Abstract
This study compared blood glucose concentrations measured with a portable blood glucometer and a validated laboratory analyzer in venous blood samples of 20 pet ferrets (Mustela putorius furo). Correlation and agreement were evaluated with a Bland-Altman plot method and Lin’s concordance correlation coefficient. Blood glucose concentrations measured with the laboratory analyzer and the glucometer ranged from 1.9 to 8.6 mmol/L and from 0.9 to 9.2 mmol/L, respectively. The glucometer had a poor agreement and correlation with the laboratory analyzer (bias, −0.13 mmol/L; level of agreement, −2.0 to 3.6 mmol/L, concordance correlation coefficient 0.665). The relative sensitivity and specificity of the portable blood glucometer for detection of hypoglycemia were 100% (95% CI: 66% to 100%) and 50% (95% CI: 20% to 80%), respectively. Positive and negative predictive values were 67% (95% CI: 39% to 87%) and 100% (95% CI: 46% to 100%), respectively. Based on these results, clinicians are advised to be cautious when considering the results from this handheld glucometer in pet ferrets, and blood glucose concentrations should be determined with a laboratory analyzer validated for this species.
Résumé
Comparaison entre un lecteur de glycémie portable pour humain et un analyseur automatisé de biochimie dans le but d’évaluer de la glycémie chez des furets domestiques (Mustela putorius furo). L’objectif de l’étude était de comparer les valeurs de glycémie mesurées par un glycomètre portable et un analyseur de laboratoire certifié pour des prélèvements sanguins veineux effectués sur 20 furets de compagnie (Mustela putorius furo). L’équivalence des méthodes a été évaluée grâce à un diagramme de Bland-Altman et au coefficient de corrélation de concordance de Lin. Les glycémies mesurées par l’analyseur de laboratoire et le glycomètre étaient respectivement comprises entre 1,9 à 8,6 mmol/L et de 0,9 à 9,2 mmol/L. Les degrés d’agrément et de corrélation entre le glycomètre et l’analyseur de laboratoire étaient faibles (biais, −0,13 mmol/L; niveau d’agrément, −2,0 à 3,6 mmol/L, coefficient de corrélation de concordance 0,665). La sensibilité et la spécificité du glycomètre concernant la détection d’hypoglycémie étaient respectivement de 100 % (95 % CI : 66–100 %) et de 50 % (95 % CI : 20–80 %) et les valeurs predictives positive et negative étaient respectivement de 67 % (95 % CI : 39–87 %) et de 100 % (95 % CI : 46–100 %). En s’appuyant sur ces résultats, l’utilisation d’un glycomètre portable devraient être réalisée avec précaution en pratique chez les furets de compagnie et les valeurs de glycémie devraient être déterminées par un analyseur de laboratoire certifié pour cette espèce.
(Traduit par les auteurs)
Introduction
Accurate and efficient assessment of blood glucose concentration is critical in clinical management of many pathological conditions in veterinary medicine, especially in ferrets (Mustela putorius furo). Insulinoma is a common disease of middle-aged to old ferrets, and represents more than 20% of the neoplasms in this species (1,2), although other disorders may also cause hypo- or hyper-glycemia (3,4). Reliable, rapid, and repeated measurements of blood glucose concentration are necessary to diagnose and manage these conditions in ferrets.
Measurements of blood glucose concentration are best determined by standardized laboratory techniques using blood (serum and plasma) biochemistry analyzers that are verified for the animal species being tested (5). However, disadvantages include the time needed to obtain the results, and, more importantly, the challenging requirement of a relatively large sample volume that may not be available due to the ferret’s small body size. During the last 20 years, many portable blood glucometers (PBGM) for humans and animals have appeared on the market. They are readily available, inexpensive, and provide immediate results while utilizing small quantities of capillary blood. The use of PBGM has been recommended in measurement of blood glucose concentration in ferrets (3,5,6); however, recent evidence showed that several brands of PBGM were unreliable in this species (7).
The purpose of this study was to assess the clinical relevance of a previously untested model of PBGM designed for human use (h-PBGM) by determining the correlation and agreement of its blood glucose measurements in ferrets with those from a laboratory analyzer. Based on our clinical experience and on current evidence (7), our hypothesis was that the h-PBGM would yield inaccurate blood glucose concentration measurements, making it unreliable for clinical use in ferrets.
Materials and methods
Ferrets
As part of the routine clinical evaluation, blood samples were collected from 20 ferrets that were presented to the Avian and Exotics Service, Ontario Veterinary College, between April 2011 and June 2012. For each sample, blood glucose was measured with a single h-PBGM and a plasma biochemistry analyzer.
Protocol
Blood samples were collected from the cranial vena cava or the external saphenous vein, using a 25-gauge needle. A drop of fresh whole blood was immediately analyzed with an h-PBGM (Contour® blood glucose monitoring system; Bayer HealthCare, Mishawaka, Indiana, USA). The remaining whole blood was then placed in tubes coated with lithium heparin (Microtainer BD Bioscience, Franklin lakes, New Jersey, USA). The heparinized sample was centrifuged within 15 min, the plasma was then separated and kept refrigerated at 4°C until the analysis was performed within 12 h (4). Plasma glucose concentration was determined with an automated chemistry analyzer (Cobass C311 analyzer; Roche Diagnostic GmbH, Indianapolis, Indiana, USA) by trained laboratory technicians, who were unaware of the results obtained from the h-PBGM.
Analyzers
The Contour® h-PBGM was evaluated in this study as this glucometer was the one used at the OVC-HSC and its reliability needed to be determined. This PBGM is based on measurement of electrical current caused by the reaction of blood glucose with the reagents (FAD glucose dehydrogenase and potassium ferricyanide) on the electrode of the strip. The generated current is proportional to the glucose concentration. The glucometer requires 0.6 μL of whole blood and gives results in 5 s in blood glucose concentration in the range of 0.6 to 33.3 mmol/L. Precision and accuracy data provided by manufacturers state a coefficient of variation < 3%. The PBGM was calibrated as directed by the manufacturers. It was consistently used in similar environmental conditions according to the manufacturer’s directions except that venous, instead of capillary, blood was taken.
The automated chemistry analyzer measures plasma glucose concentration based on a reference method via an enzymatic hexokinase oxidase reaction. Results of the reaction are detected photometrically. According to the manufacturer, lipemia, hemolysis, or icterus have no significant interference with glucose results. The analyzer was calibrated daily, using commercial quality control standards.
Data analysis
Normality of distribution for blood glucose concentration values was based on skewness and kurtosis. Normally distributed variables were described as means and standard deviations, non-normally distributed variables were described as medians and ranges. The glucose concentrations from the h-PBGM and the laboratory analyzer were classified as hypoglycemic [glucose concentration < 4.1 mmol/L, Animal Health Laboratory (AHL), University of Guelph, Guelph, Ontario], euglycemic (glucose concentration, 4.1 to 7.4 mmol/L, AHL), or hyperglycemic (glucose concentration > 7.4 mmol/L, AHL). Bias was defined as the mean difference between the 2 methods. Agreement between the PBGM and the laboratory analyzer results were evaluated graphically with a Bland-Altman plot, with a regression analysis for the average and the difference of the 2 methods, and with calculation of Lin’s concordance correlation coefficient (CCC) using a statistical software (STATA 11, Statacorp, College Station, Texas, USA) (8–10). The value of the CCC was interpreted as follows: > 0.99, almost perfect strength-of-agreement; 0.95 to 0.99, substantial strength-of-agreement; 0.90 to 0.95, moderate strength-of-agreement; < 0.90, poor strength-of-agreement (11). Current standards in human medicine recommend that the accuracy of a PBGM be within 15% of the reference value (12,13); therefore, all results were examined for the percentage of values outside of this range. The association of variables (i.e., hematocrit, red blood cell count, hemoglobin, protein level, creatinine, and time to analyzing the sample) with the difference in glucose concentrations delivered by the h-PBGM values compared to the laboratory analyzer was evaluated by linear regression modeling. Two-way interactions among the main effects were investigated first. Univariate analysis was performed and variables with a value of P < 0.20 were evaluated in a multivariable model. A given variable was retained in the multivariable model when the value of P for that variable was ≤ 0.05. All analyses, including graphical analyses to evaluate model assumptions, were performed by use of STATA 11.
Results
Plasma glucose concentrations measured by the laboratory analyzer were normally distributed and ranged from 1.9 to 8.6 mmol/L (mean ± SD, 4.60 ± 1.82 mmol/L). Blood glucose concentrations with PBGM were not normally distributed and ranged from 0.9 to 9.2 mmol/L (median, 3.3 mmol/L). Blood glucose concentrations for each method were contained respectively within the range of the h-PBGM (0.6 to 33.3 mmol/L) and of the laboratory analyzer (0.1 to 41.6 mmol/L). Seven ferrets had confirmed insulinoma, 3 ferrets were healthy, and the remaining 10 ferrets had a variety of medical conditions.
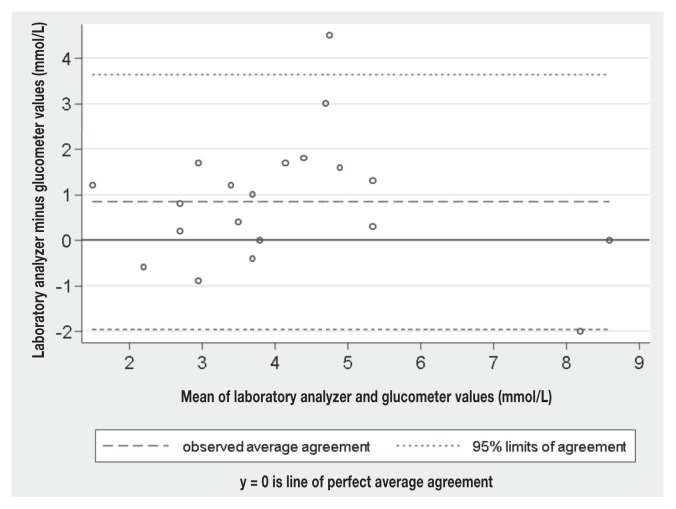
On average, the 2 methods seemed to yield similar results (bias = 0.84 mmol/L); however, the range of variation of the difference between the 2 methods (LOA, −2.0 to 3.6 mmol/L) was relatively wide in comparison to the in-house laboratory reference range (RR) of blood glucose concentration reported for ferrets (4.1 to 7.4 mmol/L). Regression analysis showed that the bias was consistent over the range of blood glucose measured (P = 0.57), which could visually be confirmed on the Bland-Altman plot (Figure 1). The CCC was 0.665, which corresponds to a poor agreement between the 2 methods (Lin’s concordance correlation coefficient, CCC < 0.90) (11). Only 6/20 (30%) of the h-PBGM values were within 15% of the laboratory analyzer values, the range recommended by current standards in human medicine (12,13).
Figure 1.
Bland-Altman difference plot of glucose concentration measures with a portable blood glucose meter and a laboratory analyzer in 20 samples of fresh whole blood and plasma from ferrets with various diseases.
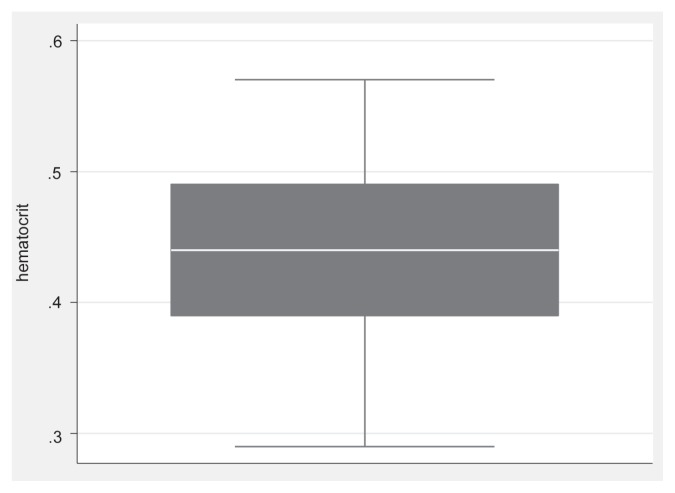
The laboratory analyzer readings showed that almost half of the ferrets (9/20) had a blood glucose value within the reference range, 1 animal was hyperglycemic, and 10/20 were below the reference range (Table 1). Based on the cohort included in this study, the prevalence for hypoglycemia in sick ferrets was thus 50% (95% CI: 27% to 72%). The relative sensitivity of the PBGM for detection of hypoglycemia was 100% (95% CI: 66% to 100%) and its relative specificity was 50% (95% CI: 20% to 80%). With the current prevalence, the positive predictive value for the PBGM was 67% (95% CI: 39% to 87%). This means that the PBGM incorrectly indicated hypoglycemia in 5/15 ferrets (33%). The negative predictive value for this test was 100% (95% CI: 46% to 100%) since the PBGM detected all hypoglycemic animals in our study. There was no statistically significant association in the difference between laboratory analyzer and h-PBGM blood glucose concentrations and any of the variables evaluated in linear regression analysis, except for the time between sampling and blood analysis with the laboratory analyzer. For every 60-minute increase in time interval, the difference between the results of the 2 methods decreases by 0.1 mmol/L (95% CI: 0 to 0.2). Hematocrit values in the study ranged from 0.29 to 0.57 (mean ± SD, 0.44 ± 0.07, RR: 0.42 to 0.68) (14); 35% (7/20) of the ferrets had an abnormally low hematocrit compared to the reference range (Figure 2).
Table 1.
Mean difference ± SD (mmol/L) bias calculated by averaging the difference between laboratory analyzer and human portable blood glucometer results for blood glucose concentrations obtained from venous blood samples in 20 ferrets (Mustela putorius furo). Blood samples were classified as hyperglycemic, hypoglycemic, or euglycemic based on the laboratory analyzer results.
| Blood sample category | ||||
|---|---|---|---|---|
|
|
||||
| Number of samples (n) | Hyperglycemic (n = 1) | Hypoglycemic (n = 10) | Euglycemic (n = 9) | Total (n = 20) |
| PBGM median (range) | 8.6 (8.6–8.6) | 2.7 (0.9–3.9) | 3.5 (2.5–9.2) | 3.3 (0.9–9.2) |
Hypoglycemic glucose concentration: <4.1 mmol/L; euglycemic glucose concentration: 4.1 to 7.4 mmol/L; hyperglycemic glucose concentration: > 7.4 mmol/L; PBGM = portable blood glucometer.
Figure 2.
Box plot of the distribution of the hematocrit values in the ferrets of the study. The outer bounds of the boxes represent the interquartile range; the median is represented by the midline.
Discussion
Overall, blood glucose concentrations measured by the h-PBGM used in this study were inconsistent compared with those from the laboratory analyzer. This is in agreement with the report of a recent study, in which 2 other h-PBGMs (AccuCheck Aviva and OneTouch Ultra 2) repeatedly underestimated blood glucose concentration in samples from ferrets (7). Utility and accuracy of some PBGM devices have been studied in dogs (15–18), cats (19), mice (20), rats (20,21), Hispaniolan Amazon parrots (22,23), rhinoceros auklets (24), white-tailed deer (25), cattle (26), sheep (26), and horses (27). The PBGM of those studies did not have an exact agreement with the automated analyzer. In our study, although the mean bias was small, the h-PBGM inconsistently and sometimes largely underestimated or, to a lesser extent, overestimated the laboratory analyzer values. Because of these important variations, no correction factor could be generated for the blood glucose concentration values of the h-PBGM. In addition, the 95% LOA (−2.0 to 3.6 mmol/L), represents too large a range to be acceptable for clinical assessment of glycemic status in ferrets.
In other species, the reliability of PBGM is controversial. Some authors concluded that glucose values with PBGM would have resulted in erroneous clinical decisions in dogs (16,18) and birds (22,24), but other studies considered that some PBGMs were acceptable for clinical use in dogs (15,17), cats (19,28), cattle (26), sheep (26), horses (27), mice (20), and rats (20). These divergent results may in part be attributed to the PBGM used, since some models have been documented to differ in their reliability (15,19). For instance, in dogs (15), cats (19), and ferrets (7), comparative studies showed that h-PBGM had a greater disagreement with laboratory analyzer values compared with PBGM designed for veterinary use (v-PBGM). In ferrets, the v-PBGM coded to test a canine blood sample had a better agreement compared with the same v-PBGM coded to test feline blood (7).
The high rate of falsely hypoglycemic ferrets diagnosed by the h-PBGM in our study is similar to the rate reported for other h-PBGM in ferrets (14% to 31%) (7). Precision and accuracy data provided by manufacturers state a coefficient of variation < 3% for the h-PBGM and coefficient of variation < 1.5% for the laboratory analyzer, respectively, which could only partially explain the variations in our values. In ferrets, a v-PBGM set to “canine” mode showed better performance since it erroneously resulted in glucose values within the hypoglycemic range in only 4% of the samples (7). However, the h-PBGM used in this study had a good sensitivity and negative predictive value, even if these values may be overestimated by the small sample of ferrets, thus suggesting the use of this h-PBGM as a fast pre-screening test for detection of hypoglycemia in ferrets.
In human studies, error grid analysis is currently considered the standard method to evaluate the clinical relevance of erroneous decisions based on PBGM values (29–31) and its use in a study with dogs has previously been described (15). However, error grid was not used in the present study due to the small sample size.
According to the manufacturers’ recommendations, whole blood was used for the PBGM and plasma was used for the laboratory analyzer. As glucose is uniformly distributed in water (H2O) components and as there is less H2O in erythrocytes than in plasma, whole blood glucose concentrations measured by some methods are lower than those of plasma (4). The PBGMs are calibrated to account for this difference, but they cannot adapt to changes in hematocrit, even though the H2O content of whole blood and thus the glucose concentration of whole blood varies with its hematocrit (4). One hypothesis for the disagreement between h-PBGM and laboratory analyzer values is that ferrets have higher hematocrit values compared with humans (mean 0.52 in ferret versus mean 0.44 in humans) (3), as previously hypothesized for birds (22,23). However, mean hematocrit in the ferrets of this study was 0.44, which is similar to human hematocrit. Lower and higher hematocrits with h-PBGM and v-PBGM have respectively a positive and a negative bias on blood glucose values in humans (32) and dogs (17). This bias varies depending on glucometer technology in humans (33) and may cause a variation from 4% to 30% of glucose concentration for every 10% change in hematocrit (12). In dogs, v-PBGM performed better than h-PBGM when hematocrit was within or above its reference interval (34), whereas h-PBGMs were more accurate in anemic dogs (34). However, in this study, the effect of hematocrit on the difference between the laboratory analyzers and the PBGM results was not statistically significant and could not explain the disagreement between the 2 methods.
In this study, there was a significant effect of the time interval on the difference between the two methods: the difference decreased when the time interval increased, which can be explained by glucose consumption by the red blood cells.
Venous blood was used in this study instead of the capillary blood recommended by the manufacturer. Because of tissue utilization of glucose, postprandial blood glucose concentrations from capillaries are typically higher than from venous blood by 1.1 to 3.9 mmol/L. When animals are fasting, this difference is reduced to 0.11 to 0.28 mmol/L (35). In this study, all ferrets were fasted before a blood sample was taken. Therefore, using venous blood instead of capillary blood is unlikely to have induced a major bias in the results.
Blood glucose concentration may have an effect on PBGM values, as PBGM may be unreliable in the hypoglycemic or severely hyperglycemic ranges (12). In dogs disagreement between h-PBGM and automated analyzer was greater at higher glucose concentration (15,17,18). Such a proportional bias was not described for v-PBGM (15). In the current study, the agreement between the h-PBGM and the laboratory analyzer seemed to improve with low blood glucose concentrations, although the limited number of samples may be responsible for this distribution.
The major limiting factor of this study was the small sample size, as a larger number of ferrets could have resulted in different LOA and in better agreement between the 2 methods. Future studies to evaluate the accuracy of any PBGM should include a larger sample size.
As hypoglycemia is a common clinical disorder in ferrets, handheld blood glucometers seemed promising to facilitate diagnosis and monitoring of this condition. These study results, in accordance with previous evidence (7), suggest that PBGMs do not yield reliable blood glucose measurements in ferrets in clinical settings. However, as the sensitivity and negative predictive values of the h-PBGM evaluated in this study were high, the use of this PBGM as a rapid preliminary screening test for detection of hypoglycemia in ferrets may be considered with caution, and low blood glucose concentrations detected by the PBGM should be confirmed by a validated laboratory analyzer. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Li XT, Fox JG, Padrid PA. Neoplastic diseases in ferrets: 574 cases (1968–1997) J Am Vet Med Assoc. 1998;212:1402–1406. [PubMed] [Google Scholar]
- 2.Willams B, Weiss C. Ferret neoplasia. In: Quesenberry KE, editor. Ferrets, Rabbits, and Rodents: Clinical Medicine. 2nd ed. St. Louis, Missouri: Saunders; 2003. pp. 91–106. [Google Scholar]
- 3.Rosenthal KL, Wyre NR. Endocrine diseases. In: Quesenberry KE, Carpenter JW, editors. Ferrets, Rabbits, and Rodents Clinical Medicine and Surgery. 3rd ed. St. Louis, Missouri: Elsevier Saunders; 2012. pp. 86–102. [Google Scholar]
- 4.Stockham SL, Scott MA. Glucose, ketoamines, and related regulatory hormones. In: Stockham SL, Scott MA, editors. Fundamentals of veterinary clinical pathology. 2nd ed. Ames, Iowa: Blackwell Publishing; 2008. pp. 712–713. [Google Scholar]
- 5.Chen S. Advanced diagnostic approaches and current medical management of insulinomas and adrenocortical disease in ferrets (Mustela putorius furo) Vet Clin North Am Exot Anim Pract. 2010;13:439–452. doi: 10.1016/j.cvex.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Lewington JH. Endocrine diseases. In: Lewington JH, editor. Ferret Husbandry, Medicine and Surgery. 2nd ed. Philadelphia, Pennsylvania: Saunders Elsevier; 2007. pp. 346–379. [Google Scholar]
- 7.Petritz OA, Antinoff N, Chen S, Kass PH, Paul-Murphy JR. Evaluation of portable blood glucose meters for measurement of blood glucose concentration in ferrets (Mustela putorius furo) J Am Vet Med Assoc. 2013;242:350–354. doi: 10.2460/javma.242.3.350. [DOI] [PubMed] [Google Scholar]
- 8.Bland JM, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. The lancet. 1986;327(8476):307–310. [PubMed] [Google Scholar]
- 9.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 10.Lin LI. Total deviation index for measuring individual agreement with application in lab performance and bioequivalence. Stat Med. 2000;19:255–270. doi: 10.1002/(sici)1097-0258(20000130)19:2<255::aid-sim293>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 11.McBride GB. A proposal for strength-of-agreement criteria for Lin’s concordance correlation coefficient: NIWA Client Report: HAM2005-062. Report to Ministry of Health, Hamilton, New Zealand. 2005 May; [Google Scholar]
- 12.Consensus Statement on Self-Monitoring of Blood-Glucose. Diabetes Care. 1987;10:95–99. [PubMed] [Google Scholar]
- 13.Molitch M, Barr J, Callahan P, et al. Consensus statement, self-monitoring of blood glucose. Diabetes Care. 1996;19:S62–S66. [Google Scholar]
- 14.Hein J, Spreyer F, Sauter-Louis C, Hartmann K. Reference ranges for laboratory parameters in ferrets. Vet Rec. 2012;171:218. doi: 10.1136/vr.100628. [DOI] [PubMed] [Google Scholar]
- 15.Johnson BM, Fry MM, Flatland B, Kirk CA. Comparison of a human portable blood glucose meter, veterinary portable blood glucose meter, and automated chemistry analyzer for measurement of blood glucose concentrations in dogs. J Am Vet Med Assoc. 2009;235:1309–1313. doi: 10.2460/javma.235.11.1309. [DOI] [PubMed] [Google Scholar]
- 16.Cohn LA, McCaw DL, Tate DJ, Johnson JC. Assessment of five portable blood glucose meters, a point-of-care analyzer, and color test strips for measuring blood glucose concentration in dogs. J Am Vet Med Assoc. 2000;216:198–202. doi: 10.2460/javma.2000.216.198. [DOI] [PubMed] [Google Scholar]
- 17.Wess G, Reusch C. Evaluation of five portable blood glucose meters for use in dogs. J Am Vet Med Assoc. 2000;216:203–209. doi: 10.2460/javma.2000.216.203. [DOI] [PubMed] [Google Scholar]
- 18.Cohen TA, Nelson RW, Kass PH, Christopher MM, Feldman EC. Evaluation of six portable blood glucose meters for measuring blood glucose concentration in dogs. J Am Vet Med Assoc. 2009;235:276–280. doi: 10.2460/javma.235.3.276. [DOI] [PubMed] [Google Scholar]
- 19.Zini E, Moretti S, Tschuor F, Reusch CE. Evaluation of a new portable glucose meter designed for the use in cats. Schweizer Archiv Fur Tierheilkunde. 2009;151:448–451. doi: 10.1024/0036-7281.151.9.448. [DOI] [PubMed] [Google Scholar]
- 20.Messier C, Kent P. Repeated blood glucose measures using a novel portable glucose meter. Physiology & Behavior. 1995;57:807–811. doi: 10.1016/0031-9384(94)00350-5. [DOI] [PubMed] [Google Scholar]
- 21.Weitgasser R, Davalli AM, Weir GC. Measurement of glucose concentrations in rats: Differences between glucose meter and plasma laboratory results. Diabetologia. 1999;42:256–256. doi: 10.1007/s001250051147. [DOI] [PubMed] [Google Scholar]
- 22.Acierno MJ, Mitchell MA, Schuster PJ, Freeman D, Guzman DSM, Tully TN. Evaluation of the agreement among three handheld blood glucose meters and a laboratory blood analyzer for measurement of blood glucose cocentration Hispaniolan Amazon parrots (Amazona ventralis) Am J Vet Res. 2009;70:172–175. doi: 10.2460/ajvr.70.2.172. [DOI] [PubMed] [Google Scholar]
- 23.Acierno MJ, Schnellbacher R, Tully TN. Measuring the level of agreement between a veterinary and a human point-of-care glucometer and a laboratory blood analyzer in Hispaniolan Amazon parrots (Amazona ventralis) J Avian Med Surg. 2012;26:221–224. doi: 10.1647/2011-038R1.1. [DOI] [PubMed] [Google Scholar]
- 24.Lieske CL, Ziccardi MH, Mazet JAK, Newman SH, Gardner IA. Evaluation of 4 handheld blood glucose monitors for use in seabird rehabilitation. J Avian Med Surg. 2002;16:277–285. [Google Scholar]
- 25.Burdick S, Mitchell MA, Neil J, Heggem B, Whittington J, Acierno MJ. Evaluation of two point-of-care meters and a portable chemistry analyzer for measurement of blood glucose concentrations in juvenile white-tailed deer (Odocoileus virginianus) J Am Vet Med Assoc. 2012;240:596–599. doi: 10.2460/javma.240.5.596. [DOI] [PubMed] [Google Scholar]
- 26.Katsoulos PD, Minas A, Karatzia MA, Pourliotis K, Christodoulopoulos G. Evaluation of a portable glucose meter for use in cattle and sheep. Vet Clin Pathol. 2011;40:245–247. doi: 10.1111/j.1939-165X.2011.00309.x. [DOI] [PubMed] [Google Scholar]
- 27.Hollis AR, Schaer BLD, Boston RC, Wilkins PA. Comparison of the Accu-Chek Aviva point-of-care glucometer with blood gas and laboratory methods of analysis of glucose measurement in equine emergency patients. J Vet Intern Med. 2008;22:1189–1195. doi: 10.1111/j.1939-1676.2008.0148.x. [DOI] [PubMed] [Google Scholar]
- 28.Wess G, Reusch C. Assessment of five portable blood glucose meters for use in cats. Am J Vet Res. 2000;61:1587–1592. doi: 10.2460/ajvr.2000.61.1587. [DOI] [PubMed] [Google Scholar]
- 29.Clarke WL, Cox D, Gonderfrederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood-glucose. Diabetes Care. 1987;10:622–628. doi: 10.2337/diacare.10.5.622. [DOI] [PubMed] [Google Scholar]
- 30.Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23:1143–1148. doi: 10.2337/diacare.23.8.1143. [DOI] [PubMed] [Google Scholar]
- 31.Krouwer JS, Cembrowski GS. A review of standards and statistics used to describe blood glucose monitor performance. J Diabetes Sci Technol. 2010;4:75–83. doi: 10.1177/193229681000400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang ZP, Lee JH, Louie RF, Kost GJ. Effects of different hematocrit levels on glucose measurements with handheld meters for point-of-care testing. Arch Pathol Lab Med. 2000;124:1135–1140. doi: 10.5858/2000-124-1135-EODHLO. [DOI] [PubMed] [Google Scholar]
- 33.Karon BS, Griesmann L, Scott R, et al. Evaluation of the impact of hematocrit and other interference on the accuracy of hospital-based glucose meters. Diabetes Technol Ther. 2008;10:111–120. doi: 10.1089/dia.2007.0257. [DOI] [PubMed] [Google Scholar]
- 34.Paul AEH, Shiel RE, Juvet F, Mooney CT, Mansfield CS. Effect of hematocrit on accuracy of two point-of-care glucometers for use in dogs. Am J Vet Res. 2011;72:1204–1208. doi: 10.2460/ajvr.72.9.1204. [DOI] [PubMed] [Google Scholar]
- 35.Sacks D. Carbohydrates. In: Burtis CA, Ashwood ER, Bruns DE, editors. Textbook of Clinical Chemistry and Molecular Diagnostic. 4th ed. St. Louis, Missouri: Elsevier Saunders; 2006. pp. 837–901. [Google Scholar]