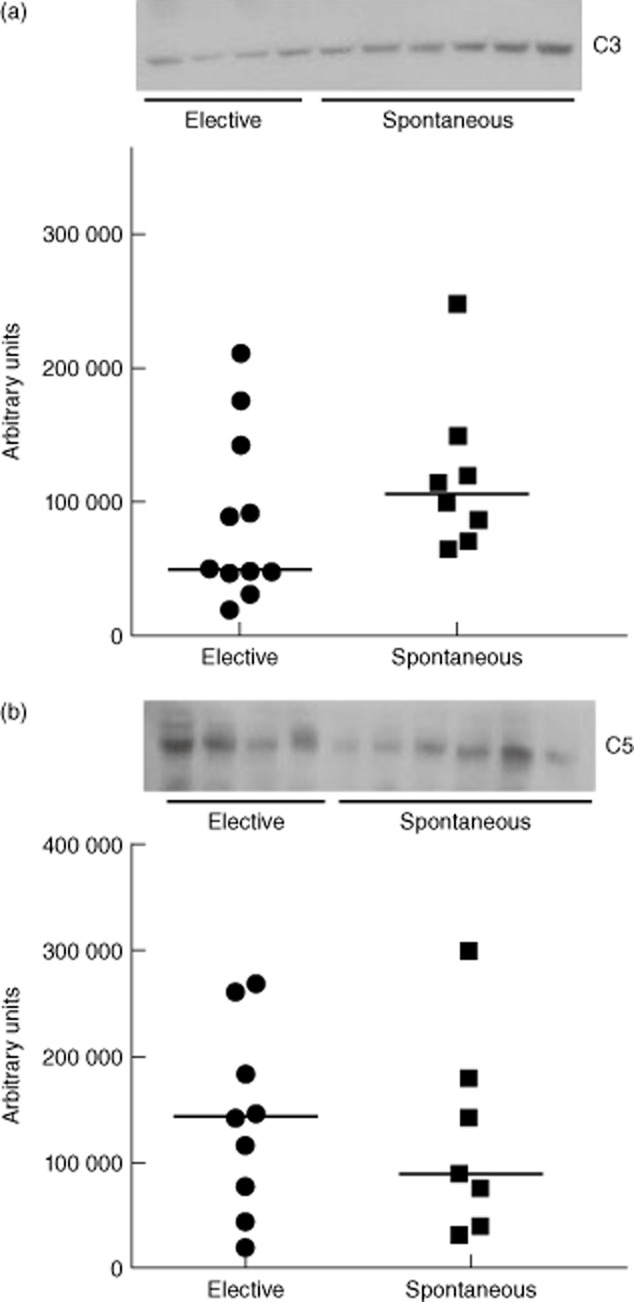
Representative Western blots for plasma C3 and C5 levels in elective and spontaneous abortion patients. Plasma C3 levels were higher in spontaneous abortion cases (a, upper panel), but the difference in median of densities of protein bands was not statistically different (a, lower panel) (P = 0·58). Plasma C5 levels were lower compared to the elective abortion patients (b, upper panel), but the difference in median of densities of protein bands was not statistically significant (P = 0·15).
mRNA expression of complement regulatory proteins CD46 and CD55 was reduced in the decidua from spontaneous abortions
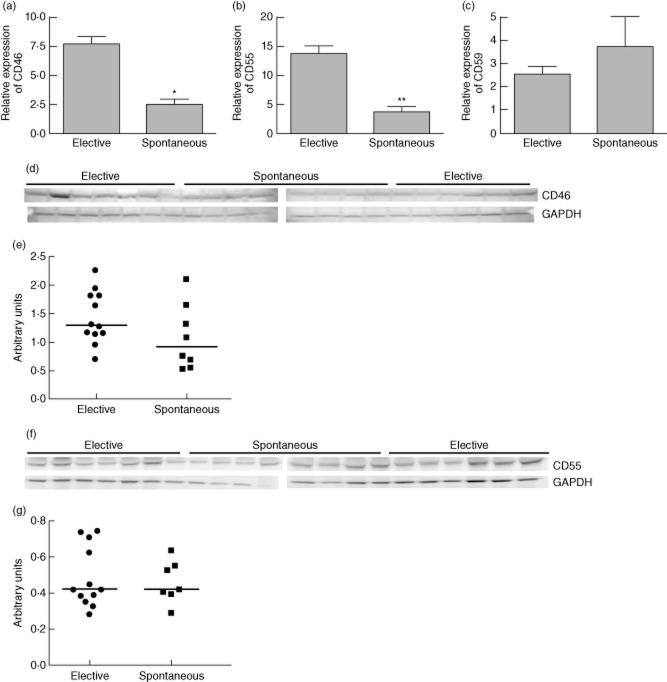
Self-cells are protected from autologous complement attack by membrane expressed complement inhibitory proteins such as CD55 or decay accelerating factor (DAF), CD46 and CD59. CD46 is a co-factor for factor I, which cleaves the C3b into inactive iC3b. The elevated levels of C5a and a corresponding trend in the levels of C5 protein in spontaneous abortion patients led us to hypothesize that CD46 expression at the feto–maternal interface was deficient in those patients, and therefore inefficient inactivation of C3b resulted in the accumulation of C3b. Accumulation of C3b generated excess C5 convertase, which cleaved additional C5 proteins to produce elevated levels of C5a. Therefore, we sought to examine the expression of CD46 at the feto–maternal interface to assess whether deficiency of CD46 was contributing to the elevated levels of plasma C5a observed in spontaneous abortion patients. The expression of CD46 mRNA was reduced significantly in the placental decidua collected from women who suffered spontaneous abortion compared to that collected from women who underwent elective abortion (Fig. 4a). Similarly, the expression of CD55, which inhibits the formation of C3 convertase, was also reduced in the decidua from spontaneous abortion patients compared to that of elective abortion patients (Fig. 4b). However, we did not observe any significant difference in the expression of terminal membrane attack complex inhibitor CD59 in the decidua from these patients (Fig. 4c). These data suggested that the deficiency of CD46 could be contributing to the elevated levels of plasma C5a in spontaneous abortion patients.
Figure 4.
mRNA and protein expression of complement regulatory proteins in the decidua from elective and spontaneous abortion patients. The mRNA expression levels of CD46 was significantly less (*P = 0·0001) in spontaneous abortion cases (n = 13) compared to elective abortion (n = 14) cases (a). The mRNA expression of CD55 was also reduced significantly (**P = 0·002) in the decidua from spontaneous abortion (n = 13) cases compared to elective abortion (n = 14) cases (b). There was no significant difference (P = 0·44) in the decidual mRNA expression of CD59 between the two groups (c). Protein levels of CD46 in the placentas from spontaneous abortion (n = 8) were reduced compared to that of elective abortions (n = 13), but not at the significant level (P = 0·15) (d,e). Protein levels of CD55 were not different (P > 0·99) between the spontaneous abortion and elective abortion groups (f,g).
In order to examine whether protein levels follow the transcript levels of CD46 and CD55 in these placentas, we assessed these protein levels by Western blot. The CD46 protein levels showed a trend of reduced expression in spontaneous abortion placentas compared to elective abortion placentas (Fig. 4d). However, the difference in the median values of CD46 band densities between the two groups was not significant (Fig. 4e). The CD55 protein levels were similar in the placentas from spontaneous and elective abortion patients (Fig. 4f,g).
Discussion
The plasma C5a levels in spontaneous abortion patients were significantly higher compared to elective abortion patients, but C3a levels were not significantly different between the two groups. The plasma levels of C3 and C5 proteins showed a corresponding trend with the C3a and C5a levels, respectively. The data obtained in the present study were consistent with previous animal studies. In a murine model of spontaneous abortion CBA X DBA/2, factor B, C3a and C5a have been shown to be required for triggering the abortion. Although complement activation at the C3 level was needed for triggering the abortion in that murine model, the C5a and C5aR interaction was extremely critical for the abortion [13]. Interestingly, when lipopolysaccharide (LPS) was used to boost the abortion in CBA X DBA/2 model, C5 but not C3 was essential for the abortion [19]. Our data were also consistent with human studies reported in the literature on the C3a and C5a levels. Richani et al. reported that women with unexplained fetal death had elevated levels of plasma C5a compared to normal pregnant women [20]. Further, they also observed that plasma C3a levels were not different between fetal death and normal pregnant groups. However, it is not clear whether the factors at the feto–maternal interface trigger the excess activation of complement cascade.
Normal pregnancy is characterized by an increase in the complement activity, as evidenced by an increase in the plasma levels of complement split products C3a, C4a and C5a in pregnant women compared to non-pregnant women. However, in normal pregnancy, plasma C3a levels correlate positively with plasma C4a and C5a levels [21]. Therefore, an imbalanced plasma C5a level in our spontaneous abortion patient group indicates pathological complement activation at the level of C5. In addition, Lynch et al. reported a significant association between elevated plasma C3a levels and various pregnancy complications, such as gestational hypertension, preterm delivery and intrauterine growth restriction. However, the association between pregnancy loss and C3a levels was not significant in their study, and our data are consistent with that report [22].
The depositions of complement split products have also been observed in the villi and decidua obtained from normal term placentas. However, despite the evidence of complement activation at the feto–maternal interface in normal pregnancy, extensive tissue injury was not observed in those placentas [23]. It was presumed that normal expression of complement regulatory proteins in placenta prevents devastating complement activation in normal pregnancy. The excess complement activation in complicated pregnancies has been associated with many pre-existing conditions such as autoimmune disease and obesity. Excess complement activity has been detected in pregnant women with circulating anti-phospholipid antibodies (aPL) [23,24]. Pregnant women who were obese before pregnancy and developed pre-eclampsia had significantly elevated levels of complement split products Bb and C3a. In the present study we do not rule out the possibility that these factors acted as a trigger for the excess complement activation, at least in some of the spontaneous abortion patients in our study group.
Earlier studies on the role of complement in pregnancy failure provided evidence for the association between circulating complement split products and miscarriage. However, the role of decreased expression levels of complement inhibitory proteins at the feto–maternal interface in spontaneous abortion was not emphasized, due probably to the lack of availability of control samples. In the present study, gestational age-matched pregnant women who decided to terminate their pregnancy because of non-medical reasons were selected as a control group and this allowed us to compare the expression of complement inhibitory proteins at the feto–maternal interface between normal and pathological pregnancy. Although the transcript levels of both CD46 and CD55 were reduced significantly in spontaneous abortion placentas, only CD46 protein levels showed a trend of decrease. The elevated plasma C5a levels but not the C3a levels in spontaneous abortion patients are consistent with the CD46 expression levels in placenta. The CD46 is an especially potent inactivator of the alternative pathway C5 convertase [25]. Further, CD46 appears to play a major role in the viability of fetus, because polymorphic and mutational variations in CD46 gene in humans have been associated with pregnancy loss, recurrent pregnancy loss and pre-eclampsia [17,26,27]. Furthermore, some of the CD46 polymorphic variations cause premature termination of CD46 synthesis [28]. Both CD55 and CD46 genes are located on chromosome 1 at position 1q32, whereas the CD59 gene is located on chromosome 11 [29,30]. Therefore, we suggest that decreased levels of both CD55 and CD46 may be related. However, decreased CD46 may play a major role in allowing C5 related complement activation in spontaneous abortion patients. Because C3a levels were unchanged, we do not believe that reduced CD55 expression at the feto–maternal interface would be involved in complement activation.
Although, in the spontaneous abortion patients, the timing of fetal death is uncertain, our results from circulating and tissue complement factors suggested that increase in C5a preceded the fetal death. More studies with an appropriate time–course and a greater number of patients are required to confirm these findings. However, we believe that the present study is significant, because this included gestational age-matched elective abortion patients for comparison with spontaneous abortion cases.
In summary, the present study revealed that systemic C5a levels were elevated and mRNA expression of CD46 at the feto–maternal interface was reduced in the spontaneous abortion patients compared to elective abortion patients without concurrent increase in the systemic C3a levels.
Acknowledgments
This work was supported by the National Institute of Health (grant number HL72650, HD57013).
Author contributions
M. B., J. D., M. B. and D. H. performed the experiments; M. B., M. S. C. and C. Y. designed the study; and M. B. and C. Y. wrote the paper.
Disclosures
The authors report no conflicts of interest.
References
- 1.Wilcox AJ, Weinberg CR, O'Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 2.Regan L, Braude PR, Trembath PL. Influence of past reproductive performance on risk of spontaneous abortion. BMJ. 1989;299:541–545. doi: 10.1136/bmj.299.6698.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chard T. Frequency of implantation and early pregnancy loss in natural cycles. Baillières Clin Obstet Gynaecol. 1991;5:179–189. doi: 10.1016/s0950-3552(05)80077-x. [DOI] [PubMed] [Google Scholar]
- 4.Branch DW. Immunologic disease and fetal death. Clin Obstet Gynecol. 1987;30:295–311. doi: 10.1097/00003081-198706000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Heinonen PK, Saarikoski S, Pystynen P. Reproductive performance of women with uterine anomalies. An evaluation of 182 cases. Acta Obstet Gynecol Scand. 1982;61:157–162. doi: 10.3109/00016348209156548. [DOI] [PubMed] [Google Scholar]
- 6.Menasha J, Levy B, Hirschhorn K, Kardon NB. Incidence and spectrum of chromosome abnormalities in spontaneous abortions: new insights from a 12-year study. Genet Med. 2005;7:251–263. doi: 10.1097/01.gim.0000160075.96707.04. [DOI] [PubMed] [Google Scholar]
- 7.Nagaishi M, Yamamoto T, Iinuma K, Shimomura K, Berend SA, Knops J. Chromosome abnormalities identified in 347 spontaneous abortions collected in Japan. J Obstet Gynaecol Res. 2004;30:237–241. doi: 10.1111/j.1447-0756.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson IM, Astedt B, Hedner U, Berezin D. Intrauterine death and circulating anticoagulant (‘antithromboplastin’) Acta Med Scand. 1975;197:153–159. doi: 10.1111/j.0954-6820.1975.tb04897.x. [DOI] [PubMed] [Google Scholar]
- 9.Stagnaro-Green A, Roman SH, Cobin RH, el-Harazy E, Alvarez-Marfany M, Davies TF. Detection of at-risk pregnancy by means of highly sensitive assays for thyroid autoantibodies. JAMA. 1990;264:1422–1425. [PubMed] [Google Scholar]
- 10.Temmerman M, Lopita MI, Sanghvi HC, Sinei SK, Plummer FA, Piot P. The role of maternal syphilis, gonorrhoea and HIV-1 infections in spontaneous abortion. Int J STD AIDS. 1992;3:418–422. doi: 10.1177/095646249200300603. [DOI] [PubMed] [Google Scholar]
- 11.Holers VM. The spectrum of complement alternative pathway-mediated diseases. Immunol Rev. 2008;223:300–316. doi: 10.1111/j.1600-065X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 12.Xu C, Mao D, Holers VM, Palanca B, Cheng AM, Molina H. A critical role for murine complement regulator Crry in fetomaternal tolerance. Science. 2000;287:498–501. doi: 10.1126/science.287.5452.498. [DOI] [PubMed] [Google Scholar]
- 13.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203:2165–2175. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De CS, Botta A, Santucci S, et al. Complementemia and obstetric outcome in pregnancy with antiphospholipid syndrome. Lupus. 2012;21:776–778. doi: 10.1177/0961203312444172. [DOI] [PubMed] [Google Scholar]
- 15.Lynch AM, Murphy JR, Byers T, et al. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am J Obstet Gynecol. 2008;198:385–389. doi: 10.1016/j.ajog.2007.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch AM, Gibbs RS, Murphy JR, et al. Complement activation fragment Bb in early pregnancy and spontaneous preterm birth. Am J Obstet Gynecol. 2008;199:354–358. doi: 10.1016/j.ajog.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salmon JE, Heuser C, Triebwasser M, et al. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLOS Med. 2011;8:e1001013. doi: 10.1371/journal.pmed.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen D, Buurma A, Goemaere NN, et al. Classical complement activation as a footprint for murine and human antiphospholipid antibody-induced fetal loss. J Pathol. 2011;225:502–511. doi: 10.1002/path.2893. [DOI] [PubMed] [Google Scholar]
- 19.Yu G, Sun Y, Foerster K, et al. LPS-induced murine abortions require C5 but not C3, and are prevented by upregulating expression of the CD200 tolerance signaling molecule. Am J Reprod Immunol. 2008;60:135–140. doi: 10.1111/j.1600-0897.2008.00605.x. [DOI] [PubMed] [Google Scholar]
- 20.Richani K, Romero R, Soto E, et al. Unexplained intrauterine fetal death is accompanied by activation of complement. J Perinat Med. 2005;33:296–305. doi: 10.1515/JPM.2005.052. [DOI] [PubMed] [Google Scholar]
- 21.Richani K, Soto E, Romero R, et al. Normal pregnancy is characterized by systemic activation of the complement system. J Matern Fetal Neonatal Med. 2005;17:239–245. doi: 10.1080/14767050500072722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch AM, Gibbs RS, Murphy JR, Giclas PC, Salmon JE, Holers VM. Early elevations of the complement activation fragment C3a and adverse pregnancy outcomes. Obstet Gynecol. 2011;117:75–83. doi: 10.1097/AOG.0b013e3181fc3afa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shamonki JM, Salmon JE, Hyjek E, Baergen RN. Excessive complement activation is associated with placental injury in patients with antiphospholipid antibodies. Am J Obstet Gynecol. 2007;196:167.e1–1675. doi: 10.1016/j.ajog.2006.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oku K, Atsumi T, Bohgaki M, et al. Complement activation in patients with primary antiphospholipid syndrome. Ann Rheum Dis. 2009;68:1030–1035. doi: 10.1136/ard.2008.090670. [DOI] [PubMed] [Google Scholar]
- 25.Seya T, Okada M, Matsumoto M, Hong KS, Kinoshita T, Atkinson JP. Preferential inactivation of the C5 convertase of the alternative complement pathway by factor I and membrane cofactor protein (MCP) Mol Immunol. 1991;28:1137–1147. doi: 10.1016/0161-5890(91)90029-j. [DOI] [PubMed] [Google Scholar]
- 26.Mohlin FC, Mercier E, Fremeaux-Bacchi V, et al. Analysis of genes coding for CD46, CD55, and C4b-binding protein in patients with idiopathic, recurrent, spontaneous pregnancy loss. Eur J Immunol. 2013;43:1617–1629. doi: 10.1002/eji.201243196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Risk JM, Flanagan BF, Johnson PM. Polymorphism of the human CD46 gene in normal individuals and in recurrent spontaneous abortion. Hum Immunol. 1991;30:162–167. doi: 10.1016/0198-8859(91)90030-d. [DOI] [PubMed] [Google Scholar]
- 28.Fremeaux-Bacchi V, Moulton EA, Kavanagh D, et al. Genetic and functional analyses of membrane cofactor protein (CD46) mutations in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2006;17:2017–2025. doi: 10.1681/ASN.2005101051. [DOI] [PubMed] [Google Scholar]
- 29.Liszewski MK, Post TW, Atkinson JP. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- 30.Forsberg UH, Bazil V, Stefanova I, Schroder J. Gene for human CD59 (likely Ly-6 homologue) is located on the short arm of chromosome 11. Immunogenetics. 1989;30:188–193. doi: 10.1007/BF02421205. [DOI] [PubMed] [Google Scholar]