Abstract
An egg count survey using environmental fecal samples obtained in spring or early summer was conducted to estimate the apparent prevalence of Toxocara vitulorum in unweaned bison calves and of other intestinal parasites in adult bison on 98 farms in Manitoba and Saskatchewan. Calf samples were pooled (maximum 5 samples per pool) by farm and positive pools were examined to determine individual T. vitulorum counts. Toxocara vitulorum eggs were found on 4 farms in Manitoba and none in Saskatchewan. Apparent herd-level prevalence estimates were 12% [95% confidence interval (95% CI): 3.4% to 28.2%] and 0% (95% CI: 0% to 5.7%) respectively. Samples from adult bison contained eggs/oocysts from trichostrongyle species, Eimeria sp., Monieza sp., Capillaria sp., Nematodirus sp. and Trichuris sp. in 100%, 95%, 72%, 13%, 13%, and 5% of herds, respectively. Strongyloides sp. were not found in any herd. Further studies are needed to assess parasite distribution patterns in bison and to evaluate the risk that T. vitulorum may pose to bison, cattle, and wildlife.
Résumé
Une enquête pour détecter Toxocara vitulorum et d’autres parasites gastro-intestinaux dans des troupeaux de bisons (Bison bison) du Manitoba et de la Saskatchewan. Une enquête sur la numération des œufs à l’aide d’échantillons fécaux environnementaux obtenus au printemps ou au début de l’été a été réalisée afin d’estimer la prévalence apparente de Toxocara vitulorum chez des veaux de bisons non sevrés et d’autres parasites intestinaux chez les bisons adultes dans 98 fermes du Manitoba et de la Saskatchewan. Les échantillons des veaux ont été regroupés (maximum de 5 échantillons par groupe) par ferme et les groupes positifs ont été examinés afin de déterminer les numérations individuelles de T. vitulorum. Des œufs de Toxocara vitulorum ont été trouvés dans 4 fermes au Manitoba et dans aucune ferme en Saskatchewan. Les estimations de prévalence apparentes au niveau du troupeau étaient de 12 % (intervalle de confiance de 95 % [IC de 95 %] : de 3,4 % à 28,2 %) et 0 % (IC de 95 % : de 0 % à 5,7 %) respectivement. Les échantillons des bisons adultes contenaient des œufs/ookystes d’espèces de trichostrongyles, Eimeria sp., Monieza sp., Capillaria sp., Nematodirus sp. et Trichuris sp. dans 100 %, 95 %, 72 %, 13 %, 13 % et 5 % des troupeaux, respectivement. Strongyloides sp. n’a pas été trouvé dans aucun troupeau. De nouvelles études sont requises pour évaluer les tendances de distribution des parasites chez les bisons et le risque que T. vitulorum puisse poser pour les bisons, le bétail et la faune.
(Traduit par Isabelle Vallières)
Introduction
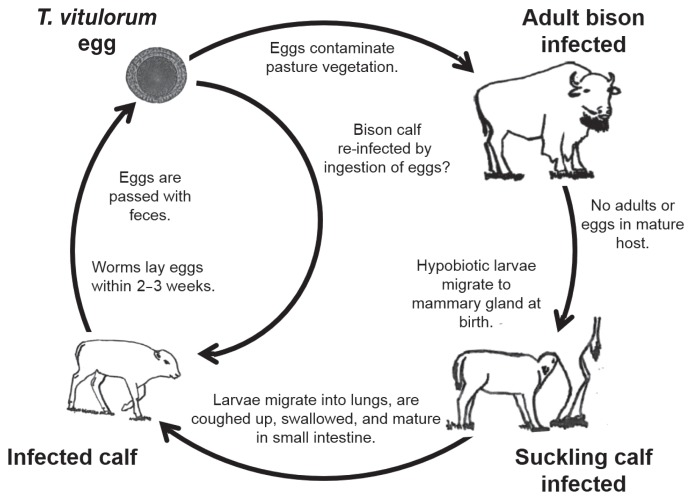
Toxocara vitulorum is a long (up to 30 cm), thick ascaridoid roundworm commonly found in water buffalo (Bubalus bubalis) and cattle (Bos taurus, Bos indicus) calves. Although T. vitulorum is most commonly found in tropical and subtropical climates (1–3) it has occasionally been reported from temperate areas including recently from cattle in the United Kingdom, the Netherlands (4), and in North American bison farmed in Belgium (5). This parasite is unusual as it does not establish patent infections in older (> 6 mo) calves and adult animals. When these older hosts ingest embryonated infective eggs from pasture the larvae hatch and migrate to muscles, liver, kidneys and other viscera where they become hypobiotic. In pregnant females these dormant larvae become active 1 to 8 d prior to parturition, migrate to the mammary glands and then into the colostrum and milk. Calves are infected via suckling and egg-producing adult worms will be present in their intestines about 3 wk after infection. These adult worms produce large numbers of eggs which are passed in calf feces onto the ground where they embryonate and become infective to other animals grazing on pasture. Mature worms remain in the calf for approximately 6 mo, at which time they are expelled. Males appear to be unaffected by the presence of hypobiotic parasites and are thought to be dead-end hosts. Figure 1 illustrates the theoretical life cycle in bison based on what is known about the parasite in water buffalo (3).
Figure 1.
The theoretical life cycle of Toxocara vitulorum in bison [after Starke-Buzetti (3)].
In June of 2011, a Manitoba bison herd with a history of ill-thrift and deaths of several young calves was investigated (6). On postmortem examination the calves were found to be heavily infected with ascaridoid roundworms subsequently identified as Toxocara vitulorum. The detection of this parasite in this herd is both notable and alarming since this was the first time that T. vitulorum had been reported in Canada. Severely affected bison calves can die from heavy worm burdens. Lesser affected calves suffer from ill-thrift, poor weight gain, and secondary health issues. Treatment of T. vitulorum infections is difficult in bison calves. In young bison calves this parasite can be eliminated during patency but this is a procedure not easily carried out under current management practices. Besides affecting the health of individual animals and the productivity of infected herds there may be potential impacts on national and international trade of live animals, as buyers may be reluctant to risk importing this parasite into their own herds.
To further investigate the significance of this discovery a study was carried out from June 1 to August 15, 2012. The primary objective of this investigation was to detect the number of bison herds infected with T. vitulorum and to estimate its apparent prevalence in calves < 6-months of age on farms in Saskatchewan and Manitoba. A secondary, opportunistic objective was to evaluate the presence of other gastrointestinal parasites in adult bison.
Materials and methods
Bison herds in the province of Manitoba were targeted for sampling because of the previous finding of T. vitulorum in this province. Inclusion of herds from outside Manitoba was restricted to Saskatchewan due to financial and logistical constraints. The sampling frame consisted of all producers in Saskatchewan (n = 173) and Manitoba (n = 59) who were registered with the Canadian Bison Association (CBA). An attempt was made to contact each producer by phone. An invitation to participate was also placed in industry publications and an invitational e-mail was sent to those producers who had an e-mail on file with the CBA. Every producer who was successfully contacted and who agreed to participate was included in the study. Samples were collected on-farm by provincial government livestock inspectors (Saskatchewan) or study personnel, or were collected and submitted by the producers themselves. Sampling personnel were provided with sampling kits consisting of sampling instructions, latex gloves, waterproof markers for sample identification, ice packs, and prepaid shipping labels. Due to the risks involved in handling bison, particularly cows with calves at foot, sampling was restricted to environmental samples i.e., fecal pats already on the ground. A target of 30 samples from different calf fecal pats and 10 samples from different adult bison fecal pats was set. If fewer calves or adults were present on the farm, then the target number of samples was to be equal to the number of animals in each class on that farm. Fecal pats were subjectively identified as from either calf or adult based primarily on size of the fecal pat and to a lesser extent on consistency. Since calving began in May and sampling was completed by August 15, 2012, most calves would have been < 90-days-old at the time of sampling.
Each sample was collected by using a clean latex glove to pick up approximately 50 g of feces from fresh pats. Gloves were then turned inside out and the opening knotted to contain the sample. Samples were shipped via courier to the Parasitology Laboratory at the Western College of Veterinary Medicine, Saskatoon, Saskatchewan. Samples were cooled by ice packs during shipment. All samples were processed and tested as soon as possible after receipt.
Calf feces were pooled by farm, with a maximum of 5 samples per pool, for the detection of T. vitulorum. All adult animal samples were tested individually. Five grams of each fecal sample or pool were used to count parasite eggs/oocysts using a Wisconsin double centrifuge fecal flotation technique (Sheather’s flotation solution) (7). Eggs and oocysts isolated using this technique were examined and identified using light microscopy (8). All calf samples from any positive pool were tested individually to determine T. vitulorum counts. Egg/oocyst counts were reported as eggs or oocysts per gram (EPG) of feces. Data were entered into an electronic spreadsheet (Microsoft Excel, 2010; Microsoft, Redmond, Washington, USA). Descriptive statistics were generated using Stata 12 statistical software (StataCorp LP, 2012. College Station, Texas, USA). Arithmetic means of egg/oocyst counts were reported.
Results
Samples were collected beginning in June through to August, 2012. Fecal samples were collected from adult bison on 34 (31% of all bison farms (9); 58% of CBA registered farms) and 64 farms [18% of all bison farms (9); 37% of CBA registered farms] (n = 1109) and from calves on 33 and 63 farms (n = 1211), with average herd sizes 131 and 173 animals (9) in Manitoba (MB) and Saskatchewan (SK), respectively. On average, 8 adult bison samples (range ± SD: 1 to 12 ± 2.2), and 13 calf samples were collected per farm (range ± SD: 1 to 30 ± 7.0). Samples were processed within 2.6 d of collection (range ± SD: 0 to 6 d ± 1.7 d).
The apparent herd-level prevalences of T. vitulorum in calf feces and other gastrointestinal parasites in adult bison feces are shown in Tables 1 and 2, respectively. Toxocara vitulorum eggs were found in the feces of calves from 4 farms in MB, but were not detected in any SK herds. The apparent prevalence of T. vitulorum in samples from these 4 herds was 77.8% (42/54). The apparent within-herd prevalence and average egg counts are shown in Table 3. The average T. vitulorum egg count for the 4 positive farms was 6051 EPG, with a range of 1 to 25 000 EPG.
Table 1.
Prevalence of Toxocara vitulorum in fecal samples collected between June 1 and August 15, 2012 from calves ≤ 3-months of age in Manitoba and Saskatchewan bison herds
| Province | Number of herds sampled | Number of samples per herd | T. vitulorum positive herds | Apparent herd-level prevalence (95% CI) |
|---|---|---|---|---|
| Saskatchewan | 63 | 12.6 | 0 | 0% (0% to 5.7%) |
| Manitoba | 33 | 12.3 | 4 | 12% (3.4% to 28.2%) |
95% CI — 95% confidence interval.
Table 2.
Prevalence of gastrointestinal parasite species and mean eggs per gram of feces in fecal samples collected between June 1 and August 15, 2012 from adult bison (≥ 1-year-old) on 98 farms in Manitoba and Saskatchewan
| GI parasite | Positive samples | Sample prevalence (%) | EPGa (SD; range) | # Positive farms | Apparent herd-level prevalence (95% CI) |
|---|---|---|---|---|---|
| Trichostrongyle | 772 | 94.0 | 132 (292; 1 to 5000) | 96 | 100% (96% to 100%) |
| Eimeria | 568 | 69.2 | 5 (84; 1 to 2000) | 93 | 97% (91% to 99%) |
| Monieza | 180 | 21.9 | 93 (183; 1 to 1000) | 70 | 73% (63% to 82%) |
| Capillaria | 21 | 2.6 | 11 (31; 1 to 145) | 13 | 14% (7% to 22%) |
| Nematodirus | 17 | 2.1 | 22 (37; 1 to 147) | 13 | 14% (7% to 22%) |
| Trichuris | 7 | 1.0 | 6.7 (6; 1 to 18) | 5 | 5% (2% to 12%) |
| Strongyloides | 0 | 0 | n/a | 0 | 0% (0% to 4%) |
Arithmetic mean egg count, standard deviation and range per gram of feces, positive samples only.
SD — standard deviation; 95% CI — 95% confidence interval. n/a — not available.
Table 3.
Prevalence of Toxocara vitulorum and mean eggs/oocytes per gram of feces in samples collected between June 1 and August 15, 2012 from bison calves ≤ 3-months of age on 4 farms in Manitoba
| Herd | Number of samples per herd | Number of positive samples | Apparent within-herd prevalence (95% CI) | EPG | SD | Range |
|---|---|---|---|---|---|---|
| 1 | 5 | 1 | 20% (1% to 72%) | 1390 | n/a | n/a |
| 2 | 21 | 16 | 76% (53% to 92%) | 4825 | 6753 | 1 to 25 000 |
| 3 | 21 | 21 | 100% (84% to 100%) | 8186 | 11170 | 2 to 25 000 |
| 4 | 7 | 4 | 57% (18% to 90%) | 914 | 1618 | 6 to 3333 |
EPG — Arithmetic mean egg count; SD — standard deviation; Range — range per gram of feces, positive samples only; n/a — not available.
Discussion
Our results for apparent within-herd prevalence and fecal egg counts for T. vitulorum in calf feces must be interpreted with caution. Because the samples were collected from the ground and not identified with specific animals it is possible that the same fecal pat was sampled more than once, thereby biasing the data used to determine within-herd prevalence. Similar sample collection methods were used for adult bison making the reported within-herd sample prevalence of each parasite suspect for the same reason (Table 2). Small sample sizes on many of the farms further reduce the precision of the results; however, the apparent herd-level prevalence data for each parasite or parasite class should be less vulnerable to the methodology used.
According to Roberts (2) treating pregnant cows is impractical due to the difficulty in accurately knowing the exact stage of pregnancy, the potential negative consequences of handling and treatment in late pregnancy, and large doses of anthelmintics required. Treatment regimens therefore focus on treatment of patent infections in young calves. The best time for treatment is in the prepatent period (10 to 16 days) of the parasite as this will preclude the deposition of large numbers of eggs into the environment, thus decreasing the risk of subsequent infection of pastured animals (10). Patent infections in bovine and water buffalo calves can be effectively treated with common avermectin class anthelmintics such as ivermectin, doramectin, moxidectin, eprinomectin, as well as fenbendazole (11–13). It is less certain which of these modern drugs are useful against the immature third-stage larvae in the calf intestine, although pyrantel and levamisole are effective (10).
Anecdotal reports indicate that treatment of patent infections in American bison calves with avermectins is effective. However, due to the difficulty in handling bison, especially when there are young calves at foot, the primary challenge remains finding a safe and efficient method of drug administration. Bison mothers (and related females) are dangerous and will aggressively defend their calves. Producers have reported the use of remote delivery of injectable drug preparations (ivermectin) through darting and application of pour-on formulations (eprinomectin) via water guns to treat calves without maternal interference, but control over the dose and certainty of adequate administration is lacking. Identification of previously treated calves and rapidly acquired avoidance behavior by the herd are also problematic.
None of the owners of herds positive with T. vitulorum observed clinical signs in their calves. However, with such high numbers of T. vitulorum eggs being excreted in calf feces there is opportunity for high infection pressure on pastures for breeding females and ultimately infection of the subsequent calves. The farm with 100% prevalence also had the highest count, with an average of 8186 EPG (range: 2 to 25 000 EPG); the next highest count was found on the farm with the next highest prevalence, with an average of 4825 EPG (range: 1 to 25 000 EPG). These counts appear very high, but are consistent with reports in other species (4,14). High worm burdens can result in diarrhea, colic, intestinal obstruction, emaciation, and death (6,15). It has been suggested that poor diet and harsh living conditions are important contributors to the clinical expression of T. vitulorum infections (2). If that is true, then it is possible that in herds with good diet and management infection may never be clinically manifested. These herds may then unknowingly disseminate this parasite throughout Canada via the movement of breeding females. The eggs may also be a source of infection for cattle via cross contamination of equipment, feed, and pasture with fecal material.
Adult bison are exposed to, and appear to carry, the same range of intestinal parasites as cattle (16). It is assumed that bison will suffer the same detrimental effects from parasitic infection as cattle. While the overall counts of egg/oocysts (other than T. vitulorum) in this study were relatively low, some individual animals had high counts, particularly for trichostrongyle species. The present study found that 100% of the bison herds sampled were infected with trichostrongyle worms. This is in agreement with a previous study conducted in Alberta from 1997 to 1999 (17). These authors indicated that their findings reflected the parasite population found in western Canadian cattle. We found that Monezia sp. (71.4%) were more prevalent than in the Alberta study (54.6%). Capillaria sp. was less prevalent (13.3%) in the current study, which is considerably lower than the 63.3% prevalence previously reported. Nematodirus and Trichuris prevalences, at 13.3% and 5.1%, respectively, were also substantially lower than in the Alberta study (50.0% and 40.0%). Penzhorn et al (18) previously reported a high prevalence of Eimeria spp. in bison, which corresponds to our current findings.
The authors have found only 1 published account of a fecal parasite survey in farmed bison (17). It was also performed on Canadian bison farms, at approximately 50° latitude. In their discussion of intestinal parasites of bison, Dies and Coupland (17) cite the work of Tessaro (19) who reviewed the pathologic conditions affecting bison and included a checklist of parasites of bison, but did not perform any fecal analyses or generate new data. Tessaro cited authors Wade et al (20) and Yamaguchi (21) as his source for information on nematodes found in bison. The former was a case report about the occurrence of ostertagiasis in 9 bison with diagnostic information from 3 farms in New York, New York. The latter is a textbook on helminths in vertebrate animals. Accordingly, there is a clear need for additional information about intestinal parasitism and its consequences in farmed bison. Ideally, multiple geographically distinct studies should be carried out on farmed or ranched bison across North America since parasite populations would no doubt reflect the disparate environmental conditions found, depending on the geographic location of the farm.
In summary, this study established the presence of T. vitulorum in 4 bison herds in Manitoba. The survey of adult bison confirmed existing information regarding parasite infection prevalence and intensity and can be used to suggest baseline parasite loads for commercial herds in western Canada. The North American bison industry is growing, with active trading of breeding animals and frequent establishment of new herds. It is easy to speculate that industry expansion might assist the rapid spread of T. vitulorum to naïve or newly established herds in western Canada. Vigilance is required to detect the occurrence of this and other parasites in bison. Monitoring should also be done in cattle, especially those that share common pasture or are otherwise in contact with known positive bison herds. Further studies will be needed to evaluate the threat that roundworms may pose to cattle (13) and wildlife (22–24), as well as their potential zoonotic risk (14).
Acknowledgments
We thank the bison producers who participated in this study, and Harry Paranjothy and the Saskatchewan Provincial Livestock Inspectors for collecting samples for the project. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
This project received support from the Canadian Bison Association, the Canadian Agricultural Adaptation Program (CAAP), the Canada-Saskatchewan Agri-Food Innovation Fund (AFIF), Manitoba Agriculture Food and Rural Initiatives (MAFRI), and the Saskatchewan Ministry of Agriculture.
References
- 1.Akyol CV. Epidemiology of Toxocara vitulorum in cattle around Bursa, Turkey. J Helminthol. 1993;67:73–77. doi: 10.1017/s0022149x00012888. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JA. Toxocara vitulorum in ruminants. Helminthol Abstr. 1993;62:151–174. [Google Scholar]
- 3.Starke-Buzetti WA. Toxocara vitulorum in livestock. In: Holland CV, Smith HV, editors. Toxocara: The Enigmatic Parasite. Cambridge, UK: CAB International; 2006. pp. 260–277. [Google Scholar]
- 4.Jones JR, Mitchell ESE, Redman E, Gilleard JS. Toxocara vitulorum infection in a cattle herd in the UK. Vet Rec. 2009;164:171–172. doi: 10.1136/vr.164.6.171. [DOI] [PubMed] [Google Scholar]
- 5.Goossens E, Dorny P, Vervaecke H, Roden C, Vercammen F, Vercruysse J. Toxocara vitulorum in American bison (Bison bison) calves. Vet Rec. 2007;160:556–557. doi: 10.1136/vr.160.16.556. [DOI] [PubMed] [Google Scholar]
- 6.Woodbury MR, Copeland S, Wagner B, Fernando C, Hill J, Clemence C. Toxocara vitulorum in a bison (Bison bison) herd from western Canada. Can Vet J. 2012;53:791–794. [PMC free article] [PubMed] [Google Scholar]
- 7.Cox DD, Todd AC. Survey of gastrointestinal parasitism in Wisconsin dairy cattle. J Am Vet Med Assoc. 1962;141:706–709. [PubMed] [Google Scholar]
- 8.Foreyt J. Veterinary Parasitology Reference Manual. 5th ed. Ames, Iowa: Iowa State University Press; 2001. pp. 1900–1989. [Google Scholar]
- 9.Census of Agriculture, selected livestock and poultry data — Canada and provinces [database on the Internet] Statistics Canada; c2012. [Last accessed June 17, 2014]. As cited by the Canadian Bison Association. Available from: http://www.canadianbison.ca/producer/resources/data_statistics.htm. [Google Scholar]
- 10.Roberts JA. Preventive treatment against toxocarosis in bovine calves. Vet Parasitol. 1992;44:111–118. doi: 10.1016/0304-4017(92)90149-4. [DOI] [PubMed] [Google Scholar]
- 11.Avcioglu H, Balkaya I. Efficacy of eprinomectin against Toxocara vitulorum in calves. Trop Anim Health Prod. 2011;43:283–286. doi: 10.1007/s11250-010-9699-7. [DOI] [PubMed] [Google Scholar]
- 12.Avcioglu H, Balkaya I. A comparison of the efficacy of subcutaneously administered ivermectin, doramectin, and moxidectin against naturally infected Toxocara vitulorum in calves. Trop An Health Prod. 2011;43:1097–1099. doi: 10.1007/s11250-011-9807-3. [DOI] [PubMed] [Google Scholar]
- 13.Davila G, Irsik M, Greiner EC. Toxocara vitulorum in beef calves in North Central Florida. Vet Parasitol. 2010;168:261–263. doi: 10.1016/j.vetpar.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Roberts JA. The extraparasitic life cycle of Toxocara vitulorum in the village environment of Sri Lanka. Vet Res Commun. 1989;13:377–388. doi: 10.1007/BF00346070. [DOI] [PubMed] [Google Scholar]
- 15.Roberts JA. The life cycle of Toxocara vitulorum in Asian buffalo (Bubalus bubalis) Intl J Parasitol. 1990;20:833–840. doi: 10.1016/0020-7519(90)90020-n. [DOI] [PubMed] [Google Scholar]
- 16.Knapp SE, Marley SE, Button SM, Rognlie MC. Bibliography and Abstracts: Parasites of the American Bison, Bison bison Linnaeus. Bozeman: Montana State University Miscellaneous Publication; 1993. p. 363. [Google Scholar]
- 17.Dies KH, Coupland RW. Prevalence of gastrointestinal helminths in domestic bison herds in northwestern Alberta. Can Vet J. 2001;42:295–296. [PMC free article] [PubMed] [Google Scholar]
- 18.Penzhorn BL, Knapp SE, Speer CA. Enteric coccidia in free-ranging American bison (Bison bison) in Montana. J Wildl Dis. 1994;30(2):267–269. doi: 10.7589/0090-3558-30.2.267. [DOI] [PubMed] [Google Scholar]
- 19.Tessaro SV. Review of the diseases, parasites and miscellaneous pathological conditions of North American bison. Can Vet J. 1989;30:416–422. [PMC free article] [PubMed] [Google Scholar]
- 20.Wade SE, Haschek WM, Georgi JR. Ostertagiosis in captive bison in New York State: Report of nine cases. Cornell Vet. 1978;69:198–205. [PubMed] [Google Scholar]
- 21.Yamaguchi S. The Nematodes of Veterbrates. Pt 1–2. III. New York: Interscience Publishing Inc; 1958. Systema helminthum. [Google Scholar]
- 22.Warren EG. Observations on the migration and development of Toxocara vitulorum in natural and experimental hosts. Intl J Parasitol. 1971;1:85–99. doi: 10.1016/0020-7519(71)90049-x. [DOI] [PubMed] [Google Scholar]
- 23.Schulte JW, Klimstra WD. Protozoan and helminth parasites of Key deer. J Wildl Manag. 1976;40:579–581. [Google Scholar]
- 24.Lucio-Forster A, Sweetman AE, Ortved K, Russell DS, Bowman DD. First report of Parascaris equorum in a white-tailed deer, Odocoileus virginianus from Mansfield, Pennsylvania, USA. Comp Parasitol. 2011;78:204–207. [Google Scholar]