Abstract
Mining operations, including crushing, grinding, smelting, refining, and tailings management, are a significant source of airborne metal and metalloid contaminants such as As, Pb and other potentially toxic elements. In this work, we show that size-resolved concentrations of As and Pb generally follow a bimodal distribution with the majority of contaminants in the fine size fraction (< 1 μm) around mining activities that include smelting operations at various sites in Australia and Arizona. This evidence suggests that contaminated fine particles (< 1 μm) are the result of vapor condensation and coagulation from smelting operations while coarse particles are most likely the result of windblown dust from contaminated mine tailings and fugitive emissions from crushing and grinding activities. These results on the size distribution of contaminants around mining operations are reported to demonstrate the ubiquitous nature of this phenomenon so that more effective emissions management and practices that minimize health risks associated with metal extraction and processing can be developed.
Keywords: Mining, Dust and Aerosol, Arsenic and Lead, Dust Transport
1. Introduction
The role of mining activities in the fate and transport of environmental contaminants is an important yet under investigated field of study (Csavina et al., 2012). Dust and aerosol produced by mining operations often contain elevated levels of metal and metalloid contaminants, including the toxic elements Pb and As (Benin et al., 1999; Bellinger, 2008; Taylor et al., 2010; Csavina et al., 2011; Mackay et al., 2013). Both Pb and As are known to have contributed to negative ecological and human health effects in surrounding communities, including elevated blood Pb levels in children (Queensland Health, 2008, 2010; Munksgaard et al., 2010, Simon and Lewis, 2010). However, the specific physiochemical nature of these exposures remains poorly understood. With dust emissions predicted to increase as climate change intensifies drought in arid and semi-arid regions and human land use increases, contaminant transport from mining operations is likely to become increasingly important in the coming decades (Breshears et al., 2012).
Two Australian mining communities of Mount Isa, Queensland, and Port Pirie, South Australia, are examples of significant contaminant emissions from smelting and mining activities. At Mount Isa, Cu, Zn, Pb and Ag mining and smelting results in the emission of significant quantities of airborne contaminants, with As and Pb emissions for 2009/2010 being 20,000 kg and 120,000 kg, respectively (Australian Department of Sustainability, 2011). With a population of 21,000, Mount Isa's most recent study reported that children aged 1–4 years had mean blood Pb levels (BLL) of 5 μg/dL, with 37% having levels > 6 μg/dL and 11.3% having levels > 10 μg/dL (Queensland Health, 2008). Pb and Zn smelting in Port Pirie is also associated with significant atmospheric pollution, with As and Pb total suspended particulate (TSP) measurements in 2009 as high as 0.25 μg/m3 and 19.7 μg/m3, respectively, taken 0.4 km from smelting activities (South Australia EPA, 2012). In 2005, 56.5% of children in Port Pirie had BLL >10 μg/dL (Simon and Lewis, 2010). Epidemiological and environmental studies have revealed that dust generation associated with mining and smelting activities largely contributes to the extensive childhood Pb poisoning within the Australian mining communities of Port Pirie and Mount Isa (Mackay et al., 2013, Taylor et al., 2013, Simon et al., 2007).
The towns of Hayden and Winkelman in Arizona have a combined population of approximately 1200. The site includes a concentrator, a smelter and tailings facilities, and it is currently administered through an Administrative Settlement Agreement and Order on Consent between EPA, ASARCO (the plant proprietor) and the Arizona Department of Environmental Quality. In 2005, soil analysis showed that arsenic, lead and copper levels exceeded their respective residential soil remediation levels (US EPA 2008). In addition, elevated concentrations of arsenic, lead, copper, chromium and cadmium have been measured in atmospheric air samples.
Many studies have explored the neurotoxic nature of Pb, especially in children who are more adversely impacted due to their early stage in neurological development and their higher relative contaminant dosage at the same concentration when compared to adults (Baghurst et al., 1992; Jusko et al., 2008; Soto-Jimenez and Flegal, 2011). Additionally, higher Pb exposures have been shown to lower academic performance and contribute to negative social outcomes related to antisocial behavior and criminality (Wright et al., 2008). Similarly to Pb, As has also shown impaired cognitive development in children and may have a synergistic toxic effect with Pb (Hwang et al. 1997; Calderón et al. 2001; Wright et al. 2006). Arsenic is also a known carcinogen and, even though the World Health Organization (WHO, 2000) has not set a safe level of atmospheric concentrations, a value of 6.6 ng/m3 has been identified as a lifetime risk level of 1:100,000. Due to the known effects of these pollutants, human Pb and As exposure should be minimized.
Dust and aerosol generated from mining operations vary in size, which is critical for physical interactions in the environment and human exposure. An important route of human exposure is the inhalation of the airborne contaminated particulate. A knowledge of the physical and chemical properties and size distribution of inhaled aerosols is necessary to completely assess risks associated with contaminant exposure (Spear et al., 1998). The size of the particle can predict the efficiency and region of deposition in the respiratory tract (Park and Wexler, 2008). Coarse particles (> 3 μm), such as those resulting from crushing and grinding of ore, deposit in the upper respiratory system and are swallowed and eliminated through the digestive system (Hinds, 1999). In contrast, fine particles (< 1 μm), such as those originating from smelting operations, are respired deep into the lungs where they may be transported directly to the blood stream and have a higher bioavailability due to their higher surface to volume ratios (Krombach et al., 1997; Park and Wexler, 2008; Valiulis et al., 2008). Particle size is also a critical characteristic for transport distance and building penetration within the adjoining environment: fine particles can travel further in the environment with residence times in the atmosphere that may reach days (Hinds, 1999). Therefore, determining the chemical composition of dust and aerosol from mining operations as a function of particle size is crucial in quantifying the potential deleterious effects on human health and the environment.
This study reports on the size-resolved As and Pb concentrations found in dust and aerosol in the Australian communities of Mount Isa and Port Pirie mentioned earlier, as well as in Hayden, Arizona. These multi-sire measurements, performed with common sampling and analysis techniques, allowed us to perform general observations on the size-fractionated contaminant concentration in the atmosphere around mining operations. In addition, we report on the use of lead isotope analysis to asses sources of contamination in the studied sites.
2. Materials and Methods
Ambient dust and aerosol sampling was carried out in the communities of Port Pirie (South Australia), Mount Isa (Queensland), and Hayden (Arizona), which are impacted by smelting activities. Samples were also collected in urban settings of Tucson (Arizona) and Sydney (New South Wales), and where mine tailings with no smelting operations are the primary source of dust and aerosol in the communities of Green Valley (Arizona) and Iron King (Arizona), for comparison purposes. An overview map of these sites can be seen in Figure 1.
Figure 1.
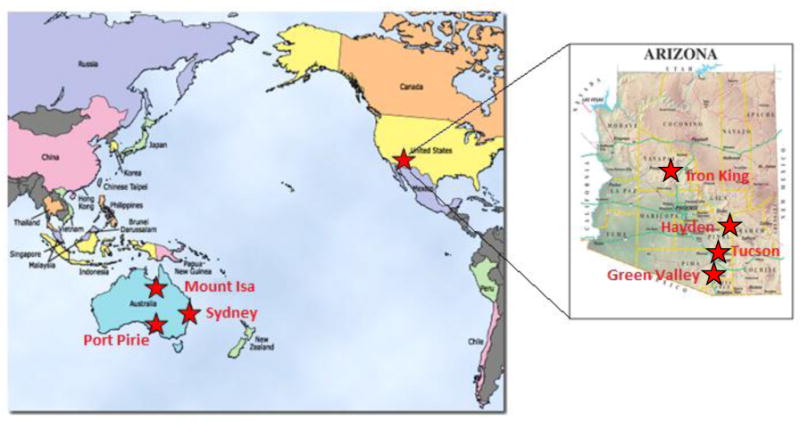
Field sites for size fractioned aerosol sampling: Mount Isa, Port Pirie and Hayden mining sources include smelting activities; Tucson and Sydney represent urban settings.
According to US EPA (2008), the Hayden smelter processes 27,400 tons of ore per day from pre mining operations in nearby Green Valley and Ray, Arizona. Copper sulfide ore is crushed and milled at the Hayden facility and concentrated by froth flotation using sulfuric acid. The waste from the flotation is sent as slurry to tailings impoundments while the concentrate is sent to the smelting facilities, which include an oxygen flash furnace, converters, anode casting, oxygen plant and acid plant. Electrostatic precipitators are used to reduce particulate matter in the air emissions from bed driers and flash furnace. The matte from the flash furnace is sent to a converter furnace to remove impurities and blister the copper. Slag waste from the smelting process is dumped in open waste stockpiles. A bag house is used to capture the converter's secondary particulates. Final refining occurs off-site and emissions from the smelter are released through a 330-m tall stack.
The Mount Isa smelter produces Pb and Cu while recovering Zn and Ag. Local underground mines provide the ore which is then crushed and refined at the surface. Ball mills grind the rock to ∼ 100 μm, the product of which is concentrated by flotation. The Cu concentrate is further processed in furnaces. The Pb concentrate is fed to a blast furnace where it is recovered as 99.6 % lead bullion. Mount Isa has an electrostatic precipitator and scrubber in the copper smelter and a bag house in the lead smelter. The plant claims to only allow emissions from operations when the prevailing winds are towards the NW direction, away from the community, as part of their pollution control strategy.
The Port Pirie smelter is primarily a Pb plant that produced 158,000 tons of Pb in 2012, but also Zn, Cu, Ag, and Au are recovered as by-products. The ore concentrate is sintered before being sent to a blast furnace. Spent slag from the furnace is sent to a Zn recovery plant before being discarded as waste. The Pb bullion is further processed to remove Cu, As, Sb, Au, and Ag before the final refining stage. Emissions are controlled by bag house, electrostatic precipitator and scrubbers, as well as mist treatment for dust suppression on processes such as ore transfer.
Sampling of atmospheric air at all sites was performed with a ten-stage micro-orifice uniform deposit impactor, MOUDI (MSP Corporation) (Marple et al., 1991). The MOUDI provides separate fractions of dust and aerosol at the 50% cut off points (d50-values) of 18, 9.9, 6.2, 3.1, 1.8, 1.0, 0.55, 0.32, 0.18, 0.10 and 0.054 μm equivalent aerodynamic diameter. Total sampling time at 30 L min-1 varied according to distance from and strength of the source. The sampling duration for Port Pirie was the shortest at 24 hours due to relatively high Pb concentrations (the sampling location was at 0.3 km from smelting operations). The MOUDI was run for 72 hours at Mount Isa (2.8 km from smelting operations). All other sites were sampled for 96 hours. At Australian sites, weather data were acquired from local Australian Environmental Protection Agency's monitoring sites, whereas at the Hayden site, weather data were acquired from a collocated weather station and data logger (Campbell Scientific CR800).
Teflon filters (PTFE membrane, 2 μm pore, filter substrate 46.2 mm, Whatman) were used as impaction substrates in the MOUDI. Filters were transferred in clean petri dishes (Micro Analytix, 800100). Field blanks were used as controls. Substrates were weighed before and after sampling using an ultra-microbalance (Mettler Toledo XP2U) according to EPA Class I equivalent methods. Once gravimetric analysis was complete, filters were extracted in sealed glass vials with trace metal grade aqua regia and sonication at 80 °C for 60 min (Harper et al., 1983). Extracted aliquots were analyzed for metal and metalloid concentrations by Inductively-Coupled Plasma Mass Spectrometry (ICP-MS, Agilent 7500ce with an Octopole Reaction System). For the analysis of total lead, the equipment was tuned for robust plasma conditions to reduce the formation of oxides to less than 2%. The plasma gas flow used was 1.5 L/min and the carrier gas flow was 1 L/min. The solutions were measured after instrument calibration, then repeatedly throughout the analytical run, after every three sample injections. Certified calibration standards from Accustandard were made with MiliQ water, 0.669 HCl (Fisher, trace-metal grade) and 0.309 M HNO3 (EMD, Omnitrace). National Institute of Standards and Technology (NIST) standard reference material (SRM 1643e trace elements in water) was analyzed with each set of data. For the analysis of lead isotopic ratios, the operating conditions were the same as those used for elemental concentration measurements. NIST SRM 981 (lead isotopic standard) was used for validation and calibration. The analytical precision of lead isotopic ratios was under 0.5 % for Pb207/Pb206 and Pb208/Pb206.
3. Results and Discussion
The size-fractionated aerosol and dust collected near the Hayden mining and smelting site clearly shows a bimodal distribution for both Pb and As, with maxima located at approximately 0.32 and 9.9 μm diameter (Figure 2). Scanning electron micrographs of fine particles (< 1 μm) show that particles containing relatively high concentrations of Pb and As are spherical (Figure 3a), which suggests that the origin of these particles is condensation from the high temperature smelter emissions. Some of the fine particles also show evidence of coagulation of two or more spheres. Coarse particles tend to be of irregular shape (Figure 3b), suggesting that they consist primarily of windblown dust. Lead and arsenic laden particles in this range may originate from mine tailings, or other exposed ore or slag.
Figure 2.
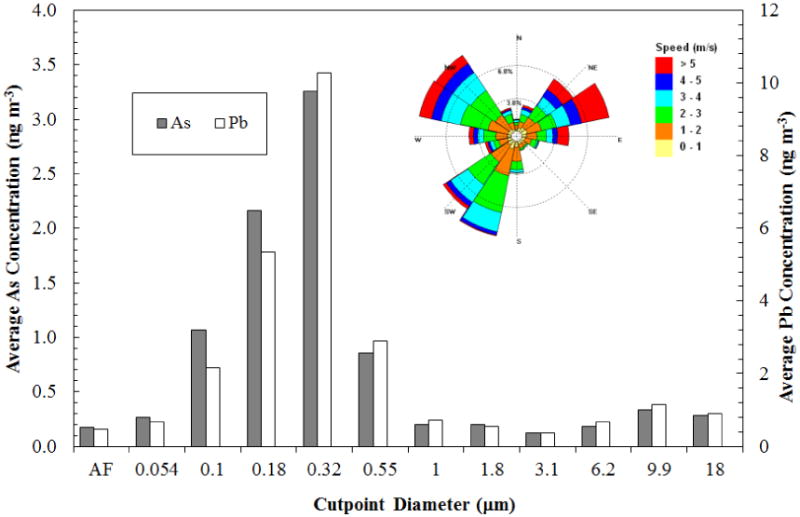
Airborne lead and arsenic content by particle size (MOUDI samples) observed at Hayden, Arizona, for a 96-h sample collected 1 km south-southeast from smelting operations with sampling starting on February 5, 2011; AF denotes after filter sample. The wind rose inset is from a co-located weather station, which shows that winds from the direction of the smelting activities (NNW) account for roughly 30% of prevailing winds during the sampling period.
Figure 3.
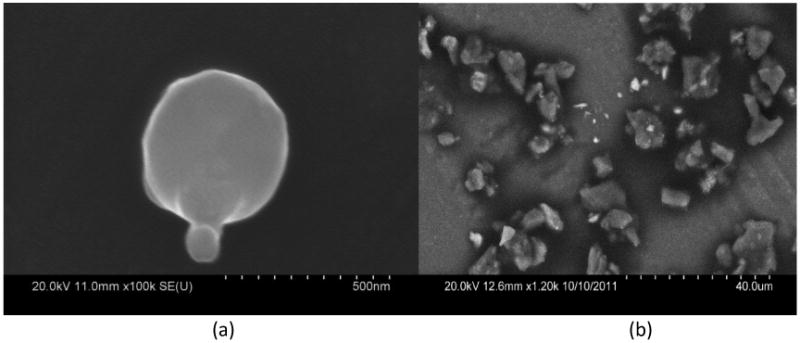
Representative Scanning Electron Microscope images from MOUDI stages (Hayden site samples) with cutpoint particle diameters of (a) 0.32 μm, particle depicted was identified to contain 10.88± 1.00 % Pb by weight by Electron Dispersive Spectroscopy; and (b) 6.2 μm.
Results from MOUDI samples collected in Mount Isa, also an active mining and smelting site, are shown in Figure 4. As was the case in Hayden, both Pb and As are concentrated in the submicron fraction, with maxima located at approximately 0.32 μm (As) and 0.55 μm (Pb). However, Figure 4 shows that the arsenic coarse mode maximum occurs at about 18 μm and a distinct coarse mode maximum for Pb is absent, although relatively high lead concentrations are observed in the coarse fractions. Similar results for Port Pirie are shown in Figure 5. Arsenic again shows a bimodal distribution with maxima at approximately 0.55 μm and 9.9 μm diameter. The Pb size distribution is significantly different from Hayden and Mount Isa. First, the highest concentration is found in the coarse mode (9.9 μm). The maximum in the fine mode is found at about 0.32 μm diameter. It should be noted that the Pb concentrations here are an order of magnitude higher than at Hayden or Mount Isa.
Figure 4.
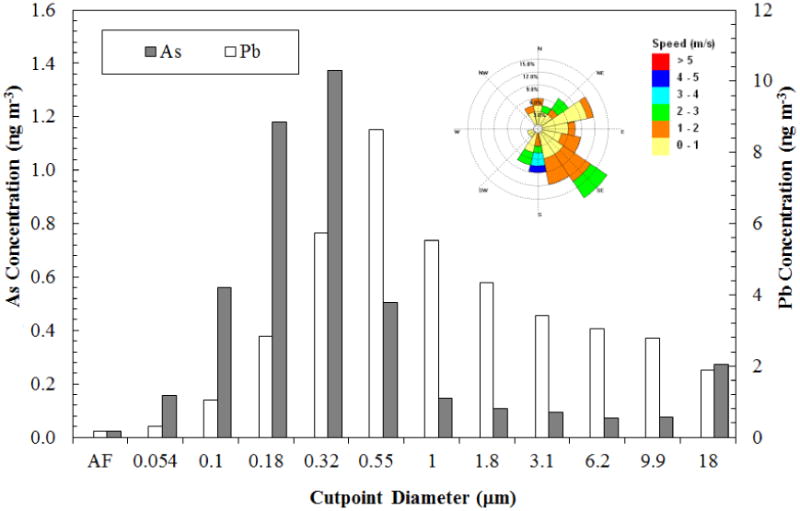
Airborne lead and arsenic content by particle size (MOUDI samples) observed at Mount Isa, New South Wales, for a 72-hour sampling period beginning on February 21, 2012. The MOUDI sampler was located 2.8 km northeast from the smelter; AF denotes after filter sample. Wind rose was from a weather station located approximately 1.5 km NNE of the smelter.
Figure 5.
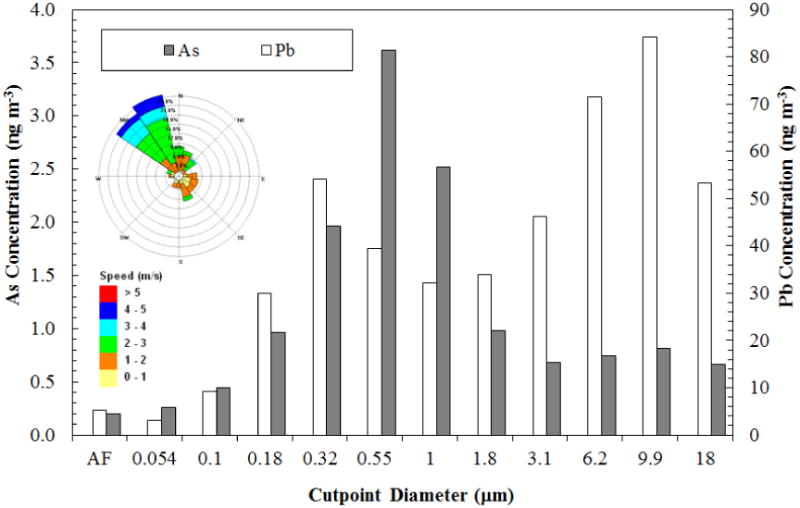
Airborne lead and arsenic content by particle size (MOUDI samples) observed at Port Pirie, South Australia for a 24-hour sampling period 0.4 km south from the smelter on April 17, 2012; AF denotes after filter sample. The wind rose is from a weather station located approximately 2.8 km south-southeast from the smelter.
Fine particles contaminated with As and Pb are thought to arise primarily from smelting operations as a result of condensation of high temperature vapors and coagulation, leading to a sub-micron maximum of contaminant concentration in the accumulation mode (Seinfeld and Pandis, 2006). On the other hand coarse particles, which are mainly windblown dust, originate either from background sources (topsoil or crust), or mine tailings or fugitive emissions from the crushing, grinding and transportation of the ore. In particular, if the lead contained in the particles originates from different sources, the isotopic composition of lead is potentially different for each source. It is known that the concentration of 206Pb, a product of the radioactive decay of 238U, increases over time at rates that are higher than those associated with changes in 207Pb and 208Pb. As a consequence, the concentration ratios 207Pb/206Pb and 208Pb/206Pb have decreased over time (Mukai et al., 2001; Komárek et al., 2008) and this type of analysis has been used widely to discriminate between anthropogenic lead sources (mined ores) and natural lead sources (derived from erosion of surface minerals) (Grousset and Quetel, 1994; Erel et al., 1997; Reuer and Weiss, 2002; among others).
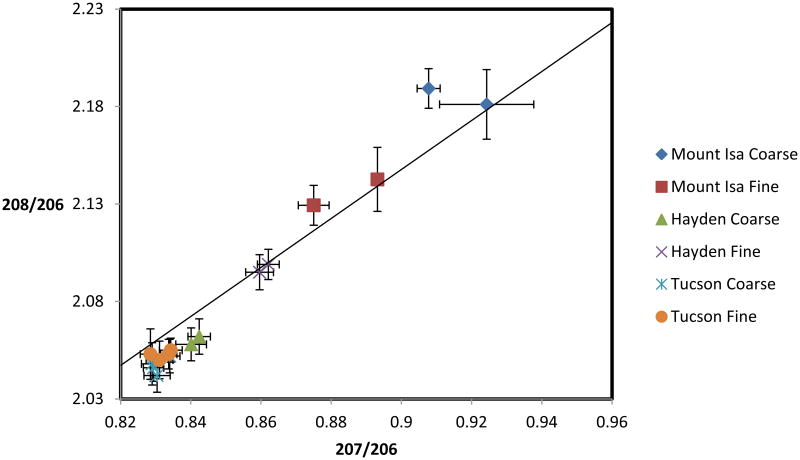
Figure 6 shows a comparison of lead isotopic ratios of samples collected in this investigation. For both the Mount Isa and Hayden sites, where active smelters operate, there is a clear difference between the isotopic ratios between coarse and fine particles, which suggests a different origin. On the other hand, at the urban control site (Tucson), there is no change in the isotopic ratios with particle size. It is interesting to note that the isotopic signature of the coarse particles in Hayden is close to the signature of the Tucson particles (100 km away), which suggests that the Hayden coarse particles are heavily influenced by the regional background while the lead in fine particles corresponds to the ore processed in the smelter. This is further confirmed by the fact that the lead isotopic ratios of the Hayden fine particles (Figure 6) are identical to the ratios reported for the Ray Mine ore, the main supplier for the Hayden smelter: 207/206: 0.86, 208/206: 2.1 (Bouse et al., 1999).
Figure 6.
Lead isotopic composition ratios of samples from various locations. Analysis samples were obtained by combining particles collected from various stages of the MOUDI sampler. Results are presented for two particle size ranges: 0.32 - 0.55 μm (fine particles) and 3.1 - 6.2 μm (coarse particles). The solid line is the growth curve, adapted from Chen et al. (2008) and developed by Cumming and Richards (1975).
The lead isotopic ratios for the Mount Isa samples also show a difference between fine and coarse particle, which suggests two different sources. The lead isotopic ratios for the Mount Isa mine ore are: 207/206: 0.96, 208/206: 2.22 (Sturges and Barrie, 1987). These ratios are appreciably different from the two particle sizes (Figure 6). However, a second source of lead in the Mount Isa region has been identified as the local crustal material, which contains relatively high concentration of lead (Mackay et al, 2013; Stacey and Kramers, 1975). The crustal lead isotope ratios are: 207/206: 0.83, 208/206: 2.05. The results in Figure 6 clearly indicate that both fine and coarse particles in the dust collected contain a mixture of crustal lead and ore lead, with the coarse fraction being closer to the ore signature. This indicates that windblown dust (coarse fraction) from tailings and ore exposure is an important contributor to atmospheric lead concentrations,
In terms of concentrations, the Hayden site samples contain 88% and 86% of the As and Pb, respectively, in the fine size fraction (< 1 μm). Similarly, in Mount Isa, fine particulate accounts for 87% of As and 61% Pb. In Port Pirie, however, 72% of the As is in the fine size fraction while only 38% of the Pb is < 1 μm. Given that both Port Pirie and Mount Isa preform grinding of the ore that will later be refined for Pb, it is expected that coarse particle fractions will be enhanced in Pb concentration. Moreover, in Port Pirie the Pb concentrate feed stock has already been ground (∼106 μm diameter) and concentrated by flotation, so it is reasonable to expect that mechanical action, including material handling and wind erosion gives rise to an enhanced concentration in the coarse mode (Jackson and Abbot, 2008), as observed here. Since Port Pirie is primarily a Pb Smelter, the fine fraction is also expected to contain relatively high Pb concentrations. While Mount Isa similarly refines Pb, the sampling site is 2.4 km further away from the activities when compared to Port Pirie. Therefore, the majority of the coarse particles have settled out of the atmosphere before reaching the receptor. For example, a 10 μm lead oxide particle has a terminal settling velocity of around 2.9 m s-1, as calculated from Stokes law, assuming a density of 9,500 kg m-3 and spherical morphology. This particle would travel 0.4 km (the distance to the Port Pirie sampling location) with a sustained wind of 1.1 m s-1 while the same particle would require a wind of 8 m s-1 to travel 2.8 km (the distance to the Mount Isa sampling location). It is important to note that some studies show the MOUDI to be less efficient at collecting coarse particles than other samplers (Cabada et al. 2004), which would introduce a low bias in the coarse particles Pb concentration. While PM10 has been shown to provide good agreement with MOUDI measurements (Csavina et al. 2011), MOUDI samples may not provide total ambient contaminant concentrations as compared with those found in total suspended particulate collection.
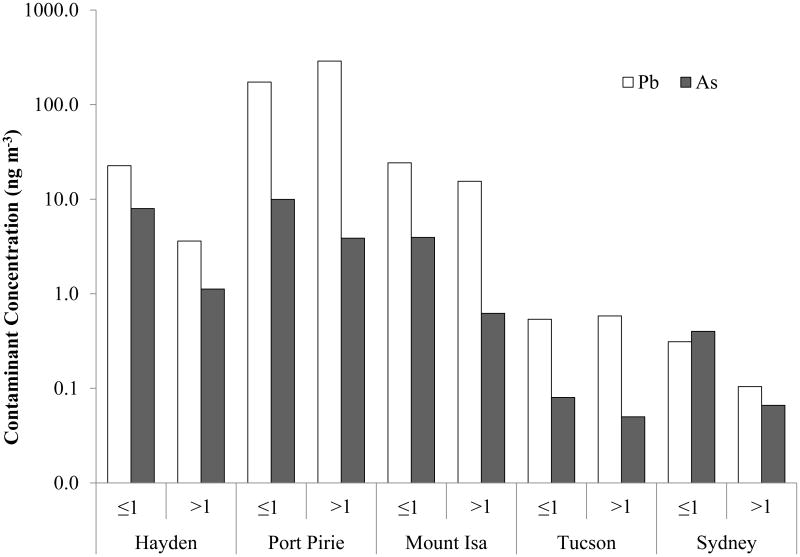
We used the same procedures to perform a field site comparison for As and Pb concentrations. MOUDI observations are presented in Figure 6, including measurements from two urban locations. The concentrations of Pb and As were summed for particles with MOUDI cutpoint diameters ≤ 1 and ≥ 1 μm. All the smelting sites have higher Pb and As concentrations in the fine fraction except for Port Pirie, as discussed above. Figure 7 indicates that smelting heavily enriches concentrations of Pb and As in the atmosphere when compared to urban samples, with a particularly high impact on the fine size fraction.
Figure 7.
Field site comparison of MOUDI observations of Pb and As concentrations summed according to two particle size fractions: < 1 μm and > 1 μm. Urban sites samples were collected for 96 hours on the following dates: Sydney – February 14, 2012; and Tucson – October 14, 2010. WHO guidelines: Pb – 500 ng/m3; As – 6.6 ng/m3 excess lifetime risk level 1:100,000.
Gravimetric analysis presented in Table 1 presents a field site comparison for the maximum mass concentration found for any individual MOUDI impactor stage. By weighing each filter (impaction stage) before and after exposures, it is possible to determine the total dust and aerosol mass collected on each stage, and thereby calculate the mass concentration (ppm) of Pb and As on each stage from the atmospheric concentration reported (Figures 2, 4 and 5). The Sydney and Tucson sites are included for comparison. The smelting sites are clearly enriched in Pb and As when compared to urban samples of Sydney and Tucson.
Table 1.
Maximum mass concentration (ppm) found for any individual MOUDI impactor stage of As and Pb for field site comparison. Hayden, Port Pirie, and Mount Isa include smelting operations; Sydney, NSW AUS and Tucson, AZ USA represent an urban sample.
| Hayden | Port Pirie | Mount Isa | Sydney | Tucson | |
|---|---|---|---|---|---|
| As (ppm) | 8,622 | 2,471 | 895 | 215 | 63 |
| Pb (ppm) | 13,173 | 36,399 | 8,922 | 148 | 302 |
4. Conclusion
To our knowledge, this study is the first comparative analysis of the size-resolved concentrations of metal and metalloids around multiple smelting sites. Results in this study show the potential of smelting operations to affect nearby ecology and human health. Mine tailings alone can be a source of contaminants when present at high concentrations, but contaminants reside primarily in the coarse size fraction that will not travel as far in the environment. However, smelting emissions can have a much broader area of impact due to the fine size of the contaminated particles. In addition, when inhalation is a route of exposure for contaminated aerosols, fine particles are respired to the lungs which are thereby transferred to the blood stream via macrophages while coarse particles are removed by the upper respiratory tract and expelled through the digestive tract leading to lower associated dose when compared to lung deposition. The smaller size fraction also has a higher bioavailability due to higher surface to volume ratio.
Acknowledgments
This work was supported by grant number P42 ES04940 from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH). The views of authors do not necessarily represent those of the NIEHS, NIH. The authors would also like to thank the Endeavour Research Fellowship Program through the Australian Government's Department of Industry, Innovation, Science, Research and Tertiary Education who supported J. Csavina's travel and stay in Australia while performing research in Port Pirie, SA, and Mount Isa, QLD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baghurst PA, McMichael AJ, et al. Environmental exposure to lead and children's intelligence at the age of seven years. The Port Pirie cohort study. New England J of Med. 1992;327:1279–1284. doi: 10.1056/NEJM199210293271805. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Lead neurotoxicity and socioeconomic status: Conceptual and analytical issues. Neurotoxicol. 2008;29:828–832. doi: 10.1016/j.neuro.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benin AL, Sargent JD, et al. High concentrations of heavy metals in neighborhoods near ore smelters in northern Mexico. Environ Health Perspect. 1999;107:279–284. doi: 10.1289/ehp.99107279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouse RM, Ruiz J, Titley S, et al. Lead isotope compositions of late Cretaceous and early Tertiary igneous rocks and sulfide minerals in Arizona: Implications for the sources of plutons and metals in porphyry copper deposits. Econ Geol Bull Soc Econ Geol. 1999;94:211–214. [Google Scholar]
- Breshears DD, Kirchner TB, et al. Modeling aeolian transport in response to succession, disturbance and future climate: Dynamic long-term risk assessment for contaminant redistribution. Aeol Res. 2012;3:445–457. [Google Scholar]
- Cabada JC, Rees S, et al. Mass size distributions and size resolved chemical composition of fine particulate matter at the Pittsburgh supersite. Atmos Environ. 2004;38:3127–3141. [Google Scholar]
- Calderón J, Navarro M, et al. Exposure to arsenic and lead and neuropsychological development in Mexican children. Environ Res. 2001;85:69–76. doi: 10.1006/enrs.2000.4106. [DOI] [PubMed] [Google Scholar]
- Chen J, Tan M, et al. Characteristics of trace elements and lead isotope ratios in PM(2.5) from four sites in Shanghai. J Hazard Mater. 2008;156:1–3. doi: 10.1016/j.jhazmat.2007.11.122. [DOI] [PubMed] [Google Scholar]
- Csavina J, Field J, et al. A review on the importance of metals and metalloids in atmospheric dust and aerosol from mining operations. Sci Total Environ. 2012;433:58–73. doi: 10.1016/j.scitotenv.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csavina J, Landázuri A, et al. Metal and metalloid contaminants in atmospheric aerosols from mining operations. Water Air Soil Poll. 2011;221:145–157. doi: 10.1007/s11270-011-0777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming GL, Richards JR. Ore lead isotope ratios in a continuously changing earth. Earth Planet Sci Lett. 1975;28:155–71. [Google Scholar]
- Department of Sustainability; Environment; Water; Population and Communities. [accessed 2011];National Pollutant Inventory. 2011 http://www.npi.gov.au.
- Erel Y, Veron A, Halicz L. Tracing the transport of anthropogenic lead in the atmosphere and in soils using isotopic ratios. Geochim Cosmochim Acta. 1997;61:3193–3204. [Google Scholar]
- Grousset FE, Quetel CR, et al. Transient Pb isotopic signatures in the western European atmosphere. Environ Sci Technol. 1994;28:1605–1608. doi: 10.1021/es00058a011. [DOI] [PubMed] [Google Scholar]
- Harper SL, Walling JF, et al. Simplex optimization of multielement ultrasonic extraction of atmospheric particulates. Anal Chem. 1983;55:1553–1557. [Google Scholar]
- Hinds WC. Aerosol Science and Technology. New York: John Wiley & Sons; 1999. [Google Scholar]
- Hwang YH, Bornschein RL, et al. Environmental arsenic exposure of children around a former copper smelter site. Environ Res. 1997;72:72–81. doi: 10.1006/enrs.1996.3691. [DOI] [PubMed] [Google Scholar]
- Jackson C, Abbot P. Keeping it South Australian - Angas base metals mine sends concentrates to Port Pirie smelter for high-end processing. MESA J. 2008;50:11–13. [Google Scholar]
- Jusko TA, Henderson CR, et al. Blood lead concentrations < 10 μu g/dL and child intelligence at 6 years of age. Environ Health Perspect. 2008;116:243–248. doi: 10.1289/ehp.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komárek M, Ettler V, et al. Lead isotopes in environmental sciences: A review. Environ Int. 2008;34:562–77. doi: 10.1016/j.envint.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Krombach F, Munzing S, et al. Cell size of alveolar macrophages: An interspecies comparison. Environ Health Perspect. 1997;105:1261–1263. doi: 10.1289/ehp.97105s51261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay AK, Taylor MP, et al. Identification of environmental lead sources and pathways in a mining and smelting town: Mount Isa, Australia. Environ Poll. 2013;180:304–311. doi: 10.1016/j.envpol.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Marple VA, Rubow KL, et al. A microorifice uniform deposit impactor (MOUDI) - description, calibration, and use. Aerosol Sci Technol. 1991;14:434–446. [Google Scholar]
- Mukai H, Machida T, et al. Lead isotope ratios in the urban air of eastern and central. Russia Atmos Environ. 2001;35:2783–93. [Google Scholar]
- Munksgaard NC, Taylor MP, et al. Recognising and responding to the obvious: the source of lead pollution at Mount Isa and the likely health impacts. Med J Australia. 2010;193:131–132. doi: 10.5694/j.1326-5377.2010.tb03827.x. [DOI] [PubMed] [Google Scholar]
- Park SS, Wexlerm AS. Size-dependent deposition of particles in the human lung at steady-state breathing. J Aerosol Sci. 2008;39:266–276. [Google Scholar]
- Queensland Health. Mount Isa Community Lead Screening Program 2006-2007: A Report into the Results of a Blood-lead Screening Program of 1-4 year Old Children in Mount Isa, Queensland, Environmental Health Services of the Tropical Population Health Network, Northern Area Health Service, Queensland Health. [accessed 2011];2008 http://www.health.qld.gov.au/ph/documents/tphn/mtisa_leadrpt.pdf.
- Reuer MK, Weiss DJ. Anthropogenic lead dynamics in the terrestrial and marine environment. Phil Trans Roy Soc London A. 2002;360:2889–2904. doi: 10.1098/rsta.2002.1095. [DOI] [PubMed] [Google Scholar]
- Seinfeld JH, Pandis SN. From air pollution to climate change. New York: Wiley; 2006. Atmospheric chemistry and physics. [Google Scholar]
- Simon D, Lewis C. Analysis of blood lead levels for 2010. [accessed 2012];Technical Paper 2010/4. 2010 http://www.sahealth.sa.gov.au/wps/wcm/connect/9b121c8046aaf0b999acfb2e504170d4/Technical+paper+Pt+Pirie+lead+2010-4-PHCC-20110427.pdf?MOD=AJPERES&CACHEID=9b121c8046aaf0b999acfb2e504170d4.
- Simon DL, Maynard EJ, et al. Living in a sea of lead-changes in blood-and hand lead of infants living near a smelter. J Exposure Anal Environ Epidemiol. 2007;17:248–259. doi: 10.1038/sj.jes.7500512. [DOI] [PubMed] [Google Scholar]
- Soto-Jimenez MF, Flegal AR. Childhood lead poisoning from the smelter in Torreon, Mexico. Environ Res. 2011;111:590–596. doi: 10.1016/j.envres.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Mitchell R, editor. South Australia Environmental Protection Agency. Ambient Air Monitoring Data for Port Pirie. South Australia Air and Noise Branch / Science and Assessment Division; 2012. [Google Scholar]
- Spear TM, Svee W, et al. Chemical speciation of lead dust associated with primary lead smelting. Environ Health Perspect. 1998;106:565–571. doi: 10.1289/ehp.98106565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey JS, Kramers JD. Approximation of terrestrial lead isotope evolution by a two-stage model. Earth Planet Sci Lett. 1975;26:207e221. [Google Scholar]
- Sturges WT, Barrie LA. Lead 206/207 isotope ratios in the atmosphere of North America as tracers of US and Canadian emissions. Nature. 329:144–146. [Google Scholar]
- Taylor MP, Mackay AK, et al. Soil Cd, Cu, Pb and Zn contaminants around Mount Isa city, Queensland, Australia: Potential sources and risks to human health. Appl Geochem. 2010;25:841–855. [Google Scholar]
- US Environmental Protection Agency. Pacific Northwest, Region 9: Superfund: Site Overviews: ASARCO Hayden Plant 2008 [Google Scholar]
- Valiulis D, Sakalys J, et al. Heavy metal penetration into the human respiratory tract in Vilnius. Lithuanian J Physics. 2008;48:349–355. [Google Scholar]
- World Health Organisation (WHO) Air Quality Guidelines for Europe. WHO Regional Publications; 2000. European Series. [PubMed] [Google Scholar]
- Wright JP, Dietrich KN, et al. Association of prenatal and childhood blood lead concentrations with criminal arrests in early adulthood. Plos Medicine. 2008;5:732–740. doi: 10.1371/journal.pmed.0050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RO, Amarasiriwardena C, et al. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicol. 2006;27:210–216. doi: 10.1016/j.neuro.2005.10.001. [DOI] [PubMed] [Google Scholar]