Abstract
The goal of this study was to determine whether a combination of local tumor irradiation and autologous T-cell transplantation can effectively treat metastatic 4T1 breast cancer in mice. BALB/c mice were injected subcutaneously with luciferase-labeled 4T1 breast tumor cells and allowed to grow for 21 days, at which time metastases appeared in the lungs. Primary tumors were treated at that time with 3 daily fractions of 20 Gy of radiation each. Although this approach could eradicate primary tumors, tumors in the lungs grew progressively. We attempted to improve efficacy of the radiation by adding autologous T-cell infusions. Accordingly, T cells were purified from the spleens of tumor-bearing mice after completion of irradiation and cryopreserved. Cyclophosphamide was administered thereafter to induce lympho-depletion, followed by T-cell infusion. Although the addition of cyclophosphamide to irradiation did not improve survival or reduce tumor progression, the combination of radiation, cyclophosphamide and autologous T-cell infusion induced durable remissions and markedly improved survival. We conclude that the combination of radiation and autologous T-cell infusion is an effective treatment for metastatic 4T1 breast cancer.
INTRODUCTION
The ability of radiation to induce remission of tumors is dependent on the injury or death of tumor cells themselves and/or the stromal and vascular cells within the tumors (1–3). A combination of DNA damage, activation of apoptosis and production of reactive oxygen species contribute to tumor remissions (1–3). In addition, radiation can be used to enhance systemic T-cell antitumor immunity that can improve therapeutic efficacy (4–23). Recent studies have shown that the ability of a single dose of radiation (20 Gy) to slow the growth of primary melanoma tumors is dependent on immune cells, since the slowing observed in wild-type mice failed to occur in immunodeficient nude mice, and slowing was abrogated by depleting the CD8+ T cells of the tumor-bearing mice with monoclonal antibodies (4, 5). Multiple smaller doses of radiation instead of the single dose were ineffective in slowing tumor growth, and chemotherapy administered after the single dose interfered with tumor regression and the associated immune response (4). Additional studies showed that radiation exposure increased tumor immunogenicity, stimulated antigen-presenting cells and promoted migration and entry of T cells into tumors (6–23).
Tumor irradiation has been combined with immunotherapy such as transduction of tumor cells with DNA-encoding immunogenic peptides, stimulatory ligands or chemokines (4, 5). The combined approach, which includes injections of dendritic cells, Flt-3 ligand or anti-CTLA4 monoclonal antibodies after radiotherapy, has been shown to induce systemic immunity in mice such that tumor growth at distant sites is slowed (12–17). Durable complete remissions with weakly immunogenic tumors were not achieved unless the tumors were small (<1 cm) and nonmetastatic (12–17).
Advances in the use of confocal radiation beams that are targeted to a tumor in 3 dimensions minimize irradiation to adjacent normal tissues [stereotactic body radiation therapy (SBRT)] and allow for administration of single doses as high as 30 Gy or up to 3 daily doses of 20 Gy each for a total of 60 Gy (24, 25). The efficacy of SBRT to induce solid tumor remission has been shown to be superior to that of fractionated irradiation with multiple small doses administered over several weeks (24, 25).
In the current study, we compared the efficacy of high-dose hypofractionated irradiation (3 × 20 Gy) alone to the combination of irradiation and autologous T-cell infusion for the treatment of metastatic 4T1 breast tumors in mice. Previous studies have shown that infusion of autologous T cells expanded ex vivo from tumor-infiltrating cells (TILs) or transfected with DNA constructs that encode T-cell antigen receptors that recognize tumor antigens can induce complete remission in patients with melanoma and lymphoid leukemias (26–28). The T-cell infusions were most effective after conditioning with lymphodepletive agents (26–28). In addition, the antitumor activity of vaccination with irradiated mouse colon tumor cells and adjuvant is markedly enhanced by autologous T-cell infusion after lymphodepletive total-body irradiation (29). The results of the current study show that the combination of local tumor irradiation and autologous T-cell infusion after lymphodepletion is more effective than irradiation alone.
MATERIALS AND METHODS
Animals
BALB/c (H-2d) wild-type female mice were purchased from Jackson Laboratories (Bar Harbor, ME). The Stanford University Committee on Animal Welfare (Administration Panel of Laboratory Animal Care) approved all mouse protocols used in this study.
Cell Lines
The 4T1 cell line was obtained from ATCC®. The 4T1-LUC/GFP cell line was lentivirally transduced (30–32).
Irradiation
Irradiation was performed with a Philips X-ray unit (200 kV, 10 mA; Philips Electronic Instruments Inc., Rahway, NJ) at a rate of 84 cGy/min with a 0.5 mm copper filter. For local tumor irradiation (LTI), unanesthetized mice were placed in lead jigs through which established tumors in the hindquarter were protruded for irradiation to an area of approximately 2 cm diameter (33).
Cell Preparation, Splenectomy and Collection of T Cells for Autologous Transplantation
Single cell suspensions from the spleen were enriched for T cells using anti-Thy1.2 columns according to previously described procedures (29). Collected T cells were cryopreserved with 10% dimethyl sulfoxide (DMSO) and frozen in liquid nitrogen.
In Vivo Bioluminescence Imaging (BLI)
In vivo BLI was performed according to the method of Edinger et al. (32).
RESULTS
Local Tumor Irradiation Alone or in Combination with Cyclophosphamide Fails to Eradicate Distant 4T1 Metastases
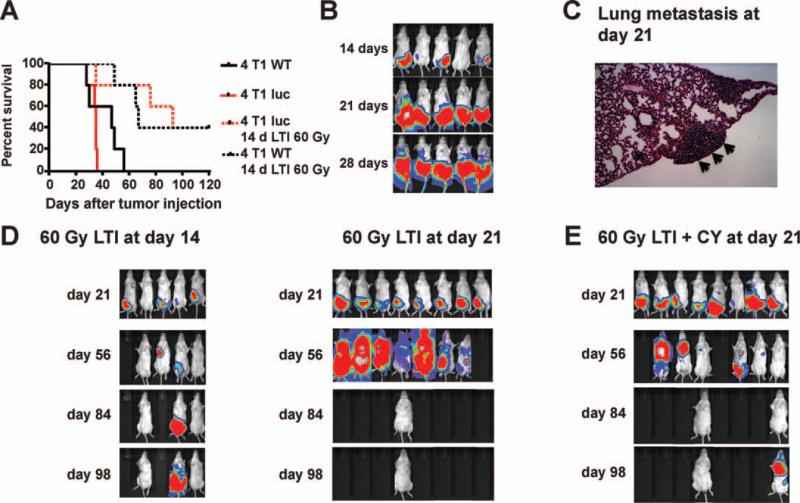
We studied the effect of high-dose irradiation on 4T1 breast cancer that metastasizes to the lungs after subcutaneous injection in BALB/c mice (34, 35). Figure 1A shows the growth of the 4T1 tumor transduced with the luciferase gene using BLI after subcutaneous injection into the right hindquarter. All mice died of tumor progression within 55 days whether or not tumor cells were transduced with the luciferase gene (Fig. 1A) and growth was similar to that reported for orthotopic injections (35). Both BLI signals above the diaphragm and histopathological analysis at day 21 showed that tumors had spread to the lungs by that time (Fig. 1B and C). The enlarged area of BLI signal below the diaphragm is associated with the growth of the primary tumor with subcutaneous local spread. When a single radiation dose of 30 Gy was administered to day 14 tumors, slowing of tumor growth was observed without development of complete remission (data not shown). However, 3 daily radiation doses of 20 Gy each resulted in complete remission in 2 out of 5 mice (Fig. 1A and D), and the mice survived for more than 100 days. After tumor remission, the irradiated skin area showed hair loss, scarring and contraction without ulceration.
FIG. 1.
Effect of local tumor irradiation alone or in combination with cyclophosphamide on growth of 4T1 breast tumors. Panel A: Survival of BALB/C mice given subcutaneous injections of 1 × 104 4T1 wild-type tumor cells and 4T1 luc+ tumor cells compared to survival of 4T1 WT or 4T1 luc+ tumor-bearing mice given 60 Gy (3 daily doses of 20 Gy each) local tumor irradiation (LTI) on days 14, 15 and 16. There were five mice in each group. Panel B: Bioluminescence imaging (BLI) of mice at serial time points after injection of 4T1 luc+ tumor cells. Note signals above diaphragm at days 21 and 28. Panel C: Representative tissue section (H&E, 1003 magnification) of lung showing tumor cell cluster (arrows) in untreated mouse. Panel D: BLI of mice given 4T1 luc+ tumor cells after 60 Gy (3 daily doses of 20 Gy each) local tumor irradiation on days 14, 15 and 16 or on days 21, 22 and 23. Blank areas indicate death of mice. Panel E: BLI of mice given 4T1 luc+ cells, 60 Gy (3 daily doses of 20 Gy each) local tumor irradiation and intraperitoneal injection of cyclophosphamide (500 mg/ kg) on day 23. There were 5–8 mice in each group in D and E.
In further studies, tumors were allowed to grow for 21 days before treatment. A single radiation dose of 30 Gy to primary tumors showed only modest slowing of local tumor growth, and had little impact on metastatic spread or survival (data not shown). When the subcutaneous tumors were irradiated with 3 daily doses of 20 Gy each, the primary tumors showed marked regression, but still spread to distant sites as shown by BLI. Seven of eight mice died by day 84, with 1 of 8 in remission (Fig. 1D). Next, we tried to augment radiotherapy with cyclophosphamide (CY) treatment. When a single intraperitoneal dose of 500 mg/kg of cyclophosphamide was administered after the third dose of local tumor irradiation, tumor growth slowed, but 6 of 8 mice died by day 84 and 1 relapsed at day 98 (Fig. 1E). This dose of cyclophosphamide depleted T cells in the spleen almost eightfold in nontumor-bearing mice. The mean ± SE absolute number of T cells in spleens of untreated animals was 29 ± 5 × 106 and was reduced to 9 ± 2 × 106 and 3.9 ± 0.7 × 106 at days 3 and 7, respectively after cyclophosphamide injection.
Local Tumor Irradiation in Combination with Cyclophosphamide and Autologous T-Cell Infusion Can Eradicate Distant 4T1 Metastases
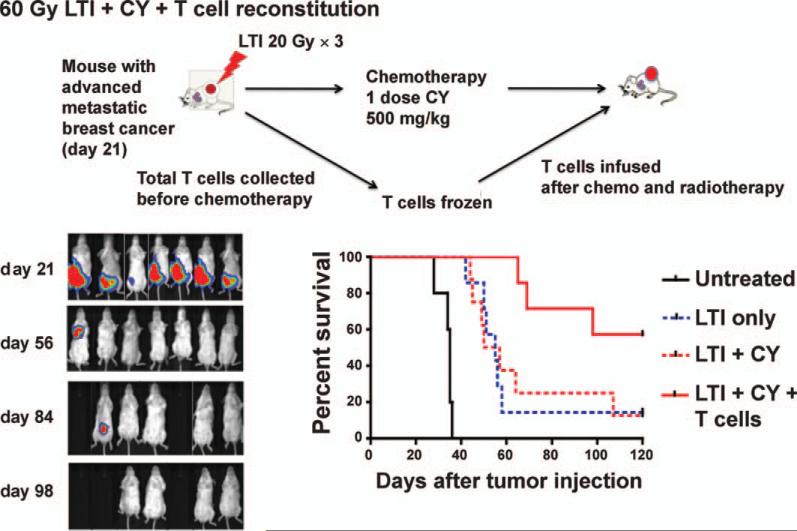
Based on the reported effect of lymphodepletive regimens to enhance T-cell immunity when given prior to T-cell infusion (27, 28), a group of tumor-bearing mice were treated with local tumor irradiation followed by collection of T cells within 24 h (Fig. 2). To collect total T cells, splenectomy was performed, and T cells were enriched using an anti-Thy1.2 mAb column and cryopreserved. Just after T-cell collection, the mice were given a single dose of 500 mg/kg cyclophosphamide. Forty-eight hours later, when the number of endogenous cells was declining, 2.5 × 106 T cells were thawed and infused intravenously. In this group, 4 of 7 mice showed no evidence of primary and metastatic tumor up to day 180. Survival of the group that had their local tumor irradiated, and were given cyclophosphamide and T cells was significantly increased according to the log rank test (P < 0.01) compared to the group that had their local tumor irradiated and were given cyclophosphamide without T cells (Fig. 2). Thus, the combination of local tumor irradiation, cyclophosphamide and T-cell therapy was more effective than either local tumor irradiation alone or local tumor irradiation in combination with cyclophosphamide.
FIG. 2.
T-cell therapy facilitates complete remissions of 4T1 metastases when used in combination with radiotherapy and chemotherapy. BALB/C mice were given 4T1 luc+ cells and local tumor irradiation and cyclophosphamide as described in Fig 1E and splenic Thy 1.2+ T cells were harvested just before cyclophosphamide injection on day 23. T cells were cryopreserved, then thawed and injected (2.5 × 106) intravenous 48 h after injection of cyclophosphamide. BLI at serial time points is shown, as well as survival of untreated and experimental groups. There were significant differences in survival between groups given local tumor irradiation alone or local tumor irradiation and cyclophosphamide vs. local tumor irradiation and cyclophosphamide and T cells (P < 0.05), but not between groups given local tumor irradiation and cyclophosphamide vs. local tumor irradiation alone (P > 0.05). There were 5–7 mice in each group.
T-Cell Subsets in Autologous Infusions
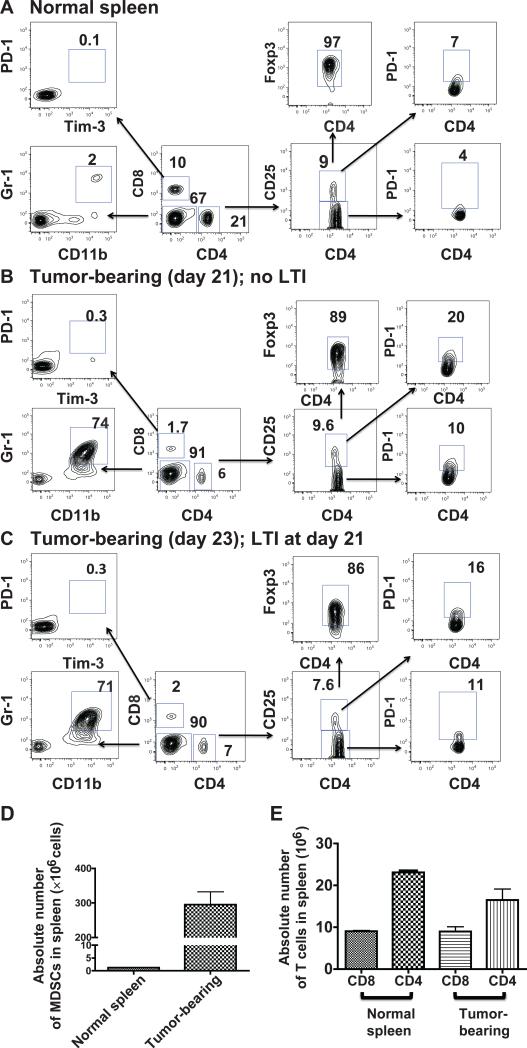
The T-cell subsets in the spleens of tumor bearing mice that were used for the autologous infusions were analyzed by immunofluorescent staining. Figure 3 compares the two-color flow cytometric analyses of spleen cells from normal mice, tumor-bearing mice at day 21 after tumor inoculation, and tumor-bearing mice immediately after 3 doses of local tumor irradiation on days 21, 22 and 23. Harvesting of T cells and enrichment using anti-Thy 1.2 columns were performed on day 23. Whereas the percentage of CD4+ and CD8+ T cells among mononuclear cells in the representative normal spleen was 21% and 10%, respectively (Fig. 3A), the percentage was reduced to about 7% and 2%, respectively in the unirradiated tumor-bearing spleen (Fig. 3B) or irradiated tumor-bearing spleen (Fig. 3C).
FIG. 3.
Analysis of T-cell subsets in spleens from normal and tumor-bearing mice. Panel A: Single cell suspensions were prepared from normal spleens and analyzed for expression of CD25, PD-1 and Tim-3 on CD4+ and CD8+ T cells and for MDSCs (CD11b+ Gr-11+). Percentages of each subset in boxes on representative two color analysis panels are shown and arrows identify gated subsets. Staining for CD11b, Gr-1, CD4 and CD8 used a mononuclear cell gate. Panel B shows the same analysis of spleens from tumor-bearing mice at day 21 after tumor induction. Panel C shows subset analysis just after local tumor irradiation 3 × 20 Gy (days 21, 22 and 23). Panel D: Absolute numbers of MDSCs in the spleens of normal and tumor-bearing (day 21) mice. Panel E: Absolute numbers of CD4+ and CD8+ T cells in normal and tumor-bearing mice (day 21).
The reduction in the percentage of CD4+ and CD8+ T cells was due to the increase in Gr-1+CD11b+ cells, from about 2% in normal mice to about 70% in the tumor-bearing mice (Fig. 3A–C). The latter cells have been identified previously as myeloid derived suppressor cells (MDSCs) (37). Figure 3D shows that on day 21 there was a massive infiltration of the MDSCs in the spleen, and the mean absolute number rose from about 2 × 106 cells per spleen to about 300 × 106 cells per spleen. Nevertheless, the mean absolute numbers of CD4+ and CD8+ T cells in the normal spleens and tumor-bearing spleens were similar to one another with about 9 × 106 CD8+ T cells and about 18–23 × 106 CD4+ T cells, respectively.
Among the CD4+ T cells, the percentages of CD4+CD25+ Tregs were similar in the normal, unirradiated tumor-bearing and irradiated tumor-bearing spleens (8–10%) (Fig. 3A–C). There was an increase in the percentage of PD-1+ cells among the CD4+CD25– cells, from about 4% in the normal spleen to about 10% in the tumor-bearing spleen. There was a somewhat larger increase in the percentage of PD-1+ cells among the CD4+CD25+ cells from about 7% to 16–20%. The percentage of CD8+ T cells that expressed the PD-1+ Tim-3+ phenotype, associated with “exhaustion” (38), was less than 1% in all three types of spleen cells. The percentage of NKT cells among T cells was similar in normal and tumor-bearing mice (data not shown). Thus, with the exception of the increase in PD-1 on CD4+CD25+ cells, the phenotypic profiles and absolute numbers of CD4+ and CD8+ T cells in tumor-bearing spleens were similar to normal.
DISCUSSION
We studied the 4T1 breast cancer model to determine whether high-dose tumor irradiation can effectively treat both primary tumors and metastases in wild-type mice. Subcutaneous injection of 4T1 tumor cells results in local tumor growth, followed by the development of metastases in the lungs (34, 35). The kinetics of this spread were confirmed in the current study by BLI using luciferase-labeled 4T1 tumor cells. Lung metastases were identified at day 21 by both imaging and histopathology. Accordingly, radiation was administered to the tumors starting on day 21. The 4T1 primary tumors were not controlled by a single radiation dose of 30 Gy, but rather required 3 daily radiation doses of 20 Gy each to induce complete remissions in day 14 tumors. However, even the 3 × 20 Gy tumor irradiation, which cured the primary tumors failed to control metastases in most mice and almost all mice died by day 84.
Since autologous T-cell infusion after lymphodepletive conditioning has been reported to be effective in treating melanoma (27, 28), we used this strategy to treat 4T1 metastases. We hypothesized that local tumor irradiation would activate T cells in the tumor and/or local lymph nodes, and that the activated T cells would disseminate in the lymphoid tissues. Immediately after tumor irradiation on days 21–23, T cells were harvested and cryopreserved. A single dose of cyclophosphamide was administered thereafter and the cryopreserved T cells were infused 2 days after the chemotherapy. The dose of cyclophosphamide chosen depleted cells in the lymphoid tissues up to eightfold. Cyclophosphamide can augment tumor immunity by altering the balance of dendritic cell subsets in the lymphoid tissues (36). In addition, our studies using the CT26 colon tumor growing in immunodeficient mice showed that neither local tumor irradiation alone nor local tumor irradiation and an infusion of syngeneic T cells could control tumor growth, but rather only the combination of local tumor irradiation plus cyclophosphamide and an infusion of T cells was very effective (unpublished results). Whereas 4T1 tumor growth and host survival were not significantly different after tumor irradiation and cyclophosphamide treatment versus tumor irradiation alone in the current study, infusion of autologous T cells after irradiation and cyclophosphamide significantly improved survival and resulted in the development of durable complete remissions.
In a previous study of the treatment of 4T1 tumors with a combination of tumor irradiation (12 or 40 Gy) on day 14 and immunotherapy with anti-CTLA4 antibody thereafter, there was a significant increase in survival of tumor-bearing mice compared to either modality alone, but there were no durable complete remissions (16, 17).
The combination of high-dose tumor irradiation followed by chemotherapy, plus the harvesting of T cells before chemotherapy for injection afterward used in the current study can be applied clinically. Although chemotherapy is likely to induce lymphopenia and/or suppress T-cell immunity induced by radiation, our cell therapy strategy circumvents this problem by restoring immunity after chemotherapy. All components of the latter procedure are in current clinical use including apheresis for harvesting of blood T cells after SBRT and before chemotherapy.
ACKNOWLEDGMENTS
This work was supported by grant number R01 CA163441 from the National Institutes of Health, from the Nishimura Fund for Cancer Research and the Hsiao Fund. We thank Dr. G. Fisher for advice, G. Letsinger for help with manuscript preparation and Dr. J. M. Brown for helpful discussions. Effort of GOA was funded in part by grant no. 2012M2B2B1055641 from the National Research Foundation of Korea.
REFERENCES
- 1.Prise KM, Schettino G, Folkard M, Held KD. New insights on cell death from radiation exposure. Lancet Oncol. 2005;6:520–8. doi: 10.1016/S1470-2045(05)70246-1. [DOI] [PubMed] [Google Scholar]
- 2.Tsai JH, Makonnen S, Feldman M, Sehgal CM, Maity A, Lee WM. Ionizing radiation inhibits tumor neovascularization by inducing ineffective angiogenesis. Cancer Biol Ther. 2005;4:1395–400. doi: 10.4161/cbt.4.12.2331. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds CP, Maurer BJ, Kolesnick RN. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004;206:169–80. doi: 10.1016/j.canlet.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–96. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–09. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 8.Liao YP, Wang CC, Butterfield LH, Economou JS, Ribas A, Meng WS, et al. Ionizing radiation affects human MART-1 melanoma antigen processing and presentation by dendritic cells. J Immunol. 2004;173:2462–9. doi: 10.4049/jimmunol.173.4.2462. [DOI] [PubMed] [Google Scholar]
- 9.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 10.Quarmby S, Hunter RD, Kumar S. Irradiation induced expression of CD31, ICAM-1 and VCAM-1 in human microvascular endothelial cells. Anticancer Res. 2000;20:3375–81. [PubMed] [Google Scholar]
- 11.Ganss R, Ryschich E, Klar E, Arnold B, Hammerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62:1462–7. [PubMed] [Google Scholar]
- 12.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10:718–26. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys. 2005;63:655–66. doi: 10.1016/j.ijrobp.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakravarty PK, Alfieri A, Thomas EK, Beri V, Tanaka KE, Vikram B, et al. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res. 1999;59:6028–32. [PubMed] [Google Scholar]
- 15.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–70. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–34. [PubMed] [Google Scholar]
- 17.Pilones KA, Kawashima N, Yang AM, Babb JS, Formenti SC, Demaria S. Invariant natural killer T cells regulate breast cancer response to radiation and CTLA-4 blockade. Clin Cancer Res. 2009;15:597–606. doi: 10.1158/1078-0432.CCR-08-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14:1848–50. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 19.Gasser S, Raulet DH. The DNA damage response arouses the immune system. Cancer Res. 2006;66:3959–62. doi: 10.1158/0008-5472.CAN-05-4603. [DOI] [PubMed] [Google Scholar]
- 20.Perez C, Hallahan DE, Geng L. Radiation induced immune response in melanoma. Intl J Radiat Biol. 2009;85:1126–36. doi: 10.3109/09553000903242099. [DOI] [PubMed] [Google Scholar]
- 21.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180:3132–9. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- 22.Mason KA, Ariga H, Neal R, Valdecanos D, Hunter N, Krieg AM, et al. Targeting toll-like receptor 9 with CpG oligodeoxynucleo-tides enhances tumor response to fractionated radiotherapy. Clin Cancer Res. 2005;11:361–9. [PubMed] [Google Scholar]
- 23.Milas L, Mason KA, Ariga H, Hunter N, Neal R, Valdecanas D, Krieg AM, Whisnant JK. CpG oligodeoxynucleotide enhances tumor response to radiation. Cancer Res. 2004;64:5074–7. doi: 10.1158/0008-5472.CAN-04-0926. [DOI] [PubMed] [Google Scholar]
- 24.Chang BK, Timmerman RD. Stereotactic body radiation therapy: a comprehensive review. Am J Clin Oncol. 2007;30:637–44. doi: 10.1097/COC.0b013e3180ca7cb1. [DOI] [PubMed] [Google Scholar]
- 25.Brown JM, Koong AC. High-dose single-fraction radiotherapy: exploiting a new biology? Int J Radiat Oncol Biol Phys. 2008;71:324–5. doi: 10.1016/j.ijrobp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Xue S, Gao L, Hart D, Gillmore R, Qasim W, Thrasher A, et al. Elimination of human leukemia cells in NOD/SCID mice by WT1-TCR gene-transduced human T cells. Blood. 2005;106:3062–7. doi: 10.1182/blood-2005-01-0146. [DOI] [PubMed] [Google Scholar]
- 27.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following nonmyeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filatenkov A, Müller AM, Tseng WW, Dejbakhsh-Jones S, Winer D, Luong R, et al. Ineffective vaccination against solid tumors can be enhanced by hematopoietic cell transplantation. J Immunol. 2009;183:7196–203. doi: 10.4049/jimmunol.0900159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breckpot K, Dullaers M, Bonehill A, van Meirvenne S, Heirman C, de Greef C, et al. Lentivirally transduced dendritic cells as a tool for cancer immunotherapy. J Gene Med. 2003;5:654–67. doi: 10.1002/jgm.400. [DOI] [PubMed] [Google Scholar]
- 31.Creusot RJ, Yaghoubi SS, Kodama K, Dang DN, Dang VH, Breckpot K, et al. Tissue-targeted therapy of autoimmune diabetes using dendritic cells transduced to express IL-4 in NOD mice. Clin Immunol. 2008;127:176–87. doi: 10.1016/j.clim.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edinger M, Cao YA, Verneris MR, Bachmann MH, Contag CH, Negrin RS. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood. 2003;101:640–8. doi: 10.1182/blood-2002-06-1751. [DOI] [PubMed] [Google Scholar]
- 33.Ahn GO, Brown MJ. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13:193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller FR, Miller BE, Heppner GH. Characterization of metastatic heterogeneity among subpopulations of a single mouse mammary tumor: heterogeneity in phenotypic stability. Invasion Metastasis. 1983;3:22–31. [PubMed] [Google Scholar]
- 35.Tao K, Fang M, Alroy J, Sahagian GG. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer. 2008;8:228–4. doi: 10.1186/1471-2407-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakahara T, Uchi H, Lesokhin AM, Avogadri F, Rizzuto GA, Hirschhorn-Cymerman D, et al. Cyclophosphamide enhances immunity by modulating the balance of dendritic cell subsets in lymphoid organs. Blood. 2010;115:4384–92. doi: 10.1182/blood-2009-11-251231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ochando JC, Chen SH. Myeloid-derived suppressor cells in transplantation and cancer. Immunol Res. 2012;54:275–85. doi: 10.1007/s12026-012-8335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–94. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]