Abstract
A novel spectro-electochemical cell for X-ray absorption spectroscopy in the tender X-ray region (TX-XAS) was designed and fabricated to investigate the electrochemical behavior of common battery materials with liquid electrolytes under in situ/operando conditions. The cell has several unique features, such as high X-ray transmittance, high signal to noise ratio, and high vacuum tightness. These features enable us quick and reliable XAS measurements. Operando P K-edge XAS measurements of an olivine-type LiFePO4 composite positive electrode were carried out to clarify its phosphorus environment during the electrochemical charging process. Results of spectral analysis show that there is no significant change in the oxidation state of phosphorus and in the coordination of the phosphate anions in the charging process, but a closer look of the consecutive XAS spectra suggests the shrinkage of the PO4 cage during the charging process, and the structural changes in a biphasic manner. These results demonstrate the usefulness of the cell for in situ/operando TX-XAS observations of light elements in practical batteries.
I. INTRODUCTION
There is a growing demand for high performance lithium ion batteries (LIBs) for electric vehicles and renewable energy storage applications. To improve performances of LIBs, it is indispensable to elucidate the mechanisms of the electrochemical reactions occurring in the battery. Among possible analytical/diagnostic methods, X-ray absorption spectroscopy (XAS), which is element specific and highly sensitive to dilute species, is a powerful and effective tool for oxidation state analysis and local structure analysis of various materials, and has been frequently applied to LIB analysis. Among the elements used in LIBs, those heavier than the third row elements in the periodic table, such as Ti, V, Mn, Fe, Co, and Ni have been mostly analyzed and the results have offered information on the redox reactions of the electrode materials used in the LIBs. These spectra are measured in the hard X-ray (HX) region and many types of electrochemical “in situ” cells have been developed for XAS experiments and used for dynamic analysis of electrode reactions of LIBs with liquid electrolytes.1–10 The electrochemical cells sealed by laminated aluminum films, being similar to that of the commercially available LIBs, can be used because of the high transmittance of the HX.7–10
For full understanding of the electrochemical mechanism in the LIBs, the information of light elements is also important. There exist many K edges of light elements (and L edges of transition metals) in the soft X-ray (SX) region below 1 keV, and the higher-energy SX region from 1 to 3 keV, recently called as “tender X-ray (TX)” region. However, in situ XAS measurements in the SX and the TX regions are much more difficult than that in the HX region, mainly due to the low transmittance of the X-rays. Accordingly, most of the SX-/TX-XAS experiments have been performed not with the transmission mode but with electron and/or fluorescence yield mode, using electrode samples taken out from a cell, i.e., under “ex situ” conditions and it is, thus, difficult to observe electrochemical reaction dynamics in a liquid electrolyte solution directly. In addition, samples for the ex situ measurement are generally washed and dried prior to the analysis, and hence the chemical condition might be changed during these preparation processes. Therefore, developing in situ methods for light element analysis is of great importance for understanding the electrochemistry in a battery.
So far, a few studies of LIB electrodes with liquid electrolyte solutions by in situ TX-XAS experiments have been reported.11–13 Totir and co-workers have performed in situ S K-edge XAS experiments using pyrite (FeS2) particle electrodes embedded in a Au foil and their original spectro-electrochemical cell.12 In this cell, they have to move the electrode for the spectroscopic measurements from the position during the charging/discharging processes, which makes obtaining reliable XAS spectra difficult. Cuisinier and co-workers have performed in situ S K-edge XAS experiments with the electrode comprised of the sulfur-imbibed robust spherical carbon shells loaded on a carbon paper.13 The XAS measurement with simultaneous charging/discharging, i.e., “operando” XAS has been carried out with their coin type in situ cell. It took about 18.5 min to get each XAFS spectrum. It could be used for the experiment with 0.1 C charge/discharge rate, but would not be applicable for an experiment with higher charging rate.
Though these cells are of importance in the in situ measurement in the TX region, it is further desirable for detailed dynamic observation to achieve higher S/N ratios and employ the measurement in high time resolutions. Furthermore, it is promising for wide application if a common composite electrode can be applied for the TX-XAS measurement.
In this paper, we report a novel spectro-electrochemical cell which enables to employ a common composite electrode and a liquid electrolyte solution and achieve in situ TX-XAS measurements with a high S/N ratio. In order to demonstrate its applicability, the composite electrode of an olivine-type LiFePO4 (LiFePO4)14–16 was used as a sample. So far, many in situ XAS studies of LiFePO4 have been carried out in the HX region to clarify the redox reactions of the iron,2–8 while SX-/TX-XAS studies of lithium, oxygen, and phosphorus have been rarely reported and, if any, they are employed ex situ.17–19 Especially, phosphorus in a LiFePO4 positive electrode has not been studied in detail because it is known that phosphorus is not the redox center in this system. However, it is predicted the presence of stable phosphorus in a LiFePO4 electrode contributes to the high cyclability and the thermal stability. We show the results of in situ P K-edge XAS measurements of the LiFePO4 electrode using the fabricated cell, and discuss about the phosphorus environment in LiFePO4 during the charging process.
II. EXPERIMENTAL
A. Spectro-electrochemical cell
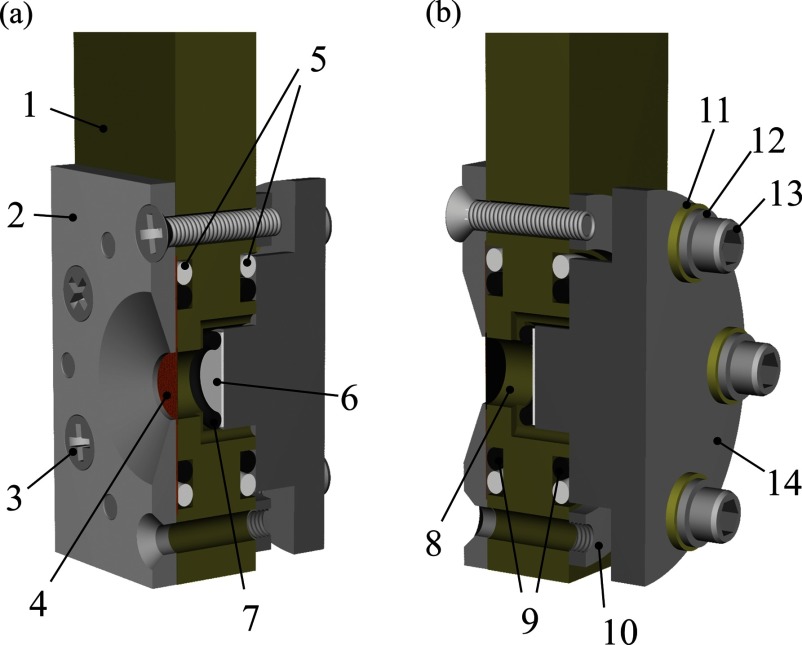
Figure 1 shows the schematic view of the fabricated spectro-electrochemical cell assembly designed for in situ TX-XAS measurement of a common composite electrode in an electrolyte solution. The aperture size in a nickel jig is 6 mm diameter. For obtaining the spectrum with a high S/N ratio, the most important component of the cell is the X-ray window. Due to the low transmittance of TX, the window has to be composed of light elements and be as thin as possible. A beryllium foil, a silicon nitride (SiN) membrane, and a polyimido (PI) film are often used as X-ray windows of liquid cells and spectro-electrochemical cells in the SX/TX region depending on the intended use.20–29 In this study, a 7.5 μm thick PI film coated with 70 nm thick aluminum was adopted as the X-ray window. A PI film was most desirable for their low background nature and mechanical/chemical toughness. The thin aluminum film on the PI worked as the current collector of the electrode and the composite electrode slurry was coated directly on the aluminum film, differently from other cells with a PI window.11–13 This structure is advantageous to reduce the distance between the window and the electrode, minimize unnecessary X-ray attenuations by the electrolyte solvent, and thus obtain the XAS spectra with high S/N ratios. In addition, the working electrode is facing to the counter electrode to minimize inhomogeneous reactive distributions in the electrode plane, being similar to the practical batteries. The fabricated cell with the battery components has also a highly vacuum-tight feature sealed by perfluoroelastomer O-rings and polytetrafluoroethylene O-rings, and hence can be used in a high vacuum.
FIG. 1.
Schematic view of the spectro-electrochemical cell assembly. (a) Diagonally forward view. (b) Diagonally backward view. 1: polyetheretherketone (PEEK) cell body; 2: nickel jig for fixing the X-ray window with the aperture of 6 mm diameter; 3: countersunk head screw; 4: aluminum coated PI film applied with a composite cathode; 5: polytetrafluoroethylene; O-rings; 6: lithium metal as counter electrode; 7: perfluoroelastomer O-ring for fixing a counter electrode; 8: electrolyte solution; 9: perfluoroelastomer O-rings; 10: stainless steel jig for temporarily fixing of nickel plate, 11: PEEK bush; 12: stainless steel washer; 13: Screw bolt; 14: stainless steel current collector for counter electrode.
In this study, the working composite electrode consisted of 70 wt.% carbon-coated olivine-type LiFePO4 (with a carbon content of 1.2 wt.%), 15 wt.% acetylene black, and 15 wt.% polyvinylidene difluoride (PVdF). The diameter of the electrode was about 8 mm. A lithium metal sheet was used as the counter electrode. A 1.0 dm−3 solution of LiClO4 dissolved in ethylene carbonate: diethyl carbonate (1:1) V/V% was used as the electrolyte solution.
B. TX-XAS conditions
All the XAS measurements were performed at the double-crystal monochromator beamline (BL-10) of the SR center, Ritsumeikan University.30 A pair of Ge(111) crystals, whose 2d value is 0.6532 nm, was used as monochromatizing crystals for the P K-edge XAS measurement. The energy of the P K-edge spectra was calibrated to the white line of FePO4 polycrystalline powder at 2153.0 eV.31 The beam size of an incident X-ray was about 6 mm (horizontal) × 3 mm (vertical) on the sample position.
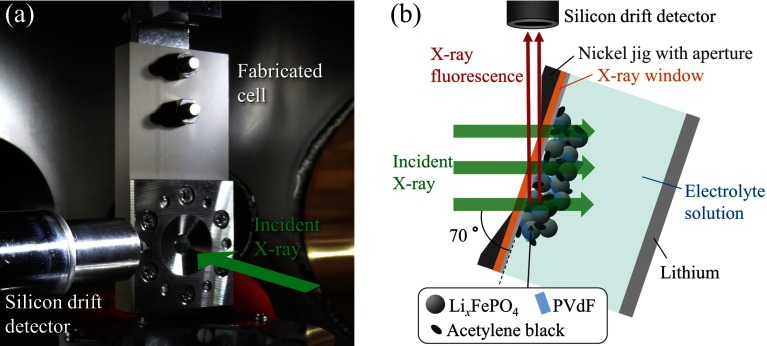
A photo of the experimental setup in the high-vacuum sample chamber is shown in Fig. 2(a). The spectro-electrochemical cell was set in the sample chamber kept less than 1 × 10−4 Pa. All the in situ XAS spectra were recorded by the partial fluorescence yield (PFY) mode, using a silicon drift detector (SDD) (Techno X Co., Ltd., Japan) and a digital X-ray processor (XIA LLC, DXP Mercury, USA). The SDD was placed beside the cell and the cell was rotated by 20° in the horizontal plane (see Fig. 2(b)). The dead time of the SDD was maintained below 10% to reduce the piling up effect by controlling the distance between the cell and the SDD. The geometry was fixed during the experiment. In the measured XAS energy region (hν = 2140–2190 eV), the theoretical transmittance of the X-ray window composed of a PI film of 7.5 μm thickness and an aluminum film of 70 nm thickness in the cell is around 68.4%–70.0% and that of the Kα fluorescence of phosphorus as a signal (about 2010 eV) is about 63.4%.32 Based on the experimental geometry shown in Fig. 2(b), the transmittances of an incident X-ray and an X-ray fluorescence are around 66.7%–68.5% and about 26.4%, respectively, due to each path length. Accordingly, the signal intensity of the in situ P K-edge XAS experiment with the PFY mode was estimated to be around 17.6%–18.1% of that obtained in the ex situ measurement (without the window). Although the signal intensity is largely lost by the window, the XAS spectra can be obtained with the high S/N ratio in a short time as shown below.
FIG. 2.
Experimental setup for in situ TX-XAS measurement. (a) Photograph in the high vacuum sample chamber. (b) Schematic top view.
In this study, a series of in situ P K-edge XAS spectra was recorded with simultaneous cell charging, i.e., the operando XAS measurements were repeatedly performed until the cell voltage reached the charge cut-off voltage at 4.0 V.
C. Spectral simulation method
The ab initio full multiple-scattering calculations were performed with the FEFF-9.03 program based on a real space Green's function approach33,34 to correlate the observed XAS spectral shapes with the atomic structures. In all the simulations, the Hedin-Lundqvist exchange and correlation potential was adopted, and the amplitude reduction factor S02 was set to 1.0. Structural parameters of both LiFePO4 and its charged structure were adopted by the result of neutron diffractions, as reported by Rousse and co-workers,35 and calculations were done for several cluster sizes with a phosphorus atom in the center in both.
III. RESULTS AND DISCUSSION
A. Evaluation of the fabricated spectro-electrochemical cell
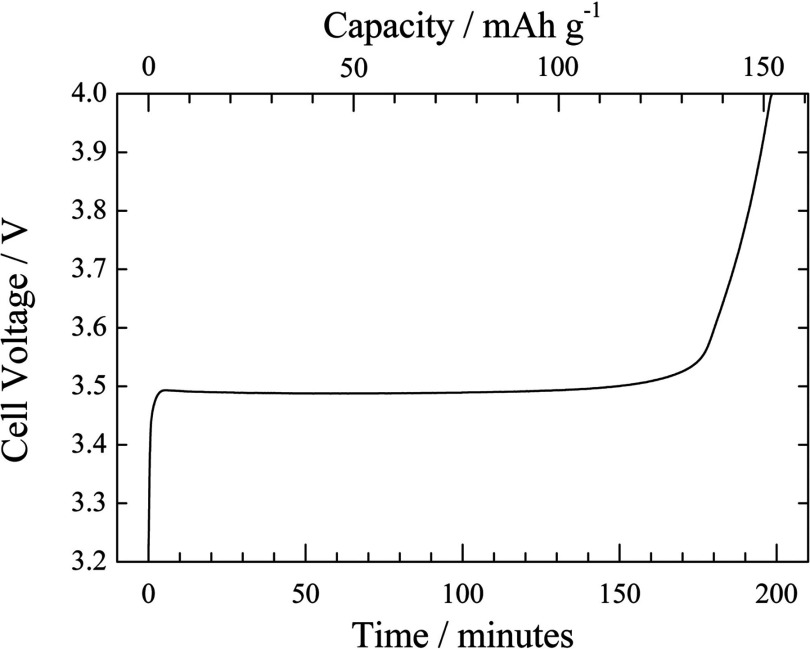
Figure 3 shows the observed voltage curve performed by the constant current charging in the operando XAS measurement with the fabricated spectro-electrochemical cell. The capacity was calculated by the constant current and the weight of active materials in the composite electrode. The cell voltage was rapidly increased after the charging started, and reached a constant value of around 3.49 V in 5 min. This voltage plateau is typical of the LiFePO4 electrode during charging process.14–16,36 The voltage was kept for ∼170 min, and then gradually increased to reach the cut-off voltage at 4.0 V in 198 min. The specific capacity at 4.0 V charged state was about 151 mAh g−1, corresponding to the utilization of 89% compared with the theoretical capacity of 170 mAh g−1. These results indicate that the fabricated cell can be used as an electrochemical cell as well as common electrochemical cells.
FIG. 3.
Observed charge curve of the LiFePO4/C positive composite electrode during operando XAFS measurement. The constant current charging was carried out and stopped at the cut-off voltage of 4.0 V.
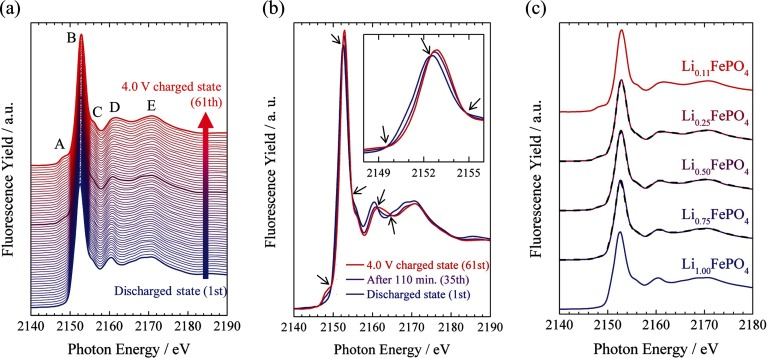
Figure 4(a) shows a series of observed P K-edge XAS spectra of the LiFePO4 composite positive electrode that were simultaneously measured with the charging operation shown in Fig. 3. All the spectra are raw I/I0 spectra without any background processing, such as subtracting the pre-edge background and normalizing at the edge-jump, and correcting self-absorption effects. With the acquisition time of 194 s for each spectrum, the S/N ratio was sufficiently high to discuss the spectral changes. The state change Δx in Li1-xFePO4 during one XAS measurement corresponds to about 0.014, which is sufficiently small to observe the spectral changes related to the delithiation process. These results indicate that XAS spectra with sufficiently high S/N ratios can be obtained in a reasonable time.
FIG. 4.
Observed P K-edge XAS spectra of the LiFePO4 composite electrode in the charging process recorded with the PFY mode. (a) Series of XAS spectra with fixed vertical offsets. Discharged state (1st), After 110 min (35th), and 4.0 V charged state (61th) are drawn by thick lines, respectively. (b) The XAS spectra of discharged state (1st), after 110 min (35th), and 4.0 V charged state (61th). Isosbestic points are shown by allows. (c) Series of XAS spectra as a function of the lithium ratio x in Li1-xFePO4 with fixed vertical offsets. Observed XAS spectra and synthetic spectra are solid and dashed lines, respectively. No data processing was performed, such as subtracting the pre-edge background and normalizing at the edge-jump, and correcting self-absorption effects for all the spectra.
Note that all the observed XAS spectra were recorded by the PFY mode and the self-absorption effect was not corrected yet. The influence of the self-absorption effect cannot be neglected in the present system, but the degree of it would not change significantly in the charge process because the density and the environment of phosphorus in the electrode were nearly unchanged. Thus, we can discuss the tendency of spectral changes semi-quantitatively.
B. About spectral changes during the charging process
As shown in Fig. 4(a), no drastic spectral change was observed in the charging process. This indicates that the coordination environment and the oxidation state of the phosphorus atoms are essentially unchanged upon charging and that the local structure of phosphorus in the LiFePO4 electrode is stable against the delithiation from the LiFePO4 electrode. However, taking a closer look at the observed spectra, some noticeable spectral changes can be found. In order to clarify the change, the XAS spectra from the discharged state (1st acquisition), after 110 min (35th acquisition), and 4.0 V charged state (61th acquisition) are shown in Fig. 4(b). Several isosbestic points (pointed by arrows in Fig. 4(b)) are observed in the spectra, indicating that two chemical species are present in the reaction system and only the component ratio of the species changes during the charging process. Previous Fe K-edge XAS and XRD results of the LiFePO4 electrode show that the charging/discharging reaction of this system proceeds in a biphasic manner between Li-rich Li1−αFePO4 and Li-poor LiβFePO4 phases.2–5,7,8,15,36 We confirmed that observed P K-edge XAS spectra can be reproduced by the linear combination of a charged state and a discharged state, as the previous works of iron in a LiFePO4 electrode. Here, discharged state and 4.0 V charged state are defined as Li1.00FePO4 and Li0.11FePO4, respectively, as a function of lithium ratio 1 − x in Li1-xFePO4 due to the specific capacity estimated in Fig. 3. Li0.75FePO4, Li0.50FePO4, and Li0.25FePO4 spectra prepared from the two observed spectra: Li1.00FePO4 and Li0.11FePO4, were synthesized and compared with those observed, respectively (see in Fig. 4(c)). As the results, all observed spectra correspond to synthetic spectra. Our results using the fabricated cell show that the phosphorus environment is essentially stable, but delicate changes in the course of delithiation might correspond to the structural changes in the biphasic manner.
C. Characterization of XAS spectra and analysis of phosphorus environment
Described above, the changes of all the XAS spectra in Fig. 4(a) are not drastic and they have similar spectral features denoted with peak A; a pre-edge peak at 2148.5 eV, peak B; a distinct peak at about 2153 eV, peak C; a shoulder of the white line at about 2155 eV, peak D; a weak peak around 2160 eV, and peak E; a broadband around 2170 eV, as typical features of phosphates.37–39 We attribute origins of these peaks in the observed spectra with support of theoretical simulations. Simulated spectra for several cluster sizes of both LiFePO4 and its delithiated form (o-FePO4) are shown in Figs. 5(a) and 5(b), respectively. The spectra of LiFePO4 and o-FePO4 with a large cluster size (radius of 9.0 Å) are compared in Fig. 5(c). Simulated spectra have four common spectral features denoted by B, C, D, and E, corresponding to those of observed spectra. As shown in Figs. 5(a) and 5(b), peak B and E can be seen in the small cluster size of radius of 2.0 Å, consists of one PO4 cage. According to the similarity in the XAS spectral study using the molecular orbital theory and the full multiple-scattering theory reported by Bokhoven and co-workers, distinct peak B and broad peak E, which come from p-d hybridization of PO4 cage with the Td symmetry, form the primal spectral shape of phosphates.40 Peak E is assigned the transition to P-O σ* anti-bonding state, so-called “shape resonance,” while peak B is assigned to non-bonding or weakly anti-bonding state, so-called “white line.” On the other hand, peaks C and D appear when the cluster size is larger (shown in Figs. 5(a) and 5(b)). It indicates that these peaks are caused by effects of multiple scatterings from the outside the PO4 cage.
FIG. 5.
Simulated P K-edge XAS spectra of olivine-type LiFePO4 and delithiated form: o-FePO4, where E-Ef denotes relative energy from the Fermi energy. (a) and (b) are simulated spectra with several cluster sizes for LiFePO4 and o-FePO4, respectively. (c) Comparison of the spectra of LiFePO4 and o-FePO4 with the size of radius 9.0 Å from the center P atom.
Here, pre-edge peak A is observed at 2148.5 eV in the observed spectrum of the 4.0 V charged state, but it is not predicted by the FEFF program based on the multiple-scattering theory. The pre-edge peak was not observed in the discharged state, but it appears and gradually gains the intensity as the charging proceeds. After the cell is charged until 4.0 V, the pre-edge peak can be observed clearly. This phenomenon is derived from the change in the electronic state of the system. According to the density functional calculations by Tang and Holzwarth,41 the Fe 3d state forms a narrow band in the higher energy level separated from the O 2p bands in LiFePO4, while the Fe 3d state is lowered and mixed with the broad O 2p band to form a strong Fe-O covalent bond in FePO4. In principle, P K-edge XAS is caused by a transition from P 1s to unoccupied P n-p states. The pre-edge peak in the P K-edge XAS spectra of FePO4 is attributed to the excitation from the initial state P 1s to the Fe(3d)-O(2p) anti-bonding states, slightly hybridized with P 3p state. Thus, increasing the component ratio of FePO4 during charging leads to the appearance of the pre-edge peak.
The peak top of the white line (peak B) is gradually shifted to the higher photon energy, while the shape resonance is also shifted more to the higher photon energy as shown in Figs. 4(a) and 4(b). These shifts correspond to the shortening of the P-O bond length.42 Four P-O bond lengths of referred structural parameters35 are 1.523, 1.540, and 1.560 Å ×2, as LiFePO4, and 1.517, 1.533, and 1.537 Å ×2 as o-FePO4, respectively. All of the P-O bond lengths shorten and get closer to each other. Simulated spectra using the same structural parameters in Fig. 5(c) also reproduce the changes of shape resonances in observed spectra. Shortening of the P-O bond lengths also means the shrinkage of the PO4 cage by delithiation. The difference of the white line intensity between observed spectra and simulated ones is derived from the not-so-high accuracy of FEFF simulations, especially in around white lines.
Above discussions of XAS spectral changes allow us to realize about phosphorus environments in the LiFePO4 electrode during charging process. We also realize that the change of the PO4 cage size by changing P-O bond lengths in the LiFePO4 plays a role as a buffer on charging/discharging, and stabilizes the delithiated LiFePO4 framework, enabling the high cyclability of the LiFePO4 electrode.
D. Advantage in this operando TX-XAS experiment
Here, we describe our experimental advantages compared with other similar experiments and an ex situ experiment. Recently, it has been reported that a LiFePO4 composite electrode in the battery has heterogeneous reactive distributions from submillimeter to a few millimeter size during the charging/discharging process.10,43 It may cause an inconsistent result between an electrochemical experiment as the averaged information of the whole electrode and an XAS experiment as if excessively focused beam compared with a sample size is used. In this experiment, incident X-rays of a relatively large beam size of about 6 mm (horizontal) × 3 mm (vertical) were irradiated to the LiFePO4 electrode (8 mm diameter) in the cell. It was estimated that about 36% of the whole electrode area was observed by TX-XAS, and hence the nearly averaged information could be obtained. Generally, it is known that not only a LiFePO4 composite electrode but also practical composite electrodes have some reactive distribution. Large errors of the linear combination fitting result using ex situ P K-edge XAS spectra reported by Yoon and co-workers19 might be caused by the heterogeneous behaviors in the LiFePO4 electrode and focused beam on the sample. For such cases, the combination of in situ experiment which enables to observe same sample position and a large beam size which enables to obtain average information of an electrode is necessary to get reliable information for the charge/discharge mechanism.
IV. CONCLUSIONS
A spectro-electrochemical cell with common composite electrodes of LIBs for operando TX-XAS experiments was designed and fabricated. For evaluation of the cell, operando P K-edge XAS measurements using the composite positive electrode of LiFePO4 were performed, and the phosphorus environment in LiFePO4 during the charging process was investigated. The phosphorus exists as PO43− in LiFePO4, and its form is maintained during the charging process. The result indicates phosphorus does not directly contribute to the oxidation reaction. The changes of observed XAS spectra, such as the pre-edge peak, the shape resonance, and the white line, indicate the hybridization between Fe(3d) and O(2p) orbitals and shortening of P-O bond lengths (the shrinkage of PO4 units size) during the charging process. It is also clarified that the phosphorus environment changes in a biphasic manner similar to the change of the iron oxidation state.
In future, it is expected that the cell is applied to other battery materials for electrochemically reactive analysis. For example, it is deduced to have applicability to the observations of redox reactions in phosphide, sulfide, and silicide electrodes, and the feature of the high S/N ratio may allow to be observed formation processes of thin solid-electrolyte interface films on the electrode surfaces with decomposition reactions of sulfide additives in electrolyte solutions.
ACKNOWLEDGMENTS
This work was supported by the Research and Development Initiative for Scientific Innovation of New Generation Battery (RISING) project from the New Energy and Industrial Technology Development Organization (NEDO), Japan.
The authors acknowledge Ms. Aiko Wakita (Panasonic Excel Staff Co., Ltd., Japan) and Mr. Takahiro Kakei (Human Resocia Co., Ltd., Japan) for technical supporting in electrochemical experiments and sample preparations, Mr. Masahiro Ogawa (SR center, Ritsumeikan University, Japan) for technical supporting in XAS experiments, and Dr. Hisao Yamashige (Toyota Motor Corporation, Japan) for many discussions about in situ techniques.
REFERENCES
- 1.Deb A., Bergmann U., Cramer S. P., and Cairns E. J., J. Appl. Phys. 97, 113523 (2005). 10.1063/1.1921328 [DOI] [Google Scholar]
- 2.Deb A., Bergmann U., Cairn E. J., and Cramer S. P., J. Synchrotron Rad. 11, 497 (2004). 10.1107/S0909049504024641 [DOI] [PubMed] [Google Scholar]
- 3.Deb A., Bergmann U., Cramer S. P., and Cairns E. J., Electrochim. Acta 50, 5200 (2005). 10.1016/j.electacta.2005.02.086 [DOI] [Google Scholar]
- 4.Leriche J. B., Hamelet S., Shu J., Morcrette M., Masquelier C., Ouvrard G., Zerrouki M., Soudan P., Belin S., Elkaim E., and Baudelet F., J. Electrochem. Soc. 157, A606 (2010). 10.1149/1.3355977 [DOI] [Google Scholar]
- 5.Inoue K., Fujieda S., Shinoda K., Suzuki S., and Waseda Y., Mater. Trans. 51, 2220 (2010). 10.2320/matertrans.M2010229 [DOI] [Google Scholar]
- 6.Balasubramanian M., Sun X., Yang X. Q., and McBreen J., J. Power Sources 92, 1 (2001). 10.1016/S0378-7753(00)00493-6 [DOI] [Google Scholar]
- 7.Orikasa Y., Maeda T., Koyama Y., Minato T., Murayama H., Fukuda K., Tanida H., Arai H., Matsubara E., Uchimoto Y., and Ogumi Z., J. Electrochem. Soc. 160, A3061 (2013). 10.1149/2.012305jes [DOI] [PubMed] [Google Scholar]
- 8.Orikasa Y., Maeda T., Koyama Y., Murayama H., Fukuda K., Tanida H., Arai H., Matsubara E., Uchimoto Y., and Ogumi Z., J. Am. Chem. Soc. 135, 5497 (2013). 10.1021/ja312527x [DOI] [PubMed] [Google Scholar]
- 9.Gustafsson T., Thomas J. O., Koksbang R., and Farrington G. C., Electrochim. Acta 37, 1639 (1992). 10.1016/0013-4686(92)80128-9 [DOI] [Google Scholar]
- 10.Liu J., Kunz M., Chen K., Tamura N., and Richardson T. J., J. Phys. Chem. Lett. 1, 2120 (2010). 10.1021/jz100634n [DOI] [Google Scholar]
- 11.Gao J., Lowe M. A., Kiya Y., and Abruna H. D., J. Phys. Chem. C 115, 25132 (2011). 10.1021/jp207714c [DOI] [Google Scholar]
- 12.Totir D. A., Antonio M. R., Schilling P., Tittsworth R., and Scherson D. A., Electrochim. Acta 47, 3195 (2002). 10.1016/S0013-4686(02)00239-6 [DOI] [Google Scholar]
- 13.Cuisinier M., Cabelguen P.-E., Evers S., He G., Kolbeck M., Garsuch A., Bolin T., Balasubramanian M., and Nazar L. F., J. Phys. Chem. Lett. 4, 3227 (2013). 10.1021/jz401763d [DOI] [Google Scholar]
- 14.Padhi A. K., Nanjundaswamy K. S., and Goodenough J. B., J. Electrochem. Soc. 144, 1188 (1997). 10.1149/1.1837571 [DOI] [Google Scholar]
- 15.Yamada A., Koizumi H., Sonoyama N., and Kanno R., Electrochem. Solid-State Lett. 8, A409 (2005). 10.1149/1.1945373 [DOI] [Google Scholar]
- 16.Tarascon J. M. and Armand M., Nature (London) 414, 359 (2001). 10.1038/35104644 [DOI] [PubMed] [Google Scholar]
- 17.Augustsson A., Zhuang G. V., Butorin S. M., Osorio-Guillen J. M., Dong C. L., Ahuja R., Chang C. L., Ross P. N., Nordgren J., and Guo J.-H., J. Chem. Phys. 123, 184717 (2005). 10.1063/1.2107387 [DOI] [PubMed] [Google Scholar]
- 18.Yoon W.-S., Chung K. Y., McBreen J., Zaghi K., and Yang X.-Q., Electrochem. Solid-State Lett. 9, A415 (2006). 10.1149/1.2216619 [DOI] [Google Scholar]
- 19.Yang S., Wang D., Liang G., Yiu Y. M., Wang J., Liu L., Sun X., and Sham T. K., Energy Environ. Sci. 5, 7007 (2012). 10.1039/c2ee03445j [DOI] [Google Scholar]
- 20.Abraham I., Horner W., Ertel T. S., and Bertagnolli H., Polyhedron 15, 3993 (1996). 10.1016/0277-5387(96)00137-4 [DOI] [Google Scholar]
- 21.Nakayama Y., Kudo Y., Oki H., Yamamoto K., Kitajima Y., and Noda K., J. Electrochem. Soc. 155, A754 (2008). 10.1149/1.2956022 [DOI] [Google Scholar]
- 22.Ertel T. S. and Bertagnolli H., Nucl. Instrum. Methods, Phys. Res. B 73, 199 (1993). 10.1016/0168-583X(93)95735-N [DOI] [Google Scholar]
- 23.Marcos E. S., Gil M., Martinez J. M., and Munoz-Paez A., Rev. Sci. Instrum. 65, 2153 (1994). 10.1063/1.1144718 [DOI] [Google Scholar]
- 24.Yang B. X. and Kirz J., Phys. Rev. B 36, 1361 (1987). 10.1103/PhysRevB.36.1361 [DOI] [PubMed] [Google Scholar]
- 25.Freiwald M., Cramm S., Eberhardt W., and Eisebitt S., J. Electron Spectrosc. Relat. Phenom. 137-140, 413 (2004). 10.1016/j.elspec.2004.02.165 [DOI] [Google Scholar]
- 26.Fuchs O., Maier F., Weinhardt L., Weigand M., Blum M., Zharnikov M., Denlinger J., Grunze M., Heske C., and Umbach E., Nucl. Instrum. Methods, Phys. Res. A 585, 172 (2008). 10.1016/j.nima.2007.10.029 [DOI] [PubMed] [Google Scholar]
- 27.Nagasaka M., Hatsui T., Horigome T., Hamamura Y., and Kosugi N., J. Electron Spectrosc. Relat. Phenom. 177, 130 (2010). 10.1016/j.elspec.2009.11.001 [DOI] [Google Scholar]
- 28.Jiang P., Chen J., Borondics F., Glans P., West M. W., Chang C., Salmeron M., and Guo J., Electrochem. Commun. 12, 820 (2010). 10.1016/j.elecom.2010.03.042 [DOI] [Google Scholar]
- 29.Arthur T. S., Glans P., Matsui M., Zhang R., Ma B., and Guo J., Electrochem. Commun. 24, 43 (2012). 10.1016/j.elecom.2012.08.018 [DOI] [Google Scholar]
- 30.Nakanishi K. and Ohta T., in Advanced Topics in Measurements, edited by Haq M. Z. (InTech, Rijeka, Croatia, 2012), pp. 43–60 [Google Scholar]
- 31.Nakanishi K. and Ohta T., J. Phys.: Condens. Matter 21, 104214 (2009). 10.1088/0953-8984/21/10/104214 [DOI] [PubMed] [Google Scholar]
- 32.Henke B. L., Gullikson E. M., and Davis J. C., At. Data Nucl. Data Tables 54, 181 (1993), also available from the web site: http://henke.lbl.gov/optical_constants/ 10.1006/adnd.1993.1013 [DOI] [Google Scholar]
- 33.Rehr J. J., Kas J. J., Prange M. P., Sorini A. P., Takimoto Y., and Vila F. D., “Ab initio theory and calculations of X-ray spectra,” C. R. Phys. 10, 548 (2009). 10.1016/j.crhy.2008.08.004 [DOI] [Google Scholar]
- 34.Rehr J. J. and Albers R. C., Rev. Mod. Phys. 72, 621 (2000). 10.1103/RevModPhys.72.621 [DOI] [Google Scholar]
- 35.Rousse G., Rodriguez-Carvaja J., Patoux S., and Masquelier C., Chem. Mater. 15, 4082 (2003). 10.1021/cm0300462 [DOI] [Google Scholar]
- 36.Yu X., Wang Q., Zhou Y., Li H., Yang X. Q., Nam K. W., Ehrlich S. N., Khalid S., and Meng Y. S., Chem. Commun. 48, 11537 (2012). 10.1039/c2cc36382h [DOI] [PubMed] [Google Scholar]
- 37.Okude N., Nagoshi M., Noro H., Baba Y., Yamamoto H., and Sasaki T. A., J. Electron Spectrosc. Relat. Phenom. 101-103, 607 (1999). 10.1016/S0368-2048(98)00341-7 [DOI] [Google Scholar]
- 38.Khare N., Martin J. D., and Hesterberg D., Geochim. Cosmochim. Acta 71, 4405 (2007). 10.1016/j.gca.2007.07.008 [DOI] [Google Scholar]
- 39.Franke R. and Hormes J., Phys. B 216, 85 (1995). 10.1016/0921-4526(95)00446-7 [DOI] [Google Scholar]
- 40.Stöhr J., NEXAFS Spectroscopy, Springer Series in Surface Science Vol. 25 (Springer, Berlin, 1992). [Google Scholar]
- 41.Tang P. and Holzwarth N. A. W., Phys. Rev. B 68, 165107 (2003). 10.1103/PhysRevB.68.165107 [DOI] [Google Scholar]
- 42.van Bokhoven J. A., Nabi T., Sambe H., Ramaker D. E., and Koningsberger D. C., J. Phys.: Condens. Matter 13, 10247 (2001). 10.1088/0953-8984/13/45/311 [DOI] [Google Scholar]
- 43.Ouvrard G., Zerrouki M., Soudan P., Lestriez B., Masquelier C., Morcrette M., Hamelet S., Belin S., Flank A., and Baudelet F., J. Power Sources 229, 16–21 (2013). 10.1016/j.jpowsour.2012.11.057 [DOI] [Google Scholar]