Abstract
The renin-angiotensin system has powerful effects in control of the blood pressure and sodium homeostasis. These actions are coordinated through integrated actions in the kidney, cardio-vascular system and the central nervous system. Along with its impact on blood pressure, the renin-angiotensin system also influences a range of processes from inflammation and immune responses to longevity. Here, we review the actions of the “classical” renin-angiotensin system, whereby the substrate protein angiotensinogen is processed in a two-step reaction by renin and angiotensin converting enzyme, resulting in the sequential generation of angiotensin I and angiotensin II, the major biologically active renin-angiotensin system peptide, which exerts its actions via type 1 and type 2 angiotensin receptors. In recent years, several new enzymes, peptides, and receptors related to the renin-angiotensin system have been identified, manifesting a complexity that was previously unappreciated. While the functions of these alternative pathways will be reviewed elsewhere in this journal, our focus here is on the physiological role of components of the “classical” renin-angiotensin system, with an emphasis on new developments and modern concepts.
Introduction
The renin-angiotensin system (RAS) is one of the major control systems for blood pressure and fluid balance. The major biologically active hormone generated by this system, angiotensin (Ang) II, is produced by sequential cleavage of peptides derived from the substrate molecule angiotensinogen (Agt). Ang II binds to specific receptors, triggering a broad range of biological actions impacting virtually every system on the body including the brain, heart, kidney, vasculature, and immune system. But a primary function of the RAS is in circulatory homeostasis, protecting body fluid volumes, and abnormal activation of the RAS can contribute to the development of hypertension, cardiac hypertrophy, and heart failure. In this regard, pharmacological inhibitors of the synthesis or activity of Ang II have proven immensely useful in cardio-vascular therapeutics. For example, angiotensin converting enzyme (ACE) inhibitors are effective and widely used for the treatment of hypertension, congestive heart failure, and kidney diseases (26,65,145,154,155,207,208,391).
Here, we will review the physiology of the classical RAS, depicted in Figure 1, focusing on its role in the kidney. For simplicity, we have organized the manuscript around the individual components of the system from protein substrate to enzymes to receptors, to highlight the integrated functions of this complex system.
Figure 1.
Classical renin-angiotensin system (RAS). Through sequential cleavage of protein substrates by specific proteases, the multi-functional peptide hormone angiotensin II is generated by the “classical” RAS. The primary substrate for the RAS is angiotensinogen. While the liver is the primary source of angiotensinogen, it is also produced in other tissues including the kidney. Renin is an aspartyl-protease that catalyzes cleavage of the 10-amino acid peptide angiotensin I from the N-terminus of the angiotensinogen molecule. In a sequential reaction, the dicarboxyl-peptidase angiotensin converting enzyme (ACE) removes 2 amino acids from the C-terminus of angiotensin I to form angiotensin II. The biological actions of the “classical” RAS are executed through high affinity binding of angiotensin II to specific angiotensin receptors. These receptors belong to the large family of G-protein coupled receptors (GPCRs) and can be separated into two pharmacological classes, AT1 and AT2, each with distinct functions linked to specific intra-cellular signaling pathways.
Angiotensinogen
Angiotensinogen (Agt) is the only known substrate of renin which cleaves a 10 amino acid peptide from its N-terminus, Ang I, which is subsequently cleaved by ACE to form Ang II, the major biologically active peptide generated by the RAS (382). Agt was first cloned in 1983 from rat liver by Ohkubo et al. (268). The human angiotensinogen (AGT) gene is located on chromosome 1, whereas the mouse Agt gene is on chromosome 8. Agt homologues are present throughout vertebrates and there is an ortholog in fish and the shark Callorhinchus milii (88,348). While the C-terminal sequences encoding Ang I are conserved across vertebrates, there is variable homology in other domains of Agt (56), resulting in species specificity to the Agt-renin reaction. For example, human Agt cannot be cleaved by mouse renin and vice-versa (43).
Agt belongs to the superfamily of noninhibitory Serpin A8 proteins, which are a large and diverse superfamily of protease inhibitors and related proteins. The signature structural elements of serpins consist of three β sheets and 8 to 9 α helices (199). Zhou and colleagues recently resolved the structure of the Agt protein by x-ray crystallography (397). This report showed that the renin cleavage site which ultimately results in the liberation of the decapeptide, Ang I, buried within the N-terminal tail of this large protein (397). When Agt is oxidized, there is a conformational change permitting access and cleavage by renin releasing Ang I as shown in Figure 2. As such, renin has a fourfold higher catalytic activity for Ang I formation when Agt is oxidized compared to the reduced form of Agt.
Figure 2.
Angiotensinogen and its complex with renin (used with permission from Zhou et al. Nature 468: 108-111, 2010). (A) Stereo image of human angiotensinogen. Serpin template in grey and helix A in purple with the A-sheet in brown, the unresolved reactive loop in red, and in dark purple the CD loop containing Cys 138. The amino-tail is in blue with the new helix A1 and a second helix A2 containing Cys18 (linked in brown to Cys 138); the terminal angiotensin I segment is in green with the renin-cleavage site shown as green and blue balls. The sequence below (same color coding) also indicates the subsequent cleavage by angiotensin converting enzyme (ACE) releasing the octapeptide angiotensin II. (B) the initiating complex formed by angiotensinogen with inactivated (Asp292Ala) renin (left), and on right superimposed on the unreacted form (brown) showing the displacement of the CD loop and the movement of the aminoterminal peptide (visible to Cys 18), into the active cleft of renin.
Studies from mice with genetic ablation of the Agt gene have provided valuable insights into the physiological functions of Agt. Mice completely deficient in Agt have a characteristic phenotype characterized by increased perinatal mortality, profound hypotension, and abnormalities of the kidney including hydronephrosis, hypertrophic lesions of renal arteries and arterioles, and an impaired ability to concentrate urine (173, 255). This phenotype is virtually identical to that of mice with deficiencies of ACE, renin, or combined deletion of type 1a angiotensin (AT1A) and type 1b (AT1B) receptors (186, 274, 362, 387) indicating that the major physiological role of Agt is its contribution to the generation of Ang II.
Agt is synthesized by hepatocytes and is secreted into the circulation after removal of the 33-amino acid signal peptide. The half-life of Agt in the plasma has been estimated to be ~5 h from iodinated-tracer studies (133, 206). However, it has yet to be defined exactly how much intact Agt versus Agt degradation product [the so-called des-(Ang I)-Agt] remain in the circulation. As such, the relative proportions of intact Agt versus des-(Ang I)-Agt in the circulation is unknown. Furthermore, it has been suggested that des-(Ang I)-Agt may have physiologic functions, including a role in angiogenesis (43). However, evidence of a physiological role for des-(Ang I)-Agt has not been apparent from studies of Agt knockout mice.
While the liver seems to be the major source of Agt in the circulation, other tissues have been reported to synthesize Agt including adipose tissue, brain, spinal cord, heart, kidney, lung, adrenal gland, large intestine, stomach, spleen, ovaries, and blood vessels (32, 37, 38). Furthermore, it has been suggested that independent regulation of levels of Agt in individual tissue compartments may form the basis of local or tissue RAS operating independently of the circulatory RAS (31, 151, 258, 269). Aspects of the nonclassical RAS is discussed in more detail in Chappell et al. (44).
Synthesis of Agt in proximal tubule of the kidney may be augmented by the chronic administration of Ang II (179), whereas high levels of Ang II inhibit the circulating RAS by suppressing release of renin at the juxtaglomerular (JG) apparatus. This has been taken as evidence of an intrarenal RAS that is regulated independently of the systemic RAS with a “feed-forward” effect impacting the function of epithelial cells along the nephron to enhance sodium reabsorption and hypertension (180). Furthermore, it has been suggested that Agt in urine primarily reflects secretion of Agt protein synthesized by proximal tubule epithelia and thus urinary Agt could be a useful biomarker for monitoring activation of the intrarenal RAS. In this regard, several studies have correlated increases in urinary excretion of Agt with hypertension and some forms of chronic kidney disease including diabetic nephropathy (168,179,182,241).
On the other hand, studies by Matsusaka and colleagues using, cell-specific gene targeting, have suggested that the liver is the primary source of Agt protein accumulating in the mouse kidney, at least in the absence of any induced disease (233). These investigators showed that specific deletion of Agt from the hepatocytes markedly reduced Agt and Ang II levels in the kidney, but did not affect urinary excretion of Agt. On the other hand, while deletion of Agt from the proximal tubule was associated with reduced urinary Agt excretion, it had no effect on renal Agt or Ang II levels. Moreover, disruption of the glomerular filtration barrier increased both tubular and urinary Agt (233). These findings suggest that under normal circumstances, most Agt and Ang II in the kidney are derived from liver whereas Agt in urine comes primarily from proximal tubule epithelia. Indeed, Nakano et al. demonstrated by multiphoton imaging that the vast majority of urinary Agt originates from the tubules rather than glomerular filtration (257). If these characteristics are modified by hypertension or kidney disease and whether there are functional differences in the utilization of Agt in the urinary compartment compared to the rest of the kidney is not clear.
A variant of the human AGT gene resulting in a methionine substitution for threonine at position 235 (M235T) has been associated with increased plasma Agt levels and with the development of hypertension (161). Interestingly, the threonine 235 is portion of the AGT sequence which is not conserved across phylogeny, and is distinct from the Ang sequences. As such, it was not clear how variation of this amino acid would affect Agt levels. A potential explanation was established when the M235T variant was found to be in tight linkage disequilibrium with another variant in the 5 untranslated region of the AGT gene (152). This second variant, a single nucleotide substitution in the promoter of the AGT gene, was associated with increased transcriptional activity of the gene (152). In humans, plasma concentrations of Agt are typically near the Michaelis constant (Km) for renin (110) so that changes in plasma concentration could have significant influence on the rate of Ang I generation at any given level of renin. Furthermore, gene titration studies in transgenic mice engineered to carry from 0 to 4 copies of the Agt gene showed an almost linear relationship between the number of Agt gene copies, plasma levels of Agt, and blood pressure, consistent with the proposed mechanism of action of the human mutation (173).
Estrogens induce AGT gene transcription in the liver (221). Plasma Agt levels increase during pregnancy and during administration of synthetic estrogens such as oral contraceptive pills (74). Furthermore, the M235T variant of the AGT gene has been associated with preeclampsia (375) and Zhou and colleagues demonstrated that patients with preeclampsia had higher circulating levels of the oxidized form of Agt in their plasma (397). In normal individuals, the ratio of reduced:oxidized Agt is 40:60. In preeclampsia this ratio falls to 30:70. As discussed above, since oxidized Agt may be a more efficient substrate for renin, both of these studies suggest that altered levels or activity of Agt might contribute to blood pressure elevation in preeclampsia.
Renin
Renin is an aspartyl protease that catalyzes the first step in the activation of the RAS. Active renin specifically cleaves the 10 amino acids from the N-terminus of Agt to form Ang I. In humans, there is an excess of Agt in serum. Likewise, ACE is ubiquitous in the endothelium and plasma (288). Accordingly, the amount of renin in the bloodstream is a key rate-limiting step determining the level of Ang II and thus the activity of the RAS. The primary source of renin in the circulation is the kidney, where its expression and secretion are tightly regulated at the JG apparatus by two distinct mechanisms: a renal baroreceptor (24, 33) and sodium chloride (NaCl) delivery to the macula densa (13, 216, 217). Through these sensing mechanisms, levels of renin in plasma can be incrementally titrated in response to changes in blood pressure and salt balance. These regulatory principles provide a basis for many of the physiological characteristics of the RAS as discussed by Kanwar et al. in the Handbook of Physiology (169).
Here, we will provide an overview of renin gene structure and expression, as well as summarize the biochemical and physiological mechanisms regulating renin expression and release in the developing and adult kidney.
Renin gene
The renin gene is highly conserved, and homologs have been identified in multiple species such as human, mice, rats, dogs, and zebra fish. Its structure is similar to that of pepsinogen, a related aspartyl protease, suggesting a common evolutionary origin (135). The human renin gene spans 12 kb of DNA on chromosome 1 and contains 10 exons separated by 9 introns (124). The transcript encodes a protein with 406 amino acids, including a pre- and a pro-segment carrying 20-23 and 43-47 amino acids, respectively (8, 149, 243). Removal of the 23 amino acid residues from the C-terminus of pre-pro-renin generates pro-renin. Active renin is generated by removal of the N-terminal peptide pro-renin fragment, presumably by proteases in the JG cells of the kidney (142). Whether intact pro-renin has a distinct physiological role remains to be determined; however, there is evidence suggesting specific contributions of the pro-renin molecule in some normal and disease states (266, 379), including evidence that pro-renin binds to and activates a specific pro-renin receptor (266).
Humans have a single renin gene located on chromosome 1. Mice, on the other hand, may have one or two renin genes depending on the strain. Mouse strains with a single renin gene have a conserved allele, Ren1c, whereas mouse strains with two renin genes have a slightly modified variant of the Ren1 allele, Ren1d, with ~99% homology to Ren1c, along with the second renin gene, Ren2, with ~97% homology to the Ren1 allele. In contrast to Ren1, which is mainly expressed in the kidney, Ren2 is also highly expressed in the submandibular gland (87). Initial reports have indicated that mice with two renin genes have differences in their plasma renin levels and blood pressure compared to mice with one renin gene (220). However, follow-up studies demonstrated that there were no differences in renin expression between strains with one or two genes (122). In an effort to determine the physiological significance of the Ren1 and Ren2 genes, Mullins and colleagues have ablated the Ren1d and Ren2 genes in mice (55, 332). Their data show that while ablation of Ren2 leads to viable and healthy mice (332), ablation of Ren1d results in altered morphology of the macula densa of the kidney distal tubule and complete absence of JG cell granulation (55).
In the rat, the renin gene is located in chromosome 13, with ~80% homology to the mouse and ~68% homology to human (96). Similar to the mouse gene, it contains 9 exons separated by 8 intervening sequences. Inbred Dahl salt hypertension sensitive (S) and inbred Dahl salt hypertension resistant (R) rats exhibit significant polymorphism in their renin genes, which have been suggested to contribute to their differences in blood pressure (372). Similar to the mouse, ablation of the renin gene in rats results in abnormal kidney morphology such as a rudimentary inner medulla, cortical interstitial fibrosis, thickening of arterial walls, and abnormally shaped glomeruli (244).
Regulation of renin
Because renin level is a key rate-limiting step in determining Ang II levels, factors regulating expression and secretion of renin have the potential to significantly impact overall activity of the RAS. Accordingly, the following sections summarize the factors controlling renin expression and release.
Renin expression during kidney development
During mouse development, renin is first detected at embyronic day 14.5 in a few scattered cells in the developing kidney (162). By embryonic day 15.5, renin is detected in the developing renal artery, interlobar arteries, and arcuate arteries of the metanephric kidney (262). Yet, lineage tracing studies using the Ren1d promoter have suggested that renin producing cells originating from mesenchymal precursors can be identified around embryonic day 11.5 days before vessel development (331). As development progresses, renin expression is found in newly developed afferent arterioles while it disappears from interlobular arteries around the time of birth (315). At all stages, renin is expressed in cells that are insulated from the inner media layer of the renal vessels (315). After birth, renin expression gradually becomes progressively restricted to the terminal portion of the afferent arterioles in the JG area. This highly plastic pattern of renin expression seems to be common during development in all mammals; however, the mechanisms regulating this switching on and off of renin expression remains poorly understood. A detailed discussion of kidney development during embryogenesis is discussed by Spitzer et al. in the Handbook of Physiology (343). Some studies have shown correlation between embryonic expression of renin and sympathetic innervation of the kidney (302), along with expression of transforming growth factor β type II receptor suggesting a possible role in regulating renin expression during development (213). In addition, genetic disruption of adrenergic receptors reduces renin expression along the developing glomerulus implicating signaling though β-adrenergic receptors (264). Similarly, deletion of the Gsα protein, a key receptor mediating the intracellular signaling of cAMP, abolishes renin expression in the developing kidney; a phenotype accompanied by aberrant formation of the preglomerular arterial tree (262).
Renin expression in the adult kidney
In the adult kidney, renin is predominantly expressed in specialized cells in the JG area termed JG cells. These cells have an epithelial-like shape and are located in the media layer of afferent arterioles at the last branching point leading to the glomerular capillary network. Cells in the medial layer of efferent arterioles or extraglomerular mesangial cells rarely express renin. Low levels of renin have been detected in the renal proximal tubule, connecting tubule, and collecting duct (300,306,307).
Renin in the juxtaglomerular cell
Despite the importance of JG cells in regulating blood pressure and electrolyte homeostasis, characterization of their molecular and cellular properties are incomplete. Based on the presence of myofilaments in their cytoplasm, it was postulated that JG cells derive from smooth muscle lineages in the kidney (169, 350). However, later studies suggested that JG cells originate from metanephric mesenchyme, migrating and incorporating into the developing afferent arterioles of the embryonic kidney, but only later acquiring smooth muscle markers (330).
JG cells are mainly defined by their unique localization in the JG region of the renal afferent arteriole and their ability to synthesize and secrete renin (325, 350). They have a large nucleus, hypertrophic rough endoplasmic reticulum and distinct Golgi apparatus. JG cells carry two types of secretory granules: large electron-dense, mature granules containing active renin, Ang peptides, and cathepsins, along with smaller, electron-lucent proto-granules containing active renin and pro-renin (94). JG cells have a distinct transcriptional signature compared to other renal cell types, consisting of arterial, interstitial cell, and pericyte markers, as well genes associated with endocrine and contractile functions (27).
In the adult kidney, environmental stimuli, such as chronic ischemia, prolonged adrenergic activation, and sodium depletion, produce an increase of the number of cells expressing renin along the afferent arteriole, in the interstitium and inside the glomerulus, in a pattern partially recapitulating embryonic distribution of renin expression (105, 107, 328) (Figure 3). This process is known as JG recruitment (328). Although the exact cellular and genetic mechanisms of this phenomenon remain poorly understood, it potentially involves dedifferentiation of arteriolar smooth muscle cells (SMCs), mesangial cells and interstitial cells from the renin cell lineage, which then reacquire the renin phenotype. Recent studies using genetically labeled renin expressing cells have indicated that the reacquisition of renin expression by arteriolar SMCs is controlled by epigenetic/transcriptional mechanisms (106,290) as well as microRNAs (miRNAs) (237); small 22-nucleotide noncoding RNAs that regulate gene expression at the posttranscriptional level. Furthermore, recent data suggest that adult stromal progenitor cells derived from bone marrow and kidney can express renin and acquire JG-like characteristics upon stimulation with LXRα and/or cAMP (235, 371). Moreover, in vivo, sodium depletion results in activation of cells that express mesenchymal stem cell markers in the kidney suggesting that renal progenitor cells might contribute to JG recruitment (371).
Figure 3.
Renin expression (used with permission from Gomez et al. Kidney Int., 2009, 460-462). During embryonic development, renin-expressing cells (depicted above as yellow with black dots) can be found along the intrarenal arteries, the glomeruli and the interstitium. This pattern is progressively restricted and in the adult kidney renin can be only found in a few cells in the juxtaglomerular (JG) area. However, in response to various physiological stimuli such as severe sodium depletion, renal cells can reacquire renin expression (recruitment). In these cases, renin expression can be observed mainly in cells along the afferent arteriole as well as in the interstitium, the mesangium, glomerular capsule, and efferent arteriole.
Renin in the distal nephron
Although the JG area is the main localization of renin expression in the adult kidney, more recent studies suggest that under certain conditions, renin expression can also be detected in distal nephron segments (300, 307). For example, chronic Ang II infusion increases renin mRNA and protein levels in principal cells from connecting tubules and collecting ducts (300,307) and this up regulation is prevented by AT1 receptor blockade (301). By contrast, Ang II induced hypertension is accompanied by reduced levels of renin expression in the JG area. Additionally, renin expression in the distal nephron is also increased in experimental models of diabetes and chronic kidney disease (294). These effects are mediated via the PR91 receptor (also called SUCNR1) which binds succinate, a tricarboxylic acid cycle intermediate, that can rapidly accumulate in local tissues when energy supply and demand are out of balance. The PR91 receptor is expressed in the luminal membrane of macula densa cells of the JG apparatus in close proximity to renin-producing granular cells, the cortical thick ascending limb, as well as in the cortical and inner medullary collecting duct cells (306). These findings open the possibility of a new mechanism for local control of renin synthesis and release by endogenous metabolic intermediates (293).
Renin in mast cells
Mast cells can also express and secrete active renin (200,222, 336). Because of the ubiquity of mast cells, they provide a unique paradigm for understanding local renin-angiotensin systems in all tissues (336). Release of renin by cardiac mast cells can be induced by ischemia resulting in release of norepinephrine and generation of cardiac arrhythmias (222). Furthermore, kidney mast cells can also make chymase which can augment Ang II production and microvascular function in diabetes (286). Still the physiological importance of these mechanisms remains to be determined.
Processing and secretion of renin in JG cells
Renin is initially synthesized as a pre-pro-renin protein. After cleavage of the pre-fragment, pro-renin is transferred to the Golgi. From there, pro-renin can be immediately secreted by the constitutive pathway or sorted to the dense-core secretory granules for regulated exocytosis (94). It appears that release of pro-renin through the constitutive pathway depends directly on the levels of renin synthesis per se, including levels of transcription per cell and the total number of renin-producing cells (299). Acute stimulation of renin release involves exocytosis of mature renin secretory granules that contain active renin only; whereas chronic stimulation results in release of both pro-renin and renin into the circulation (359).
The sorting of proteins to dense core secretory granules for regulated exocytosis involves a number of different mechanisms allowing interaction of the granules with the cell membrane, enzymatic cleavage of pro-renin, modulation of the local pH or other factors favoring aggregation of granule material (324). The correct sorting of pro-renin to the regulated secretory pathway depends on the presence of a paired basic amino acid site in the pro-renin molecule, which serves as a protease-processing site. As cathepsins are co-localized with pro-renin in the secretory granules, it was suggested that these proteases might be responsible for the conversion of pro-renin to renin (113). However, renin processing was not impaired in cathepsin B deficient mice suggesting that cathepsin B is not essential for renin processing (239).
Studies in renin knockout mice suggest that glycosylation is crucial for pro-renin sorting into the dense core secretory granules. Ren1 and Ren2 proteins differ in their glycosylation patterns and studies using knock out mice for Ren1 or Ren2 gene show differences in their renin processing. Indeed, deletion of the Ren2 gene alone did not cause an apparent phenotype (332). In contrast, the kidneys in Ren1 deficient mice showed a very low number or complete absence of dense-core renin vesicles (55). It has been postulated that glycosylation differences may be responsible for these phenotypic differences (55).
Renin release also depends on polarization state of the membrane potential of the JG cell. Depolarization results in suppression of renin release whereas hyperpolarization increases the levels of renin exocytosis (40). In that context, inhibition of renin release by Ang II or release of renin stimulated by cAMP depends on changes in membrane potential mediated by K+ channels expressed by JG cells. JG cell membranes also contain NKCC1 and calcium (Ca2+)-activated chloride channels, which might also mediate inhibition of renin by Ang II and perhaps other vasoconstrictors. Increased intracellular free calcium, which typically triggers secretion in other secretory cells, inhibits renin secretion. This unique feature of renin secretion is commonly referred to as the “calcium paradox” (9,114).
Intracellular signals controlling renin release
As shown in Figure 4, renin expression and release at the cellular level is controlled by three main intracellular mediators: cAMP, cGMP, and Ca2+, which we will discuss individually below.
Figure 4.
Overview of the major signaling pathways involved in regulation of renin. Figure is reproduced, with permission, from Schnermann and Briggs, 2012; Kidney Int 81, 529-538. Details about the effects of each pathway (stimulatory or inhibitory) are described in the text. A1AR, A1 adenosine receptor subtype; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; eNOS, endothelial nitric oxide synthase; nNOS, neuronal nitric oxide synthase; NO, nitric acid; PACAP, pituitary adenylate cyclase-activating polypeptide; PDE3, phosphodiesterase 3; PGE2, prostaglandin E2.
The cyclic AMP pathway in renin release
cAMP is an important second messenger mediating intra-cellular signal transduction in several biological processes. Stimulation of seven transmembrane receptors coupled to Gs proteins triggers the activation of adenylyl cyclases, which in turn generate cAMP from adenosine triphosphate. cAMP then activates protein kinase A, which phosphorylates a number of other proteins such as transcription factors of the cAMP response element-binding protein/activating transcription factor-1 (CREB/ATF1) family, regulating gene expression and hormonal release (196). cAMP is degraded by phosphodiesterases, which therefore limit cAMP action. The cellular concentration of cAMP is determined by a balance between the rates of cAMP synthesis by adenylyl cyclases and cAMP degradation by phosphodiesterases (Fig. 4).
Abundant evidence indicates that cAMP is the main regulator of renin release. For example, direct activation of adenyl cyclase with forskolin triggers release of renin by JG cells (114). Similar effects are observed following exposure to cAMP analogs (92). Extracellular ligands, such prostaglandin E2 (PGE2), prostacyclin I2 (PGI2), and adrenergic hormones, acting via their respective Gs-coupled receptors in renin producing cells, stimulate adenyl cyclase activity, cAMP production and renin release. Accordingly, mice with specific genetic deletion of the Gsα gene in JG cells display lower plasma renin levels and reduced JG cell renin stores (Fig. 5) (47). Moreover, renin secretion in response to isoproterenol or PGE2 is similarly diminished in JG cells isolated from Gsα deficient mice (2, 47). Conversely, administration of selective inhibitors of the phosphodiesterases (PDE)-3 and PDE-4 increases renin secretion (50,51,191). Yet, the molecular pathways downstream of cyclic AMP stimulating renin secretion are not well defined and further research is required.
Figure 5.
Renin mRNA in kidney cortex and kidney medulla from Gsα-deficient mice. (Used with permission from Chen et al. Am J Physiol Renal Physiol 2007, F27-F37) RC/FF mice (n 6) relative to control animals (n 12; 9 RR/GG and 6 RR/FF) as determined by quantitative are given for comparisons between genotypes. PCR. Significances are given for comparisons between genotypes.
Calcium in renin release
While cAMP is the dominant second messenger for renin secretion, Ca2+ appears to modulate the integrated activities of the enzymes that balance cAMP synthesis and degradation (Fig. 4) (9). In contrast to cAMP, increased levels of intracellular Ca2+ inhibit renin release (114). For example, exposure of JG cells to agents such as 1,2-bis(o-aminophenoxy)ethane-N,N,N,N-tetraacetic acid, which deplete intracellular Ca2+ stores, stimulates renin release (276), whereas exposure to thapsigargan, which increases intracellular Ca2+ levels, blocks renin release (327). Moreover, several mediators with putative actions to inhibit renin release, such as vasopressin (193), Ang II (248), and endothelins (305), increase intracellular Ca2+ concentrations in JG cells. The inhibitory effect of Ca2+ on renin release appears to be mediated by protein kinase C (193). Schweda and colleagues have shown that Ca2+ can also block cAMP generation through Ca2+ inhibitable adenylyl-cyclase V (114).
Nitric oxide and the cyclic GMP pathway in regulation of renin
Increases in intracellular levels of cGMP in JG cells can be triggered by various stimuli such as nitric oxide (NO) and atrial natriuretic peptide (Fig. 4) (190). Depending on the circumstances, they can either inhibit (131) or stimulate renin release (322). NO stimulation in vivo consistently results in increased renin release (194), and the endothelial form of nitric oxide synthase (eNOS) is required for renin cell recruitment (263). It has been suggested that NO stimulates renin release through the formation of cGMP, which can inhibit PDEs, thereby attenuating cAMP hydrolysis (93,191). In parallel, cGMP can potentially suppress renin secretion through activation of cGMP regulated protein kinase type II (cGKII) and inhibition of renin by cGMP agonists is lost in mice with targeted disruption of cGKII (368). Levels of cGKII are subject to regulation in the JG cell (98), but the impact of this regulation on control of renin release has not been established.
Regulation of renin by microRNAs
Endogenous miRNAs, noncoding RNA species 18 to 22 nucleotides in length, play an important role in cell differentiation and homeostasis by modulating target gene expression (238). miRNAs are encoded within the genome and initially are transcribed as primary miRNA precursors (about 100-1000 nucleotides in length). These precursor transcripts are then released in the cytoplasm and processed by the sequential activity of the RNase III-type endonucleases Drosha and Dicer to produce mature miRNAs of 21 to 22 nucleotides in length (175). Sequeira-Lopez and associates showed that deletion of Dicer specifically in the renin cell lineage resulted in disappearance of renin-producing cells from the kidney, reduced renin expression, and hypotension (329), indicating a role for miRNAs in renin regulation (329). Subsequent work identified miR-330 and miR-125 as important determinants of JG cell identity (237). In humans, miR-663 and miR-181a are differentially expressed in kidneys of hypertensive males, and these miRNAs bind to the 5 untranslated region of the renin (REN) mRNA and regulate its expression (227). In addition, a number of miRNAs have been shown to regulate the expression of genes involved in β-cell exocytosis (218), but whether any of these miRNAs affect renin release is not clear (329).
Local control of renin release
As discussed above, renin-producing cells are localized to the distal part of the afferent arteriole, adjacent or in close proximity to macula densa. Physiological control for renin synthesis and release by these cells is regulated by three main pathways.
Renal perfusion pressure (renal baroreceptor)
Numerous studies have shown that renin secretion is inversely related to renal perfusion pressure or pulse amplitude (24,120,176,267,339). As such, the baroreceptor is an independent mechanism for controlling renin, residing within the kidney and clearly separate from regulation by the sympathetic nervous system (120). Yet, identification of its precise nature has been elusive. Various models have been proposed to explain the mechanism for pressure sensing and consequent signal transduction including direct stretch of the JG cells due to transmural pressure across the afferent arteriole (33,91) or indirect pathways involving secondary release of autocoids (277). Some of these candidate soluble factors include NO (178,191,292), and prostanoids (67,103), which are stimulatory, or endothelins, which are inhibitory (251). Gene targeting in the mouse has been utilized to examine the role of some of these mediators in the baroreceptor response. In one study, genetic deletion of eNOS had no effect on renin release in response to changes in renal perfusion pressure (10). On the other hand, the absence of the prostacyclin (IP) receptor, the single known receptor for PGI2 conferred substantial resistance to hypertension and hyperreninemia after unilateral renal artery stenosis (95). This suggests an absolute requirement for PGI2 in triggering renin release after baroreceptor activation, but a number of questions remain concerning the mechanism and cell lineages controlling synthesis of key mediators such as PGI2, and the cellular targets for these mediators affecting renin release (89).
The baroreceptor mechanism also appears to depend on extracellular Ca2+ concentration. For example, Kurtz and colleagues demonstrated that when the extracellular level of Ca2+ is lowered, the inhibitory effect of renal perfusion pressure on renin release is abolished (320). A potential mediator in this process may involve connexin proteins, which form gap junctions between JG cells and the adjacent endothelial cells. Disruption of connexin40 (Cx40) in the mouse, either through gene deletion or point mutation, results in hyperreninemia, hypertension, and loss of pressure control of renin release (185,219,367), similar to the effect observed when extracellular Ca2+ concentration is lowered (320). Yet, mice with renin cell-specific deletion of Cx40 maintain the ability to recruit additional renin producing cells in response to salt depletion and ACE inhibition, whereas this response is eliminated in mice with global deletion of C×40. These observations suggest that gap junction coupling is not required for recruitment of additional renin cells during homeostatic challenges, but it appears to be necessary for proper localization of these cells both under basal conditions and during prolonged renin stimulation (83).
NaCl reabsorption at the macula densa
The second major pathway for physiological regulation of renin is the macula densa mechanism whereby cells at the macula densa sense a reduction in chloride ions in the filtrate of the distal tubule, triggering renin release (216). In this circumstance, release of renin and the consequent generation of Ang II are believed to serve as a mechanism for enhancing renal sodium reabsorption in states of fluid volume depletion. The anatomical association of the macula densa with the JG apparatus stimulated the first speculation by Goormaghtigh of its physiological function (109). The macula densa is made up of specialized epithelial cells at the terminal portion of the thick ascending limb. Their basolateral membrane is in contact with glomerular mesangial cells, which, in turn, are contiguous with granular cells in the JG apparatus (13). The role of the macula densa in renin regulation was initially hypothesized by Vander in 1967 (340, 353, 363) and there is now general consensus that this mechanism provides a control of renin secretion that is directly determined by NaCl delivery to the distal nephron (340, 353). Moreover, several studies indicate that chloride flux through the NKCC2 regulates the signaling pathways linked to renin secretion (229). Increased chloride delivery to the macula densa inhibits, whereas reduced chloride delivery stimulates renin release (184,217,320).
In addition to the well-studied NKCC2 transporter, the sodium/hydrogen exchanger isoform 2 (NHE2) expressed on the apical surface of the macula densa also plays a role in renin release, perhaps through its effect on macula densa cell volume. A study by Peti-Peterdi and colleagues demonstrated that NHE2-deficient mice have significantly increased renal tissue renin activity and plasma renin concentration (121). These changes were associated with increased renal expression of cortical cyclo-oxygenase 2 (COX-2) and membrane-associated PGE synthase (mPGES), suggesting that the mechanisms responsible for increased renin levels are maculadensa specific, since these mice have been characterized to have normal blood pressure (201).
Several candidate signaling pathways linking distal tubule solute concentration to control of renin have been proposed. These include adenosine, NO, and prostanoids. The most compelling evidence suggests that macula densa stimulation of renin involves the activation of COX-2 (125). Constitutive expression of COX-2 at high levels in the macula densa, generate abundant PGE2 (318, 325). PGE2 then activates a prostaglandin E2 (EP) receptor on granular cells in the JG apparatus to stimulate renin release (325). The EP4 receptor is likely the major EP receptor that mediates the actions of PGE2 in this process. Facemire et al. demonstrated that EP4 receptor deficient mice display a ~70% reduction in renal renin expression and plasma renin concentration compared to wild-type mice after treatment with furosemide (84). By contrast, deletion of EP2 receptors in mice had no effect on renin stimulation by furosemide. Interestingly, this study suggested that the source of PGE2 in this pathway is not dependent on mPGES1 or mPGES2. The capacity for PGE2 to directly stimulate renin secretion has been long recognized (11,376). Moreover, studies using specific inhibitors and COX-2 deficient mice have clearly demonstrated the importance of COX-2 in the macula densa pathway (48, 123). In addition, the activity of various components of this system has been demonstrated in the isolated perfused macula densa segments (295) and JG cell lines (388). However, at least one study (95) has failed to confirm a nonredundant role for individual EP receptors for PGE2 in furosemide-stimulated renin release in vivo.
Initial evidence suggesting a role for adenosine in macula densa signaling came from studies using the selective A1 adenosine receptor antagonist 8-cyclopentyl-1, 3-dipropylxanthine. These studies demonstrated that renin release decreases as luminal NaCl concentration drops and this response is abrogated by blocking the A1 adenosine receptor (377). Later studies using A1 adenosine receptor deficient mice confirmed the role of adenosine is primarily restricted to the arm mediating the inhibition of renin release. In A1 adenosine receptor deficient mice, renin-inhibitory actions of enhanced sodium chloride delivery to the macula densa are blocked, whereas stimulation of renin secretion caused by reduced sodium chloride transport at the macula densa are unaffected (174).
Macula densa cells express high levels of neuronal nitric oxide synthase (nNOS) (249,378). The role of NO in regulation of renin was first tested using nonselective inhibitors of NO synthesis, which attenuated renin release stimulated by reduced luminal NaCl concentrations (128, 352). The specific role of the individual NOS isoforms has been examined using mice with targeted deletion of nNOS or eNOS. In these studies, activation of the macula densa pathway was achieved by administration of NKCC2 blocking loop diuretics in vivo and in isolated perfused mouse kidneys. Deficiency of either nNOS or eNOS alone did not significantly affect macula densa-dependent renin secretion (41), while nonspecific NOS blockade attenuated renin stimulation by loop diuretics. This suggests that NO plays a permissive rather than a primary role in the macula densa control of renin release (41). However, as discussed above, eNOS appears to be required for new recruitment of renin-producing cells to the afferent arteriole (263), highlighting the complicated role of NO in renin regulation.
β1-adrenergic stimulation in renin release
The capacity for sympathetic nerve activation to stimulate renin has been long recognized. For example, β-adrenergic receptors are abundant in the JG apparatus of kidneys from various species (205). Furthermore, numerous studies have demonstrated that β-adrenergic agonists stimulate renin release (171). Chronic renal nerve activation also stimulates renin (138,139) along with its affects to modulate renal blood flow and tubular function. In experiments controlling for these factors, a clear relationship between increasing renal sympathetic nerve activity and renin secretion is maintained (75, 177). However, as discussed above, renal denervation does not abolish the capacity of the baroreceptor (21, 22) or macula densa mechanisms to stimulate renin (90, 137, 360). Accordingly, it appears likely that β-sympathetic tone has a modulatory, rather than primary role in the regulation of renin.
Short loop feedback: Regulation of renin by Ang II
AT1 receptors are also highly expressed in the JG apparatus and Ang II may exert an inhibition of renin release by the short-loop feedback mechanism. Treatment with RAS antagonists or genetic ablation of the AT1A receptor in mice increases renin mRNA expression and causes JG apparatus expansion with recruitment renin-containing cells (354). In addition, infusion of Ang II into isolated perfused kidneys inhibits renin release (192). AT1 receptors couple to Gq proteins and activation of these receptors by ligand increases intracellular Ca2+ concentrations in JG cells (53, 326). As discussed above, the inhibitory effect of Ca2+ on renin release appears to be mediated by protein kinase C since stimulation of protein kinase C inhibits renin secretion, whereas blockade of protein kinase C attenuates the inhibitory effect on renin secretion (52,54,193). There is also evidence that the effects of Ca2+ on renin release are mediated in part by a calmodulin-dependent process, since inhibition of calmodulin activity stimulates renin secretion (70, 285). On the other hand, in kidney cross transplantation experiments with AT1A receptor deficient mice indicated that stimulation of renin expression was more directly associated with reduced blood pressure rather than direct modulation by AT1 receptors (60).
Genomic regulation of renin expression
The multiple levels of control for release of renin protein at the JG apparatus are accompanied by a parallel, complex regulation of renin gene expression. The regulatory region of the renin gene has been studied extensively in vitro utilizing a renin expressing cell line isolated from a mouse renal tumor (termed As4.1 cells) (335) coupled with in vivo studies utilizing transgenic mouse lines. These systems have identified two regions acting in conjunction to control renin expression, including a proximal promoter element (284,297) and a 242-base pair enhancer region.
The proximal renin promoter refers to the 5′-regulatory sequence located between base pairs −197 and −50. This region is necessary for maximal renin expression (25, 297) with sequences that are highly homologous in human, mouse, and rat renin including a conserved TATA box. It also contains a number of important cis-regulatory elements including an E26 transformation-specific (Ets)-binding site, homeobox (HOX)/pre-B cell leukemia transcription factor (PBX)-binding site, a CRE that binds transcription factors such as CREB/ATF1, and a putative binding site for an actin-related protein 1 homolog A (ARP-1) termed chicken ovalbumin upstream promoter transcription factor II (COUP-TFII) (25,183). Additional important sites include a cAMP negative regulatory element (CNRE) involved in cAMP effects modulated by the nuclear receptor liver X receptor-α (LXRα). Although mice lacking LXRα show reduced basal renin levels and attenuated response to adrenergic stimulation (242), in vivo deletion of the LXRα binding motif within the mouse promoter had no effect on either basal expression or the regulation of the renin gene (349). More recently, a retinol binding protein (RBP)-J/Su(H)/Lag1 site involved in the Notch-signaling pathway has also been identified in the proximal renin promoter region. Notably, deletion of the RBP-J, the common downstream effector of all Notch receptors, reduced renin expression under normal and sodium-depleted conditions, and lowered blood pressure (39).
The 242-bp enhancer element is located at −2866 to −2625 in the mouse Ren1c gene. Within the enhancer there are three distinct DNA binding sites that are conserved between mouse and human. These elements are essential for renin expression (282) comprising: (i) a CRE recognized by CREB, the cAMP responsive element modulator, and nuclear factor kappa B (Nf-κB) (358); (ii) an E-box that binds upstream stimulatory factors 1 and 2 (USF-1/2) (282); and (iii) two TGACCT motifs, constituting hormone response elements (HRE) (334). These sites can be bound by the retinoic acid receptors/retinoic X receptors promoting renin expression (334), as well as a nuclear orphan receptor (EAR2) and vitamin D receptor with inhibitory effects on the enhancer (212,214). Interestingly, the human renin HRE differs from the mouse sequences both in the spacer between the HREs and the proximal HRE. This difference seems to account for the lower trans-activating capacity of the human kidney renin enhancer and may contribute to the dramatic differences in renin levels between mice and humans (~1000 vs. ~3 ng Ang I/mL/h, respectively) (156).
The transcription factor peroxisome proliferator-activated receptor-γ (PPAR-γ) can also bind the enhancer region through a canonical PPAR response element (356), and play an important role in renin regulation. Indeed, selective reduction of PPAR-γ expression reduces renin expression (73). Still, there are might be differences between species as the effects of PPAR-γ in human renin transcription seem to be also dependent on a proximal promoter palindromic repeat with 3-bp spacer (Pal3) site (357). Finally, there are several important binding sites for transcriptional regulators such as nuclear transcription factor Y (NF-Y), Wilms’ tumor suppressor, nuclear factor I (NFI) and specificity protein (Sp)1/Sp3 located both in enhancer and proximal renin promoter (104). Mutations of the NFI/Sp1/Sp3 binding sequences almost completely eliminates activity of the renin promoter, indicating a crucial role for these transcription factors in the control of renin gene expression (283). It remains to be clarified whether these multiple renin promoter-binding sites are redundant or have different functions. Nonetheless, the importance of the enhancer in renin expression in vivo is controversial. In transgenic mice that express human renin from a 160-kb P1 artificial chromosome, Zhou et al. showed that the enhancer of the human renin gene is not critical for the stimulation of renin gene expression by ACE inhibition (398). Moreover, the enhCRE and CNRE sites were shown to be dispensable for the cell-specific expression of the human renin gene in the afferent arteriole (72). This contrasts with other studies using mice lacking an endogenous renin locus (226) or transgenic mice where expression of green fluorescent protein is driven by renin regulatory elements lacking the enhancer region (104). In both cases, the enhancer region appeared to be necessary for full activation of renin transcription by ACE inhibition alone or in combination with low salt diet. These discrepancies might reflect species differences in regulation of renin between humans and mice or experimental differences in the specific regions of the enhancer that were deleted. On the other hand, rather than regulating absolute levels of promoter activity, it has been also postulated that the enhancer acts like an on/off switch allowing renin transcription (245). We suggest that further experiments are necessary to dissect these mechanisms and to determine their physiological importance.
Angiotensin converting enzyme
ACE is a dicarboxypeptidase that generates the vasoactive peptide Ang II by cleaving 2 amino acids from the c-terminus of the inactive precursor Ang I (58). There are two distinct forms of ACE, somatic and testicular, both generated by alternative splicing of a single gene (81,144,197). A testis-specific form of ACE is generated from the ACE gene through alternative transcription, beginning at the 13th exon of the ACE gene, 7.2 kb downstream of the translation start site of somatic ACE in mice (141). Somatic tissues splice from exon 12 to exon 14 and thus remove exon 13 as if it were intronic. In the male germ cell, transcription is initiated at exon 13, providing testis ACE with its unique 66 amino-acid N-terminal sequence (140). The remainder of the protein corresponds exactly to the carboxyl half of somatic ACE. Mice lacking testis ACE have a phenotype of impaired fertility do to defective migration of sperm into the oviduct and reduced binding to the zona pellucida (252). These studies clearly demonstrated the key role for testis ACEs in male fertility in mice. While reduced fertility has not been described in male patients on ACE-inhibitors, the molecular mechanism by which testis ACE promotes fertility is not clear, including whether it depends on carboxypeptidase activity. In contrast, somatic ACE is expressed as an ectoenzyme on the surface of endothelial cells throughout the body and is particularly abundant in lung, intestine, choroid plexus, placenta, and on brush border membranes in the kidney. A soluble form of ACE that circulates in plasma is formed by enzymatic cleavage of tissue-bound ACE at its transmembrane domain (12). Since its original description, additional physiological roles for ACE have been reported and were recently reviewed by Bernstein (15); these will be described briefly below.
As with other components of the RAS, molecular variants of the human ACE gene have been proposed as candidate genes in hypertension, cardiovascular, and kidney diseases (316). Insertion (I) and deletion (D) polymorphisms of the human ACE gene are common and have been associated with significantly altered levels of ACE in plasma (303, 366). In some cohorts but not others, these ACE gene variants have been linked to differing susceptibilities to hypertension, cardiovascular, and renal diseases (316,347). The strongest association between ACE polymorphisms and disease exists for diabetic nephropathy (265, 374). In a meta-analysis of published studies, homozygosity for I allele was associated with significant risk reduction for diabetic nephropathy (265) and in a separate study, protection from the I allele was also observed in expanded renal outcomes (374). Conversely, the D allele has been associated with increased risk of diabetic renal disease in both type1 and type 2 diabetes (160). Finally, studies in mice have also demonstrated this effect (143). Diabetic mice with three copies of the Ace gene had a 25% increase in ACE levels and more proteinuria than mice with fewer copies of the Ace gene. However, the human ACE locus has not been emerged as a susceptibility locus in a series of genome-wide association studies of large cohorts of patients with hypertension, diabetic nephropathy and other cardiovascular diseases (311).
The actions of ACE to generate Ang II from Ang I are central to its biological functions as Ang II is the major effector molecule of the RAS. In addition to Ang I, there are other biologically active peptide substrates for ACE. Perhaps the most important of these is bradykinin (225). ACE degrades bradykinin into an inactive peptide, representing a significant biological pathway for bradykinin metabolism in vivo (30); in older literature, ACE was referred to as kininase II. Since bradykinin has vasodilator and natriuretic properties (225), it has been suggested that one mechanism of blood pressure reduction with ACE inhibition is blockade of this kininase activity. This was clearly demonstrated by Brown and associates, who showed that the antihypertensive efficacy of ACE inhibitors is attenuated by simultaneous administration of a bradykinin receptor antagonist (Fig. 6) (97), (15, 16). Beyond a role in blood pressure lowering, bradykinin acting via its receptors may also play a role in modifying kidney injury in diabetes. For example, studies in mice lacking bradykinin receptors show that they have dramatic acceleration of albuminuria and glomerular pathology, indicating a protective effect of bradykinin in diabetic nephropathy (164-167, 361). These studies suggest that ACE activity has the potential to modulate renal disease in diabetes via degradation of bradykinin, and that one potential mechanism underlying the benefits of ACE inhibition in diabetic nephropathy (207, 209) might be potentiation of bradykinin effects.
Figure 6.
Attenuation of the antihypertensive efficacy of ACE inhibitors with the B2 bradykinin receptor antagonist icatibant. (Used with permission from Gainer et al. N Engl J Med 339: 1285-1292, 1998.) Mean arterial pressures (MAP) were measured over 250 min in hypertensive patients treated with placebo, ACE inhibitor alone, ACE inhibitor + icatibant, and angiotensin receptor blocker (ARB). The largest blood pressure reduction was seen with ACE inhibitor alone, and this was attenuated when icatibant was given along with the ACE inhibitor. The extent of blood pressure lowering was intermediate and equivalent in the groups receiving the ARB or ACE inhibitor + icatibant.
Gene targeting in mice has proven a very useful tool in deciphering gene function and many of the functions of RAS components were clarified in this way (351). The effort to dissect functions of ACE is a prime example with a large body of work to support its diverse physiological roles (15). Work largely by the Bernstein group has expanded the function of ACE to include diverse roles such as hematopoiesis, major histocompatibility class I peptide processing, resistance to tumors and infection, in addition to normal kidney development and male fertility and is nicely described in their detailed review (15,351).
Using genome-based strategies, homologues of ACE have been identified (76, 355, 393, 394). One of these, ACE2, exhibits more than 40% identity at the protein level with the catalytic domain of ACE (76, 355). Similar to ACE, ACE2 is expressed on the surface of certain endothelial cell populations. However, compared to the ubiquitous distribution of ACE, the expression pattern of ACE2 is more limited with most abundant expression in kidney followed by heart and testis (76, 355), although the physiological importance of ACE2 in other tissues such as the brain, adipose, and pancreas is emerging (19, 20, 77, 344, 384, 385). Their substrate specificities also differ; ACE2 hydrolyzes Ang II with high efficiency, but has much lower activity against Ang I (76,365). Hydrolysis of Ang II by ACE2 (or prolyl endopeptidases) generates Ang (1-7), a biologically active 7-amino acid peptide (Asp-Arg-Val-Tyr-Ile-His-Pro). Ang (1-7) subsequently acts upon the Mas receptor (312) and has several opposing actions to Ang II, such as the release of vasodilatory products such as NO, PGE2, and bradykinin (256). Accumulating evidence indicates that this peptide causes vasodilation, natriuresis, and may promote reduced blood pressures (85). It has been further suggested that ACE2 may be the major pathway for synthesis of Ang 1-7 (86). Thus, the functions of ACE2 may be determined by its distinct actions to metabolize Ang II and to generate Ang 1-7. Chappell has a detailed discussion of the nonclassical RAS in the Handbook of Physiology (44).
ACE2 was originally identified and cloned from a cDNA library prepared from ventricular tissue of a patient with heart failure (76). Initial studies in separate lines of ACE2-deficient mice suggested a role for ACE2 in cardiac function (59, 386) and in blood pressure regulation (115). Taken together, these studies using mouse lines with targeted deletion of the Ace2 gene have provided a number of contributions to understanding the role of ACE2 in cardiovascular functions (116). Despite a lack of uniformity in certain phenotypes, some common themes have emerged from these studies. ACE2 appears to have only modest effects on baseline cardiovascular functions and blood pressure control, but these effects can be substantially modulated by genetic and, perhaps, environmental factors. On the other hand, the activity of ACE2 may have more profound effects on susceptibility to pathological states such as hypertension and cardiac hypertrophy. Recent work by many groups has demonstrated roles for ACE2 in renal diseases such as diabetic and nondiabetic kidney disease (279,341,380,383) and hypertension (278,396) in both experimental models and human cohorts. Furthermore, ACE2 was also identified as a receptor for the severe acute respiratory syndrome coronavirus and in acute lung diseases (187-189) and shown to have a role in the gastrointestinal tract to regulate amino acid transport (29,126,291).
A third member of the ACE gene family, collectrin, was identified as a gene that is upregulated in the sub-total nephrectomy model of chronic kidney disease (393). Collectrin is highly homologous to the transmembrane portion of ACE2, but lacks the carboxypeptidase domain (393). Its physiological functions are emerging (66, 223, 224); and appear to regulate amino acid transport by the kidney and intestines (29,108). A recent report by Cechova et al. suggests collectrin directly regulates l-arginine uptake in endothelial cells leading to endothelial NOS coupling. Lack of collectrin led to vascular dysfunction, exaggerated salt sensitivity and impaired pressure natriuresis and hypertension (42).
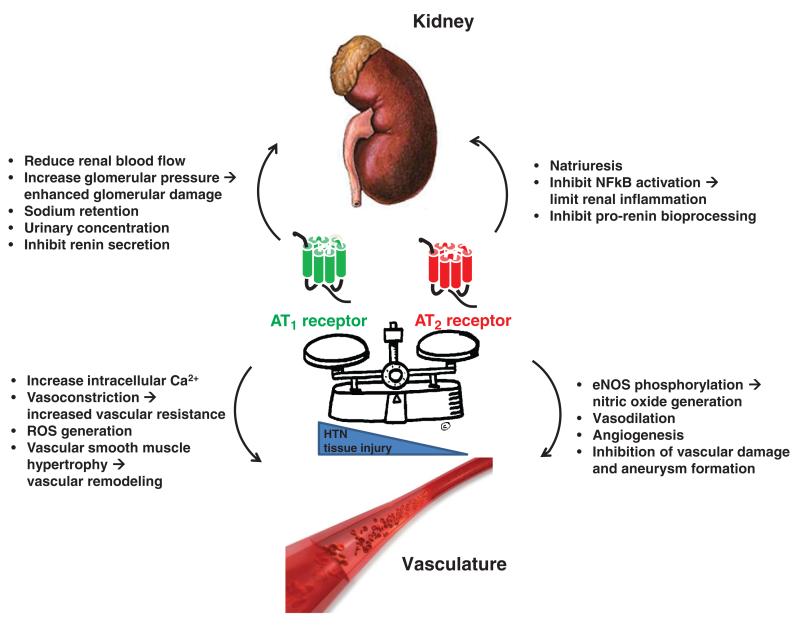
Angiotensin receptors
The biological actions of Ang II are mediated by cell surface receptors that belong to the large family of 7 trans-membrane receptors (145,354). The angiotensin receptors can be divided into two pharmacological classes: AT1 and AT2, based on their differential affinities for various nonpeptide antagonists (Fig. 1). Studies using these antagonists suggested that most of the classically recognized functions of the RAS are mediated by AT1 receptors including regulation of TG feedback (319), stimulation of renal tubular sodium reabsorption, release of aldosterone from the adrenal glomerulosa, SMC contraction, and stimulation of hypothalamic thirst sensors (354). Gene targeting studies have confirmed these conclusions (62).
AT1 receptors from a number of species have been cloned (150,253,313) and two subtypes, designated AT1A and AT1B, have been identified in rat (158, 159, 163, 310) and mouse (314). In the classical view, AT1 receptors signal through Gαq-linked signaling pathways involving phospholipase C, inositol triphosphate (IP3), and increases in intracellular Ca2+ (69). However, the AT1 receptor has also been linked to Janus kinase and signal transducer and activator of transcription activation (228), as well as β-arrestin-dependent pathways linked to extracellular signal-regulated kinases (ERK) activation (1, 333). In addition, other studies have shown that the AT1 receptor has the capacity to transactivate the epidermal growth factor receptor (80). This pathway may contribute to chronic kidney injury and renal epithelial cell hypertrophy (46,198).
The murine AT1 receptors are products of separate genes and share substantial sequence homology (28,159,163). AT1A receptors predominate in most organs except the adrenal gland and regions of the CNS, where AT1B expression may be more prominent (28, 99, 215). Commercially available antibodies to the AT1 receptor are unreliable, so precise characterization of receptor distribution requires gene expression analysis coupled with radioligand binding studies (132). The murine AT1B isoform was also recently detected within the glomerular podocyte, and pharmacologic blockade of this isoform afforded protection from proteinuria and renal injury in a mouse model of autoimmune nephritis, thereby directly illustrating the detrimental actions of glomerular AT1 receptors in mediating progressive kidney disease (63). An early report that the AT1B receptor was detected in humans has not been confirmed (181), and only a single AT1 receptor gene was identified in the sequence of the human genome. Thus, the murine AT1A receptor is considered to be the closest homologue to this single human AT1 receptor.
The binding signatures of the AT1A and AT1B receptors are virtually identical (49), making it difficult to discriminate their in vivo functions pharmacologically. Experiments using gene targeting have provided insights into the discrete functions of the two AT1 receptor genes (270,271,274). Although the AT1B receptor has a unique role to mediate thirst responses (68), AT1A receptors have the predominant role in determining the level of blood pressure (157, 234, 346) and in mediating vasoconstrictor responses (157, 270). The phenotype of markedly reduced blood pressures and profound sodium sensitivity in mice lacking the AT1A receptor (157,272) under-scores its importance in blood pressure control. Moreover, similarities in blood pressure reduction with genetic deletion versus blockade of the AT1 receptor confirm that hypotension seen in global AT1A “knockout” mice is not due to a developmental abnormality (157). Conversely, overexpression of the AT1A receptor in gene titration experiments yields step-wise increases in blood pressure corresponding to gene copy number (173), whereas constitutive activation of the AT1A receptor leads to hypertension and both cardiac and renal fibrosis (18). AT receptors may also have a role to promote aging since mice with genetic deficiency of AT1A receptors have increased longevity (14).
AT1A receptors are expressed in all of the key organ systems involved in coordinately determining the level of blood pressure, including the kidney, vasculature, adrenal gland, heart, and both central and peripheral nervous systems. A renal cross-transplantation approach using wild-type and Agtr1a−/− kidneys (Fig. 7) illustrated equal and non-redundant contributions of AT1 receptors in kidney and non-renal, systemic AT1 receptors to the maintenance of normal blood pressure (61), suggesting that AT1 receptors inside and outside of the kidney work together in maintaining blood pressure and preventing circulatory collapse in nonhypertensive mice. The importance of AT1 receptors to regulating renal sodium handling even at baseline was evidenced in that only the transplanted animals lacking AT1 receptors within in the kidney had significant increases in blood pressure during salt-loading. These studies also informed our understanding of renin mRNA regulation in the kidney, as renin expression was dramatically elevated in the hypotensive transplanted animals lacking AT1 receptors in all tissues, but was not significantly increased in the “kidney knockout” mice lacking AT1 receptors only within the kidney. According to these experiments, blood pressure rather than short-loop feedback through activation of renal AT1 receptors is the more prominent regulator of renin generation at least at the RNA level (61).
Figure 7.
Kidney cross-transplantation groups. Wild-type (+/+) or AT1A (−/−) receptor-deficient mice were transplanted with kidneys from wild-type or AT1A−/− mice. Group I animals (Wild-type) had a full complement of AT1A receptors. Group II animals (Kidney KOs) expressed AT1A receptors only outside the kidney. Group III animals (Systemic KOs) expressed AT1A receptors only within the kidney. Group IV animals (Total KOs) completely lacked AT1A receptors.
The same kidney cross-transplantation technique coupled with the chronic Ang II infusion model of hypertension confirmed a dominant contribution of AT1 receptors in the kidney to promoting sodium retention and blood pressure elevation in hypertension (60). As shown in Figure 8, after 2 weeks of Ang II infusion, the blood pressure elevation in the “systemic knockout” that retained AT1 receptor expression only within the kidney recapitulated that seen in transplanted and non-transplanted wild-type controls. Conversely, the “kidney knockout” mice that lacked AT1 receptors in the kidney showed only a diminutive and ephemeral increase in blood pressure during chronic Ang II infusion (Fig. 8). Thus, AT1 receptors in the kidney were both necessary and sufficient to fully manifest Ang II-dependent hypertension (60). These studies further illustrated that activation of renal AT1 receptors drives blood pressure elevation through sodium retention and intravascular volume expansion. Specifically, the hypertensive “wild-type” and “systemic knockout” cohorts that expressed AT1 receptors in the kidney demonstrated blunted renal sodium excretion following the initiation of chronic Ang II infusion coupled with an increase in total body weight compared to the “kidney knockout” mice that lacked renal AT1 receptors and remained normotensive during chronic Ang II infusion (Fig. 8). In subsequent studies, challenging the cross-transplanted animals with a low salt diet during chronic Ang II infusion illustrated that the major component of blood pressure elevation mediated through renal AT1 receptor stimulation is indeed a consequence of sodium retention. However, a nontrivial component also accrues directly from the increase in renal vascular resistance due to AT1 receptor activation, independent of renal sodium handling (64).
Figure 8.
Blood pressures and urinary sodium excretion during chronic Ang II infusion in mice after kidney cross-transplantation. (A) Daily, 24-h blood pressures in the experimental groups before (“pre”) and during 21 days of Ang II infusion (*, P ≤ 0.03 vs. Wild-type; §, P < 0.008 vs. Systemic KO; †, P < 0.006–0.0001 vs. Wild-type). (B) Cumulative sodium excretion during the first 5 days of Ang II infusion. (§, P < 0.02 vs. Kidney KO and P = 0.03 vs. Total KO; ‡, P = 0.03 vs. Kidney KO and Total KO). (C) Change in body weights after 5 days of Ang II infusion. (*, P = 0.03 vs. “pre”; #, P = 0.05 vs. “pre”).
Subsequent generation of mice with conditional deletion of AT1A receptors from epithelia of the proximal tubule of the kidney showed that AT1 receptors in this cell lineage play a nonredundant role in maintaining blood pressure homeostasis and in the pathogenesis of Ang II-dependent hypertension (Fig. 9). These actions are mediated, at least in part, through modulation of solute and fluid reabsorption by the proximal tubule and by controlling abundance of sodium transporters including the NHE3 antiporter and the sodium phosphate cotransporter (117,211,304). These in vivo findings were consistent with earlier in vitro studies showing that Ang II acting through AT1 receptors on the basolateral surface of proximal tubules promotes sodium reabsorption by coordinately stimulating the sodium-proton antiporter on the luminal membrane along with the sodium-potassium ATPase on the basolateral surface (57, 100, 323). These actions also result in enhanced basolateral sodium bicarbonate flux (100). Thus, activation of AT1 receptors in the renal proximal tubule stimulates sodium and fluid reabsorption, leading to intravascular volume expansion and blood pressure elevation.
Figure 9.
AT1A receptors in the proximal tubule promote hypertension (A) With infusion of ang II (1000 ng/kg/min), BPs increased significantly in both control and PTKO mice but the hypertensive response to angiotensin II was significantly attenuated in the PTKOs (**; P < 0.001). (B) The mean increase in BP during the angiotensin II infusion was significantly less in the PTKOs (23 ± 3 mmHg) compared to controls (black bars; 38 ± 5 mmHg, *; P 0.0005). (C) Cumulative sodium balance was significantly lower in the PTKOs (n = 8) than controls (n = 7, *; P = 0.046) during the first 3 days of Ang II infusion. Error bars represent SEM.
In addition to the proximal tubule, AT1 receptors are expressed on epithelial cells across the entire nephron. For example, they are expressed on the luminal and basolateral membranes of the epithelium in the medullary thick ascending limb (287, 298). However, their effects on activity of NKCC2 are inconsistent and appear to depend on local Ang II levels (4, 203). At lower concentrations of Ang II, inhibition of NKCC2 may occur (4, 203), whereas stimulation of NKCC2 may be seen at higher concentrations (4). Functions of AT1 receptors in more distal segments of the nephron have also been examined. In cortical and outer medullary collecting ducts, activation of AT1 receptors stimulates sodium-hydrogen exchange by increasing the density of the vacuolar sodium-hydrogen ATPase in the apical membrane of the type A intercalated cell, which in turn leads to an increase in bicarbonate reabsorption (7, 204, 289, 309). AT1 receptors also modulate the activity of pendrin, a sodium-independent chloride transporter expressed by type B intercalated cells. Chronic activation of AT1 receptors shifts pendrin distribution from the subapical space to the apical membrane leading to enhanced chloride reabsorption. These effects of AT1 receptors to augment pendrin activity raise blood pressure in vivo (364). On the apical membrane of the principal cells in the cortical collecting duct, luminal Ang II stimulates amiloride-sensitive sodium transport by increasing activity of the epithelial sodium channel through an AT1 receptor-dependent mechanism (296,370). However, preliminary studies suggest that cell-specific deletion of AT1 receptors from principal cells does not have a significant impact on baseline blood pressure (345).
Along with their impact on sodium handing, AT1 receptors also seem to play a critical role in controlling urinary concentrating mechanisms. The complete absence of AT1 receptors in mice doubly deficient in AT1A and AT1B receptors is associated with atrophy of the renal papilla, polyuria, and a marked urinary concentrating defect (274, 275). A similar phenotype is seen in Agt and ACE-deficient mice indicating that Ang II acting through AT1 receptors is essential for maintenance of the inner medulla and its functions to promote urinary concentration (82, 172). Similarly, AT1A receptor deficient mice with preservation of inner medullary architecture also have a urinary concentrating defect due to a relative resistance to vasopressin, and this defect can be reproduced in wild-type mice by administration of an AT1 receptor blocker (273). AT1 receptor blockade also blunts the maximal urine concentrating capacity in 1-desamino-8-D-arginine vasopressin-challenged rats and this effect is associated with reduced expression of aquaporins-1 and -2 (195). In the medullary collecting duct, Ang II upregulates gene expression for the V2 vasopressin receptor and the apical membrane targeting of the aquaporin-2 channel (210, 345, 373, 381). These effects are mediated through a protein kinase A-dependent pathway (381). Moreover, cell-specific deletion of AT1 receptors from the collecting duct is sufficient to cause a urinary concentrating defect (345). In this case, epithelial levels of aquaporin-2 protein were significantly diminished in the inner and outer medulla, whereas localization to the apical membrane was unaffected. Thus, direct effects of AT1 receptors in epithelial cells of the collecting duct modulate aquaporin-2 levels and these actions are required to achieve maximal urinary concentration.
AT1 receptors are widely expressed in vascular tissues and when activated by Ang II cause potent vasoconstriction. Stimulation of AT1 receptors in vascular SMCs initiates a signaling cascade including increased intracellular Ca2+ concentration and alterations in the cytoskeleton, inducing contraction with consequent increases in vascular resistance (112). This response is virtually absent in mice deficient in both the AT1A and AT1B receptor isoforms, confirming the importance of AT1 receptors in this response (157,274). The vasoconstrictor actions of Ang II play a central role in maintaining circulatory homeostasis in a number of tissues, including the kidney. In the kidney, the hemodynamic actions of Ang II impact renal blood flow, glomerular filtration rate, excretion of salt and water, and progression of renal damage in disease states. Infusion of Ang II increases filtration fraction. Accordingly, it has been suggested that AT1 receptor activation in the glomerular microvasculature causes greater constriction of the efferent compared to the afferent arteriole (254,321). However, experimental studies are not in complete agreement on this point. Work by Navar and colleagues indicates that the rise in filtration fraction occur in parallel with concomitant increases in resistance of both the afferent and efferent arterioles (35,36). On the other hand, Denton et al. showed that despite preferential constriction of the afferent over the efferent arterioles in response to Ang II, resistance was higher on the efferent side due to smaller resting luminal dimensions of efferent arterioles (71). This is discussed in great detail by Navar et al. in the renal microcirculation section of the Handbook of Physiology (260). In hypertensive states, such as chronic Ang II infusion, the increased glomerular pressure from AT1 receptor stimulation can promote renal injury (23, 79, 261). The apparent actions of Ang II to promote increased glomerular hydrostatic pressure in diabetes were a major rationale for using ACE inhibitors in diabetic nephropathy (392).
Outside of the kidney, the role of vascular AT1 receptors in promoting direct vascular damage is complex. Along with their effects on vascular tone, AT1 receptors may also stimulate growth and hypertrophy of SMCs (101), thereby directly contributing to vascular remodeling in hypertension. It has been suggested that nonhemodynamic actions of AT1 receptors, including enhanced generation of reactive oxygen species (ROS) may promote changes in vascular structure that perpetuate the development of hypertension (153). Further-more, angiotensin receptor blockers (ARBs) reverse vascular remodeling in patients with hypertension suggesting a direct role for vascular AT1 receptors in this process (317). On the other hand, deletion of AT1 receptors from SMC lineages reduced oxidative stress in vascular tissue but did not affect the degree of vascular remodeling induced by hypertension (342), indicating a key role for pressure in hypertensive vascular injury (60,342).
Cardiovascular control centers in the central nervous system (CNS) also have a powerful capacity to influence blood pressure and fluid homeostasis through activation of the sympathetic nervous system. Within the CNS, AT1A receptors mediate the systemic pressor effects of Ang II whereas activation of AT1B receptors stimulates the drinking response and thereby regulates water balance through a central neurogenic mechanism (68). The pressor response is mediated through stimulation of AT1A receptors along the subfornical organ (SFO)-rostral ventrolateral medulla pathway leading to local Ras-related C3 botulinum toxin substrate 1 (Rac1)-dependent generation of ROS (399, 400). Accordingly, targeted expression and/or stimulation of AT1A receptors directly within the rostral ventrolateral medulla can raise blood pressure independently of other systemic or renal AT1 receptors (3,45). Inhibition of endoplasmic reticulum stress within the SFO by infusing tauroursodeoxycholic acid or by enhancing local activity of an endoplasmic reticulum chaperone glucose-related protein (GRP78) abrogates the chronic hypertensive response to Ang II (390). Thus, some of the effects of Ang II in the SFO to drive blood pressure elevation occur through potentiation of endoplasmic reticulum stress.
Ang II mediates damage to the heart and kidney in part by stimulating inflammatory responses (246,247). Furthermore, activation of T lymphocyte populations during chronic Ang II infusion has the capacity to modulate the chronic hypertensive response (6,118). Although Ang II can directly enhance lymphocyte proliferation in vitro (136, 259), recent studies have uncovered potentially immunosuppressive effects of AT1 receptors on mononuclear cells in the setting of hypertension (64,395). For example, deletion of AT1 receptors solely from T lymphocytes promotes T cell differentiation toward a proinflammatory “Th1” phenotype, leading to exaggerated levels of albuminuria and glomerular podocyte damage in Ang II-induced hypertension (395). Thus, whether Ang II activates the immune system through direct stimulation of AT1 receptors on inflammatory cells remains controversial. Alternative mechanisms through which Ang II mediates immune activation may involve AT1 receptor activation in the CNS and/or the endothelium (130,230).
Pharmacological and genetic studies have thus confirmed that virtually all of the classically recognized functions of the RAS are mediated by AT1 receptors (Fig. 10). However, exploring the physiological role of AT2 receptors has received considerable attention in recent studies particularly as new specific AT2 receptor agonists have been identified and are being considered for clinical use (369). AT2 receptors are found in abundance during fetal development (111, 240) but their expression generally falls after birth. Nevertheless, persistent AT2 receptor expression can be detected in several adult tissues including the kidney, adrenal gland and the brain, and absolute levels of AT2 receptor expression may be modulated by Ang II and certain growth factors (147). Likewise, it has been suggested that AT2 receptor expression may be increased in pathological states.
Figure 10.
The myriad actions of angiotensin receptors in the kidney and vasculature. In cardiovascular control centers, the effects of AT2 receptor stimulation oppose and ameliorate the prohypertensive and proinflammatory effects of AT1 receptor stimulation.
AT2 receptors appear to signal by coupling to Gαi2 and Gαi3 proteins (17). Using site-directed mutagenesis, the intermediate portion of the third intracellular loop of the AT2 receptor was found to be necessary for normal receptor signaling (127, 202). Moreover, it has been suggested that activation of AT2 receptors stimulates bradykinin, NO, and cGMP (34, 337), and these pathways may mediate actions of the receptor to promote natriuresis and blood pressure lowering. In vitro data further indicate that AT2 receptor activation similarly directs angiogenesis in the hypoxic heart via downstream stimulation of the bradykinin B2 receptor and NO generation (250). Phosphorylation of eNOS underlies the increase in NO bioavailability in the vasculature following AT2 receptor activation (134, 389). AT2 receptor stimulation counteracts AT1 receptor-induced constriction in the vasculature by inhibiting activation of the phospholipase D signaling pathway (5). Finally, there is evidence that AT2 receptors signal via nonclassical eicosanoid signaling pathways. For example, hydroxy-eicosatetraenoic acids (HETEs) act as second messengers for AT2 receptors in the kidney, leading to ERK 1/2 phosphorylation (78). Moreover, AT2 receptors are upregulated during inflammation, and AT2 stimulation in this setting reduces organ damage both by inhibiting epoxyeicosatrienoic acid synthesis and by limiting activation of NF-κB (308).
Targeted disruption of the mouse Agtr2 gene did not cause a dramatically abnormal phenotype at baseline. However, these animals clearly manifest increased sensitivity to the pressor actions of Ang II (129, 148) and to Ang II-induced vascular damage that promotes aortic aneurysm progression (119). One of the AT2 deficient lines manifested increased baseline blood pressure and heart rate (129). Inter-estingly, behavioral changes were also observed in AT2-deficient mice; they had decreased spontaneous movements and rearing activity and impaired drinking response to water deprivation (129, 148). Transgenic mice that overexpress the AT2 receptor gene under control of a cardiac-specific promoter have decreased sensitivity to AT1-mediated pressor and chronotropic actions (231). Moreover, the pressor actions of Ang II are significantly attenuated in these transgenic mice. This attenuation was completely reversed following pretreatment with a specific AT2 receptor antagonist. Some target effects of AT2 receptor activation may relate to renin regulation. Stimulation of AT2 receptors inhibits the processing of pro-renin in JG cells, which in turn diminishes active renin content (146,338).
More recent studies with compound 21 (C21), a putative specific AT2 receptor agonist, suggest that AT2 receptor activation may limit progressive cardiovascular and renal damage in a mirror image of the actions of AT1 receptors to promote such injury. For example, treatment with C21 provides neuro-protection to stroke-prone rats, limits the extent of the infarct zone in a model of myocardial ischemia, and mitigates renal inflammation and proteinuria in hypertension (102,170,236). The renoprotective effects of C21 may relate to increases in NO bioavailability accruing from AT2 receptor stimulation (232). In contrast to the effects of AT1 receptors in the kidney to drive sodium retention, activation of renal AT2 receptors by Ang II augments natriuresis (280). Indeed, natriuresis mediated by activation of the renal dopamine D1 receptor may require recruitment of AT2 receptors to the apical membranes of epithelial cells in the proximal tubule (281). Taken together, these data are consistent with a primary function of the AT2 receptor to negatively modulate the actions of the AT1 receptor.
Conclusion
Because of their efficacy in a wide range of disorders from hypertension to heart failure to kidney disease, pharmacological inhibitors of the RAS are widely used in clinical medicine and understanding of their optimal use in patients is still evolving. While it has been more than 100 years since the discovery of renin, basic research on the RAS continues to unlock novel features of its functions in normal physiology and in disease. During the past two decades, the use of transgenic animals has been particularly fruitful in providing a rich molecular overlay of the physiological actions of the RAS. In this chapter, we have attempted to provide an in-depth overview of the physiological functions of the RAS, with a focus on more recent studies where molecular mechanisms of RAS functions have been defined. In the context of the robust nature of current research into the “classical RAS,” we also anticipate significant advances in a number of areas in the future. These include emerging data on the structural biology of RAS components, new understanding of the complexity of angiotensin receptor signaling, and the development of novel agonists and biased ligands for manipulating RAS activity in vivo. Along with advancing the scientific field, such understanding should facilitate translational approaches for improving the safety and efficacy of RAS inhibition in the clinic.
References
- 1.Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest. 2004;114:504–511. doi: 10.1172/JCI21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldehni F, Tang T, Madsen K, Plattner M, Schreiber A, Friis UG, Hammond HK, Han PL, Schweda F. Stimulation of renin secretion by catecholamines is dependent on adenylyl cyclases 5 and 6. Hypertension. 2011;57:460–468. doi: 10.1161/HYPERTENSIONAHA.110.167130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen AM, Dosanjh JK, Erac M, Dassanayake S, Hannan RD, Thomas WG. Expression of constitutively active angiotensin receptors in the rostral ventrolateral medulla increases blood pressure. Hypertension. 2006;47:1054–1061. doi: 10.1161/01.HYP.0000218576.36574.54. [DOI] [PubMed] [Google Scholar]
- 4.Amlal H, LeGoff C, Vernimmen C, Soleimani M, Paillard M, Bichara M. ANG II controls Na(+)-K (NH4)-2Cl-cotransport via 20-HETE and PKC in medullary thick ascending + +limb. Am J Physiol. 1998;274:C1047–C1056. doi: 10.1152/ajpcell.1998.274.4.C1047. [DOI] [PubMed] [Google Scholar]
- 5.Andresen BT, Romero GG, Jackson EK. AT2 receptors attenuate AT1 receptor-induced phospholipase D activation in vascular smooth muscle cells. J Pharmacol Exp Ther. 2004;309:425–431. doi: 10.1124/jpet.103.061846. [DOI] [PubMed] [Google Scholar]
- 6.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Par-adis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57:469–476. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 7.Barreto-Chaves ML, Mello-Aires M. Effect of luminal angiotensin II and ANP on early and late cortical distal tubule HCO3-reabsorption. Am J Physiol. 1996;271:F977–F984. doi: 10.1152/ajprenal.1996.271.5.F977. [DOI] [PubMed] [Google Scholar]
- 8.Baxter JD, Hsueh WA1. Human prorenin. Hypertension. 1991;17(4):469–477. doi: 10.1161/01.hyp.17.4.469. [DOI] [PubMed] [Google Scholar]
- 9.Beierwaltes WH. The role of calcium in the regulation of renin secretion. Am J Physiol Renal Physiol. 2010;298:F1–F11. doi: 10.1152/ajprenal.00143.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beierwaltes WH, Potter DL, Shesely EG. Renal baroreceptor-stimulated renin in the eNOS knockout mouse. Am J Physiol Renal Physiol. 2002;282:F59–F64. doi: 10.1152/ajprenal.0144.2001. [DOI] [PubMed] [Google Scholar]
- 11.Beierwaltes WH, Schryver S, Sanders E, Strand J, Romero JC. Renin release selectively stimulated by prostaglandin I2 in isolated rat glomeruli. Am J Physiol. 1982;243:F276–F283. doi: 10.1152/ajprenal.1982.243.3.F276. [DOI] [PubMed] [Google Scholar]
- 12.Beldent V, Michaud A, Wei L, Chauvet MT, Corvol P. Proteolytic release of human angiotensin-converting enzyme. Localization of the cleavage site. J Biol Chem. 1993;268:26428–26434. [PubMed] [Google Scholar]
- 13.Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci U S A. 2003;100:4322–4327. doi: 10.1073/pnas.0736323100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L, Cassis P, Morigi M, Coffman TM, Remuzzi G. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernstein KE, Ong FS, Blackwell WL, Shah KH, Giani JF, Gonzalez-Villalobos RA, Shen XZ, Fuchs S, Touyz RM. A modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzyme. Pharmacol Rev. 2013;65:1–46. doi: 10.1124/pr.112.006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernstein KE, Shen XZ, Gonzalez-Villalobos RA, Billet S, Okwan-Duodu D, Ong FS, Fuchs S. Different in vivo functions of the two catalytic domains of angiotensin-converting enzyme (ACE) Curr Opin Pharmacol. 2011;11:105–111. doi: 10.1016/j.coph.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry C, Touyz R, Dominiczak AF, Webb RC, Johns DG. Angiotensin receptors: Signaling, vascular pathophysiology, and interactions with ceramide. Am J Physiol Heart Circ Physiol. 2001;281:H2337–H2365. doi: 10.1152/ajpheart.2001.281.6.H2337. [DOI] [PubMed] [Google Scholar]
- 18.Billet S, Bardin S, Verp S, Baudrie V, Michaud A, Conchon S, Muffat-Joly M, Escoubet B, Souil E, Hamard G, Bernstein KE, Gasc JM, Elghozi JL, Corvol P, Clauser E. Gain-of-function mutant of angiotensin II receptor, type 1A, causes hypertension and cardiovascular fibrosis in mice. J Clin Invest. 2007;117:1914–1925. doi: 10.1172/JCI28764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bindom SM, Hans CP, Xia H, Boulares AH, Lazartigues E. Angiotensin I-converting enzyme type 2 (ACE2) gene therapy improves glycemic control in diabetic mice. Diabetes. 2010;59:2540–2548. doi: 10.2337/db09-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bindom SM, Lazartigues E. The sweeter side of ACE2: physiological evidence for a role in diabetes. Mol Cell Endocrinol. 2009;302:193–202. doi: 10.1016/j.mce.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blaine EH, Davis JO. Evidence for a renal vascular mechanism in renin release: New observations with graded stimulation by aortic constriction. Circ Res. 1971;28(Suppl 2):118–126. doi: 10.1161/01.res.28.5.ii-118. [DOI] [PubMed] [Google Scholar]
- 22.Blaine EH, Davis JO, Prewitt RL. Evidence for a renal vascular receptor in control of renin secretion. Am J Physiol. 1971;220:1593–1597. doi: 10.1152/ajplegacy.1971.220.6.1593. [DOI] [PubMed] [Google Scholar]
- 23.Blantz RC, Konnen KS, Tucker BJ. Angiotensin II effects upon the glomerular microcirculation and ultrafiltration coefficient of the rat. J Clin Invest. 1976;57:419–434. doi: 10.1172/JCI108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bock HA, Hermle M, Brunner FP, Thiel G. Pressure dependent modulation of renin release in isolated perfused glomeruli. Kidney Int. 1992;41:275–280. doi: 10.1038/ki.1992.39. [DOI] [PubMed] [Google Scholar]
- 25.Borensztein P, Germain S, Fuchs S, Philippe J, Corvol P, Pinet F. cis-regulatory elements and trans-acting factors directing basal and cAMP-stimulated human renin gene expression in chorionic cells. Circ Res. 1994;74:764–773. doi: 10.1161/01.res.74.5.764. [DOI] [PubMed] [Google Scholar]
- 26.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 27.Brunskill EW, Sequeira-Lopez MLS, Pentz ES, Lin E, Yu J, Aronow BJ, Potter SS, Gomez RA. Genes that confer the identity of the renin cell. J Am Soc Nephrol. 2011;267 doi: 10.1681/ASN.2011040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burson JM, Aguilera G, Gross KW, Sigmund CD. Differential expression of angiotensin receptor 1A and 1B in mouse. Am J Physiol. 1994;267:E260–E267. doi: 10.1152/ajpendo.1994.267.2.E260. [DOI] [PubMed] [Google Scholar]
- 29.Camargo SM, Singer D, Makrides V, Huggel K, Pos KM, Wagner CA, Kuba K, Danilczyk U, Skovby F, Kleta R, Penninger JM, Verrey F. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology. 2009;136:872–882. doi: 10.1053/j.gastro.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell DJ, Alexiou T, Xiao HD, Fuchs S, McKinley MJ, Corvol P, Bernstein KE. Effect of reduced angiotensin-converting enzyme gene expression and angiotensin-converting enzyme inhibition on angiotensin and bradykinin peptide levels in mice. Hypertension. 2004;43:854–859. doi: 10.1161/01.HYP.0000119190.06968.f1. [DOI] [PubMed] [Google Scholar]
- 31.Campbell DJ, Habener JF. Angiotensinogen gene is expressed and differentially regulated in multiple tissues of the rat. J Clin Invest. 1986;78:31–39. doi: 10.1172/JCI112566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell DJ, Habener JF. Cellular localization of angiotensinogen gene expression in brown adipose tissue and mesentery: Quantification of messenger ribonucleic acid abundance using hybridization in situ. Endocrinology. 1987;121:1616–1626. doi: 10.1210/endo-121-5-1616. [DOI] [PubMed] [Google Scholar]
- 33.Carey RM, McGrath HE, Pentz ES, Gomez RA, Barrett PQ. Biomechanical coupling in renin-releasing cells. J Clin Invest. 1997;100:1566–1574. doi: 10.1172/JCI119680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carey RM, Wang ZQ, Siragy HM. Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. Hypertension. 2000;35:155–163. doi: 10.1161/01.hyp.35.1.155. [DOI] [PubMed] [Google Scholar]
- 35.Carmines PK, Morrison TK, Navar LG. Angiotensin II effects on microvascular diameters of in vitro blood-perfused juxtamedullary nephrons. Am J Physiol. 1986;251:F610–F618. doi: 10.1152/ajprenal.1986.251.4.F610. [DOI] [PubMed] [Google Scholar]
- 36.Carmines PK, Perry MD, Hazelrig JB, Navar LG. Effects of pre-glomerular and postglomerular vascular resistance alterations on filtration fraction. Kidney Int. 1987;20:S229–S232. [PubMed] [Google Scholar]
- 37.Cassis LA, Lynch KR, Peach MJ. Localization of angiotensinogen messenger RNA in rat aorta. Circ Res. 1988;62:1259–1262. doi: 10.1161/01.res.62.6.1259. [DOI] [PubMed] [Google Scholar]
- 38.Cassis LA, Saye J, Peach MJ. Location and regulation of rat angiotensinogen messenger RNA. Hypertension. 1988;11:591–596. doi: 10.1161/01.hyp.11.6.591. [DOI] [PubMed] [Google Scholar]
- 39.Castellanos Rivera RM, Monteagudo MC, Pentz ES, Glenn ST, Gross KW, Carretero O, Sequeira-Lopez MLS, Gomez RA. Transcriptional regulator RBP-J regulates the number and plasticity of renin cells. Physiol Genomics. 2011;43:1021–1028. doi: 10.1152/physiolgenomics.00061.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castrop H, Hocherl K, Kurtz A, Schweda F, Todorov V, Wagner C. Physiology of kidney renin. Physiol Rev. 2010;90:607–673. doi: 10.1152/physrev.00011.2009. [DOI] [PubMed] [Google Scholar]
- 41.Castrop H, Schweda F, Mizel D, Huang Y, Briggs J, Kurtz A, Schnermann J. Permissive role of nitric oxide in macula densa control of renin secretion. Am J Physiol Renal Physiol. 2004;286:F848–F857. doi: 10.1152/ajprenal.00272.2003. [DOI] [PubMed] [Google Scholar]
- 42.Cechova S, Zeng Q, Billaud M, Mutchler S, Rudy CK, Straub AC, Chi L, Chan FR, Hu J, Griffiths R, Howell NL, Madsen K, Jensen BL, Palmer LA, Carey RM, Sung SS, Malakauskas SM, Isakson BE, Le TH. Loss of collectrin, an angiotensin-converting enzyme 2 homolog, uncouples endothelial nitric oxide synthase and causes hypertension and vascular dysfunction. Circulation. 2013;128:1770–1780. doi: 10.1161/CIRCULATIONAHA.113.003301. [DOI] [PubMed] [Google Scholar]
- 43.Celerier J, Cruz A, Lamande N, Gasc JM, Corvol P. Angiotensinogen and its cleaved derivatives inhibit angiogenesis. Hypertension. 2002;39:224–228. doi: 10.1161/hy0202.103441. [DOI] [PubMed] [Google Scholar]
- 44.Chappell MC. Nonclassical renin-angiotensin system and renal function. Compr Physiol. 2012;2:2733–2752. doi: 10.1002/cphy.c120002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen D, Bassi JK, Walther T, Thomas WG, Allen AM. Expression of angiotensin type 1A receptors in C1 neurons restores the sympathoexcitation to angiotensin in the rostral ventrolateral medulla of angiotensin type 1A knockout mice. Hypertension. 2010;56:143–150. doi: 10.1161/HYPERTENSIONAHA.110.151704. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Chen JK, Neilson EG, Harris RC. Role of EGF receptor activation in angiotensin II-induced renal epithelial cell hypertrophy. J Am Soc Nephrol. 2006;17:1615–1623. doi: 10.1681/ASN.2005111163. [DOI] [PubMed] [Google Scholar]
- 47.Chen L, Kim SM, Oppermann M, Faulhaber-Walter R, Huang Y, Mizel D, Chen M, Lopez ML, Weinstein LS, Gomez RA, Briggs JP, Schnermann J. Regulation of renin in mice with Cre recombinase-mediated deletion of G protein Gsalpha in juxtaglomerular cells. Am J Physiol Renal Physiol. 2007;292:F27–F37. doi: 10.1152/ajprenal.00193.2006. [DOI] [PubMed] [Google Scholar]
- 48.Cheng H, Harris R. Angiotensin converting enzyme inhibitor-mediated increases in renal renin expression are not seen in cyclooxygenase-2 knockout mice. J Am Soc Nephrol. 1999;10:343A. [Google Scholar]
- 49.Chiu A, Dunscomb J, McCall D, Benfield P, Baubonis W, Sauer B. Characterization of angiotensin AT1A receptor isoform by it ligand binding signature. Regul Pept. 1993;44:141–147. doi: 10.1016/0167-0115(93)90237-3. [DOI] [PubMed] [Google Scholar]
- 50.Chiu N, Park I, Reid IA. Stimulation of renin secretion by the phosphodiesterase IV inhibitor rolipram. J Pharmacol Exp Ther. 1996:1073–1077. [PubMed] [Google Scholar]
- 51.Chiu YJ, Hu SH, Reid IA. Inhibition of phosphodiesterase III with milrinone increases renin secretion in human subjects. J Pharmacol Exp Ther. 1999:16–19. [PubMed] [Google Scholar]
- 52.Churchill MC, Churchill PC. 12-0-Tetradecanoylphorbol 13-acetate inhibits renin secretion of rat renal cortical slices. J Hyperten. 1984;2:25–28. [Google Scholar]
- 53.Churchill PC. Second messengers in renin secretion. Am J Physiol Renal Physiol. 1985;249:F175–F184. doi: 10.1152/ajprenal.1985.249.2.F175. [DOI] [PubMed] [Google Scholar]
- 54.Churchill PC, Rossi NF, Churchill MC, Ellis VR. Effect of melittin on renin and prostaglandin E2 release from rat renal cortical slices. J Physiol. 1990;428:233–241. doi: 10.1113/jphysiol.1990.sp018209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clark AF, Sharp MG, Morley SD, Fleming S, Peters J, Mullins JJ. Renin-1 is essential for normal renal juxtaglomerular cell granulation and macula densa morphology. J Biol Chem. 1997;272:18185–18190. doi: 10.1074/jbc.272.29.18185. [DOI] [PubMed] [Google Scholar]
- 56.Clouston WM, Evans BA, Haralambidis J, Richards RI. Molecular cloning of the mouse angiotensinogen gene. Genomics. 1988;2:240–248. doi: 10.1016/0888-7543(88)90008-0. [DOI] [PubMed] [Google Scholar]
- 57.Cogan MG. Angiotensin II: A powerful controller of sodium transport in the early proximal tubule. Hypertension. 1990;15:451–458. doi: 10.1161/01.hyp.15.5.451. [DOI] [PubMed] [Google Scholar]
- 58.Corvol P, Williams TA, Soubrier F. Peptidyl dipeptidase A: Angiotensin I-converting enzyme. Methods Enzymol. 1995;248:283–305. doi: 10.1016/0076-6879(95)48020-x. [DOI] [PubMed] [Google Scholar]
- 59.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 60.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crowley SD, Tharaux PL, Audoly LP, Coffman TM. Exploring type I angiotensin (AT1) receptor functions through gene targeting. Acta Physiol Scand. 2004;181:561–570. doi: 10.1111/j.1365-201X.2004.01331.x. [DOI] [PubMed] [Google Scholar]
- 63.Crowley SD, Vasievich MP, Ruiz P, Gould SK, Parsons KK, Pazmino AK, Facemire C, Chen BJ, Kim HS, Tran TT, Pisetsky DS, Barisoni L, Prieto-Carrasquero MC, Jeansson M, Foster MH, Coffman TM. Glomerular type 1 angiotensin receptors augment kidney injury and inflammation in murine autoimmune nephritis. J Clin Invest. 2009;119:943–953. doi: 10.1172/JCI34862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crowley SD, Zhang J, Herrera M, Griffiths R, Ruiz P, Coffman TM. Role of AT1 receptor-mediated salt retention in angiotensin II-dependent hypertension. Am J Physiol Renal Physiol. 2011;301:F1124–F1130. doi: 10.1152/ajprenal.00305.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dahlof B. Left ventricular hypertrophy and angiotensin II antagonists. AM J Hyperten. 2001;14:174–182. doi: 10.1016/s0895-7061(00)01257-7. [DOI] [PubMed] [Google Scholar]
- 66.Danilczyk U, Sarao R, Remy C, Benabbas C, Stange G, Richter A, Arya S, Pospisilik JA, Singer D, Camargo SM, Makrides V, Ramadan T, Verrey F, Wagner CA, Penninger JM. Essential role for collectrin in renal amino acid transport. Nature. 2006;444:1088–1091. doi: 10.1038/nature05475. [DOI] [PubMed] [Google Scholar]
- 67.Data JL, Gerber JG, Crump WJ, Frolich JC, Hollifield JW, Nies AS. The prostaglandin system. A role in canine baroreceptor control of renin release. Circ Res. 1978;42:454–458. doi: 10.1161/01.res.42.4.454. [DOI] [PubMed] [Google Scholar]
- 68.Davisson RL, Oliverio MI, Coffman TM, Sigmund CD. Divergent functions of angiotensin II receptor isoforms in the brain. J Clin Invest. 2000;106:103–106. doi: 10.1172/JCI10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 70.Della Bruna R, Pinet F, Corvol P, Kurtz A. Calmodulin antagonists stimulate renin secretion and inhibit renin synthesis in vitro. Am J Physiol. 1992;262:F397–F402. doi: 10.1152/ajprenal.1992.262.3.F397. [DOI] [PubMed] [Google Scholar]
- 71.Denton KM, Anderson WP, Sinniah R. Effects of angiotensin II on regional afferent and efferent arteriole dimensions and the glomerular pole. Am J Physiol Regul Integr Comp Physiol. 2000;279:R629–R638. doi: 10.1152/ajpregu.2000.279.2.R629. [DOI] [PubMed] [Google Scholar]
- 72.Desch M, Harlander S, Neubauer B, Gerl M, Germain S, Castrop H, Todorov VT. cAMP target sequences enhCRE and CNRE sense low-salt intake to increase human renin gene expression in vivo. Pflugers Arch. 2011;461:567–577. doi: 10.1007/s00424-011-0956-z. [DOI] [PubMed] [Google Scholar]
- 73.Desch M, Schreiber A, Schweda F, Madsen K, Friis UG, Weather-ford ET, Sigmund CD, Sequeira Lopez ML, Gomez RA, Todorov VT. Increased renin production in mice with deletion of peroxisome proliferator-activated receptor-gamma in juxtaglomerular cells. Hypertension. 2010;55:660–666. doi: 10.1161/HYPERTENSIONAHA.109.138800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deschepper CF. Angiotensinogen: Hormonal regulation and relative importance in the generation of angiotensin II. Kidney Int. 1994;46:1561–1563. doi: 10.1038/ki.1994.446. [DOI] [PubMed] [Google Scholar]
- 75.DiBona GF. Neural regulation of renal tubular sodium reabsorption and renin secretion. Fed Proc. 1985;44:2816–2822. [PubMed] [Google Scholar]
- 76.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 77.Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R373–R381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dulin NO, Alexander LD, Harwalkar S, Falck JR, Douglas JG. Phospholipase A2-mediated activation of mitogen-activated protein kinase by angiotensin II. Proc Natl Acad Sci U S A. 1998;95:8098–8102. doi: 10.1073/pnas.95.14.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dworkin LD, Ichikawa I, Brenner BM. Hormonal modulation of glomerular function. Am J Physiol. 1983;244:F95–F104. doi: 10.1152/ajprenal.1983.244.2.F95. [DOI] [PubMed] [Google Scholar]
- 80.Eguchi S, Numaguchi K, Iwasaki H, Matsumoto T, Yamakawa T, Utsunomiya H, Motley ED, Kawakatsu H, Owada KM, Hirata Y, Marumo F, Inagami T. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J Biol Chem. 1998;273:8890–8896. doi: 10.1074/jbc.273.15.8890. [DOI] [PubMed] [Google Scholar]
- 81.Ehlers MR, Fox EA, Strydom DJ, Riordan JF. Molecular cloning of human testicular angiotensin-converting enzyme: The testis isozyme is identical to the C-terminal half of endothelial angiotensin-converting enzyme. Proc Natl Acad Sci U S A. 1989;86:7741–7745. doi: 10.1073/pnas.86.20.7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Esther C, Howard T, Marino E, Goddard J, Capecchi M, Bernstein K. Mice lacking angiotensin converting enzyme have low blood pressure, renal pathology, and reduced male fertility. LabInvest. 1996;74:953–965. [PubMed] [Google Scholar]
- 83.Facemire CS, Gurley SB. Minding the gap: Connexin 40 at the heart of renin release. J Am Soc Nephrol. 2011;22:985–986. doi: 10.1681/ASN.2011040395. [DOI] [PubMed] [Google Scholar]
- 84.Facemire CS, Nguyen M, Jania L, Beierwaltes WH, Kim HS, Koller BH, Coffman TM. A major role for the EP4 receptor in regulation of renin. Am J Physiol Renal Physiol. 2011;301:F1035–F1041. doi: 10.1152/ajprenal.00054.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ferrario C, Brosnihan K, Diz D, Jaiswal N, Khosla M, Milsted A, Tallant E. Angiotensin-(1-7): a new hormone of the angiotensin system. Hypertension. 1991;18:III-126–III-133. doi: 10.1161/01.hyp.18.5_suppl.iii126. [DOI] [PubMed] [Google Scholar]
- 86.Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289:H2281–H2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Field LJ, McGowan RA, Dickinson DP, Gross KW. Tissue and gene specificity of mouse renin expression. Hypertension. 1984;6:597–603. doi: 10.1161/01.hyp.6.4.597. [DOI] [PubMed] [Google Scholar]
- 88.Fournier D, Luft FC, Bader M, Ganten D, Andrade-Navarro MA. Emergence and evolution of the renin-angiotensin-aldosterone system. J Mol Med. 2012;90:495–508. doi: 10.1007/s00109-012-0894-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Francois H, Coffman TM. Prostanoids and blood pressure: Which way is up? J Clin Invest. 2004;114:757–759. doi: 10.1172/JCI22929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fray JC. Stretch receptor model for renin release with evidence from perfused rat kidney. Am J Physiol. 1976;231:936–944. doi: 10.1152/ajplegacy.1976.231.3.936. [DOI] [PubMed] [Google Scholar]
- 91.Fray JC. Regulation of renin secretion by calcium and chemiosmotic forces: (patho) physiological considerations. Biochim Biophys Acta. 1991;1097:243–262. doi: 10.1016/0925-4439(91)90078-n. [DOI] [PubMed] [Google Scholar]
- 92.Friis UG, Jensen BL, Aas JK, Skott O. Direct demonstration of exocytosis and endocytosis in single mouse juxtaglomerular cells. Circ Res. 1999:929–936. doi: 10.1161/01.res.84.8.929. [DOI] [PubMed] [Google Scholar]
- 93.Friis UG, Jensen BL, Sethi S, Andreasen D, Hansen PB, Skøtt O. Control of renin secretion from rat juxtaglomerular cells by cAMP-specific phosphodiesterases. Circ Res. 2002;90:996–1003. doi: 10.1161/01.res.0000017622.25365.71. [DOI] [PubMed] [Google Scholar]
- 94.Friis UG, Madsen K, Stubbe J, Hansen PBL, Svenningsen P, Bie P, Skøtt O, Jensen BL. Regulation of renin secretion by renal juxtaglomerular cells. Pflugers Arch. 2012;465:25–37. doi: 10.1007/s00424-012-1126-7. [DOI] [PubMed] [Google Scholar]
- 95.Fujino T, Nakagawa N, Yuhki K, Hara A, Yamada T, Takayama K, Kuriyama S, Hosoki Y, Takahata O, Taniguchi T, Fukuzawa J, Hasebe N, Kikuchi K, Narumiya S, Ushikubi F. Decreased susceptibility to renovascular hypertension in mice lacking the prostaglandin I2 receptor IP. J Clin Invest. 2004;114:805–812. doi: 10.1172/JCI21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fukamizu A, Nishi K, Cho T, Saitoh M, Nakayama K, Ohkubo H, Nakanishi S, Murakami K. Structure of the rat renin gene. J Mol Biol. 1988;201:443–450. doi: 10.1016/0022-2836(88)90151-9. [DOI] [PubMed] [Google Scholar]
- 97.Gainer JV, Morrow JD, Loveland A, King DJ, Brown NJ. Effect of bradykinin-receptor blockade on the response to angiotensin-converting-enzyme inhibitor in normotensive and hypertensive subjects. N Engl J Med. 1998;339:1285–1292. doi: 10.1056/NEJM199810293391804. [DOI] [PubMed] [Google Scholar]
- 98.Gambaryan S, Ḧausler C, Markert T, Pöhler D, Jarchau T, Walter U, Haase W, Kurtz A, Lohmann SM. Expression of type II cGMP-dependent protein kinase in rat kidney is regulated by dehydration and correlated with renin gene expression. J Clin Invest. 1996;98:662–670. doi: 10.1172/JCI118837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gasc JM, Shanmugam S, Sibony M, Corvol P. Tissue-specific expression of type 1 angiotensin II receptor subtypes. An in situ hybridization study. Hypertension. 1994;24:531–537. doi: 10.1161/01.hyp.24.5.531. [DOI] [PubMed] [Google Scholar]
- 100.Geibel J, Giebisch G, Boron WF. Angiotensin II stimulates both Na()-H mal+ + exchange and Na+/HCO3-cotransport in the rabbit proxitubule. Proc Natl Acad Sci U S A. 1990;87:7917–7920. doi: 10.1073/pnas.87.20.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Geisterfer A, Peach M, Owens G. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988;62:749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- 102.Gelosa P, Pignieri A, Fandriks L, de Gasparo M, Hallberg A, Banfi C, Castiglioni L, Turolo L, Guerrini U, Tremoli E, Sironi L. Stimulation of AT2 receptor exerts beneficial effects in stroke-prone rats: Focus on renal damage. J Hypertens. 2009;27:2444–2451. doi: 10.1097/HJH.0b013e3283311ba1. [DOI] [PubMed] [Google Scholar]
- 103.Gerber JG, Keller RT, Nies AS. Prostaglandins and renin release: The effect of PGI2, PGE2, and 13,14-dihydro PGE2 on the baroreceptor mechanism of renin release in the dog. Circ Res. 1979;44:796–799. doi: 10.1161/01.res.44.6.796. [DOI] [PubMed] [Google Scholar]
- 104.Glenn ST, Jones CA, Gross KW, Pan L. Control of rene gene expression. Pfliigers Archiv. 2012;465:13–21. doi: 10.1007/s00424-012-1110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gomez RA, Chevalier RL, Everett AD, Elwood JP, Peach MJ, Lynch KR, Carey RM. Recruitment of renin gene-expressing cells in adult rat kidneys. Am J Physiol. 1990;259:F660–F665. doi: 10.1152/ajprenal.1990.259.4.F660. [DOI] [PubMed] [Google Scholar]
- 106.Gomez RA, Pentz ES, Jin X, Cordaillat M, Sequeira-Lopez MLS. CBP and p300 are essential for renin cell identity and morphological integrity of the kidney. Am J Physiol Heart CircPhysiol. 2009;296:H1255–H1262. doi: 10.1152/ajpheart.01266.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gomez RA, Lopez MLS. Who and where is the renal baroreceptor?: The connexin hypothesis. Kidney Int. 2009;75:460–462. doi: 10.1038/ki.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gonzalez-Villalobos RA, Shen XZ, Bernstein EA, Janjulia T, Taylor B, Giani JF, Blackwell WL, Shah KH, Shi PD, Fuchs S, Bernstein KE. Rediscovering ACE: Novel insights into the many roles of the angiotensin-converting enzyme. J Mol Med. 2013 doi: 10.1007/s00109-013-1051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Goormaghtigh NJ. Facts in favor of an endocrine function of the renal arterioles. Pathol Bacteriol. 1945;57:392–393. [Google Scholar]
- 110.Gould AB, Green D. Kinetics of the human renin and human substrate reaction. Cardiovasc Res. 1971;5:86–89. doi: 10.1093/cvr/5.1.86. [DOI] [PubMed] [Google Scholar]
- 111.Grady E, Sechi L, Griffn C, Schambelan M, Kalinyak J. Expression of AT2 receptors in the developing rat fetus. J Clin Invest. 1991;88:921–933. doi: 10.1172/JCI115395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Griendling KK, Ushio-Fukai M, Lassegue B, Alexander RW. Angiotensin II signaling in vascular smooth muscle. New concepts. Hypertension. 1997;29:366–373. doi: 10.1161/01.hyp.29.1.366. [DOI] [PubMed] [Google Scholar]
- 113.Gross KW, Gomez RA, Sigmund CD. Twists and turns in the search for the elusive renin processing enzyme: Focus on ‘Cathepsin B is not the processing enzyme for mouse prorenin’. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1209–R1211. doi: 10.1152/ajpregu.00188.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grunberger C, Obermayer B, Klar J, Kurtz A, Schweda F. The calcium paradoxon of renin release: Calcium suppresses renin exocytosis by inhibition of calcium-dependent adenylate cyclases AC5 and AC6. Circ Res. 2006;99:1197–1206. doi: 10.1161/01.RES.0000251057.35537.d3. [DOI] [PubMed] [Google Scholar]
- 115.Gurley SB, Allred A, Le TH, Griffiths R, Mao L, Philip N, Haystead TA, Donoghue M, Breitbart RE, Acton SL, Rockman HA, Coffman TM. Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J Clin Invest. 2006;116:2218–2225. doi: 10.1172/JCI16980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gurley SB, Coffman TM. Angiotensin-converting enzyme 2 gene targeting studies in mice: Mixed messages. Exp Physiol. 2008;93:538–542. doi: 10.1113/expphysiol.2007.040014. [DOI] [PubMed] [Google Scholar]
- 117.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metabol. 2011;13:469–475. doi: 10.1016/j.cmet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, Chen Y, Modiri AN, Judge DP, Dietz HC. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 2011;332:361–365. doi: 10.1126/science.1192152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 1990;70:1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 121.Hanner F, Chambrey R, Bourgeois S, Meer E, Mucsi I, Rosivall L, Shull GE, Lorenz JN, Eladari D, Peti-Peterdi J. Increased renal renin content in mice lacking the Na /H Renal + + exchanger NHE2. Am J Physiol Physiol. 2008;294:F937–F944. doi: 10.1152/ajprenal.00591.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hansen PB, Yang T, Huang Y, Mizel D, Briggs J, Schnermann J. Plasma renin in mice with one or two renin genes. Acta Physiol Scand. 2004;181:431–437. doi: 10.1111/j.1365-201X.2004.01315.x. [DOI] [PubMed] [Google Scholar]
- 123.Harding P, Sigmon DH, Alfie ME, Huang PL, Fishman MC, Beierwaltes WH, Carretero OA. Cyclooxygenase-2 mediates increased renal renin content induced by low-sodium diet. Hypertension. 1997;29:297–302. doi: 10.1161/01.hyp.29.1.297. [DOI] [PubMed] [Google Scholar]
- 124.Hardman JA, Hort YJ, Catanzaro DF, Tellam JT, Baxter JD, Morris BJ, Shine J. Primary structure of the human renin gene. DNA. 1984;3:457–468. doi: 10.1089/dna.1.1984.3.457. [DOI] [PubMed] [Google Scholar]
- 125.Harris RC, Breyer MD. Physiological regulation of cyclooxygenase-2 in the kidney. Am J Physiol Renal Physiol. 2001;281:F1–F11. doi: 10.1152/ajprenal.2001.281.1.F1. [DOI] [PubMed] [Google Scholar]
- 126.Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hayashida W, Horiuchi M, Dzau VJ. Intracellular third loop domain of angiotensin II type-2 receptor. Role in mediating signal transduction and cellular function. J Biol Chem. 1996;271:21985–21992. doi: 10.1074/jbc.271.36.21985. [DOI] [PubMed] [Google Scholar]
- 128.He XR, Greenberg SG, Briggs JP, Schnermann JB. Effect of nitric oxide on renin secretion. II. Studies in the perfused juxtaglomerular apparatus. Am J Physiol. 1995;268:F953–F959. doi: 10.1152/ajprenal.1995.268.5.F953. [DOI] [PubMed] [Google Scholar]
- 129.Hein L, Barsh GS, Pratt RE, Dzau VJ, Kobilka BK. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature. 1995;377:744–747. doi: 10.1038/377744a0. [DOI] [PubMed] [Google Scholar]
- 130.Henke N, Schmidt-Ullrich R, Dechend R, Park J-K, Qadri F, Wellner M, Obst M, Gross V, Dietz R, Luft FC, Scheidereit C, Muller DN. Vascular endothelial cell-Specific NF-κB suppression attenuates hypertension-induced renal damage. Circ Res. 2007;101:268–276. doi: 10.1161/CIRCRESAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- 131.Henrich WL, McAllister EA, Smith PB, Campbell WB. Guanosine 3′,5′-cyclic monophosphate as a mediator of inhibition of renin release. Am J Physiol. 1988;255:F474–F478. doi: 10.1152/ajprenal.1988.255.3.F474. [DOI] [PubMed] [Google Scholar]
- 132.Herrera M, Sparks MA, Alfonso-Pecchio AR, Harrison-Bernard LM, Coffman TM. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type 1 receptor protein. Hypertension. 2013;61:253–258. doi: 10.1161/HYPERTENSIONAHA.112.203679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hilgenfeldt U. Half-life of rat angiotensinogen: Influence of nephrectomy and lipopolysaccharide stimulation. Mol Cell Endocrinol. 1988;56:91–98. doi: 10.1016/0303-7207(88)90012-3. [DOI] [PubMed] [Google Scholar]
- 134.Hiyoshi H, Yayama K, Takano M, Okamoto H. Angiotensin type 2 receptor-mediated phosphorylation of eNOS in the aortas of mice with 2-kidney, 1-clip hypertension. Hypertension. 2005;45:967–973. doi: 10.1161/01.HYP.0000164571.77710.19. [DOI] [PubMed] [Google Scholar]
- 135.Hobart PM, Fogliano M, O’Connor BA, Schaefer IM, Chirgwin JM. Human renin gene: Structure and sequence analysis. Proc Natl Acad Sci U S A. 1984;81:5026–5030. doi: 10.1073/pnas.81.16.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2009;296:R208–R216. doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hofbauer KG, Zschiedrich H, Hackenthal E, Gross F. Function of the renin-angtiotensin system in the isolated perfused rat kidney. Circ Res. 1974:I-193–I-202. [Google Scholar]
- 138.Holdaas H, DiBona GF, Kiil F. Effect of low-level renal nerve stimulation on renin release from nonfiltering kidneys. Am J Physiol. 1981;241:F156–F161. doi: 10.1152/ajprenal.1981.241.2.F156. [DOI] [PubMed] [Google Scholar]
- 139.Holdaas H, Langard O, Eide I, Kiil F. Mechanism of renin release during renal nerve stimulation in dogs. Scand J Clin Lab Invest. 1981;41:617–625. doi: 10.3109/00365518109090506. [DOI] [PubMed] [Google Scholar]
- 140.Howard T, Balogh R, Overbeek P, Bernstein KE. Sperm-specific expression of angiotensin-converting enzyme (ACE) is mediated by a 91-base-pair promoter containing a CRE-like element. Mol Cell Biol. 1993;13:18–27. doi: 10.1128/mcb.13.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Howard TE, Shai SY, Langford KG, Martin BM, Bernstein KE. Transcription of testicular angiotensin-converting enzyme (ACE) is initiated within the 12th intron of the somatic ACE gene. Mol Cell Biol. 1990;10:4294–4302. doi: 10.1128/mcb.10.8.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hsueh WA, Baxter JD. Human prorenin. Hypertension. 1991;17:469–477. doi: 10.1161/01.hyp.17.4.469. [DOI] [PubMed] [Google Scholar]
- 143.Huang W, Gallois Y, Bouby N, Bruneval P, Heudes D, Belair MF, Krege JH, Meneton P, Marre M, Smithies O, Alhenc-Gelas F. Genetically increased angiotensin I-converting enzyme level and renal complications in the diabetic mouse. Proc Natl Acad Sci U S A. 2001;98:13330–13334. doi: 10.1073/pnas.231476798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hubert C, Houot AM, Corvol P, Soubrier F. Structure of the angiotensin I-converting enzyme gene. Two alternate promoters correspond to evolutionary steps of a duplicated gene. J Biol Chem. 1991;266:15377–15383. [PubMed] [Google Scholar]
- 145.Husain A, Graham R. Drugs, Enzymes and Receptors of the Renin-Angiotensin System: Celebrating a Century of Discovery. Harwood Academic; Sidney: 2000. [Google Scholar]
- 146.Ichihara A, Hayashi M, Hirota N, Okada H, Koura Y, Tada Y, Kaneshiro Y, Tsuganezawa H, Saruta T. Angiotensin II type 2 receptor inhibits prorenin processing in juxtaglomerular cells. Hypertens Res. 2003;26:915–921. doi: 10.1291/hypres.26.915. [DOI] [PubMed] [Google Scholar]
- 147.Ichiki T, Kambayashi Y, Inagami T. Multiple growth factors modulate mRNA expression of angiotensin II type-2 receptor in R3T3 cells. Circ Res. 1995;77:1070–1076. doi: 10.1161/01.res.77.6.1070. [DOI] [PubMed] [Google Scholar]
- 148.Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, Niimura F, Ichikawa I, Hogan BL, Inagami T. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995;377:748–750. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
- 149.Imai T, Miyazaki H, Hirose S, Hori H, Hayashi T, Kageyama R, Ohkubo H, Nakanishi S, Murakami K. Cloning and sequence analysis of cDNA for human renin precursor. Proc Natl Acad Sci U S A. 1983;80:7405–7409. doi: 10.1073/pnas.80.24.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Inagami T, Iwai N, Sasaki K, Yamamo Y, Bardhan S, Chaki S, Guo DF, Furuta H. Cloning, expression and regulation of angiotensin II receptors. J Hypertens. 1992;10:713–716. [PubMed] [Google Scholar]
- 151.Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest. 1990;85:417–423. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Inoue I, Nakajima T, Williams CS, Quackenbush J, Puryear R, Powers M, Cheng T, Ludwig EH, Sharma AM, Hata A, Jeunemaitre X, Lalouel JM. A nucleotide substitution in the promoter of human angiotensinogen is associated with essential hypertension and affects basal transcription in vitro. J Clin Invest. 1997;99:1786–1797. doi: 10.1172/JCI119343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Intengan H, Schiffrin E. Vascular remodeling in hypertension: Roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 154.Investigators TS, The SOLVD Investigators Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:725–727. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 155.Investigators TS, The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 156.Itani HA, Liu X, Pratt JH, Sigmund CD. Functional characterization of polymorphisms in the kidney enhancer of the human renin gene. Endocrinology. 2007;148:1424–1430. doi: 10.1210/en.2006-1381. [DOI] [PubMed] [Google Scholar]
- 157.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci U S A. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Iwai N, Inagami T. Identification of two subtypes in the rat type I angiotensin receptor. FEBS Letts. 1992;298:257–260. doi: 10.1016/0014-5793(92)80071-n. [DOI] [PubMed] [Google Scholar]
- 159.Iwai N, Inagami T, Ohmichi N, Nakamura Y, Saeki Y, Kinoshita M. Differential regulation of rat AT1a and AT1b receptor mRNA. Biochem Biophys Res Commun. 1992;188:298–303. doi: 10.1016/0006-291x(92)92384-a. [DOI] [PubMed] [Google Scholar]
- 160.Jacobsen P, Tarnow L, Carstensen B, Hovind P, Poirier O, Parving HH. Genetic variation in the Renin-Angiotensin system and progression of diabetic nephropathy. J Am Soc Nephrol. 2003;14:2843–2850. doi: 10.1097/01.asn.0000092139.19587.51. [DOI] [PubMed] [Google Scholar]
- 161.Jeunemaitre X, Soubrier F, Kotelevtsev YV, Lifton RP, Williams CS, Charru A, Hunt SC, Hopkins PN, Williams RR, Lalouel JM, Corvol P. Molecular basis of human hypertension: Role of angiotensinogen. Cell. 1992;71:169–180. doi: 10.1016/0092-8674(92)90275-h. [DOI] [PubMed] [Google Scholar]
- 162.Jones CA, Sigmund CD, McGowan RA, Kane-Haas CM, Gross KW. Expression of murine renin genes during fetal development. Mol Endocrinol. 1990;4:375–383. doi: 10.1210/mend-4-3-375. [DOI] [PubMed] [Google Scholar]
- 163.Kakar S, Riel K, Neill J. Differential expression of angiotensin II receptor subtype mRNAs (AT-1A and AT-1B) in the brain. Biochem Biophys Res Comm. 1992;185:688–692. doi: 10.1016/0006-291x(92)91680-o. [DOI] [PubMed] [Google Scholar]
- 164.Kakoki M, Kizer CM, Yi X, Takahashi N, Kim HS, Bagnell CR, Edgell CJ, Maeda N, Jennette JC, Smithies O. Senescence-associated phenotypes in Akita diabetic mice are enhanced by absence of bradykinin B2 receptors. J Clin Invest. 2006;116:1302–1309. doi: 10.1172/JCI26958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kakoki M, Smithies O. The kallikrein-kinin system in health and in diseases of the kidney. Kidney Int. 2009;75:1019–1030. doi: 10.1038/ki.2008.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kakoki M, Sullivan KA, Backus C, Hayes JM, Oh SS, Hua K, Gasim AM, Tomita H, Grant R, Nossov SB, Kim HS, Jennette JC, Feldman EL, Smithies O. Lack of both bradykinin B1 and B2 receptors enhances nephropathy, neuropathy, and bone mineral loss in Akita diabetic mice. Proc Natl Acad Sci U S A. 2010;107:10190–10195. doi: 10.1073/pnas.1005144107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kakoki M, Takahashi N, Jennette JC, Smithies O. Diabetic nephropathy is markedly enhanced in mice lacking the bradykinin B2 receptor. Proc Natl Acad Sci U S A. 2004;101:13302–13305. doi: 10.1073/pnas.0405449101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kamiyama M, Zsombok A, Kobori H. Urinary angiotensinogen as a novel early biomarker of intrarenal renin-angiotensin system activation in experimental type 1 diabetes. J Pharmacol Sci. 2012;119:314–323. doi: 10.1254/jphs.12076fp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kanwar YS, Venkatachalam MA. Comprehensive Physiology. John Wiley & Sons, Inc; 2010. Ultrastructure of Glomerulus and Juxtaglomerular Apparatus. [Google Scholar]
- 170.Kaschina E, Grzesiak A, Li J, Foryst-Ludwig A, Timm M, Rompe F, Sommerfeld M, Kemnitz UR, Curato C, Namsolleck P, Tschöpe C, Hallberg A, Alterman M, Hucko T, Paetsch I, Dietrich T, Schnack-enburg B, Graf K, Dahlöf B, Kintscher U, Unger T, Steckelings UM. Angiotensin II type 2 receptor stimulation: A novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation. 2008;118:2523–2532. doi: 10.1161/CIRCULATIONAHA.108.784868. [DOI] [PubMed] [Google Scholar]
- 171.Keeton TK, Campbell WB. The pharmacologic alteration of renin release. Pharmacol Rev. 1980;32:81–227. [PubMed] [Google Scholar]
- 172.Kihara M, Umemura S, Sumida Y, Yokoyama N, Yabana M, Nyui N, Tamura K, Murakami K, Fukamizu A, Ishii M. Kidney Int Genetic deficiency of angiotensinogen produces an impaired urine concentrating ability in mice. 1998;53:548. doi: 10.1046/j.1523-1755.1998.00801.x. [DOI] [PubMed] [Google Scholar]
- 173.Kim HS, Krege JH, Kluckman KD, Hagaman JR, Hodgin JB, Best CF, Jennette JC, Coffman TM, Maeda N, Smithies O. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci U S A. 1995;92:2735–2739. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kim SM, Mizel D, Huang YG, Briggs JP, Schnermann J. Adenosine as a mediator of macula densa-dependent inhibition of renin secretion. Am J Physiol Renal Physiol. 2006;290:F1016–F1023. doi: 10.1152/ajprenal.00367.2005. [DOI] [PubMed] [Google Scholar]
- 175.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 176.Kirchheim H, Ehmke H, Persson P. Physiology of the renal baroreceptor mechanism of renin release and its role in congestive heart failure. Am J Cardiol. 1988;62:68E–71E. doi: 10.1016/s0002-9149(88)80015-8. [DOI] [PubMed] [Google Scholar]
- 177.Kirchheim HR, Gross R, Hackenberg HM, Hackenthal E, Huber J. Autoregulation of renin release and its modification by renal sympathetic nerves in conscious dogs. Kidney Inst. 1981;20:152. [Google Scholar]
- 178.Knoblich PR, Freeman RH, Villarreal D. Pressure-dependent renin release during chronic blockade of nitric oxide synthase. Hypertension. 1996;28:738–742. doi: 10.1161/01.hyp.28.5.738. [DOI] [PubMed] [Google Scholar]
- 179.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 181.Konishi H, Kuroda S, Inada Y, Fujisawa Y. Novel subtype of human angiotensin II type 1 receptor: cDNA cloning and expression. Biochem Biophys Res Commun. 1994;199:467–474. doi: 10.1006/bbrc.1994.1252. [DOI] [PubMed] [Google Scholar]
- 182.Konishi Y, Nishiyama A, Morikawa T, Kitabayashi C, Shibata M, Hamada M, Kishida M, Hitomi H, Kiyomoto H, Miyashita T, Mori N, Urushihara M, Kobori H, Imanishi M. Relationship between urinary angiotensinogen and salt sensitivity of blood pressure in patients with IgA nephropathy. Hypertension. 2011;58:205–211. doi: 10.1161/HYPERTENSIONAHA.110.166843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Konoshita T, Fuchs S, Makino Y, Wakahara S, Miyamori I. A proximal direct repeat motif characterized as a negative regulatory element in the human renin gene. J Cell Biochem. 2007;102:1043–1050. doi: 10.1002/jcb.21341. [DOI] [PubMed] [Google Scholar]
- 184.Kotchen TA, Galla JH, Luke RG. Failure of NaHCO3 and KHCO3 to inhibit renin in the rat. Am J Physiol. 1976;231:1050–1056. doi: 10.1152/ajplegacy.1976.231.4.1050. [DOI] [PubMed] [Google Scholar]
- 185.Krattinger N, Capponi A, Mazzolai L, Aubert JF, Caille D, Nicod P, Waeber G, Meda P, Haefliger JA. Connexin40 regulates renin production and blood pressure. Kidney Int. 2007;72:814–822. doi: 10.1038/sj.ki.5002423. [DOI] [PubMed] [Google Scholar]
- 186.Krege J, John S, Langenbach L, Hodgin J, Hagaman J, Bachman E, Jennette J, O’Brien D, Smithies O. Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature. 1995;375:146–149. doi: 10.1038/375146a0. [DOI] [PubMed] [Google Scholar]
- 187.Kuba K, Imai Y, Ohto-Nakanishi T, Penninger JM. Trilogy of ACE2: A peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128:119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kuba K, Imai Y, Rao S, Jiang C, Penninger JM. Lessons from SARS: Control of acute lung failure by the SARS receptor ACE2. J Mol Med. 2006;84:814–820. doi: 10.1007/s00109-006-0094-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kurtz A, Della Bruna R, Pfeilschifter J, Taugner R, Bauer C. Atrial natriuretic peptide inhibits renin release from juxtaglomerular cells by a cGMP-mediated process. Proc Natl Acad Sci U S A. 1986;83:4769–4773. doi: 10.1073/pnas.83.13.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kurtz A, Götz KH, Hamann M, Wagner C. Stimulation of renin secretion by nitric oxide is mediated by phosphodiesterase 3. Proc Natl Acad Sci U S A. 1998;95:4743–4747. doi: 10.1073/pnas.95.8.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kurtz A, Penner R. Angiotensin II induces oscillations of intracellular calcium and blocks anomalous inward rectifying potassium current in mouse renal juxtaglomerular cells. Proc Natl Acad Sci U S A. 1989;86:3423–3427. doi: 10.1073/pnas.86.9.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kurtz A, Pfeilschifter J, Hutter A, Bührle C, Nobiling R, Taugner R, Hackenthal E, Bauer C. Role of protein kinase C in inhibition of renin release caused by vasoconstrictors. Am J Physiol. 1986;250:C563–C571. doi: 10.1152/ajpcell.1986.250.4.C563. [DOI] [PubMed] [Google Scholar]
- 194.Kurtz A, Wagner C. Role of nitric oxide in the control of renin secretion. Am J Physiol. 1998;275:F849–F862. doi: 10.1152/ajprenal.1998.275.6.F849. [DOI] [PubMed] [Google Scholar]
- 195.Kwon T-H,, Nielsen J, Knepper MA, Frokiaer J, Nielsen S. Angiotensin II AT1 receptor blockade decreases vasopressin-induced water reabsorption and AQP2 levels in NaCl-restricted rats. Am J Physiol Renal Physiol. 2005;288:F673–F684. doi: 10.1152/ajprenal.00304.2004. [DOI] [PubMed] [Google Scholar]
- 196.Lalli E, Sassone-Corsi P. Signal transduction and gene regulation: The nuclear response to cAMP. J Biol Chem. 1994;269:17359–17362. [PubMed] [Google Scholar]
- 197.Langford KG, Shai SY, Howard TE, Kovac MJ, Overbeek PA, Bernstein KE. Transgenic mice demonstrate a testis-specific promoter for angiotensin-converting enzyme. J Biol Chem. 1991;266:15559–15562. [PubMed] [Google Scholar]
- 198.Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: A new therapeutic approach. Nat Med. 2005;11:867–874. doi: 10.1038/nm1275. [DOI] [PubMed] [Google Scholar]
- 199.Law RH, Zhang Q, McGowan S, Buckle AM, Silverman GA, Wong W, Rosado CJ, Langendorf CG, Pike RN, Bird PI, Whisstock JC. An overview of the serpin superfamily. Genome Biol. 2006;7:216. doi: 10.1186/gb-2006-7-5-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Le TH, Coffman TM. A new cardiac MASTer switch for the renin-angiotensin system. J Clin Invest. 2006;116:866–869. doi: 10.1172/JCI28312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ledoussal C, Lorenz JN, Nieman ML, Soleimani M, Schultheis PJ, Shull GE. Renal salt wasting in mice lacking NHE3 Na /H exchanger but not in mice lacking NHE2. Am J Physiol Renal Physiol. 2001;281:F718–F727. doi: 10.1152/ajprenal.2001.281.4.F718. [DOI] [PubMed] [Google Scholar]
- 202.Lehtonen JY, Daviet L, Nahmias C, Horiuchi M, Dzau VJ. Analysis of functional domains of angiotensin II type 2 receptor involved in apoptosis. Mol Endocrinol. 1999;13:1051–1060. doi: 10.1210/mend.13.7.0303. [DOI] [PubMed] [Google Scholar]
- 203.Lerolle N, Bourgeois S, Leviel F, Lebrun G, Paillard M, Houillier P. Angiotensin II inhibits NaCl absorption in the rat medullary thick ascending limb. Am J Physiol Renal Physiol. 2004;287:F404–F410. doi: 10.1152/ajprenal.00265.2003. [DOI] [PubMed] [Google Scholar]
- 204.Levine DZ, Iacovitti M, Buckman S, Burns KD. Role of angiotensin II in dietary modulation of rat late distal tubule bicarbonate flux in vivo. J Clin Invest. 1996;97:120–125. doi: 10.1172/JCI118378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lew R, Summers RJ. The distribution of beta-adrenoceptors in dog kidney: An autoradiographic analysis. Eur J Pharmacol. 1987;140:1–11. doi: 10.1016/0014-2999(87)90627-3. [DOI] [PubMed] [Google Scholar]
- 206.Lewicki JA, Printz JM, Printz MP. Clearance of rabbit plasma angiotensinogen and relationship to CSF angiotensinogen. Am J Physiol. 1983;244:H577–H585. doi: 10.1152/ajpheart.1983.244.4.H577. [DOI] [PubMed] [Google Scholar]
- 207.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD, The Collaborative Study Group The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 208.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 209.Lewis EJ, Lewis JB. ACE inhibitors versus angiotensin receptor blockers in diabetic nephropathy: Is there a winner? J Am Soc Nephrol. 2004;15:1358–1360. [PubMed] [Google Scholar]
- 210.Li C, Wang W, Rivard CJ, Lanaspa MA, Summer S, Schrier RW. Molecular mechanisms of angiotensin II stimulation on aquaporin-2 expression and trafficking. Am J Physiol Renal Physiol. 2011;300:F1255–F1261. doi: 10.1152/ajprenal.00469.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li XC, Hopfer U, Zhuo JL. AT1 receptor-mediated uptake of angiotensin II and NHE-3 expression in proximal tubule cells through a microtubule-dependent endocytic pathway. Am J Physiol Renal Physiol. 2009;297:F1342–F1352. doi: 10.1152/ajprenal.90734.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Li YC, Kong J, Wei M, Chen Z-F, Liu SQ, Cao L-P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Liu A, Ballermann BJ. TGF-beta type II receptor in rat renal vascular development: Localization to juxtaglomerular cells. Kidney Int. 1998;53:716–725. doi: 10.1046/j.1523-1755.1998.00810.x. [DOI] [PubMed] [Google Scholar]
- 214.Liu X, Huang X, Sigmund CD. Identification of a nuclear orphan receptor (Ear2) as a negative regulator of renin gene transcription. Circ Res. 2003;92:1033–1040. doi: 10.1161/01.RES.0000071355.82009.43. [DOI] [PubMed] [Google Scholar]
- 215.Llorens-Cortes C, Greenberg B, Huang H, Corvol P. Tissular expression and regulation of type 1 angiotensin II receptor subtypes by quantitative reverse transcriptase-polymerase chain reaction analysis. Hypertension. 1994;24:538–548. doi: 10.1161/01.hyp.24.5.538. [DOI] [PubMed] [Google Scholar]
- 216.Lorenz JN, Weihprecht H, He XR, Skott O, Briggs JP, Schnermann J. Effects of adenosine and angiotensin on macula densa-stimulated renin secretion. Am J Physiol. 1993;265:F187–F194. doi: 10.1152/ajprenal.1993.265.2.F187. [DOI] [PubMed] [Google Scholar]
- 217.Lorenz JN, Weihprecht H, Schnermann J, Skott O, Briggs JP. Renin release from isolated juxtaglomerular apparatus depends on macula densa chloride transport. Am J Physiol. 1991;260:F486–F493. doi: 10.1152/ajprenal.1991.260.4.F486. [DOI] [PubMed] [Google Scholar]
- 218.Lovis P, Gattesco S, Regazzi R. Regulation of the expression of components of the exocytotic machinery of insulin-secreting cells by microR-NAs. Biol Chem. 2008;389:305–312. doi: 10.1515/BC.2008.026. [DOI] [PubMed] [Google Scholar]
- 219.Lubkemeier I, Machura K, Kurtz L, Neubauer B, Dobrowolski R, Schweda F, Wagner C, Willecke K, Kurtz A. The connexin 40 A96S mutation causes renin-dependent hypertension. J Am Soc Nephrol. 2011;22:1031–1040. doi: 10.1681/ASN.2010101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lum C. Cardiovascular and renal phenotype in mice with one or two renin genes. Hypertension. 2003;43:79–86. doi: 10.1161/01.HYP.0000107401.72456.50. [DOI] [PubMed] [Google Scholar]
- 221.Lynch KR, Peach MJ. Molecular biology of angiotensinogen. Hypertension. 1991;17:263–269. doi: 10.1161/01.hyp.17.3.263. [DOI] [PubMed] [Google Scholar]
- 222.Mackins CJ, Kano S, Seyedi N, Scḧafer U, Reid AC, Machida T, Silver RB, Levi R. Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion. J Clin Invest. 2006;116:1063–1070. doi: 10.1172/JCI25713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Malakauskas SM, Kourany WM, Zhang XY, Lu D, Stevens RD, Koves TR, Hohmeier HE, Muoio DM, Newgard CB, Le TH. Increased insulin sensitivity in mice lacking collectrin, a downstream target of HNF-1alpha. Mol Endocrinol. 2009;23:881–892. doi: 10.1210/me.2008-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Malakauskas SM, Quan H, Fields TA, McCall SJ, Yu MJ, Kourany WM, Frey CW, Le TH. Aminoaciduria and altered renal expression of luminal amino acid transporters in mice lacking novel gene collectrin. Am J Physiol Renal Physiol. 2007;292:F533–F544. doi: 10.1152/ajprenal.00325.2006. [DOI] [PubMed] [Google Scholar]
- 225.Margolius HS. Kallikreins and kinins. Molecular characteristics and cellular and tissue responses. Diabetes. 1996;45(Suppl 1):S14–S19. doi: 10.2337/diab.45.1.s14. [DOI] [PubMed] [Google Scholar]
- 226.Markus MA, Goy C, Adams DJ, Lovicu FJ, Morris BJ. Renin enhancer is crucial for full response in Renin expression to an in vivo stimulus. Hypertension. 2007;50:933–938. doi: 10.1161/HYPERTENSIONAHA.107.096891. [DOI] [PubMed] [Google Scholar]
- 227.Marques FZ, Campain AE, Tomaszewski M, Zukowska-Szczechowska E, Yang YHJ, Charchar FJ, Morris BJ. Gene expression profiling reveals renin mRNA overexpression in human hypertensive kidneys and a role for microRNAs. Hypertension. 2011;58:1093–1098. doi: 10.1161/HYPERTENSIONAHA.111.180729. [DOI] [PubMed] [Google Scholar]
- 228.Marrero M, Schieffer B, Paxton W, Heerdt L, Berk B, Delafontaine P, Bernstein K. Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature. 1995;375:247–250. doi: 10.1038/375247a0. [DOI] [PubMed] [Google Scholar]
- 229.Martinez-Maldonado M, Gely R, Tapia E, Benabe JE. Role of macula densa in diuretics-induced renin release. Hypertension. 1990;16:261–268. doi: 10.1161/01.hyp.16.3.261. [DOI] [PubMed] [Google Scholar]
- 230.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res. 2010;107:263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Masaki H, Kurihara T, Yamaki A, Inomata N, Nozawa Y, Mori Y, Murasawa S, Kizima K, Maruyama K, Horiuchi M, Dzau VJ, Takahashi H, Iwasaka T, Inada M, Matsubara H. Cardiac-specific overexpression of angiotensin II AT2 receptor causes attenuated response to AT1 receptor-mediated pressor and chronotropic effects. J Clin Invest. 1998;101:527–535. doi: 10.1172/JCI1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Matavelli LC, Huang J, Siragy HM. Angiotensin AT2 receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension. 2011;57:308–313. doi: 10.1161/HYPERTENSIONAHA.110.164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, Nishiyama A, Ichikawa I. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012;23:1181–1189. doi: 10.1681/ASN.2011121159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Matsusaka T, Nishimura H, Utsunomiya H, Kakuchi J, Niimura F, Inagami T, Fogo A, Ichikawa I. Chimeric mice carrying ‘regional’ targeted deletion of the angiotensin type 1A receptor gene. Evidence against the role for local angiotensin in the in vivo feedback regulation of renin synthesis in juxtaglomerular cells. J Clin Invest. 1996;98:1867–1877. doi: 10.1172/JCI118988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Matsushita K, Zhang Z, Pratt RE, Dzau VJ. Molecular mechanism of juxtaglomerular cell hyperplasia: A unifying hypothesis. J Am Soc Hypertens. 2007;1:164–168. doi: 10.1016/j.jash.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 236.McCarthy CA, Vinh A, Callaway JK, Widdop RE. Angiotensin AT2 receptor stimulation causes neuroprotection in a conscious rat model of stroke. Stroke. 2009;40:1482–1489. doi: 10.1161/STROKEAHA.108.531509. [DOI] [PubMed] [Google Scholar]
- 237.Medrano S, Monteagudo MC, Sequeira-Lopez MLS, Pentz ES, Gomez RA. Two microRNAs -miR-330 and miR-125b-5p-mark the juxta-glomerular cell and balance its smooth muscle phenotype. Am J Physiol Renal Physiol. 2012;302:F29–F37. doi: 10.1152/ajprenal.00460.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell. 2012;148:1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mercure C, Lacombe M-J, Khazaie K, Reudelhuber TL. Cathepsin B is not the processing enzyme for mouse prorenin. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1212–R1216. doi: 10.1152/ajpregu.00830.2009. [DOI] [PubMed] [Google Scholar]
- 240.Millan M, Carvallo P, Izumi S-I, Zemel S, Catt K, Aguilera G. Novel sites of expression of functional angiotensin II receptors in the late gestation fetus. Science. 1989;244:1340–1342. doi: 10.1126/science.2734613. [DOI] [PubMed] [Google Scholar]
- 241.Mills KT, Kobori H, Hamm LL, Alper AB, Khan IE, Rahman M, Navar LG, Liu Y, Browne GM, Batuman V, He J, Chen J. Increased urinary excretion of angiotensinogen is associated with risk of chronic kidney disease. Nephrol Dial Transplant. 2012;27:3176–3181. doi: 10.1093/ndt/gfs011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Morello F, de Boer RA, Steffensen KR, Gnecchi M, Chisholm JW, Boomsma F, Anderson LM, Lawn RM, Gustafsson JK, Lopez-Ilasaca M, Pratt RE, Dzau VJ. Liver X receptors alpha and beta regulate renin expression in vivo. J Clin Invest. 2005;115:1913–1922. doi: 10.1172/JCI24594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Morales, et al. Journal of Molecular Biology. 2012;421(1):100–111. doi: 10.1016/j.jmb.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 244.Moreno C, Hoffman M, Stodola TJ, Didier DN, Lazar J, Geurts AM, North PE, Jacob HJ, Greene AS. Creation and characterization of a renin knockout rat. Hypertension. 2011;57:614–619. doi: 10.1161/HYPERTENSIONAHA.110.163840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Morris BJ. Fluorescence activated cell sorting of transiently transfected As4.1 cells shows renin enhancer directs on/off switching of renin promoter in vitro. Clin Exp Pharmacol Physiol. 2008;35:367–371. doi: 10.1111/j.1440-1681.2008.04879.x. [DOI] [PubMed] [Google Scholar]
- 246.Muller DN, Dechend R, Mervaala EMA, Park J-K, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H, Luft FC. NF-kappa B Inhibition Ameliorates Angiotensin II-Induced Inflammatory{ Damage } in Rats. Hypertension. 2000;35:193–201. doi: 10.1161/01.hyp.35.1.193. [DOI] [PubMed] [Google Scholar]
- 247.Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol. 2002;161:1679–1693. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Müller MWH, Todorov V, Kr̈amer BK, Kurtz A. Angiotensin II inhibits renin gene transcription via the protein kinase C pathway. Pfiiugers Archiv. 2002;444:499–505. doi: 10.1007/s00424-002-0835-8. [DOI] [PubMed] [Google Scholar]
- 249.Mundel P, Bachmann S, Bader M, Fischer A, Kummer W, Mayer B, Kriz W. Expression of nitric oxide synthase in kidney macula densa cells. Kidney Int. 1992;42:1017–1019. doi: 10.1038/ki.1992.382. [DOI] [PubMed] [Google Scholar]
- 250.Munk VC, Sanchez de Miguel L, Petrimpol M, Butz N, Banfi A, Eriksson U, Hein L, Humar R, Battegay EJ. Angiotensin II induces angiogenesis in the hypoxic adult mouse heart in vitro through an AT2-B2 receptor pathway. Hypertension. 2007;49:1178–1185. doi: 10.1161/HYPERTENSIONAHA.106.080242. [DOI] [PubMed] [Google Scholar]
- 251.Munter K, Hackenthal E. The effects of endothelin on renovascular resistance and renin release. J Hypertens Suppl. 1989;7:S276–S277. doi: 10.1097/00004872-198900076-00134. [DOI] [PubMed] [Google Scholar]
- 252.Muro Y, Okabe M. Mechanisms of fertilization-a view from the study of gene-manipulated mice. J Androl. 2011;32:218–225. doi: 10.2164/jandrol.110.010900. [DOI] [PubMed] [Google Scholar]
- 253.Murphy TJ, Alexander RW, Griendling KK, Runge MS, Bernstein KE. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature. 1991;351:233–236. doi: 10.1038/351233a0. [DOI] [PubMed] [Google Scholar]
- 254.Myers BD, Deen WM, Brenner BM. Effects of norepinephrine and angiotensin II on the determinants of glomerular ultrafiltration and proximal tubule fluid reabsorption in the rat. Circ Res. 1975;37:101–110. doi: 10.1161/01.res.37.1.101. [DOI] [PubMed] [Google Scholar]
- 255.Nagata M, Tanimoto K, Fukamizu A, Kon Y, Sugiyama F, Yagami K, Murakami K, Watanabe T. Nephrogenesis and renovascular development in angiotensinogen-deficient mice. Lab Invest. 1996;75:745–753. [PubMed] [Google Scholar]
- 256.Nakamoto H, Ferrario CM, Fuller SB, Robaczewski DL, Winicov E, Dean RH. Angiotensin-(1-7) and nitric oxide interaction in renovascular hypertension. Hypertension. 1995;25:796–802. doi: 10.1161/01.hyp.25.4.796. [DOI] [PubMed] [Google Scholar]
- 257.Nakano D, Kobori H, Burford JL, Gevorgyan H, Seidel S, Hitomi H, Nishiyama A, Peti-Peterdi J. Multiphoton imaging of the glomerular permeability of angiotensinogen. J Am Soc Nephrol. 2012;23:1847–1856. doi: 10.1681/ASN.2012010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Naruse K, Takii Y, Inagami T. Immunohistochemical localization of renin in luteinizing hormone-producing cells of rat pituitary. Proc Natl Acad Sci U S A. 1981;78:7579–7583. doi: 10.1073/pnas.78.12.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Nataraj C, Oliverio MI, Mannon RB, Mannon PJ, Audoly LP, Amuchastegui CS, Ruiz P, Smithies O, Coffman TM. Angiotensin II regulates cellular immune responses through a calcineurin-dependent pathway. J Clin Invest. 1999;104:1693–1701. doi: 10.1172/JCI7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Navar LG, Arendshorst WJ, Pallone TL, Inscho EW, Imig JD, Bell PD. Comprehensive Physiology. Supplement 9. John Wiley & Sons, Inc.; 2011. The renal microcirculation; pp. 550–683. [Google Scholar]
- 261.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev. 1996;76:425–536. doi: 10.1152/physrev.1996.76.2.425. [DOI] [PubMed] [Google Scholar]
- 262.Neubauer B, Machura K, Chen M, Weinstein LS, Oppermann M, Sequeira Lopez ML, Gomez RA, Schnermann J, Castrop H, Kurtz A, Wagner C. Development of vascular renin expression in the kidney critically depends on the cyclic AMP pathway. Am J Physiol Renal Physiol. 2009;296:F1006–F1012. doi: 10.1152/ajprenal.90448.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Neubauer B, Machura K, Kettl R, Lopez ML, Friebe A, Kurtz A. Endothelium-derived nitric oxide supports renin cell recruitment through the nitric oxide-sensitive guanylate cyclase pathway. Hypertension. 2013;61:400–407. doi: 10.1161/HYPERTENSIONAHA.111.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Neubauer B, Machura K, Schnermann J, Wagner C. Renin expression in large renal vessels during fetal development depends on functional 1/2-adrenergic receptors. Am J Physiol Renal Physiol. 2011;301:F71–F77. doi: 10.1152/ajprenal.00443.2010. [DOI] [PubMed] [Google Scholar]
- 265.Ng DP, Tai BC, Koh D, Tan KW, Chia KS. Angiotensin-I converting enzyme insertion/deletion polymorphism and its association with diabetic nephropathy: A meta-analysis of studies reported between 1994 and 2004 and comprising 14,727 subjects. Diabetologia. 2005;48:1008–1016. doi: 10.1007/s00125-005-1726-2. [DOI] [PubMed] [Google Scholar]
- 266.Nguyen G. The (pro)renin receptor in health and disease. Ann Med. 2010;42:13–18. [PubMed] [Google Scholar]
- 267.Nobiling R, Munter K, Buhrle CP, Hackenthal E. Influence of pulsatile perfusion upon renin release from the isolated perfused rat kidney. Pflugers Arch. 1990;415:713–717. doi: 10.1007/BF02584010. [DOI] [PubMed] [Google Scholar]
- 268.Ohkubo H, Kageyama R, Ujihara M, Hirose T, Inayama S, Nakanishi S. Cloning and sequence analysis of cDNA for rat angiotensinogen. Proc Natl Acad Sci U S A. 1983;80:2196–2200. doi: 10.1073/pnas.80.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ohkubo H, Nakayama K, Tanaka T, Nakanishi S. Tissue distribution of rat angiotensinogen mRNA and structural analysis of its heterogeneity. J Biol Chem. 1986;261:319–323. [PubMed] [Google Scholar]
- 270.Oliverio M, Madsen K, Best C, Ito M, Maeda N, Smithies O, Coffman T. The type 1A receptor for angiotensin II is not essential for renal growth and development. Am J Phyiol. 1997;274:F43–F50. doi: 10.1152/ajprenal.1998.274.1.F43. [DOI] [PubMed] [Google Scholar]
- 271.Oliverio MI, Best CF, Kim HS, Arendshorst WJ, Smithies O, Coffman TM. Angiotensin II responses in AT1A receptor-deficient mice: A role for AT1B receptors in blood pressure regulation. Am J Physiol. 1997;272:F515–F520. doi: 10.1152/ajprenal.1997.272.4.F515. [DOI] [PubMed] [Google Scholar]
- 272.Oliverio MI, Best CF, Smithies O, Coffman TM. Regulation of sodium balance and blood pressure by the AT(1A) receptor for angiotensin II. Hypertension. 2000;35:550–554. doi: 10.1161/01.hyp.35.2.550. [DOI] [PubMed] [Google Scholar]
- 273.Oliverio MI, Delnomdedieu M, Best CF, Li P, Morris M, Callahan MF, Johnson GA, Smithies O, Coffman TM. Abnormal water metabolism in mice lacking the type 1A receptor for ANG II. Am J Physiol Renal Physiol. 2000;278:F75–F82. doi: 10.1152/ajprenal.2000.278.1.F75. [DOI] [PubMed] [Google Scholar]
- 274.Oliverio MI, Kim HS, Ito M, Le T, Audoly L, Best CF, Hiller S, Kluckman K, Maeda N, Smithies O, Coffman TM. Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II. Proc Natl Acad Sci U S A. 1998;95:15496–15501. doi: 10.1073/pnas.95.26.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Oliverio MI, Madsen K, Best CF, Ito M, Maeda N, Smithies O, Coffman TM. Renal growth and development in mice lacking AT1A receptors for angiotensin II. Am J Physiol. 1998;274:F43–F50. doi: 10.1152/ajprenal.1998.274.1.F43. [DOI] [PubMed] [Google Scholar]
- 276.Ortiz-Capisano MC, Ortiz PA, Harding P, Garvin JL, Beierwaltes WH. Decreased intracellular calcium stimulates renin release via calcium-inhibitable adenylyl cyclase. Hypertension. 2007;49:162–169. doi: 10.1161/01.HYP.0000250708.04205.d4. [DOI] [PubMed] [Google Scholar]
- 277.Osborn JL, Kopp UC, Thames MD, DiBona GF. Interactions among renal nerves, prostaglandins, and renal arterial pressure in the regulation of renin release. Am J Physiol. 1984;247:F706–F713. doi: 10.1152/ajprenal.1984.247.5.F706. [DOI] [PubMed] [Google Scholar]
- 278.Oudit GY, Crackower MA, Backx PH, Penninger JM. The role of ACE2 in cardiovascular physiology. Trends Cardiovasc Med. 2003;13:93–101. doi: 10.1016/s1050-1738(02)00233-5. [DOI] [PubMed] [Google Scholar]
- 279.Oudit GY, Liu GC, Zhong J, Basu R, Chow FL, Zhou J, Loibner H, Janzek E, Schuster M, Penninger JM, Herzenberg AM, Kassiri Z, Scholey JW. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes. 2010;59:529–538. doi: 10.2337/db09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Padia SH, Kemp BA, Howell NL, Fournie-Zaluski M-C, Roques BP, Carey RM. Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor-mediated natriuresis in rats. Hypertension. 2008;51:460–465. doi: 10.1161/HYPERTENSIONAHA.107.103242. [DOI] [PubMed] [Google Scholar]
- 281.Padia SH, Kemp BA, Howell NL, Keller SR, Gildea JJ, Carey RM. Mechanisms of dopamine D1 and angiotensin type 2 receptor interaction in natriuresis. Hypertension. 2012;59:437–445. doi: 10.1161/HYPERTENSIONAHA.111.184788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Pan L, Black TA, Shi Q, Jones CA, Petrovic N, Loudon J, Kane C, Sigmund CD, Gross KW. Critical roles of a cyclic AMP responsive element and an E-box in regulation of mouse renin gene expression. J Biol Chem. 2001;276:45530–45538. doi: 10.1074/jbc.M103010200. [DOI] [PubMed] [Google Scholar]
- 283.Pan L, Glenn ST, Jones CA, Gronostajski RM, Gross KW. Regulation of renin enhancer activity by nuclear factor I and Sp1/Sp3. Biochim Biophys Acta. 2003;1625:280–290. doi: 10.1016/s0167-4781(03)00016-2. [DOI] [PubMed] [Google Scholar]
- 284.Pan L, Jones CA, Glenn ST, Gross KW. Identification of a novel region in the proximal promoter of the mouse renin gene critical for expression. Am J Physiol Renal Physiol. 2004;286:F1107–F1115. doi: 10.1152/ajprenal.00319.2003. [DOI] [PubMed] [Google Scholar]
- 285.Park CS, Honeyman TW, Chung ES, Lee JS, Sigmon DH, Fray JC. Involvement of calmodulin in mediating inhibitory action of intracellular Ca2+ on renin secretion. Am J Physiol. 1986;251:F1055–F1062. doi: 10.1152/ajprenal.1986.251.6.F1055. [DOI] [PubMed] [Google Scholar]
- 286.Park S, Bivona BJ, Ford SM, Jr., Xu S, Kobori H, de Garavilla L, Harrison-Bernard LM. Direct evidence for intrarenal chymase-dependent angiotensin II formation on the diabetic renal microvas-culature. Hypertension. 2013;61:465–471. doi: 10.1161/HYPERTENSIONAHA.111.202424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Paxton WG, Runge M, Horaist C, Cohen C, Alexander RW, Bernstein KE. Immunohistochemical localization of rat angiotensin II AT1 receptor. Am J Physiol. 1993;264:F989–F995. doi: 10.1152/ajprenal.1993.264.6.F989. [DOI] [PubMed] [Google Scholar]
- 288.Peach MJ. Renin-angiotensin system: Biochemistry and mechanisms of action. Physiol Rev. 1977;57:313–370. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- 289.Pech V, Zheng W, Pham TD, Verlander JW, Wall SM. Angiotensin II activates H+-ATPase in type A intercalated cells. J Am Soc Nephrol. 2008;19:84–91. doi: 10.1681/ASN.2007030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Pentz ES, Cordaillat M, Carretero OA, Tucker AE, Sequeira-Lopez MLS, Gomez RA. Histone acetyl transferases CBP and p300 are necessary for maintenance of renin cell identity and transformation of smooth muscle cells to the renin phenotype. Am J Physiol Heart Circ Physiol. 2012;302:H2545–H2552. doi: 10.1152/ajpheart.00782.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Perlot T, Penninger JM. ACE2 - from the renin angiotensin system to gut microbiota and malnutrition. Microbes Infect. 2013;15:866–876. doi: 10.1016/j.micinf.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Persson PB, Baumann JE, Ehmke H, Hackenthal E, Kirchheim HR, Nafz B. Endothelium-derived NO stimulates pressure-dependent renin release in conscious dogs. Am J Physiol. 1993;264:F943–F947. doi: 10.1152/ajprenal.1993.264.6.F943. [DOI] [PubMed] [Google Scholar]
- 293.Peti-Peterdi J, Gevorgyan H, Lam L, Riquier-Brison A. Metabolic control of renin secretion. Pfiiugers Archiv. 2013;465:53–58. doi: 10.1007/s00424-012-1130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Peti-Peterdi J, Kang JJ, Toma I. Activation of the renal renin-angiotensin system in diabetes-new concepts. Nephrol Dial Transplant. 2008;23:3047–3049. doi: 10.1093/ndt/gfn377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Peti-Peterdi J, Komlosi P, Fuson AL, Guan Y, Schneider A, Qi Z, Redha R, Rosivall L, Breyer MD, Bell PD. Luminal NaCl delivery regulates basolateral PGE2 release from macula densa cells. J Clin Invest. 2003;112:76–82. doi: 10.1172/JCI18018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 297.Petrovic N, Black TA, Fabian JR, Kane C, Jones CA, Loudon JA, Abonia JP, Sigmund CD, Gross KW. Role of proximal promoter elements in regulation of renin gene transcription. J Biol Chem. 1996;271:22499–22505. doi: 10.1074/jbc.271.37.22499. [DOI] [PubMed] [Google Scholar]
- 298.Poumarat JS, Houillier P, Rismondo C, Roques B, Lazar G, Paillard M, Blanchard A. The luminal membrane of rat thick limb expresses AT1 receptor and aminopeptidase activities. Kidney Int. 2002;62:434–445. doi: 10.1046/j.1523-1755.2002.00453.x. [DOI] [PubMed] [Google Scholar]
- 299.Pratt RE, Flynn JA, Hobart PM, Paul M, Dzau VJ. Different secretory pathways of renin from mouse cells transfected with the human renin gene. J Biol Chem. 1988;263:3137–3141. [PubMed] [Google Scholar]
- 300.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutiérrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F632–F637. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Pupilli C, Gomez RA, Tuttle JB, Peach MJ, Carey RM. Spatial association of renin-containing cells and nerve fibers in developing rat kidney. Pediatr Nephrol. 1991;5:690–695. doi: 10.1007/BF00857873. [DOI] [PubMed] [Google Scholar]
- 303.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Riquier-Brison AD, Leong PK, Pihakaski-Maunsbach K, McDonough AA. Angiotensin II stimulates trafficking of NHE3, NaPi2, and associated proteins into the proximal tubule microvilli. Am J Physiol Renal Physiol. 2010;298:F177–F186. doi: 10.1152/ajprenal.00464.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ritthaler T, Scholz H, Ackermann M, Riegger G, Kurtz A, Krämer BK. Effects of endothelins on renin secretion from isolated mouse renal juxtaglomerular cells. Am J Physiol. 1995;268:F39–F45. doi: 10.1152/ajprenal.1995.268.1.F39. [DOI] [PubMed] [Google Scholar]
- 306.Robben JH, Fenton RA, Vargas SL, Schweer H, Peti-Peterdi J, Deen PMT, Milligan G. Localization of the succinate receptor in the distal nephron and its signaling in polarized MDCK cells. Kidney Int. 2009;76:1258–1267. doi: 10.1038/ki.2009.360. [DOI] [PubMed] [Google Scholar]
- 307.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 308.Rompe F, Artuc M, Hallberg A, Alterman M, Ströder K, ThöneReineke C, Reichenbach A, Schacherl J, Dahlöf B, Bader M, Alenina N, Schwaninger M, Zuberbier T, Funke-Kaiser H, Schmidt C, Schunck W-H, Unger T, Steckelings UM. Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor κB. Hypertension. 2010;55:924–931. doi: 10.1161/HYPERTENSIONAHA.109.147843. [DOI] [PubMed] [Google Scholar]
- 309.Rothenberger F, Velic A, Stehberger PA, Kovacikova J, Wagner CA. Angiotensin II stimulates vacuolar H -ATPase activity in renal acid-secretory intercalated cells from the +outer medullary collecting duct. J Am Soc Nephrol. 2007;18:2085–2093. doi: 10.1681/ASN.2006070753. [DOI] [PubMed] [Google Scholar]
- 310.Sandberg K, Ji H, Clark AJ, Shapira H, Catt KJ. Cloning and expression of a novel angiotensin II receptor subtype. J Biol Chem. 1992;267:9455–9458. [PubMed] [Google Scholar]
- 311.Sandholm N, Salem RM, McKnight AJ, Brennan EP, Forsblom C, Isakova T, McKay GJ, Williams WW, Sadlier DM, Makinen VP, Swan EJ, Palmer C, Boright AP, Ahlqvist E, Deshmukh HA, Keller BJ, Huang H, Ahola AJ, Fagerholm E, Gordin D, Harjutsalo V, He B, Heikkila O, Hietala K, Kyto J, Lahermo P, Lehto M, Lithovius R, Osterholm AM, Parkkonen M, Pitkaniemi J, Rosengard-Barlund M, Saraheimo M, Sarti C, Soderlund J, Soro-Paavonen A, Syreeni A, Thorn LM, Tikkanen H, Tolonen N, Tryggvason K, Tuomilehto J, Waden J, Gill GV, Prior S, Guiducci C, Mirel DB, Taylor A, Hosseini SM, Group DER, Parving HH, Rossing P, Tarnow L, Ladenvall C, Alhenc-Gelas F, Lefebvre P, Rigalleau V, Roussel R, Tregouet DA, Maestroni A, Maestroni S, Falhammar H, Gu T, Mollsten A, Cimponeriu D, Ioana M, Mota M, Mota E, Serafinceanu C, Stavarachi M, Hanson RL, Nelson RG, Kretzler M, Colhoun HM, Panduru NM, Gu HF, Brismar K, Zerbini G, Hadjadj S, Marre M, Groop L, Lajer M, Bull SB, Waggott D, Paterson AD, Savage DA, Bain SC, Martin F, Hirschhorn JN, Godson C, Florez JC, Groop PH, Maxwell AP. New susceptibility loci associated with kidney disease in type 1 diabetes. PLoS Genet. 2012;8:e1002921. doi: 10.1371/journal.pgen.1002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Santos RA, Simoes e, Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Sasaki K, Yamano Y, Bardhan S, Iwai N, Murray JJ, Hasegawa M, Matsuda Y, Inagami T. Cloning and expression of a complementary DNA encoding a bovine adrenal angiotensin II type-1 receptor. Nature. 1991;351:230–233. doi: 10.1038/351230a0. [DOI] [PubMed] [Google Scholar]
- 314.Sasamura H, Hein L, Krieger JE, Pratt RE, Kobilka BK, Dzau VJ. Cloning, characterization, and expression of two angiotensin receptor (AT-1) isoforms from the mouse genome. Biochem Biophys Res Commun. 1992;185:253–259. doi: 10.1016/s0006-291x(05)80983-0. [DOI] [PubMed] [Google Scholar]
- 315.Sauter A, Machura K, Neubauer B, Kurtz A, Wagner C. Development of renin expression in the mouse kidney. Kidney Int. 2007;73:43–51. doi: 10.1038/sj.ki.5002571. [DOI] [PubMed] [Google Scholar]
- 316.Sayed-Tabatabaei FA, Oostra BA, Isaacs A, van Duijn CM, Witteman JC. ACE polymorphisms. Circ Res. 2006;98:1123–1133. doi: 10.1161/01.RES.0000223145.74217.e7. [DOI] [PubMed] [Google Scholar]
- 317.Schiffrin EL, Park JB, Intengan HD, Touyz RM. Correction of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation. 2000;101:1653–1659. doi: 10.1161/01.cir.101.14.1653. [DOI] [PubMed] [Google Scholar]
- 318.Schnermann J, Homer W. Smith Award lecture. The juxtaglomerular apparatus: From anatomical peculiarity to physiological relevance. J Am Soc Nephrol. 2003;14:1681–1694. doi: 10.1097/01.asn.0000069221.69551.30. [DOI] [PubMed] [Google Scholar]
- 319.Schnermann J, Ḧaberle DA, Davis JM, Thurau K. Tubuloglomerular feedback control of renal vascular resistance. Comprehensive Physiology. 2011:1675–1705. [Google Scholar]
- 320.Scholz H, Gotz KH, Hamann M, Kurtz A. Differential effects of extra-cellular anions on renin secretion from isolated perfused rat kidneys. Am J Physiol. 1994;267:F1076–F1081. doi: 10.1152/ajprenal.1994.267.6.F1076. [DOI] [PubMed] [Google Scholar]
- 321.Schor N, Ichikawa I, Brenner BM. Glomerular adaptations to chronic dietary salt restriction or excess. Am J Physiol. 1980;238:F428–F436. doi: 10.1152/ajprenal.1980.238.5.F428. [DOI] [PubMed] [Google Scholar]
- 322.Schricker K, Kurtz A. Liberators of NO exert a dual effect on renin secretion from isolated mouse renal juxtaglomerular cells. Am J Physiol. 1993;265:F180–F186. doi: 10.1152/ajprenal.1993.265.2.F180. [DOI] [PubMed] [Google Scholar]
- 323.Schuster VL, Kokko JP, Jacobson HR. Angiotensin II directly stimulates sodium transport in rabbit proximal convoluted tubules. J Clin Invest. 1984;73:507–515. doi: 10.1172/JCI111237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Schweda F, Friis U, Wagner C, Skott O, Kurtz A. Renin release. Physiology (Bethesda) 2007;22:310–319. doi: 10.1152/physiol.00024.2007. [DOI] [PubMed] [Google Scholar]
- 325.Schweda F, Klar J, Narumiya S, Niising RM, Kurtz A. Stimulation of renin release by prostaglandin E2 is mediated by EP2 and EP4 receptors in mouse kidneys. Am J Physiol Renal Physiol. 2004;287:F427–F433. doi: 10.1152/ajprenal.00072.2004. [DOI] [PubMed] [Google Scholar]
- 326.Schweda F, Kurtz A. Cellular mechanism of renin release. Acta Physiol Scand. 2004;181:383–390. doi: 10.1111/j.1365-201X.2004.01309.x. [DOI] [PubMed] [Google Scholar]
- 327.Schweda F, Riegger GA, Kurtz A, Kr̈amer BK. Store-operated calcium influx inhibits renin secretion. Am J Physiol Renal Physiol. 2000;279:F170–F176. doi: 10.1152/ajprenal.2000.279.1.F170. [DOI] [PubMed] [Google Scholar]
- 328.Sequeira-Lopez MLS, Gomez RA. Novel mechanisms for the control of renin synthesis and release. Curr Hypertens Rep. 2010;12:26–32. doi: 10.1007/s11906-009-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Sequeira-Lopez MLS, Weatherford ET, Borges GR, Monteagudo MC, Pentz ES, Harfe BD, Carretero O, Sigmund CD, Gomez RA. The microRNA-processing enzyme dicer maintains juxtaglomerular cells. J Am Soc Nephrol. 2010;21:460–467. doi: 10.1681/ASN.2009090964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell. 2004;6:719–728. doi: 10.1016/s1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- 331.Sequeira Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA. Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol. 2001;281:F345–F356. doi: 10.1152/ajprenal.2001.281.2.F345. [DOI] [PubMed] [Google Scholar]
- 332.Sharp MG, Fettes D, Brooker G, Clark AF, Peters J, Fleming S, Mullins JJ. Targeted inactivation of the Ren-2 gene in mice. Hypertension. 1996;28:1126–1131. doi: 10.1161/01.hyp.28.6.1126. [DOI] [PubMed] [Google Scholar]
- 333.Shenoy SK, Lefkowitz RJ. Angiotensin II-stimulated signaling through G proteins and beta-arrestin. Sci STKE. 2005;2005:cm14. doi: 10.1126/stke.3112005cm14. [DOI] [PubMed] [Google Scholar]
- 334.Shi Q, Gross KW, Sigmund CD. Retinoic acid-mediated activation of the mouse renin enhancer. J Biol Chem. 2001;276:3597–3603. doi: 10.1074/jbc.M008361200. [DOI] [PubMed] [Google Scholar]
- 335.Sigmund CD, Okuyama K, Ingelfinger J, Jones CA, Mullins JJ, Kane C, Kim U, Wu CZ, Kenny L, Rustum Y. Isolation and characterization of renin-expressing cell lines from transgenic mice containing a renin-promoter viral oncogene fusion construct. J Biol Chem. 1990;265 [PubMed] [Google Scholar]
- 336.Silver RB, Reid AC, Mackins CJ, Askwith T, Schaefer U, Herzlinger D, Levi R. Mast cells: A unique source of renin. Proc Natl Acad Sci U S A. 2004;101:13607–13612. doi: 10.1073/pnas.0403208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Siragy HM, Jaffa AA, Margolius HS, Carey RM. Renin-angiotensin system modulates renal bradykinin production. Am J Physiol. 1996;271:R1090–R1095. doi: 10.1152/ajpregu.1996.271.4.R1090. [DOI] [PubMed] [Google Scholar]
- 338.Siragy HM, Xue C, Abadir P, Carey RM. Angiotensin subtype-2 receptors inhibit renin biosynthesis and angiotensin II formation. Hypertension. 2005;45:133–137. doi: 10.1161/01.HYP.0000149105.75125.2a. [DOI] [PubMed] [Google Scholar]
- 339.Skinner SL, McCubbin JW, Page IH. Control of renin Secretion. Circ Res. 1964;15:64–76. doi: 10.1161/01.res.15.1.64. [DOI] [PubMed] [Google Scholar]
- 340.Skott O, Briggs JP. Direct demonstration of macula densa-mediated renin secretion. Science. 1987;237:1618–1620. doi: 10.1126/science.3306925. [DOI] [PubMed] [Google Scholar]
- 341.Soler MJ, Wysocki J, Batlle D. ACE2 alterations in kidney disease. Nephrol Dial Transplant. 2013 doi: 10.1093/ndt/gft320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Sparks MA, Parsons KK, Stegbauer J, Gurley SB, Vivekanandan-Giri A, Fortner CN, Snouwaert J, Raasch EW, Griffiths RC, Haystead TA, Le TH, Pennathur S, Koller B, Coffman TM. Angiotensin II type 1A receptors in vascular smooth muscle cells do not influence aortic remodeling in hypertension. Hypertension. 2011;57:577–585. doi: 10.1161/HYPERTENSIONAHA.110.165274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Spitzer A, Schwartz GJ. Comprehensive Physiology. Supplement 25. John Wiley & Sons, Inc.; 2011. The kidney during development; pp. 475–544. [Google Scholar]
- 344.Sriramula S, Cardinale JP, Lazartigues E, Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res. 2011;92:401–408. doi: 10.1093/cvr/cvr242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Stegbauer J, Gurley SB, Sparks MA, Woznowski M, Kohan DE, Yan M, Lehrich RW, Coffman TM. AT1 receptors in the collecting duct directly modulate the concentration of urine. J Am Soc Nephrol. 2011;22:2237–2246. doi: 10.1681/ASN.2010101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Sugaya T, Nishimatsu S, Tanimoto K, Takimoto E, Yamagishi T, Imamura K, Goto S, Imaizumi K, Hisada Y, Otsuka A, Uchida H, Sugiura M, Fukuta K, Fukamizu A, Murakami K. Angiotensin II type 1a receptor-deficient mice with hypotension and hyperreninemia. J Biol Chem. 1995;270:18719–18722. doi: 10.1074/jbc.270.32.18719. [DOI] [PubMed] [Google Scholar]
- 347.Takahashi N, Smithies O. Human genetics, animal models and computer simulations for studying hypertension. Trends Genet. 2004;20:136–145. doi: 10.1016/j.tig.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 348.Takei Y, Joss JM, Kloas W, Rankin JC. Identification of angiotensin I in several vertebrate species: its structural and functional evolution. Gen Comp Endocrinol. 2004;135:286–292. doi: 10.1016/j.ygcen.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 349.Tanimoto K, Sugiura A, Kanafusa S, Saito T, Masui N, Yanai K, Fukamizu A. A single nucleotide mutation in the mouse renin promoter disrupts blood pressure regulation. J Clin Invest. 2008;118:1006–1016. doi: 10.1172/JCI33824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Taugner R, Hackenthal E. The Juxtaglomerular Apparatus: Structure and Function. Springer Verlag; Heidelberg, Germany: 1989. [Google Scholar]
- 351.Tharaux P-L, TM C, Coffman TM. Transgenic mice as a tool to study the renin-angiotensin system. Contrib Nephrol. 2001;135:72–91. doi: 10.1159/000060158. [DOI] [PubMed] [Google Scholar]
- 352.Tharaux PL, Dussaule JC, Pauti MD, Vassitch Y, Ardaillou R, Chatziantoniou C. Activation of renin synthesis is dependent on intact nitric oxide production. Kidney Int. 1997;51:1780–1787. doi: 10.1038/ki.1997.245. [DOI] [PubMed] [Google Scholar]
- 353.Thurau K, Schnermann J, Nagel W, Horster M, Wahl M. Composition of tubular fluid in the macula densa segment as a factor regulating the function of the juxtaglomerular apparatus. Circ Res. 1967;21(Suppl 2):79–90. [PubMed] [Google Scholar]
- 354.Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- 355.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 356.Todorov VT, Desch M, Schmitt-Nilson N, Todorova A, Kurtz A. Peroxisome proliferator-activated receptor-gamma is involved in the control of renin gene expression. Hypertension. 2007;50:939–944. doi: 10.1161/HYPERTENSIONAHA.107.092817. [DOI] [PubMed] [Google Scholar]
- 357.Todorov VT, Desch M, Schubert T, Kurtz A. The Pal3 promoter sequence is critical for the regulation of human renin gene transcription by peroxisome proliferator-activated receptor-gamma. Endocrinology. 2008;149:4647–4657. doi: 10.1210/en.2008-0127. [DOI] [PubMed] [Google Scholar]
- 358.Todorov VT, Völkl S, Miiller M, Bohla A, Klar J, Kunz-Schughart LA, Hehlgans T, Kurtz A. Tumor necrosis factor-alpha activates NFkappaB to inhibit renin transcription by targeting cAMP-responsive element. J Biol Chem. 2004;279:1458–1467. doi: 10.1074/jbc.M308697200. [DOI] [PubMed] [Google Scholar]
- 359.Toffelmire EB, Slater K, Corvol P, Menard J, Schambelan M. Response of plasma prorenin and active renin to chronic and acute alterations of renin secretion in normal humans. Studies using a direct immunoradiometric assay. J Clin Invest. 1989;83:679–687. doi: 10.1172/JCI113932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Tokumori Y, Kurahashi A, Murakami J, Mokuda GO, Ikeda T, Takeda A, Tominaga M, Mashib H. Biphasic renin release from perfused rat kidney. Horm Metab Res. 1983;15:310–311. doi: 10.1055/s-2007-1018706. [DOI] [PubMed] [Google Scholar]
- 361.Tomita H, Sanford RB, Smithies O, Kakoki M. The kallikrein-kinin system in diabetic nephropathy. Kidney Int. 2012;81:733–744. doi: 10.1038/ki.2011.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Tsuchida S, Matsusaka T, Chen X, Okubo S, Niimura F, Nishimura H, Fogo A, Utsunomiya H, Inagami T, Ichikawa I. Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest. 1998;101:755–760. doi: 10.1172/JCI1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Vander AJ. Control of renin release. Physiol Rev. 1967;47:359–382. doi: 10.1152/physrev.1967.47.3.359. [DOI] [PubMed] [Google Scholar]
- 364.Verlander JW, Hong S, Pech V, Bailey JL, Agazatian D, Matthews SW, Coffman TM, Le T, Inagami T, Whitehill FM, Weiner ID, Farley DB, Kim YH, Wall SM. Angiotensin II acts through the angiotensin 1a receptor to upregulate pendrin. Am J Physiol Renal Physiol. 2011;301:F1314–F1325. doi: 10.1152/ajprenal.00114.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 366.Villard E, Tiret L, Visvikis S, Rakotovao R, Cambien F, Soubrier F. Identification of new polymorphisms of the angiotensin I-converting enzyme (ACE) gene, and study of their relationship to plasma ACE levels by two-QTL segregation-linkage analysis. Am J Hum Genet. 1996;58:1268–1278. [PMC free article] [PubMed] [Google Scholar]
- 367.Wagner C, de Wit C, Kurtz L, Grunberger C, Kurtz A, Schweda F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res. 2007;100:556–563. doi: 10.1161/01.RES.0000258856.19922.45. [DOI] [PubMed] [Google Scholar]
- 368.Wagner C, Pfeifer A, Ruth P, Hofmann F, Kurtz A. Role of cGMP-kinase II in the control of renin secretion and renin expression. J Clin Invest. 1998;102:1576–1582. doi: 10.1172/JCI4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Wan Y, Wallinder C, Plouffe B, Beaudry H, Mahalingam AK, Wu X, Johansson B, Holm M, Botoros M, Karlen A, Pettersson A, Nyberg F, Fandriks L, Gallo-Payet N, Hallberg A, Alterman M. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J Med Chem. 2004;47:5995–6008. doi: 10.1021/jm049715t. [DOI] [PubMed] [Google Scholar]
- 370.Wang DH, Du Y, Yao A, Hu Z. Regulation of type 1 angiotensin II receptor and its subtype gene expression in kidney by sodium loading and angiotensin II infusion. J Hypertens. 1996;14:1409–1415. doi: 10.1097/00004872-199612000-00004. [DOI] [PubMed] [Google Scholar]
- 371.Wang H, Gomez JA, Klein S, Zhang Z, Seidler B, Yang Y, Schmeck-peper J, Zhang L, Muramoto GG, Chute J, Pratt RE, Saur D, Mirotsou M, Dzau VJ. Adult renal mesenchymal stem cell-like cells contribute to juxtaglomerular cell recruitment. J Am Soc Nephrol. 2013;24:1263–1273. doi: 10.1681/ASN.2012060596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Wang SM, Rapp JP. Structural differences in the renin gene of Dahl salt-sensitive and salt-resistant rats. Mol Endocrinol. 1989;3:288–294. doi: 10.1210/mend-3-2-288. [DOI] [PubMed] [Google Scholar]
- 373.Wang W, Li C, Summer S, Falk S, Schrier RW. Interaction between vasopressin and angiotensin II in vivo and in vitro: Effect on aquaporins and urine concentration. Am J Physiol Renal Physiol. 2010;299:F577–F584. doi: 10.1152/ajprenal.00168.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Wang Y, Ng MC, So WY, Tong PC, Ma RC, Chow CC, Cockram CS, Chan JC. Prognostic effect of insertion/deletion polymorphism of the ace gene on renal and cardiovascular clinical outcomes in Chinese patients with type 2 diabetes. Diabetes care. 2005;28:348–354. doi: 10.2337/diacare.28.2.348. [DOI] [PubMed] [Google Scholar]
- 375.Ward K, Hata A, Jeunemaitre X, Helin C, Nelson L, Namikawa C, Farrington PF, Ogasawara M, Suzumori K, Tomoda S, Berrebi S, Sasaki M, Corvol P, Lifton RP, Lalouel J-M. A molecular variant of angiotensinogen associated with preeclampsia. Nat Genet. 1993;4:59–61. doi: 10.1038/ng0593-59. [DOI] [PubMed] [Google Scholar]
- 376.Webber PC, Larsson C, Anggard E, Hamberg M, Corey EJ, Nicolaou KC, Samuelsson B. Stimulation of renin release from rabbit renal cortex by arachidonic acid and prostaglandin endoperoxides. Circ Res. 1976;39:868–874. doi: 10.1161/01.res.39.6.868. [DOI] [PubMed] [Google Scholar]
- 377.Weihprecht H, Lorenz JN, Schnermann J, Skott O, Briggs JP. Effect of adenosine1-receptor blockade on renin release from rabbit isolated perfused juxtaglomerular apparatus. J Clin Invest. 1990;85:1622–1628. doi: 10.1172/JCI114613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Wilcox CS, Welch WJ. Macula densa nitric oxide synthase: Expression, regulation, and function. Kidney Int. 1998;67:S53–S57. doi: 10.1046/j.1523-1755.1998.06711.x. [DOI] [PubMed] [Google Scholar]
- 379.Wilson DM, Luetscher JA. Plasma prorenin activity and complications in children with insulin-dependent diabetes mellitus. N Engl J Med. 1990;323:1101–1106. doi: 10.1056/NEJM199010183231604. [DOI] [PubMed] [Google Scholar]
- 380.Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, Backx PH, Penninger JM, Herzenberg AM, Scholey JW. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol. 2007;171:438–451. doi: 10.2353/ajpath.2007.060977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Wong NL, Tsui JK. Angiotensin II upregulates the expression of vaso-pressin V2 mRNA in the inner medullary collecting duct of the rat. Metabolism. 2003;52:290–295. doi: 10.1053/meta.2003.50047. [DOI] [PubMed] [Google Scholar]
- 382.Wu C, Lu H, Cassis LA, Daugherty A. Molecular and pathophysiological features of angiotensinogen: A mini review. N Am J Med Sci (Boston) 2011;4:183–190. doi: 10.7156/v4i4p183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao HD, Bernstein KE, Coffman TM, Chen S, Batlle D. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55:2132–2139. doi: 10.2337/db06-0033. [DOI] [PubMed] [Google Scholar]
- 384.Xia H, Suda S, Bindom S, Feng Y, Gurley SB, Seth D, Navar LG, Lazartigues E. ACE2-mediated reduction of oxidative stress in the central nervous system is associated with improvement of autonomic function. PloS one. 2011;6:e22682. doi: 10.1371/journal.pone.0022682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Xu P, Sriramula S, Lazartigues E. ACE2/ANG-(1-7)/Mas pathway in the brain: The axis of good. Am J Physiol Regul Integr Comp Physiol. 2011;300:R804–R817. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Yamamoto K, Ohishi M, Katsuya T, Ito N, Ikushima M, Kaibe M, Tatara Y, Shiota A, Sugano S, Takeda S, Rakugi H, Ogihara T. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension. 2006;47:718–726. doi: 10.1161/01.HYP.0000205833.89478.5b. [DOI] [PubMed] [Google Scholar]
- 387.Yanai K, Saito T, Kakinuma Y, Kon Y, Hirota K, Taniguchi-Yanai K, Nishijo N, Shigematsu Y, Horiguchi H, Kasuya Y, Sugiyama F, Yagami K, Murakami K, Fukamizu A. Renin-dependent cardiovascular functions and renin-independent blood-brain barrier functions revealed by renin-deficient mice. J Biol Chem. 2000;275:5–8. doi: 10.1074/jbc.275.1.5. [DOI] [PubMed] [Google Scholar]
- 388.Yang T, Endo Y, Huang YG, Smart A, Briggs JP, Schnermann J. Renin expression in COX-2-knockout mice on normal or low-salt diets. Am J Physiol Renal Physiol. 2000;279:F819–F825. doi: 10.1152/ajprenal.2000.279.5.F819. [DOI] [PubMed] [Google Scholar]
- 389.Yayama K, Hiyoshi H, Imazu D, Okamoto H. Angiotensin II stimulates endothelial NO synthase phosphorylation in thoracic aorta of mice with abdominal aortic banding via type 2 receptor. Hypertension. 2006;48:958–964. doi: 10.1161/01.HYP.0000244108.30909.27. [DOI] [PubMed] [Google Scholar]
- 390.Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, Iadecola C, Mark AL, Davisson RL. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest. 2012;122:3960–3964. doi: 10.1172/JCI64583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 392.Zatz R, Dunn BR, Meyer TW, Anderson S, Rennke HG, Brenner BM. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest. 1986;77:1925–1930. doi: 10.1172/JCI112521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Zhang H, Wada J, Hida K, Tsuchiyama Y, Hiragushi K, Shikata K, Wang H, Lin S, Kanwar YS, Makino H. Collectrin, a collecting duct-specific transmembrane glycoprotein, is a novel homolog of ACE2 and is developmentally regulated in embryonic kidneys. J Biol Chem. 2001;276:17132–17139. doi: 10.1074/jbc.M006723200. [DOI] [PubMed] [Google Scholar]
- 394.Zhang H, Wada J, Kanwar YS, Tsuchiyama Y, Hiragushi K, Hida K, Shikata K, Makino H. Screening for genes up-regulated in 5/6 nephrectomized mouse kidney. Kidney Int. 1999;56:549–558. doi: 10.1046/j.1523-1755.1999.00561.x. [DOI] [PubMed] [Google Scholar]
- 395.Zhang JD, Patel MB, Song YS, Griffiths R, Burchette J, Ruiz P, Sparks MA, Yan M, Howell DN, Gomez JA, Spurney RF, Coffman TM, Crowley SD. A novel role for type 1 angiotensin receptors on T lymphocytes to limit target organ damage in hypertension. Circ Res. 2012;110:1604–1617. doi: 10.1161/CIRCRESAHA.111.261768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Zhong JC, Ye JY, Jin HY, Yu X, Yu HM, Zhu DL, Gao PJ, Huang DY, Shuster M, Loibner H, Guo JM, Yu XY, Xiao BX, Gong ZH, Penninger JM, Oudit GY. Telmisartan attenuates aortic hypertrophy in hypertensive rats by the modulation of ACE2 and profilin-1 expression. Regul Pept. 2011;166:90–97. doi: 10.1016/j.regpep.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 397.Zhou A, Carrell RW, Murphy MP, Wei Z, Yan Y, Stanley PL, Stein PE, Broughton Pipkin F, Read RJ. A redox switch in angiotensinogen modulates angiotensin release. Nature. 2010;468:108–111. doi: 10.1038/nature09505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Zhou X, Sigmund CD. Chorionic enhancer is dispensable for regulated expression of the human renin gene. Am J Physiol Regul Integr Comp Physiol. 2008;294:R279–R287. doi: 10.1152/ajpregu.00780.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res. 2004;95:532–539. doi: 10.1161/01.RES.0000139957.22530.b9. [DOI] [PubMed] [Google Scholar]
- 400.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]