Harris et al.(1) apparently overlooked the central finding of our analysis(2), that n-6 specific PUFA interventions and mixed n-3/n-6 PUFA interventions have significantly different effects on risk of non-fatal myocardial infarction (MI) and CHD death in heterogeneity analysis (P=0·02). Given this clear statistical distinction, it is not valid to assess these two heterogeneous interventions together as a single unit(3). This key finding of heterogeneity calls into question the validity of interpreting results of any pooled analysis of the effects of mixtures of n-3 and n-6 PUFA to support advice specific to n-6 PUFA. Yet Harris et al. have repeatedly cited(4 – 8) three such pooled analyses, that did not make this essential distinction, as the primary evidence base for American Heart Association (AHA) advice to maintain or increase intake of n-6 PUFA: (1) the meta-analysis of randomised controlled trials (RCT) and CHD outcomes by Mozaffarian et al.(8); (2) the analysis of prospective observational cohorts by Jakobsen et al.(9); and (3) the analysis of intermediate risk factors by Mensink et al.(10). Each of these analyses evaluated the effects of increasing n-3 and n-6 PUFA together (Tables 1 and 2), without specificity for n-6 linoleic acid (LA), as we recently reviewed in detail(11). In relying on these non-specific PUFA data, rather than data selective to n-6 LA, Harris et al.(1) and the AHA Advisory Group were willing to assume that the modest but ‘remarkably consistent’ CHD benefits were attributable to n-6 LA, rather than to n-3 EPA + DHA and/or α-linolenic acid (ALA). We were unwilling to make this assumption without valid data evaluating the effects of selectively increasing LA.
Table 1.
Pooled analyses cited as the primary evidence base for CHD benefits of n-6 linoleic acid (LA): no clear distinction made between n-3 and n-6 PUFA species (adapted with permission from Ramsden et al.(11))*
| Pooled analysis | Outcome | Quote defining the independent variable | Exposure variable(s) | Mixed n-3/n-6 PUFA† or n-6 specific PUFA‡ |
|---|---|---|---|---|
| Mozaffarian et al.(8) Meta-analysis of RCT | Non-fatal MI + CHD death | ‘[total] PUFA consumption as a replacement for SFA’ | LA + ALA + EPA and DHA | Mixed (n-3 + n-6) |
| Jakobsen et al.(9)§ Pooled analysis of eleven observational cohorts | CHD events + CHD death | ‘[total] PUFAs; including both n-3 and n-6 fatty acids, also known as omega-3 and omega-6 fatty acids; primarily n-6 linoleic acid’ | LA + ALA + EPA and DHA | Mixed (n-3 + n-6) |
| Mensink et al.(10) Meta-analysis of RCT | LDL-cholesterol | ‘total PUFAs may be considered to equal the n-6 PUFAs with 18 carbon atoms (linoleic acid plus some α-linolenic acid)’ | LA + ALA | Mixed (n-3 + n-6) |
RCT, randomised controlled trial; MI, myocardial infarction; ALA, α-linolenic acid.
None of these pooled analyses evaluated the specific effects of n-6 LA or n-6 PUFA; however, their concordant benefits have been attributed to n-6 LA.
Mixed n-3/n-6 PUFA indicates RCT interventions that substantially increased both n-3 and n-6 PUFA, or exposure variables in observational cohorts that included both n-3 and n-6 PUFA.
n-6 Specific PUFA indicates RCT interventions that increased n-6 LA without substantially increasing n-3 PUFA.
Table 2.
Limited capacity of food-frequency questionnaires in the Nurses’ Health Study (NHS) to disentangle respective intakes of n-3 α-linolenic acid (ALA) and n-6 linoleic acid (LA) (adapted with permission from Ramsden et al.(11))*
| FFQ year | Specific oils and/or brand names identified?
|
|||
|---|---|---|---|---|
| Oils | Margarines | Shortenings, mayonnaise, salad dressings, packaged foods | Evaluating ‘usual use’ of fats and oils in home-prepared meals | |
| 1980† (sixty-one-item) | No | No | No | No specific brand-name questions |
| 1984‡ (116-item) | Partial | No | No | What type of fat do you usually use for frying and sautéing?|| |
| What type of fat do you usually use for baking?|| | ||||
| What form of margarine do you usually use? (e.g. tub or stick) | ||||
| What type of cooking oil do you usually use? (specify type and brand) | ||||
| 1986§ (131-item) | Partial | Partial | No | Kind of fat most often used at home for baking:|| |
| Kind of fat most often used at home for frying and sautéing:|| | ||||
| What form of margarine do you usually use? (brand and type) | ||||
| Usual type of cooking oil: (brand and type) | ||||
| 1990§ | Partial | Partial | No | What type of fat do you usually use for frying and sautéing at home?|| |
| What type of fat do you usually use for baking at home?|| | ||||
| What form of margarine do you usually use? (e.g. Promise Extra Light) | ||||
| What type of cooking oil do you usually use at home? (e.g. Mazola Corn Oil) | ||||
| 1994§ | Partial | Partial | No | What kind of fat is usually used for frying and sautéing at home?|| |
| What kind of fat is usually used for baking at home?|| | ||||
| What form of margarine do you usually use? (e.g. Land O’ Lakes Country Morning Blend Light) | ||||
| What type of cooking oil do you usually use at home (e.g. Wesson Corn Oil)? | ||||
| 1998§ | Partial | Partial | No | What kind of fat is usually used for frying and sautéing at home?||?¶ |
| What kind of fat is usually used for baking at home?|| | ||||
| What form of margarine do you usually use? (e.g. Parkay Corn Oil Spread) | ||||
| What type of cooking oil do you usually use at home (e.g. Mazola Corn Oil)? | ||||
Harris et al.(1) cited the 20-year follow-up of the NHS(29), which reported a protective association between dietary n-6 LA and CHD risk as evidence for CHD benefits of LA. However, the FFQ used in the NHS have limited ability to disentangle the respective intakes of n-3 ALA and n-6 LA, especially in packaged food items and in the approximately 40 % of PUFA eaten away from home(21), as recently reviewed(11). The exact methodology used to estimate the absolute and relative intakes of ALA and LA from these limited FFQ data has not been published.
The sixty-one-item 1980 NHS FFQ that provided baseline data for the report that dietary n-6 LA is associated with lower CHD risk(29) did not identify any specific oils or brand names of any food items.
The revised 116-item 1984 NHS FFQ providing baseline data for the report that dietary ALA is associated with lower risk of fatal CHD(30) did not capture specific brand names for any of the three reported primary sources of ALA (mayonnaise, oil and vinegar salad dressing, and margarine).
The revised 131-item 1986 FFQ, which did not capture brand names or specific oils used in shortenings, mayonnaises, salad dressings, or packaged foods, was used thereafter.
Indicates a general question about fat sources (for example, real butter, margarine, vegetable oil, vegetable shortening, lard), without the identification of specific brand names or specific vegetable oils in ingredient lists.
The 1998 revision is the first FFQ listing olive oil as an option for the fat ‘usually used’ for cooking at home.
Our meta-analysis of four n-6 specific PUFA RCT data-sets with 9569 participants(2) is therefore unique because it is the first analysis to compile the detailed dietary fatty acid data necessary to evaluate the CHD effects of selectively increasing n-6 LA (in place of trans-fatty acids and SFA). These n-6 specific PUFA data, which were not available to Mozaffarian et al.(8), were obtained via an extensive search of published and unpublished data sources enabling identification of the specific study oils and n-3 and n-6 PUFA content of RCT diets. Of the total RCT participants (n 11 275), 85 % were in the n-6 specific PUFA RCT (n 9569); therefore only 15 % of all RCT participants (n 1706) were in the mixed n-3/n-6 PUFA RCT. Harris et al.(1) seem preoccupied with the results of the latter 15 % of RCT participants, which they interpret as ‘confirming’ that ‘vegetable oils rich in n-6 PUFA’ lower CHD(1). However, they apparently dismiss our pivotal finding that there was no indication of benefit in any of the four n-6 specific PUFA datasets representing 85 % of the total RCT participants.
There was a non-significant, but relatively consistent, signal toward harm in each of these four n-6 specific PUFA datasets (Figs. 1 and 2), as well as a pooled analysis for both CHD outcomes and death from all causes, despite substitution of n-6 LA for trans-fatty acids and SFA, which are generally considered atherogenic. By highlighting only the two n-6 specific PUFA RCT (three datasets) that provided corn oil(12,13), and ignoring the unfavourable results of the fourth n-6 specific PUFA RCT dataset that provided safflower-seed oil(14,15), Harris et al. have inaccurately portrayed our n-6 specific PUFA evaluation of four datasets and 9569 participants as a ‘two-trial analysis’ with ‘limited statistical power’ useful for ‘hypothesis generation but insufficient for deriving meaningful conclusions’. This fourth dataset, the Sydney Diet Heart Study (SDHS)(14), reported a 49 % increase in risk of death in the n-6 specific intervention group that approached significance (risk ratio (RR) 1·49 (95 % CI 0·95, 2·34); P=0·08). Nearly all SDHS deaths were attributed to CHD (91 %); however, non-fatal MI and CHD death were not reported by group. Using reported SDHS data, the lowest possible increase in CHD death in the SDHS was + 26 % (RR 1·26 (95 % CI 0·79, 2·02); P=0·33); the highest possible increase was + 90 % (RR 1·90 (95 % CI 1·17, 3·10); P=0·01). Therefore, the 13 % increase in CHD risk from only three of the four n-6 specific PUFA interventions underestimates potential harm. In modelling the equitable assumption that the same percentage of SDHS deaths (91 %) were CHD deaths in each group, the risk of CHD death was increased by 49 % (RR 1·49 (95 % CI 0·93, 2·40); P=0·10), and the pooled 28 % increase in risk of CHD death from n-6 specific PUFA interventions was nearly significant (RR 1·28 (95 % CI 0·96, 1·71); P=0·09). Pooled data from all four n-6 specific PUFA datasets (n 9569; 85 % of all RCT participants) provide a relatively consistent, albeit non-significant, trend toward increased risks for CHD death (+28 %) (RR 1·28 (95 % CI 0·96, 1·71); P=0·09), total CHD events (+23 %) (RR 1·23 (95 % CI 0·94, 1·61); P=0·13), and death from all causes (+16 %) (RR 1·16 (95 % CI 0·95, 1·42); P=0·15). With no indication of benefit in any n-6 specific PUFA RCT or the pooled analysis of all four datasets, and a relatively consistent signal in the opposite direction for each dataset and for the pooled analysis, these data provide no justification for population-wide advice to maintain or increase n-6 PUFA consumption.
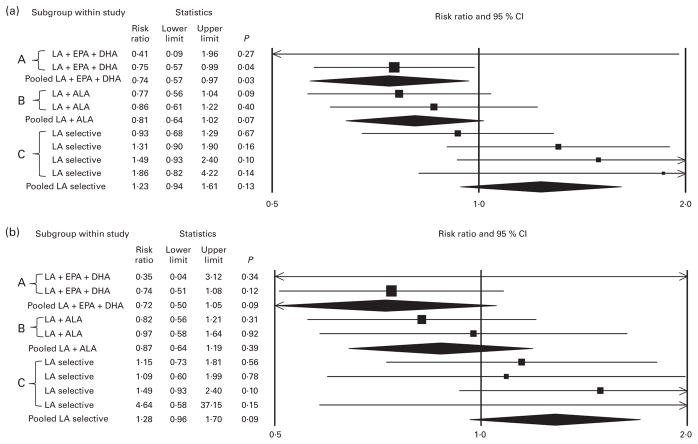
Fig. 1.
No indication of benefit, and a signal toward harm, in non-fatal myocardial infarction (MI) + CHD death (a) and in CHD death (b) shown by n-6 specific PUFA interventions. PUFA replaced a combination of trans-fatty acids and SFA in each randomised controlled trial (RCT). ‘A’ indicates mixed n-3/n-6 PUFA RCT datasets increasing n-3 EPA + DHA and n-6 linoleic acid (LA); ‘B’ indicates mixed n-3/n-6 PUFA RCT datasets increasing n-3 α-linolenic acid (ALA) and n-6 LA; ‘C’ indicates n-6 specific PUFA RCT datasets selectively increasing n-6 LA. Each of two RCT datasets increasing LA + EPA and DHA showed a trend toward benefit; pooled analysis showed significant reduction in non-fatal MI + CHD death (risk ratio (RR) 0·74 (95 % CI 0·57, 0·97); P=0·03), and trends toward reduction in CHD death (RR 0·72 (95 % CI 0·50, 1·05); P=0·09) and death from all causes (RR 0·73 (95 % CI 0·52, 1·04); P=0·08) (Fig. 2). Each of the two RCT datasets increasing LA + ALA, and their pooled analysis showed a trend toward reduced risk of non-fatal MI + CHD death (RR 0·81 (95 % CI 0·64, 1·02); P=0·07), and signals toward reduction in death from CHD (RR 0·87 (95 % CI 0·64, 1·19); P=0·39) and all-cause mortality (RR 0·96 (95 % CI 0·83, 1·12); P=0·62) (Fig. 2). The four individual n-6 specific PUFA RCT datasets, and pooled analysis of all four datasets showed a relatively consistent trend toward increased risk of non-fatal MI + CHD death (RR 1·23 (95 % CI 0·94, 1·61); P=0·13), death from CHD (RR 1·28 (95 % CI 0·96, 1·70); P=0·09), and of death from all causes (RR 1·16 (95 % CI 0·95, 1·42); P=0·15) (Fig. 2). The structure of these RCT data suggest that CHD benefits previously attributed to n-6 PUFA or total PUFA may have been due to substantial increases in n-3 PUFA, particularly n-3 EPA + DHA, and that the accompanying increases in n-6 LA attenuated these benefits. Calculated RR, 95 % CI and P values include data from all eight datasets included in the primary analysis. As explained in the text, the Sydney Diet Heart Study (SDHS) reported a 49 % increased risk of CHD with an n-6 specific intervention (RR 1·49 (95 % CI 0·95, 2·34); P=0·08). Only total deaths were reported for each group; however, 91 % of these total deaths were attributed to CHD. Therefore, the pooled analysis of only three of the four n-6 specific RCT datasets without the SDHS underestimates the potential harm of n-6 PUFA. The CHD event data in Fig. 1 model the assumption that 91 % of total deaths in each group were due to CHD, allowing for a more accurate estimation of the CHD effects of replacing trans- and saturated fats with n-6 LA because it includes all four n-6 specific PUFA datasets. Based on reported SDHS data for total deaths and CHD death, the lowest possible pooled increase in risk of CHD death from the four n-6 specific PUFA datasets was +21 % (RR 1·21 (95 % CI 0·91, 1·60); P=0·20); the highest possible increase was +40 % (RR 1·40 (95 % CI 0·97, 2·02); P=0·07).
Fig. 2.
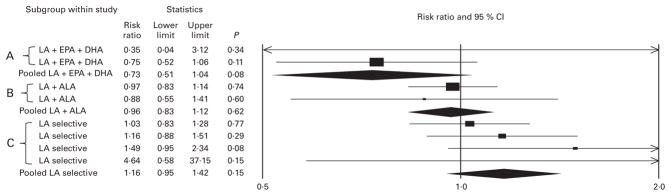
No indication of benefit, and a signal toward harm, in deaths from all causes shown by n-6 specific PUFA interventions. PUFA replaced a combination of trans-fatty acids and SFA in each randomised controlled trial (RCT). ‘A’ indicates mixed n-3/n-6 PUFA RCT datasets increasing n-3 EPA + DHA and n-6 linoleic acid (LA); ‘B’ indicates mixed n-3/n-6 PUFA RCT datasets increasing n-3 α-linolenic acid (ALA) and n-6 LA; ‘C’ indicates n-6 specific PUFA RCT datasets selectively increasing n-6 LA. All RCT reported total death. RCT data follow the same pattern as observed for CHD events and CHD deaths. Pooled analysis of two datasets increasing LA +EPA and DHA showed a trend toward reduced risk of death (risk ratio (RR) 0·73 (95 % CI 0·52, 1·04); P=0·08); two datasets increasing LA + ALA showed no difference (RR 0·96 (95 % CI 0·83, 1·12); P=0·62); four datasets selectively increasing n-6 LA in place of trans- and saturated fats showed a signal toward increased risk of death (RR 1·16 (95 % CI 0·95, 1·42); P=0·15).
Harris et al.(1) have not fully appreciated the essential distinction between n-3 and n-6 PUFA species in attributing benefits from our pooled analysis of mixed n-3/n-6 PUFA RCT datasets to ‘vegetable oils rich in n-6 PUFA’ or simply ‘soybean oil’, despite substantial increases in n-3 EPA + DHA in two(16 – 18) of the four datasets. The experimental dieters in the Oslo Diet Heart Study (ODHS) were actually provided with ‘substantial quantities of Norwegian sardines canned in cod liver oil’(16) and not simply ‘encouraged’ as Harris et al. state here(1) and elsewhere(4,19,20). Oslo dieters consumed about 5 g EPA + DHA per d (thirty times the average US intake)(21); therefore it is not valid to attribute CHD benefits to LA or soybean oil. After exclusion of the ODHS and the St Thomas Atherosclerosis Regression Study (STARS), the remaining two datasets(22 – 24) that substantially increased both n-3 ALA and n-6 LA from soybean oil without increasing n-3 EPA + DHA have a non-significant signal toward benefit, and lack the signal toward harm of the n-6 specific PUFA interventions.
The structure of all RCT data is shown in Fig. 1 and Fig. 2, as follows: (A) mixed n-3/n-6 interventions including EPA + DHA are beneficial; (B) mixed n-3/n-6 interventions including ALA provide an intermediate signal toward benefit; and (C) interventions specific to n-6 LA provide no indication of benefit and a relatively consistent signal toward harm. Given this consistent pattern for CHD deaths and total CHD events (Fig. 1), and for deaths from all causes (Fig. 2), it is tempting to speculate that CHD benefits previously attributed to n-6 PUFA or total PUFA in general(4,6 – 8,25) were actually due to substantial increases in n-3 PUFA in general, and n-3 EPA + DHA in particular, and that the accompanying increases in n-6 LA attenuated these benefits. If this testable hypothesis is correct, lowering dietary n-6 LA is likely to potentiate the benefits of n-3 PUFA and/or have independent CHD benefits. However, we have no RCT data evaluating the effects of lowering n-6 LA as a controlled variable on clinical CHD outcomes. This is clearly a critical evidence gap with major public health implications. The next logical step is to evaluate whether lowering dietary n-6 LA as a controlled variable to about 2 % of energy (consistent with evolutionary(26) and historical US diets(27)) reduces the risk of CHD in a large RCT. This can be accomplished via substitution of a high-oleic version of sunflower-seed oil (3·6 g LA per 100 g)(28) for a typical high-LA version of the same oil (65·7 g LA per 100 g)(28) with otherwise identical background diets.
This project was supported by the intramural research program of the National Institute on Alcohol Abuse and Alcoholism. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health.
Footnotes
The authors declare that they have no conflicts of interest.
Contributor Information
Christopher E. Ramsden, Email: chris.ramsden@nih.gov, Section on Nutritional Neurosciences, Laboratory of Membrane Biochemistry and Biophysics, National Institute on Alcohol Abuse, National Institutes of Health, Bethesda, MD, USA.
Joseph R. Hibbeln, Section on Nutritional Neurosciences, Laboratory of Membrane Biochemistry and Biophysics, National Institute on Alcohol Abuse, National Institutes of Health, Bethesda, MD, USA
Sharon F. Majchrzak-Hong, Section on Nutritional Neurosciences, Laboratory of Membrane Biochemistry and Biophysics, National Institute on Alcohol Abuse, National Institutes of Health, Bethesda, MD, USA
John M. Davis, Department of Psychiatry, School of Medicine, University of Illinois, Chicago, IL, USA
References
- 1.Harris WS, Brouwer I, Mozaffarian D. n-6 Fatty acids and risk for CHD: consider all the evidence. Br J Nut. 2011 doi: 10.1017/S000711451100105X. (epublication ahead of print version) [DOI] [PubMed] [Google Scholar]
- 2.Ramsden CE, Hibbeln JR, Majchrzak SF, et al. n-6 Fatty acid-specific and mixed polyunsaturate dietary interventions have different effects on CHD risk: a meta-analysis of randomised controlled trials. Br J Nutr. 2010;104:1586–1600. doi: 10.1017/S0007114510004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matt GE, Cook TD. Threats to the validity of research synthesis. In: Cooper HM, Hedges LV, editors. The Handbook of Research Synthesis. New York: Russell Sage Foundation; 1994. pp. 503–518. [Google Scholar]
- 4.Harris WS, Mozaffarian D, Rimm E, et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119:902–907. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 5.Harris WS. Linoleic acid and coronary heart disease. Prostaglandins Leukot Essent Fatty Acids. 2008;79:169–171. doi: 10.1016/j.plefa.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Kris-Etherton P, Harris WS. Dietary omega-6 poly-unsaturated fatty acids - important for heart health. Clin Nutr Insight. 2009;35:1–5. [Google Scholar]
- 7.Kris-Etherton P, Fleming J, Harris WS. The debate about n-6 polyunsaturated fatty acid recommendations for cardiovascular health. J Am Diet Assoc. 2010;110:201–204. doi: 10.1016/j.jada.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7:e1000252. doi: 10.1371/journal.pmed.1000252. (epublication 23 March 2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jakobsen MU, O’Reilly EJ, Heitmann BL, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89:1425–1432. doi: 10.3945/ajcn.2008.27124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mensink RP, Zock PL, Kester AD, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 11.Ramsden CE, Hibbeln JR, Majchrzak SF. All PUFAs are not created equal: absence of CHD benefit specific to linoleic acid in randomized controlled trials and prospective observational cohorts. World Rev Nutr Diet. 2011 doi: 10.1159/000327789. (In the Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frantz ID, Jr, Dawson EA, Ashman PL, et al. Test of effect of lipid lowering by diet on cardiovascular risk. The Minnesota Coronary Survey. Arteriosclerosis. 1989;9:129–135. doi: 10.1161/01.atv.9.1.129. [DOI] [PubMed] [Google Scholar]
- 13.Frantz ID, Jr, Keys A. Research Grant Application: Effect of a Dietary Change on Human Cardiovascular Disease. The Minnesota Coronary Survey 1967 [Google Scholar]
- 14.Woodhill JM, Palmer AJ, Leelarthaepin B, et al. Low fat, low cholesterol diet in secondary prevention of coronary heart disease. Adv Exp Med Biol. 1978;109:317–330. doi: 10.1007/978-1-4684-0967-3_18. [DOI] [PubMed] [Google Scholar]
- 15.Woodhill JM, Palmer AJ, Blacket RB. Dietary habits and their modification in a coronary prevention programme for Australians. Food Technol Aust. 1969;21:264–271. [Google Scholar]
- 16.Leren P. The effect of plasma cholesterol lowering diet in male survivors of myocardial infarction. A controlled clinical trial. Acta Med Scand Suppl. 1966;466:1–92. [PubMed] [Google Scholar]
- 17.Watts GF, Jackson P, Burke V, et al. Dietary fatty acids and progression of coronary artery disease in men. Am J Clin Nutr. 1996;64:202–209. doi: 10.1093/ajcn/64.2.202. [DOI] [PubMed] [Google Scholar]
- 18.Watts GF, Jackson P, Mandalia S, et al. Nutrient intake and progression of coronary artery disease. Am J Cardiol. 1994;73:328–332. doi: 10.1016/0002-9149(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 19.Ramsden CE, Hibbeln JR, Lands WE. Letter to the Editor re: Linoleic acid and coronary heart disease. In: Harris WS, editor. Prostaglandins Leukot Essent Fatty Acids. Vol. 80. 2008. p. 77. [DOI] [PubMed] [Google Scholar]
- 20.Ramsden C. A misrepresented meta-analysis. [accessed March 2011];Letter to Circulation. 2009 http://www.americanheart.org/downloadable/heart/1256648338750Omega6letterswresp.pdf.
- 21.US Department of Agriculture Agricultural Research Service. [accessed March 2011];Nutrient Intakes from Food: Mean Amounts Consumed per Individual, by Gender and Age, What We Eat in America, National Health and Nutrition Examination Survey (NHANES 2007–2008) 2010 http://www.ars.usda.gov/ba/bhnrc/fsrg.
- 22.Medical Research Council. Controlled trial of soya-bean oil in myocardial infarction. Lancet. 1968;28:693–699. [PubMed] [Google Scholar]
- 23.Hiscock E, Dayton S, Pearce ML, et al. A palatable diet high in unsaturated fat. J Am Diet Assoc. 1962;40:427–431. [PubMed] [Google Scholar]
- 24.Dayton S, Pearce ML. Diet high in unsaturated fat. A controlled clinical trial. Minn Med. 1969;52:1237–1242. doi: 10.1161/01.cir.40.1s2.ii-1. [DOI] [PubMed] [Google Scholar]
- 25.Katan MB. Omega-6 polyunsaturated fatty acids and coronary heart disease. Am J Clin Nutr. 2009;89:1283–1284. doi: 10.3945/ajcn.2009.27744. [DOI] [PubMed] [Google Scholar]
- 26.Kuipers RS, Luxwolda MF, Dijck-Brouwer DA, et al. Estimated macronutrient and fatty acid intakes from an East African Paleolithic diet. Br J Nutr. 2010;104:1666–1687. doi: 10.1017/S0007114510002679. [DOI] [PubMed] [Google Scholar]
- 27.Blasbalg T, Hibbeln JR, Ramsden CE, et al. Am J Clin Nut. 2011. Changes in omega-6 and omega-3 fatty acid consumption in the US during the 20th century. (epublication ahead of print version 2 March 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Department of Agriculture Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 23. [accessed 29 January 2011];Nutrient Data Laboratory Home Page. 2010 http://www.ars.usda.gov/nutrientdata.
- 29.Oh K, Hu FB, Manson JE, et al. Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the Nurses’ Health Study. Am J Epidemiol. 2005;161:672–679. doi: 10.1093/aje/kwi085. [DOI] [PubMed] [Google Scholar]
- 30.Hu FB, Stampfer MJ, Manson JE, et al. Dietary intake of α-linolenic acid and risk of fatal ischemic heart disease among women. Am J Clin Nutr. 1999;69:890–897. doi: 10.1093/ajcn/69.5.890. [DOI] [PubMed] [Google Scholar]