Abstract
This study evaluated the psychological mechanisms underlying imitation of facial actions in young infants. A novel aspect of the study was that it used a nonoral gesture that had not been tested before (head movement), as well as a tongue-protrusion gesture. Results showed imitation of both displays. Imitation was not limited to the intervals during which the experimenter’s movements were displayed; Ss also imitated from memory after the display had stopped. The results established that newborn imitation is not constrained to a few privileged oral movements. The findings support Meltzoff and Moore’s hypothesis that early imitation is mediated by an active cross-modal matching process. A common representational code may unite the perception and production of basic human acts.
There is a rekindling of interest in the origins and early development of imitation in infants. This rekindling has been engendered, in part, by the reports of imitation in early infancy (Flavell, 1985). The proposition has been offered that there exists at birth some primitive capacity for matching the acts of others (Meltzoff & Moore, 1977, 1983a). Such an ability would be an important building block for subsequent social and cognitive development.
Meltzoff and Moore’s (1977) report of neonatal imitation sparked a two-pronged discussion—one concerning the existence of the effect and the other concerning the psychological mechanism that might mediate it. The first of these issues, that of existence, has now been addressed in numerous studies. After an initial period of debate, the findings of neonatal imitation have been confirmed and extended in at least eight independent laboratories since 1977 (Abravanel & Sigafoos, 1984; Field, Woodson, Greenberg, & Cohen, 1982; Fontaine, 1984; Heimann & Schaller, 1985; Jacobson, 1979; Kaitz, Meschulach-Sarfaty, Auerbach, & Eidelman, 1988; Reissland, 1988; Vinter, 1986). These studies include work done both in this country and cross-culturally as well—in Sweden, Switzerland, Israel, and Nepal. The caveat is that early imitation is more easily induced using certain eliciting conditions (Meltzoff & Moore, 1983b) and, moreover, there may be individual differences in the proclivity to imitate (Heimann & Schaller, 1985). These factors may contribute to the fact that imitative behavior is not, by any author’s report, something as automatically or readily triggered as a Moro reflex or palmar grasp. Nonetheless, the basic phenomenon reported by Meltzoff and Moore—that a certain set of adult gestures will elicit matching responses by young infants—seems repeatable by many independent investigators testing infants in different settings. A key question now concerns the second issue raised earlier—that of explicating the psychological mechanisms underlying this early behavior.
One account of early imitation proposed by Meltzoff and Moore (1977, 1983a, 1983b, 1985) is that the behavior under study—for example, tongue protrusion—might be a prepackaged motor program that simply is tripped or “released” by the adult’s behavior. This view has garnered some support (Abravanel & Sigafoos, 1984; Jacobson, 1979; Kaitz et al., 1988). The view predicts that there will be a limited set of special actions that are triggered and that neonates will not have a generative capacity to produce behavioral matches across a wide range of modeled behaviors. Exactly how many different behaviors are “too many” for the releaser model to incorporate is not established, but all theorists agree that at some point it becomes unparsimonious for the releaser model to hold that every new behavior that is imitated is mediated by an innate releasing mechanism (IRM). Most theorists also agree that the imitation of certain acts, such as particular oral movements, could be due to IRMs, whereas the imitation of more arbitrary acts (such as wiggling one’s toes) is unlikely to be amenable to such an explanation. No precise specification of the set of human acts that are predicted to be mediated by IRMs is currently available.
The preceding analysis suggests that it would be informative to explore the range of different acts that can be imitated by neonates and, in particular, to go beyond acts involving the oral region in tests of neonatal imitation. If it could be demonstrated that the same infants who succeeded in imitating tongue protrusion failed to imitate a new non-oral behavior that was within their visual and motor capacity, the result would fit well with the type of constraints predicted by a releasing mechanism view; conversely, if infants succeeded at both gestures, it would raise the possibility that a more general matching mechanism may need to be contemplated.
Young infants have motor control over their head movements if their heads are well supported. Indeed, Piaget reported that at least two infants he observed during sensorimotor Stage 2 (1 to 4 months of age) imitated head movements that were presented by an adult. Neither Piaget nor other theorists have tried to explain this observation in terms of an IRM; more often it has been considered an unexplained anomaly. An experimental demonstration of the imitation of head movements by newborns would contribute to the literature in two ways: (a) On basic empirical grounds, it would extend the range of gestures beyond elementary lip and tongue movements, and (b) on theoretical grounds, it would motivate discussion as to whether something else, besides simple releasers, should be considered to account for instances of early imitation.
This study investigated whether or not newborn infants could imitate head movements. The subjects were shown both tongue protrusions and head movements in a repeated-measures design, with the specific aim of conducting a replication of the tongue-protrusion effect in newborns and using these same subjects to test a new non-oral gesture that had not previously been examined under experimental conditions in infants this young.
Method
Subjects
The following predetermined factors were adopted as minimum criteria for admitting infants into the study: (a) less than 72 hr old, (b) full-term (greater than 36 weeks’ gestation), (c) normal birthweight (2.5–4.5 kg), (d) fed within the last 3 hr and no rooting or other signs of hunger for 5 min immediately prior to testing, and (e) wide-eyed, alert, and behaviorally calm for 5 min immediately prior to testing.
The subjects were 40 healthy newborn infants with no known visual, motor, or mental abnormalities. The mean age at the time of test was 40.55 hr (range = 13.37–67.33, SD = 16.79). The mean birthweight was 3.66 kg (SD = 0.43 kg). The mean gestational age at birth according to hospital records was 40.49 weeks (SD = 1.36). The mean 1-min Apgar score was 7.90 (SD = 1.43). The mean 5-min Apgar score was 8.85 (SD = 0.48), with the lowest score being a single infant who received a 7. Of the 40 subjects, 18 were boys and 22 were girls. The maternity ward served primarily middle- and upper-middle-class Whites: Of the 40 subjects, 38 were White, 1 was Hispanic, and 1 was Asian. All of the mothers were between 20 and 36 years of age (M = 29.20, SD = 3.77).
Testing began on 53 additional newborns who did not complete the study for the following reasons: crying (36%), falling asleep (23%), spitting or choking uncontrollably (19%), hiccuping (13%), or having a bowel movement during the test session (9%). This loss rate is typical of previous studies conducted with newborns (e.g., Kessen, Salapatek, & Haith, 1972; Mendelson & Haith, 1976; Salapatek & Kessen, 1966). The specification that an infant was sleeping, crying, and so forth was not made by the experimenter during the test itself, but by an independent judge who evaluated the infant’s state from videotape and was kept uninformed about the infant’s test condition.
Test Room
The test took place in a newborn laboratory that had been set up adjacent to the nursery and was isolated from the sound of other crying babies. Inside the room the infants were tested within a large, black-lined test chamber (2.0 m × 1.5 m). The room lights were extinguished during the test. A small light fastened above (25 cm) and behind (15 cm) the infant was used to spotlight the experimenter’s face. To reduce reflectance from his body, the experimenter wore a black gown made from the same material as lined the test chamber. The luminance was approximately 0.6 log cd/m2 at the experimenter’s face and −1.3 log cd/m2 on the black background 30 cm to the right of the experimenter’s face. The cameras used to record the session were located outside the test chamber, with holes in the back wall only large enough for the lenses to poke through. The infants showed no tendency to fixate the camera lenses during the test. An assistant silently focused the camera on the subject’s face at the beginning of each session and readied the video decks, which were also housed outside the test chamber within a sound-dampening box to avoid any auditory distractions.
Apparatus
To obtain high-quality video recordings under the low illumination levels required, we used an infrared-sensitive video camera (Telemation TMC-1100SD with a 4352H silicon diode pickup tube) and a Pichel IR-75 infrared illuminator. This camera and a corresponding video recorder were devoted solely to recording a close-up picture of the infant’s face. The camera was focused on the infant’s lips, and the resulting picture encompassed the area from the top of the head to about 2.5 cm below the chin. A second camera (Sony 3260) and video recorder were used to record a mirror reflection of the experimenter’s face; this reflection was obtained from a small mirror that was placed behind and to the left of the infant.
The experiment was electronically timed. The timer consisted of a character generator, the output of which was digitally displayed in a small box located directly above (5 cm) the infant’s head. The output of the character generator was also simultaneously fed into both video machines, such that the elapsed time (in 0.10-s increments) was mixed in as a permanent reference on the video records.
Design and Procedure
The infants were carefully handled so that they did not see the experimenter (the “stimulus” in the experiment) until the test began. For the test the infants were placed in a specially padded infant seat that comfortably supported their trunks and shoulders. The seat supported them in a semi-upright posture, raised approximately 30° off of the horizontal. Once the infant was placed in the seat, the experimenter slowly moved a small white cloth (46 cm × 15 cm) in the spotlight before the infant’s eyes for at least 20 s. As soon as the infant fixated the cloth while in a quiet, alert state, the experimenter did the following: (a) removed the cloth, (b) put his face in the spotlight at a distance of approximately 25 cm from the infant’s eyes, (c) waited for the signal from the camera operator as to which randomly determined test condition to use, and (d) when he received it, simultaneously activated the experimental clock and commenced modeling the first gesture. This procedure ensured that the subject’s test condition was not known by the experimenter until the moment the test began. From that time on, the test was entirely time locked.
Each infant was shown both a tongue-protrusion and a head-movement display in a repeated-measures design, with each infant acting as his or her own control. The order of stimulus presentation was counterbalanced such that half the subjects saw the tongue-protrusion display first and the head-movement display second, and the other half saw the gestures in the reverse order. In previous work with newborns it was reported that attention and responsivity were maximized if a burst of adult gesturing was alternated with an interval in which the adult remained passive—what we called a “burst-pause” procedure (Meltzoff & Moore, 1983a). The present experiment capitalized on that type of design. The temporal structure of the test is depicted in Figure 1. As shown, the overall test was 8 min in duration. It was composed of two 4-min demonstration periods (one for the demonstration of tongue protrusions and the other for the demonstration of head movements). Each of these periods consisted of twelve 20-s intervals such that the experimenter alternately demonstrated the gestures for 20 s, then assumed a passive face for 20 s, and so on. At the end of the first 4-min period, the identical procedure was repeated using the new gesture. The gestures were performed in a standardized fashion, at the rate of four times in a 20-s interval with a 1-s interact interval. In brief, the experiment followed a predetermined time schedule, and there were no breaks or pauses during the test.
Figure 1.
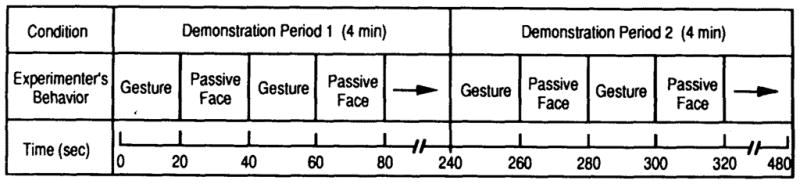
Schematic diagram of the temporal structure of the test. (The arrows indicate that these alternating intervals were repeated for the specified time.)
The adult tongue-protrusion gesture consisted of protruding and withdrawing the tongue between closed lips at the specified rate. The adult head-movement gesture consisted of a full rotation of the head in a clockwise direction in the frontal plane. The head did not quite trace a circular path, but traced more of an ellipse. Taking the adult’s nose as a referent, the path traced was an ellipse with a lateral extent (along the x-axis) of approximately 13 cm and a vertical extent of approximately 5 cm. The act was simply a comfortable, full rotation of the adult’s head.
Scoring
The video records of the infants’ behavior were close-ups of the face and, hence, did not contain a record of the gesture shown to the infants. The eighty 4-min periods (40 subjects × 2 gestures each) were scored from video in random order by an independent coder who was kept uninformed about which gesture had been shown to the infant in any given period. The scorer reviewed the videotapes in real time, slow motion, and, if necessary, even frame-by-frame. The scorer’s instructions were to record the occurrences of all instances of infant tongue protrusions and head movements, making reference to the time code that was on the screen. The scorer was provided with operational definitions of the target behaviors.
The operational definition for the onset of tongue protrusion was a clear forward thrust of the tongue such that the tongue tip crossed the back edge of the lower lip. For those cases in which the tongue was being retracted but was not yet behind the lip when a second tongue thrust occurred, the first tongue protrusion was terminated with the initiation of the second. An infant head movement was scored if the behavior met either of two criteria. Criterion 1 was that there was a large “lateral movement of the head” such that it swept horizontally in a smooth movement from one side of the midline to the other. To be counted as a lateral head movement in this scoring system, it was not enough simply to move the head slightly, but rather to perform a head movement that extended from one side across the midline to the other side. Criterion 2 involved “head rotations” by the infant. In this case the head had to be moved in both the x and y directions, such that it traced an arc. The minimum arc that needed to be executed to count as a head rotation was 90°; in other words, the infant’s head needed to move not only horizontally but also vertically in one smooth, continuous motion. The direction of the head rotation was noted as being either clockwise or counterclockwise. Excluded from analysis was any infant behavior (tongue or head movement) that occurred during occasional yawning, sneezing, choking, or spitting.
Both intra- and interobserver reliabilities were assessed, the former by having the original scorer rescore a randomly selected 15% of the data, and the latter by having a second scorer, who was also blind to the gestures shown to the infant in any given period, score 10% of the data. The intrascorer assessments were conducted more than 1 week after the data had been scored the first time, and the scorer was kept unaware of the trials to be used to assess reliability, which has the potential for fostering high scoring precision throughout all the trials (Reid, 1970). Pearson correlations were used to assess reliability. The rs for the intraobserver assessments were .98 for tongue protrusion and .93 for head movement, and the rs for the interscorer assessments were .98 for tongue protrusion and .95 for head movement.
Results
The results show that infants systematically matched the adult display shown to them. Table 1 presents the data for five infant behaviors as a function of the two adult displays. As shown, the number of infant tongue protrusions was significantly greater when infants were shown the adult tongue-protrusion display (M = 6.73, SD = 7.66) than when those same infants were shown the head-movement display (M = 4.67, SD = 5.62), z = −2.62, p < .01, using a Wilcoxon matched-pairs signed-ranks test. Similarly, infants produced almost twice as many head movements (M = 8.33, SD = 5.84) when shown the adult head-movement display than when shown the tongue display (M = 4.68, SD = 4.32), z = −3.76, p < .001, Wilcoxon test. The data were most appropriately analyzed using nonparametric statistics, given the variance in the behavioral frequencies of the newborns and the level of measurement that could be justified in this study (Siegel, 1956). However, to check the stability of the effects across different statistical assumptions, the data were also reanalyzed using parametric statistics. The results were highly comparable. There were a significantly greater number of infant tongue protrusions when infants were shown the tongue-protrusion display than when shown the head-movement display, t(39) = −2.57, p < .05. Similarly, there were significantly more head movements when infants were shown the head-movement display than when shown the tongue display, t(39) = −4.23, p < .001.1
Table 1.
Mean Number of Infant Behaviors as a Function of the Two Adult Displays
| Infant behavior | Adult display
|
|||
|---|---|---|---|---|
| Tongue protrusion
|
Head movement
|
|||
| M | SD | M | SD | |
| Tongue protrusions | 6.73 | 7.66 | 4.67 | 5.62 |
| Head movementsa | 4.68 | 4.32 | 8.33 | 5.84 |
| Counterclockwise rotations | 0.48 | 0.75 | 1.53 | 1.69 |
| Clockwise rotations | 0.97 | 1.42 | 2.00 | 1.97 |
| Lateral movements | 3.23 | 3.66 | 4.80 | 4.34 |
The sum of the three subdivisions equals the number of total head movements.
A depiction of the imitation effect at the level of individual subjects is provided by taking into account both infant behaviors (tongue protrusions and head movements) simultaneously. With regard to tongue protrusions, each infant can produce a greater frequency of tongue protrusions to the adult tongue-protrusion display (indicated as a “+”) or to the adult head-movement display (indicated as a “−“), or can produce an equal frequency of tongue protrusions to both displays (indicated as a “0”). Similarly, for head movements, each infant can produce a greater frequency of head movements to the head-movement display (+) or to the tongue-protrusion display (−), or can produce an equal frequency to both (0). Infants who produce more head movements to the head-movement display and more tongue protrusions to the tongue display were classified as “++” responders, and so on. An exhaustive categorization of the 40 subjects in terms of their individual response patterns during the test is given in the first line of data in Table 2. A one-sample chi-square test shows that the distribution of the 40 subjects across the response patterns cannot be accounted for by chance, χ2(7, N = 40) = 56.40, p < .001. The hypothesis of imitation is most stringently tested by comparing the number of infants falling into the most extreme cells of this distribution (++ vs. −−). The subjects who were categorized as ++ had, by definition, systematically switched their behavior and matched both adult displays. Conversely, infants who were categorized as −− had systematically mismatched both displays. Under the null hypothesis, there is an equal probability of infants falling in either the ++ or the −− category. The data reveal 20 infants with the ++ profile as compared to only 2 with the −− profile, p < .001 using a binomial test, thus providing strong support for the hypothesis of imitation.
Table 2.
Number of Infants Displaying Each of Nine Possible Response Patterns as a Function of Test Period
| Test period b | Response patternsa
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ++ | +0 | 0+ | +− | −+ | 00 | 0− | −0 | −− | |
| Whole test (8 min) | 20 | 4 | 2 | 7 | 2 | 0 | 1 | 2 | 2 |
| Gesture periods (4 min) | 13 | 8 | 3 | 4 | 5 | 1 | 0 | 3 | 3 |
| Passive-face periods (4 min) | 17 | 3 | 2 | 8 | 0 | 3 | 1 | 3 | 3 |
The response patterns are shown as ordered pairs depicting the two infant behaviors in the order: tongue protrusions, head movements. (+) indicates a matching response, (−) indicates a mismatching response, (0) indicates an equal response to both displays.
The entire test was 8 min in duration. The data were also recast, isolating only the gesture periods alone (4 min) and the passive-face periods alone (4 min). Each row in the table sums to 40 because there were 40 subjects in the study.
Up to this point infant head-movement behavior has been considered as a broad category of response, but the scoring system allowed us to subdivide infant head movements into three types (as delineated in the scoring criteria section): clockwise rotation, counterclockwise rotation, and lateral head movement. These subdivisions are interesting to examine for two reasons. First and foremost, they provide a more qualitative perspective on the nature of the infant’s response. Second, they provide some data relevant for exploring the mechanisms mediating the head-movement response. In particular, one might ask whether infant tracking responses were mediating the effects. Might the infants be making head movements of their own as they visually tracked the adult’s moving head, in a sense being “perceptually tethered” to the adult’s movements? Piaget (1962) developed this hypothesis in some detail. In the present study, Piagetian “perceptual tethering” should primarily cause the infants to make counterclockwise movements (as taken from their perspective, because of the left-right reversal engendered by the participants facing each other and the adult moving his head in a clockwise fashion).2
The data in Table 1 reveal that there were indeed significantly more counterclockwise head rotations in response to the adult head-movement display than to the adult tongue-protrusion display, z = −3.21, p < .005, Wilcoxon test. However, this type of infant head movement does not explain all the data. There were also significantly more clockwise head rotations in response to the adult head-movement display (M = 2.00, SD = 1.97) than to the tongue display (M = 0.97, SD = 1.42), z = −2.77, p < .01, Wilcoxon test. Clockwise rotations literally match what the adult did in terms of an anatomical act. Similarly, there were significantly more lateral head movements to the adult head-movement display than to the adult tongue display, z = −2.85, p < .005, Wilcoxon test. These data suggest that although head movements due to strict perceptual tethering probably occur (the counterclockwise effects were significant as predicted by this model), they are not the sole basis for infants’ head-movement responses.
Gesture Periods Only
We next addressed the question of whether infants’ responses were limited to the intervals when the adult was presenting a moving display, or whether infants also showed evidence of imitation during the subsequent passive-face periods—even though the adult was not presenting his gesture and indeed was providing only a stationary target to fixate during these periods. To assess this, the data from the entire test (reported above) were broken down into the time intervals during which the adult was actually presenting the gestures (hereafter called “gesture periods”) and the intervals during which the adult was presenting a passive face (“passive-face periods”). Reference to Figure 1 shows that the 20-s gesture periods in the test were alternated with 20-s passive-face periods.
Table 3 isolates the data obtained during the adult gesture periods alone, 4 min of test time, ignoring for the moment any responses during the passive-face periods. As shown, infants responded with more tongue protrusions when witnessing the adult tongue display (M = 3.18, SD = 3.90) than when witnessing the adult head-movement display (M = 2.15, SD = 3.09), z = −1.71, p < .05, Wilcoxon test, one-tailed. Similarly, infants responded with more head movements when witnessing adult head movements (M = 4.73, SD = 3.86) than when witnessing tongue protrusions (M = 2.23, SD = 2.25), z = −3.40, p < .001, Wilcoxon test. The consistency of the imitative effect at the level of individual subjects is noteworthy. The second line of data in Table 2 presents a categorization of the 40 subjects according to their individual response profiles. Once again the extreme cells most directly test the hypothesis of imitation, because it is equiprobable under the null hypothesis that infants will consistently match (++) or consistently mismatch (−−) the two adult displays. The results identify 13 individuals who conformed to the ++ pattern and only 3 with the −− pattern, p < .05, using a binomial test.
Table 3.
Mean Number of Infant Behaviors as a Function of the Two Adult Displays During the Gesture Periods Only
| Infant behavior | Adult display
|
|||
|---|---|---|---|---|
| Tongue protrusion
|
Head movement
|
|||
| M | SD | M | SD | |
| Tongue protrusions | 3.18 | 3.90 | 2.15 | 3.09 |
| Head movementsa | 2.23 | 2.25 | 4.73 | 3.86 |
| Counterclockwise rotations | 0.23 | 0.42 | 1.02 | 1.21 |
| Clockwise rotations | 0.55 | 0.82 | 1.18 | 1.30 |
| Lateral movements | 1.45 | 1.92 | 2.53 | 2.62 |
The sum of the three subdivisions equals the number of total head movements.
Subdividing the data according to the different types of infant head movements was also informative. Not surprisingly, there were more counterclockwise head movements (ones potentially due to perceptual tethering) when infants were watching the adult head-movement display than when watching the tongue display, z = −3.47, p < .001, Wilcoxon test. The data also reveal that the clockwise head movements were discriminative, z = −2.28, p < .05, Wilcoxon test, as were the lateral head movements, z = −2.14, p < .05, Wilcoxon test.
Passive-Face Periods Only
The next step in the analysis was to examine the data from the passive-face periods alone. Note that for this analysis the visual stimulus present in front of the subject was identical whether the infant had previously witnessed adult tongue protrusion or head movement. There were no adult movements to follow visually, only a stationary face to fixate. The question under test was whether infants would imitate the adult display that they had just seen in the previous gesture period, or whether the differential responding obtained during the gesture periods fell to chance during these adult passive-face intervals.
The data in Table 4 show that the infants produced more tongue protrusions during the passive-face intervals after the adult tongue-protrusion display (M = 3.55, SD = 4.02) than they did in the passive-face intervals after the head-movement display (M = 2.52, SD = 3.11), z = −2.54, p < .05. Similarly, infants produced more head movements in the passive-face intervals after seeing head movement (M = 3.60, SD = 2.95) than in the passive-face intervals after seeing tongue protrusion (M = 2.45, SD = 2.95), z = −3.14, p < .005, Wilcoxon test. The breakdown of the 40 subjects according to their individual response profiles is presented in the third line of data in Table 2. The strongest test of imitation derives from the extreme cells, which isolate subjects who either matched (++) or mismatched (−−) both the adult displays. The results identify 17 subjects who displayed the ++ profile versus 3 who displayed the −− profile, p < .001, using a binomial test, thus providing strong support for the imitation hypothesis even during the adult passive-face intervals.
Table 4.
Mean Number of Infant Behaviors as a Function of the Two Adult Displays During the Passive-Face Periods Only
| Infant behavior | Adult display
|
|||
|---|---|---|---|---|
| Tongue protrusion
|
Head movement
|
|||
| M | SD | M | SD | |
| Tongue protrusions | 3.55 | 4.02 | 2.52 | 3.11 |
| Head movementsa | 2.45 | 2.95 | 3.60 | 2.95 |
| Counterclockwise rotations | 0.25 | 0.54 | 0.50 | 0.75 |
| Clockwise rotations | 0.43 | 0.81 | 0.83 | 1.20 |
| Lateral movements | 1.77 | 2.43 | 2.27 | 2.25 |
The sum of the three subdivisions equals the number of total head movements.
It is of relevance to theory to decompose the infants’ total head-movement scores into their component types, because during these adult passive-face intervals the infants have no movements to follow visually. The data obtained during these passive-face intervals revealed that the infants’ counterclockwise rotations (potential repetitions or continuations of the tethered tracking responses) were not significantly different between the head-movement and tongue-protrusion trials, although there was a trend toward more such responses to the head movements, z = −1.48. Of equal interest are the clockwise rotations, the ones that were opposite those that would have been engendered by direct perceptual tethering of the adult, but were anatomically matched to the adult’s act. Infants produced more of these clockwise head movements in the passive-face intervals following the adult head-movement display (M = 0.83, SD = 1.20) than in the passive-face intervals following the tongue-protrusion display (M = 0.43, SD = 0.81), z = −1.91, p = .056, Wilcoxon test. The number of lateral head movements was also greater after the adult head-movement display than after adult tongue protrusion, z = −2.11, p < .05.
Further Analysis of the Passive-Face Periods
The passive-face intervals were broken down still further to provide an even more microanalytic assessment of the potential role of infant tracking responses. We asked whether infants could imitate head movements during a passive-face period even if they had made no previous tracking movements during the adult’s display itself. In other words, could infants inaugurate an imitative head-movement response having exhibited no previous “tracking”? To address this very specific question, analyses were conducted on head-movement responses that met two criteria: (a) They had to occur during a passive-face period (as above), and (b) the infant could not have already produced any head movement during the adult demonstration. The first criterion ensured that the infant was not presently tracking the adult (because the adult was physically stationary during the passive-face intervals). The second criterion ensured that the infant had not yet performed such a tracking head movement in a previous gesture period (and therefore could not merely be continuing or repeating that movement during the passive-face interval).
For this very specific microanalysis, a subset of the passive-face data reported above was used. In the previous analysis, the behavior in all the passive-face intervals was tallied with no restrictions. The present analysis was more restrictive, and in accordance with criterion (b) above, as soon as an infant made a head movement of any type during a gesture period, the subsequent behavior was ignored on the grounds that it might potentially be a repetition of this tracking response. For example, if an infant made a head movement in the third gesture period of a given display, only those responses that had occurred in the first two passive-face intervals were tallied. Because infants remained inactive for differing numbers of gesture periods, and therefore had different numbers of passive-face periods that contributed data, the data were expressed in terms of the number of behaviors per period. More specifically, an infant might perform a total of four head movements during the course of four criterial passive-face periods; this would be expressed as a “1” (4/4). Another infant might perform four head movements in six criterial passive-face periods, and this would be expressed as “.66” (4/6).
The results of this microanalysis show that infants produced a higher rate of head movements in response to the adult head-movement display (M = 1.01, SD = 1.47) than they did to the tongue-protrusion display (M = 0.33, SD = 0.54), z = −2.10, p < .05, Wilcoxon test. These data provide empirical support for the notion that infants can observe the adult without moving their own heads, and then on the basis of this perception (but not on the basis of any concomitant action) can subsequently produce a matching response.
Discussion
The overall pattern of the data is in line with that predicted from a hypothesis of infant imitation. The newborns produced significantly more tongue protrusions in response to an adult tongue-protrusion display than they did in response to an adult head-movement display. Similarly, these same infants produced more head movements in response to an adult head-movement display than in response to an adult tongue-protrusion display. This overall imitative pattern was further broken down along two dimensions: the time intervals in which the responses were produced (the adult-gesture vs. passive-face intervals) and the morphology of the infants’ responses. It was found that the imitative effects were strong both during the adult’s gesturing and during the subsequent passive-face intervals and that the effects were present within each subdivision of the head-movement code. From a purely empirical standpoint, these findings extend previous reports in two ways: They show matching of a non-oral act, and they provide more detail about the morphology and temporal characteristics of the matching response than has heretofore been available.
What inferences can be drawn from these matching effects? The detailed pattern of results affords information about the mechanisms that may mediate this behavior. The data showed that infants not only matched what they presently saw, but what they had previously seen (passive-face results). This is of particular relevance to the question of what mechanism mediates the head-movement response. If infants had been constrained to matching the adult only during the gestural demonstration, it would invite the notion that infant tracking responses underlie this behavior. The argument would be that the infant was, in a sense, “perceptually tethered” to the adult during the display; in the very act of following the adult’s demonstration, the infant is caused to produce a like response. Indeed, this notion of perceptual tethering was developed by Piaget (1962) as a possible account of head-movement imitation that occurred prior to about 8 months old; after that age his theory postulated that head movements could be imitated without such tethering.
This idea of tethering makes sense, but can it account for all the data reported here, or might some other mechanism also be invoked? The present experiment was designed to check whether infants were constrained to performing head movements in synchrony with the adult, as if tethered to his movements. Recall that in the present design the adult did not continuously perform head movements, as is sometimes done in tests of imitation. Had such a design been used, it would have been difficult to evaluate the tethering hypothesis. However, this experiment was designed to include preset intervals in which the adult halted and remained stationary for the infant to fixate. The data showed significant matching not only during the gesture period, but during the passive-face periods. Thus, the view that the head-movement response is solely attributable to infants being tethered to the adult during his display is not supported by the data.
However, it may be worthwhile to broaden the argument and move beyond the idea of strict perceptual tethering. One might suggest a theory in which infants tend to repeat an activity that they previously performed during the perception of the model (cf. Piaget, 1962). According to this view, the infant head-movement matching would be initiated as a tracking response, and then the infant would persevere in this movement after the adult had stopped. This theory would hold that although direct tracking is not wholly sufficient to account for the head movements reported here (because an account needs to be added about why the tracking act is repeated in the presence of a stationary face to fixate), nonetheless, tracking is a necessary condition to initiate the response in the first instance. For the sake of distinguishing this view from the narrower one described above, we will hereafter refer to the two as Tracking 1 (perceptual tethering) and Tracking 2 (repetition of tracking in the absence of a moving stimulus).
Tracking 2 is difficult to assess empirically, because it takes as axiomatic that a head movement, even one that is initiated in the presence of a stationary target, is ultimately caused by prior tracking activity. As a theoretical matter, it would be possible to evaluate this model by preventing tracking movements during the display period altogether—for example, by outfitting the subject with a head brace—but that is not something that can be appropriately done with newborns. Instead, we evaluated this hypothesis by performing a microanalysis of two aspects of the infants’ responses—their morphology and temporal characteristics.
Regarding morphology, the primary responses of interest became the ones in the clockwise direction, because they would have been opposite those entailed by direct perceptual tethering. The data showed that infants produced significantly more of these clockwise head movements to the adult head-movement display than to adult tongue protrusions, even during the passive-face intervals. Thus, the notion that infants simply persevered in the same movement that they would have made while perceptually tethered or bound to the adult did not receive support.
The second microanalytic approach was that of examining the temporal characteristics of the imitative response and, in particular, whether imitation could be initiated without any prior tracking. The head-brace experiment was not conducted (we would predict positive results); however, the data were reanalyzed to isolate a naturally occurring analog. There were instances in which infants did not perform head movements during the gesture period—the infants being transfixed by the display. Analysis of these instances showed a significant head-movement response during the passive-face intervals that followed. In other words, infants were able to initiate their matching response, to begin it for the first time, even after the adult movement had ceased. This suggests that tracking is not a necessary condition for eliciting this response; something more than tracking needs to be invoked to explain the entire pattern of results.
This conclusion should not necessarily be surprising, because it has always been the case that a tracking hypothesis would need to be coupled with another mechanism to cover the full set of results reported in this experiment and others. That is, newborns also match a tongue-protrusion display. Watching a tongue move may lead to eye movements, but such perceptual activity is not intrinsically related to the target response under test (tongue movements). Some mechanism other than tracking must be invoked in the case of tongue protrusion to link an infant’s response to a visual display. Might not that mechanism also subserve the matching of head movements?
In addition to the tracking explanation, three candidate mechanisms for neonatal imitation have been outlined in the literature: early learning, innate releasing mechanisms (IRMs), and visual–motor equivalence mapping. The current experiment was conducted with newborns with a mean postnatal age of 41 hr. The findings of imitation in this age group are in line with five recent studies that have reported imitation within the newborn period (Field et al., 1982; Kaitz et al., 1988; Meltzoff & Moore, 1983a; Reissland, 1988; Vinter, 1986). It can be concluded from this work that training by caretakers probably is not a necessary condition for imitation; apparently infants from the earliest age are capable of motor matching of selected acts.
The debate, then, may be tentatively cast between the visual–motor equivalence mapping and IRM models. The IRM model is more plausible the shorter the list of items that can be imitated and the more automatic and stereotypic the response. If the data had shown that young infants could imitate only tongue protrusions and nothing else, this view would certainly gain favor. However, the IRM model becomes strained as the list becomes longer and the acts more “arbitrary.” This study directly addresses the question of whether newborns are constrained to mimicking only tongue movements, and the answer appears to be no. These data add to the previous reports of early imitation of hand movements (Meltzoff & Moore, 1977; Vinter, 1986) and a variety of different facial movements by several investigators. This pattern of findings provides a motivation for hypothesizing that something other than simple releasers may be involved.
The account of neonatal imitation offered by Meltzoff and Moore (1977, 1983a, 1983b, 1985) suggests that neonates have some underlying ability to recognize and use the equivalences between body movements they see and acts of their own. We believe that intermodal equivalence mapping is at the heart of the problem of infant imitation. According to Piaget’s theory, adult head-movement gestures provide infants with essential learning experiences in constructing visual–motor equivalences, because infants would be perceptually tethered to the adult and would often duplicate the act as part and parcel of perceiving it. This type of natural tethering was believed to be a developmental precursor to later forms of imitation, such as tongue protrusion, that did not entail tethering. However, given the many reports of tongue-protrusion imitation in newborns and the current findings of newborn head-movement duplication in the passive-face periods, it is unparsimonious to maintain that perceptual tethering is a necessary developmental precursor to the onset of other forms of motor imitation. Rather than infants needing to construct gradually the very first links between body transformations they see and like body transformations of their own, it seems worthwhile to inquire whether some such primitive capacity may be part of infants’ initial state.
One possibility is that infants can represent human movement patterns they see and ones they perform using the same internal code. The perception of an act may be registered in such a way that it can be used directly for the execution of a motor plan. In this view, the motor plans activated in imitation are not innately programmed units that exist at birth, waiting dormant, as it were, for an adult to trigger or “release” them (as per the IRM model). Rather, the infant actively uses the adult’s act as a model or guide against which to fashion motor output. This hypothesis helps to make sense of the fact that infants often initiate imitation during the passive-face intervals after the gesturing has ceased. One key motivation for early imitation may be the infants’ detection of a mismatch between the current perceptual field (the adult’s passive face) and the infant’s stored representation of the now-absent gesture (Meltzoff, Kuhl, & Moore, in press). We are thus proposing that early imitation is mediated by a process of active intermodal mapping (AIM). rather than a series of IRMs with attendant fixed-action patterns, and that imitation is but one manifestation of an underlying representational system that unites the perception and production of human acts within the same framework. At a fundamental level, imitation is tied to a network of skills in other domains (Bower. 1982) and, particularly, to speech-motor phenomena, which also involve early perception-production links (Kuhl & Meltzoff, 1982, 1988: Meltzoff & Kuhl, 1989; Meltzoff et al., in press).
For theory building, it would now be particularly interesting to test the length of delay that can be tolerated between the perception of an act and its reproduction. In this study, infants were shown to imitate in passive-face intervals immediately following adult gestures. Might infants imitate after a longer delay? This would help address questions concerning the perseverance-of-tracking idea, and would also provide further information about possible temporal constraints on the effect. To flesh out more fully the nature and limits of the notion of equivalence mapping, one would want to explore the resolution in the link between perception and action. For example, one could investigate whether young infants are able to analyze the display sufficiently to imitate two variants of an act, such as two different types of head movements. Also of relevance would be the conduct of developmental studies using a variety of different gestures. The IRM model is often associated with the view that early behavioral matching exists in neonates and then “drops out” of the infant’s repertoire after an initial period. However, this notion is usually put forward with reference to the tongue-protrusion effect. We believe that any apparent “dropout” of early imitation is more likely due to changes in motivation or in the meaning of the display for the infant than in an across-the-board loss in competence. This predicts that infants of a given age who stopped matching particular acts would still imitate others, which again highlights the importance for theory of testing a range of gestures developmentally, rather than a single gesture in a single situation.
To date, the majority of the experiments on early imitation have tested for the raw existence of the basic phenomenon, with a focus on infant tongue protrusion. It now seems worthwhile to move to a next phase in the research and to design studies directed at five broader questions that will need to be addressed before a comprehensive theory can be offered: the generality of the effect, the characteristics of the effective stimulus, the mechanisms underlying this behavior, its meaning for the infant, and its social and functional significance in development.
Acknowledgments
This research was supported by a grant from the National Institute of Child Health and Human Development (HD-22514).
We gratefully acknowledge the assistance of Calle Fisher and Craig Harris and thank Patricia Kuhl for helpful comments on an earlier draft of this article.
Footnotes
These main effects are further broken down in the remainder of the Results section. Within these smaller subdivisions of the data, the assumptions of parametric tests clearly are not fulfilled; thus, only non-parametric statistics are used hereafter.
We say primarily because one could argue that infant tracking movements may be more general and less unidirectional than those predicted by strict perceptual tethering. However, the strict tethering account is addressed first, and in later sections other variants of the tracking hypothesis are examined. (See Further Analysis of the Passive-Face Periods in this section and, also, the Discussion section.)
References
- Abravanel E, Sigafoos AD. Exploring the presence of imitation during early infancy. Child Development. 1984;55:381–392. [PubMed] [Google Scholar]
- Bower TGR. Development in infancy. 2. San Francisco: Freeman; 1982. [Google Scholar]
- Field TM, Woodson R, Greenberg R, Cohen D. Discrimination and imitation of facial expressions by neonates. Science. 1982;218:179–181. doi: 10.1126/science.7123230. [DOI] [PubMed] [Google Scholar]
- Flavell JH. Cognitive development. 2. Englewood Cliffs, NJ: Prentice-Hall; 1985. [Google Scholar]
- Fontaine R. Imitative skills between birth and six months. Infant Behavior and Development. 1984;7:323–333. [Google Scholar]
- Heimann M, Schaller J. Imitative reactions among 14–21 days old infants. Infant Mental Health Journal. 1985;6:31–39. [Google Scholar]
- Jacobson SW. Matching behavior in the young infant. Child Development. 1979;50:425–430. [PubMed] [Google Scholar]
- Kaitz M, Meschulach-Sarfaty O, Auerbach J, Eidelman A. A reexamination of newborn’s ability to imitate facial expressions. Developmental Psychology. 1988;24:3–7. [Google Scholar]
- Kessen W, Salapatek P, Haith M. The visual response of the human newborn to linear contour. Journal of Experimental Child Psychology. 1972;13:9–20. doi: 10.1016/0022-0965(72)90003-3. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Meltzoff AN. The bimodal perception of speech in infancy. Science. 1982;218:1138–1141. doi: 10.1126/science.7146899. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Meltzoff AN. Speech as an intermodal object of perception. In: Yonas A, editor. Perceptual development in infancy: The Minnesota Symposia on Child Psychology. Vol. 20. Hillsdale, NJ: Erlbaum; 1988. pp. 235–266. [Google Scholar]
- Meltzoff AN, Kuhl PK. Infants’ perception of faces and speech sounds: Challenges to developmental theory. In: Zelazo PR, Barr R, editors. Challenges to developmental paradigms. Hillsdale. NJ: Erlbaum; 1989. pp. 67–91. [Google Scholar]
- Meltzoff AN, Kuhl PK, Moore MK. Perception and the control of action in newborns and young infants: Toward a new synthesis. In: Weiss MJ, Zelazo PR, editors. Newborn attention: Biological constraints and the influence of experience. Norwood, NJ: Ablex; (in press) [Google Scholar]
- Meltzoff AN, Moore MK. Imitation of facial and manual gestures by human neonates. Science. 1977;198:75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Newborn infants imitate adult facial gestures. Child Development. 1983a;54:702–709. [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. The origins of imitation in infancy: Paradigm, phenomena, and theories. In: Lipsitt LP, editor. Advances in infancy research. Vol. 2. Norwood, NJ: Ablex; 1983b. pp. 265–301. [Google Scholar]
- Meltzoff AN, Moore MK. Cognitive foundations and social functions of imitation and intermodal representation in infancy. In: Mehler J, Fox R, editors. Neonate cognition: Beyond the blooming, buzzing confusion. Hillsdale, NJ: Erlbaum; 1985. pp. 139–156. [Google Scholar]
- Mendelson MJ, Haith MM. The relation between audition and vision in the human newborn. Monographs of the Society for Research in Child Development. 1976;41(4) Serial No. 167. [PubMed] [Google Scholar]
- Piaget J. Play, dreams and imitation in childhood. New York: Norton; 1962. [Google Scholar]
- Reid JB. Reliability assessment of observation data: A possible methodological problem. Child Development. 1970;41:1143–1150. [Google Scholar]
- Reissland N. Neonatal imitation in the first hour of life: Observations in rural Nepal. Developmental Psychology. 1988;24:464–469. [Google Scholar]
- Salapatek P, Kessen W. Vision scanning of triangles by the human newborn. Journal of Experimental Child Psychology. 1966;13:155–167. doi: 10.1016/0022-0965(66)90090-7. [DOI] [PubMed] [Google Scholar]
- Siegel S. Nonparametric statistics for the behavioral sciences. New York: McGraw-Hill; 1956. [Google Scholar]
- Vinter A. The role of movement in eliciting early imitations. Child Development. 1986;57:66–71. [Google Scholar]


