Abstract
Objective(s):
Neurotoxicity of anticancer drugs complicates treatment of cancer patients. Vinblastine (VBL) is reported to induce motor and cognitive impairments in patients receiving chronic low-dose regimen.
Materials and Methods:
The effects of VBL treatment on motor, learning and memory functions of male and female Wistar rats were studied by behavioral related tests. Animals were given chronic intraperitoneal injections of VBL (0.2 mg/kg/week for 5 weeks) from postnatal day 23 to 52. Motor function was evaluated using grasping test and balancing was evaluated by the rotarod. Spatial learning and memory and anxiety-like behavior were determined using Morris water maze (MWM) task and open field test, respectively.
Results:
Administration of VBL caused severe damage to motor and balance function of male rats in comparison to female rats treated with VBL and rats treated with saline. Memory and locomotion were affected in both male and female rats compared with saline treated rats, while a sex difference was also observed in these parameters; male rats showed more impairment compared with female ones. Both male and female rats showed cognitive impairments in MWM task and no sex differences were observed in these functions.
Conclusion:
Results revealed that VBL is a potent neurotoxic agent and despite the profound effect of VBL on motor and cognitive functions, it seems that male rats are more susceptible to motor deficits induced by VBL.
Keywords: Anticancer, Learning and memory, Motor function, Vinblastine
Introduction
Impairments in cognitive and motor functions are associated with chemotherapy treatment in most cancer survivors (1). One of the best-known classes of these agents is the dimeric Vinca alkaloids. Vinca alkaloids are one of anticancer agents in current use (2). Vinblastine (VBL), as an anticancer drug from Vinca alkaloids family which is useful for treatment of malignant tumors, could penetrate in the blood-brain barrier sufficiently so as to have a therapeutic effect on CNS mass lesions(3). VBL is potentially a useful therapeutic option in Langerhans cell histiocytosis with CNS mass lesions, especially for those with inoperable lesions or multiple lesions. In addition, VBL is well tolerated and can be delivered for a prolonged time(3). Despite therapeutic effects of Vinca alkaloids, some neurotoxic effects have been reported (4, 5). Previous data show that VBL, like the other Vinca alkaloids, binds to tubulin and induces an accumulation of cells in mitosis (6, 7). Compounds interfering with microtubule function form an important class of anticancer agents, widely used in combination chemotherapy regimens for treating many cancers as well as leukemias (8, 9).
It is recognized that chemotherapy can have a profound toxicity for the nervous system. Long term studies have established that chemotherapy may unfavorably affect the development of higher order cerebral abilities and cognitive skills and also may impair learning and memory (10, 11).
The Vinca alkaloids compounds, such as VBL and vincristine, are well-known for inducing chronic sensory neuropathies (12, 13), but their motor and cognition neurotoxicities and sex differences are less recognized. Therefore, the purpose of the present study is to evaluate (1) whether hippocampal and cerebellar behavioral dysfunction occurs after intraperitoneal (IP) administration of VBL, and (2) the relationship between sex and behavior deficits subsequent to chronic VBL treatment, in rats.
To study the effect of VBL on the general health, the body mass and mortality rate of the animals were measured 23, 30, 37, 44, and 52 days after birth.
Materials and Methods
Male and primiparous female Wistar rats, weighing 200–250 g, were purchased from Pasture Institute (Tehran, Iran) and maintained in the Animal House, Kerman Neuroscience Research Center.
Pairs of females were then placed with single male rats in the late afternoon. Vaginal smears or plugs were examined the following morning at 9:00 AM. The day in which sperm was found, was designated as the gestation day of 0 (GD 0). Pups were weaned at 21 days of age. In the beginning of the experiment, animals were weight-matched and randomly assigned into motor and cognitive experimental groups with four subgroups: Groups I and II consisting of male and female saline groups were administered normal saline (0.2 ml) intraperitoneally (IP) for 5 weeks; Groups III and IV consisted of male and female VBL groups treated with VBL (IP, 0.2 mg/kg for 5 weeks from postnatal day 23 to 52, 1 injection for each week). VBL was purchased from Sobhan chemotherapeutic (0.5 mg/ml solution, EBEWE Pharma, Austria). Animal discomfort was minimized, each group consisted of a minimum of twelve animals (n=12). The care of laboratory animals followed the guiding principles for care and use of laboratory animals of the Neuroscience Research Center of Kerman Medical University and the study protocol was approved by the animal ethics committee of this institution (Code: EC/KNRC/91-183). Animals were kept in standard conditions: temperature of 22 ± 2° C and a 12-hr dark-light cycle. They had free access to food and water. We housed similarly treated female rats in the same cage to coordinate their sexual cycles. Measurements were taken during daylight (between 8:00 and 16:00 hr). Sex-dependent motor and cognitive behaviors were examined 5 weeks after the first injection using cerebellum-dependent, motor learning and hippocampal functional tasks and four behavioral tasks, the grasping, the rotarod, the open field, and Morris water maze task were chosen to evaluate these functions.
Behavioral studies
Grasping (hanging) test
For assessment of muscle strength and balance, this test was performed according to Van Wijk et al (2008) and Shabani et al (2013). Each rat was suspended with both forepaws on a horizontal steel wire (80 cm long, diameter 7 mm). The animal was held in a vertical position when its front paws were placed in contact with the wire. When the rat grasped the wire, it was released, and the latency to fall was recorded with a stopwatch. Each animal was given three trials with a 30 min inter-trial rest interval (14, 15).
Rotarod test
We used the accelerating rotating rod (Hugo Sachs Electronik, Germany) to analyze the effects of VBL on motor coordination and balance skills. The rotarod accelerated from a minimum speed of 10 to a maximum speed of 60 rpm. Rats were given three trials using maximum time of 300 sec with a 30 min inter-trial rest interval. The length of time each animal was able to maintain its balance walking on top of the moving rod was recorded (16, 17).
Open field test
The open field test was used as described in previous studies (18). Briefly, at 52 days of age the horizontal and vertical activities of the male and female rats were recorded for a period of 5 min and then analyzed using Ethovision software (version 7.1). The apparatus consisted of a square arena (90×90×20 [H] cm) made of Plexiglas and its floor was divided by lines into 16 squares that allowed the definition of central and peripheral parts. At the beginning of the session, each rat was placed in the centre of the arena and its activity was recorded for 5 min and the following behavioral parameters were then scored: frequency of rearing, total distance moved (TDM, Cm), velocity (Cm/sec), time spent in center and perimeter zone (sec) (19), total duration of mobility (sec), and immobility (s). At the end of each session, rats were removed from the open field and the experimental chamber was thoroughly cleaned with a damp cloth and dried (20).
Morris water maze task
The testing procedure was basically the same as that described by Shabani et al (2012). The experimental apparatus consisted of a circular water tank (140 Cm wide and 45 Cm high). A platform, either visible or submerged, (15 Cm wide and 35 Cm high) was placed 1.5 cm above or below the surface of the water. To assess for gross physical, sensory, motor, or motivational impairments, 8 rats in each group were first trained in a task with a visible escape platform. The water temperature was 21–23°C. Data collection was automated by a video image motion analyzer (Ethovision, Noldus Information Technology, The Netherlands). In a single training protocol each rat completed three blocks (each block consisted of four successive trials with four different releasing points) separated by a 30 min resting period. All of the experimental groups were tested during the lights on period between 8:00 and 12:00 hr. On each trial, rats were randomly released into the water from one of the four quadrants with their face toward the wall of the maze. During acquisition, the location of the platform remained constant and rats were allowed to swim for duration of 60 sec to find the hidden platform. After the animal found the platform, it was allowed to remain there for 20 – 30 sec and was then moved to an animal cage to wait 20 – 30 sec before the start of the next trial. The time and distance needed to find the hidden platform were collected and analyzed later. A single probe trial was given 2 hr after the last training trial to test the spatial memory in the water maze. In this trial the platform was removed and the rat was allowed to swim for 60 sec. The time and distance spent in the target quadrant (quadrant 4) were analyzed as a measure of spatial memory retention (21, 22).
Histological study
Under deep anesthesia, the animals were sacrificed and their brains removed and immersed in 10% buffered formaldehyde at 4 °C overnight. The brains were processed by the standard method for light microscopy study. Coronal sections 1.6–2.8 mm posterior to bregma were cut at a thickness of 5 μm using a microtome. Neuronal damage in the cerebellum and hippocampus were assessed by staining sections with cresyl fast violet (Nissl staining) (23).
Statistical analysis method
The results obtained are expressed as means ± SEM. Two-way ANOVAs for repeated measures were calculated for rotarod and the learning phase of the Morris water maze. The grasping test, motor activity and probe data were analyzed by one-way ANOVA. Individual comparisons were calculated using Tukey’s comparisons test, where appropriate. Sex differences data were analyzed by unpaired-sample t-test. Data are expressed as means±SEM of twelve animals per group. Statistical significance was defined at P<0.05. All computations were made using the SPSS software package (version 16.0).
Results
Effect of VBL on muscle coordination and balancing
Animals in the male VBL group showed a decreased latency to fall in grasping test compared to saline males (F(3, 38) = 9.12, P<0.01) and the female VBL group (F(3, 38) = 4.65, P<0.05), but these parameters did not change significantly in VBL treated female rats compared with female saline group (Figure 1.A). VBL in male rats induced a significant reduction in time spent on accelerating rotarod compared with female VBL rats (F(3, 38) = 3.91, P<0.05) and the saline group (F(3, 38) = 7.42, P<0.01) (Figure 1.B). These results indicate decreased muscle strength and motor balance in male VBL treated rats.
Figure 1.
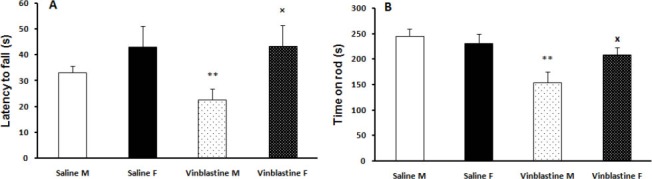
Effect of Vinblastine (VBL) on muscle strength and coordination in grasping test (A) and rotarod test (B)., **P< 0.01, * P< 0.05 as compared with saline and male groups respectively. VBL did decrease muscle strength and time spent on rod compared with saline only in the male group. A significant difference was also observed between male and female groups. Values are expressed as mean±SEM (n=12 for each group)
Effect of VBL on explorative and anxiety related behaviors
The open-field test was performed to examine locomotor activity and anxiety-related behavior. Our results showed that VBL administration did alter some of the parameters evaluated in this test and male rats were more susceptible to changes induced by VBL. The rearing number (male, F(3, 38) = 11.8, P< 0.001; female, F(3, 38) = 8.17, P<0.01) and total distance moved (male and female, F(3, 38) = 14.02, P< 0.001) were decreased in VBL group compared with saline group, while animals in the female VBL group showed an increased rearing number and total distance moved compared with male VBL treated rats (P<0.05) (Figure 2.A, 2.B). There were no significant differences in the time spent in center and periphery (indicating no difference in anxiety related behavior) and speed (Figure 2.C, 2.D, and 2.E). There were no significant differences in duration of mobility (F(3, 38) = .24, P=0.17) and immobility (F(3, 38) = .69, P=0.3) among the saline and VBL-treated groups (data not shown).
Figure 2.
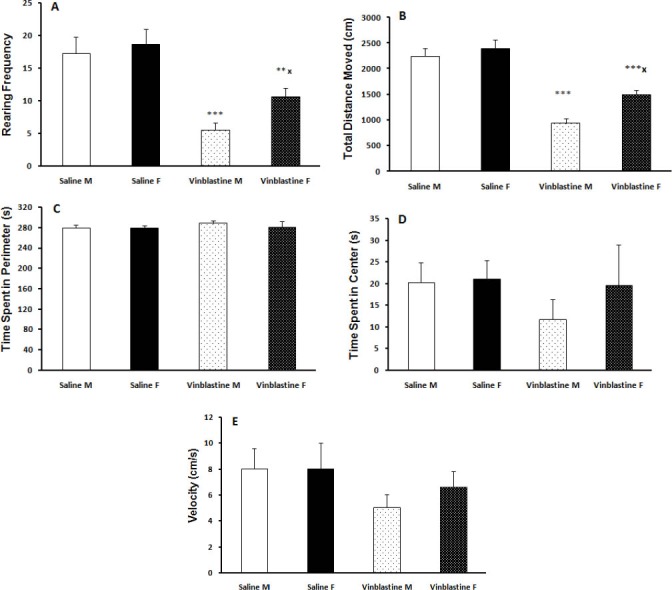
Vinblastine significantly reduced rearing frequency (A) and TDM (B). Female treated rats had significant differences in rearing frequency and TDM with male treated rats. There were no significant differences in time spent in perimeter (C), time spent in center (D), and velocity (E) among the four groups. ** P< 0.01, *** P< 0.001 as compared with the control group. * P< 0.05 as compared with the male group
Learning and memory alterations induced by VBL in Morris water maze test
VBL increased swimming distance (Figure 3A) and swimming time (Figure 3B) to reach the platform in male and female rats in block 2 (F(3, 38) = 9.63, P< 0.001) and block 3 (F(3, 38) = 6.19, P< 0.01) compared with male and female rats receiving saline (learning trial). Swimming speed was not different among the 4 groups (Figure 3C). In the probing session, swimming percentage in correct quadrant (F(3, 38) = 3.27, P< 0.05) and swimming time percentage spent in correct quadrant was significantly decreased in male and female rats receiving VBL compared with saline treated rats (F(3, 38) = 2.91, P<0.05) (Figure 3D, 3E). There was no difference between two sexes in MWM (P> 0.05). There was no significant difference between the saline and VBL groups in crossing number in the correct quadrant (Figure 3F).
Figure 3.
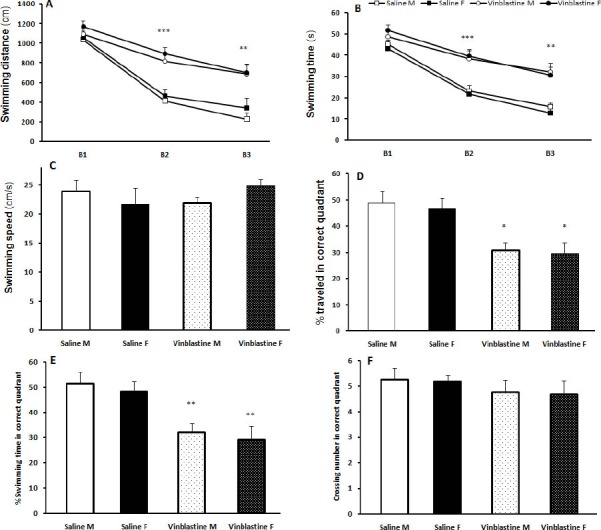
Hidden platform training in the Morris water maze acquisition after 5 weeks treatment with saline or vinblastine (VBL). VBL treated rats showed increased distance travelled (A) and time spent (B) to reach the hidden platform compared with the saline treated rats. There were no significant differences in swimming speed (C) and crossing numbers in the correct quadrant (F) in the probe test. In the probe test, the path length traveled (D) and percent swimming time (E) by VBL rats in the correct quadrant were significantly less than the saline groups. * P< 0.05,** P< 0.01, *** P< 0.001 as compared with the saline group
Histological evaluation
Light microscopy study of cerebellum and hippocampus sections showed normal morphology in the saline treated group. The Purkinje and granule cells in cerebellum sections and dentate gyrus, CA1, CA2, and CA3 of hippocampal sections in saline treated groups were intact. Meanwhile, severe injury in Purkinje cells of cerebellum sections and pyramidal neurons of hippocampal CA2 and CA3 sectors in VBL-treated groups was evident (Figure 4A, 4B). Cell population in Purkinje (Figure 4A), CA2 and CA3 (Figure 4B) sections in the vinblastine-treated groups showed neurodegenerative changes including shrunken nuclei and dark cytoplasm in these cells (Figure 4).
Figure 4.
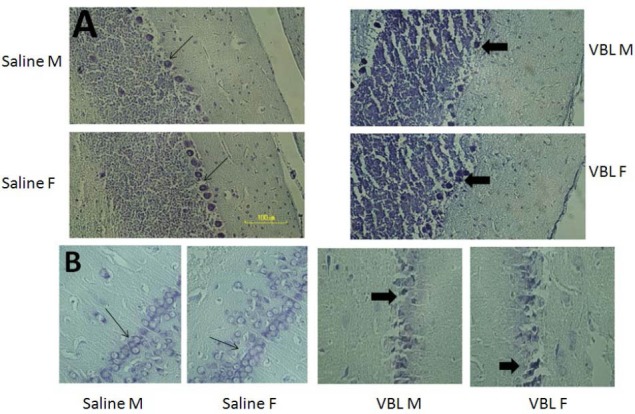
Neurons from the cerebellar cortex and hippocampus 5 weeks after exposure to Vinblastin (VBL). Most neurons from the cerebellum (A), and the hippocampus (B) in saline-treated rats have normal morphology (arrows), but for the VBL-treated groups, several degenerated cells (arrowheads) can be seen with shrinkaged nuclei and dark cytoplasm
Effects of adolescent exposure to VBL on mortality rate and body mass
In the present study, no differences were observed in weight between saline and VBL groups on the 1st day of experimental procedure, while long-term exposure to VBL 0.2 mg/kg (the 2nd, 3rd: P<0.01 and 4th: P<0.001 weeks) significantly decreased body weight in the male VBL group and in the 3rd and 4th (P<0.01) weeks in female VBL rats (Table 1).
Table 1.
Effect of vinblastine (VBL) treatment on body weight and mortality rate of different groups of rats
| Body weight (g) | Mortality rate | |||||
|---|---|---|---|---|---|---|
| Group | aPND 23 | PND 30 | PND 37 | PND 45 | PND 52 | |
| bSaline M | 48.2±4.01 | 80.7±4.2 | 115.5±6.1 | 150.1±5.8 | 167.8±8.1 | 1 |
| cSaline F | 45.8±3.3 | 71.5±3.3 | 105.6±4.9 | 123.7±7.6 | 129±8.3 | 0 |
| d VBL M | 46.6±2.7 | 74.1±7.1 | 75.04±6.3** | 100.5±6.2** | 110.2±6.5*** | 3 |
| e VBL F | 48.1±5.3 | 70.5±6.6 | 96.8±8.1 | 97.9±4.5** | 105.9±5.1** | 2 |
No differences were observed in weight between saline and VBL groups on the 1st day of experimental procedure, while long-term exposure to VBL 0.2 mg/kg (the 2nd, 3rd, and 4th weeks) significantly decreased body weight in the male VBL group and in the 3rd and 4th weeks in female VBL rats. **, *** P< 0.01, P< 0.001, indicate statistically significant differences between male and female VBL treated rats compared with saline groups, respectively, (n=at least 12 rats in each group). a: Postnatal Day, b: Saline Male c: Saline Female, d: VBL Male and e: VBL Female
Discussion
VBL impaired motor and learning functions in male and female rats; yet, male rats were more susceptible to motor impairments induced by VBL compared with female rats, and these findings implicate a sex-dependent motor deficit observed in rats after chronic exposure to VBL.
Although the toxic effects of these agents still remains a major limitation to their usage, vincristine is the most studied agent of this family and it is proven to have a high neurotoxic profile. The most important side effect of this drug is sensory loss and peripheral neuropathy (12), with the mechanism similar to colchicines; it disrupts microtubules and interferes with axonal and dendritic transport (5). Wright et al reported that two vinblastine derivatives (S-12363 and S-12362) prevented microtubule assembly and differed in their ability to induce the formation of tubulin paracrystals and in the stability of the paracrystals (24). Excitotoxic mechanisms and caspase-mediated cell death contribute to the neurotoxicity of anticancer compounds. Previous in vitro studies have focused on neurotoxicity of Vinca alkaloids in sympathetic neurons and motor neurons (25, 26). The excitotoxic component of anti-cancer drug neurotoxicity may relate to disruption of mitochondrial energy metabolism, resulting oxidative stress, and increased vulnerability of neurons to physiological glutamate concentrations (27, 28).
In this study, we have shown neurotoxic effect of VBL on motor and cognitive functions in male and female rats; as predicted, this agent could impair these functions. The most probable reason for this observation might be the same mechanism observed by Goldschmidt and steward (1989) for other Vinca alkaloids: disruption in axonal and dendritic transport (29). To our knowledge, the exact mechanism of VBL-induced neuropathy is not clear yet and this subject must be addressed in further studies.
Purkinje cells (PCs) play a crucial role in the movement control, and damages to these neurons would induce balance issues and movement deficits (16, 30). One possible mechanism for motor impairments observed in this study might be the consequent damage to PCs after chronic exposure to VBL, as observed in histologic evaluation.
Male rats were more susceptible than female rats to the VBL-induced impairments in motor function. We previously have observed sex differences in neurotoxic effects of anti-cancer agents on motor and cognitive functions (31). Shabani et al (2012) demonstrated the sex-dependence effect of vincristine on the developing nervous system (4). Vincristine induced impairments in motor and cognitive functions of both male and female rats, while male rats were more susceptible to the toxic effect of vincristine, a finding which is consistent with the present study. This difference between male and female rats might be due to sexual dimorphism in brain organization and function. Joseph and Levine (2003) have shown the sexual dimorphism in PKC signaling in vincristine-induced peripheral neuropathy and interestingly, female rats showed an estrogen-dependent sexual dimorphism in mechanical hyperalgesia (32).
Male rats were more susceptible to VBL-induced disruption in muscle strength and deficits in the maintenance of body balance than female rats. On the other hand, in female VBL-treated rats, no impairment was found in the grip-strength and motor balance tests, suggesting that muscular strength and motor balance in male rats is probably more susceptible to the effects of VBL than in female rats. Snell et al (2009) reported that the damage to vermis causes deficits in the maintenance of body balance, which appears as a difficulty in holding the head steady and the trunk erect (33). These impairments were seen in VBL-treated rats, which can be attributed to cerebellar dysfunction, probably derived from damage to the cerebellar vermis. Chemotherapeutic toxicity is affected by sex, and females are more prone to developing signs of toxicity (34), but in this study, male rats were more susceptible to the effects of VBL. We do not know the reason for this difference with clinical studies, but one possible cause might be sexual dimorphism in receptors and neurotransmitters affected by VBL (32), further research would clarify the exact mechanism and help us to develop better anti-cancer drugs noting sexual dimorphism. Meanwhile, it is difficult to determine with certainty the causes of the observed sex differences in our study. However, it is suggested that these differences may be related to sexual dimorphism in brain organization and function. Evidence exists for sex differences in many aspects of brain structure, neurotransmitter systems, and neuroendocrine regulation (35–37). It has been shown that receptor affinity of glucocorticoids in male rats is twice that in female rats (38). In addition, sex-dependent differences in brain development as well as in receptor densities have been reported for several monoamine neurotransmitter systems (39).
Cognitive function was impaired in rats receiving VBL, regardless of sex difference. This finding is consistent with our previous study showing that vincristine affects cognitive function of rats receiving chronic dosages of this anti-cancer agent (4). Patients receiving anti-cancer drugs show impairments in cognitive functions, and even some sex-differences have been observed in clinical research. Waber et al (1990) have shown cognitive impairments in visuoperceptual skills in patients treated for Acute Lymphoblastic Leukemia (ALL). Females were more susceptible to the chemotherapies and showed more severe cognitive impairments in comparison to males (40). Although we did not observe such sex-difference in cognitive function, the finding that cognitive processes is affected by anti-cancer agents is consistent with this study.
Since previous studies demonstrate that Vinka alkaloids might not pass blood-brain barrier (BBB), our study shows a new evidence of CNS involvement in Vinka alkaloids neurotoxicity. By using histologic methods, we have shown that neurons in CNS (cerebellum and hippocampus) are affected by VBL. Whether this is a direct effect of VBL or is it a result of VBL metabolism is a question which might be addressed in further research.
In conclusion, we have shown CNS involvement in neurotoxicity induced by VBL and furthermore, we have shown that sex might play an important role in response to treatment with VBL, with male rats being more susceptible to the toxic effects of this anti-cancer agent.
Acknowledgment
The present manuscript is the product of a research project that was approved by the Kerman University of Medical Sciences, Kerman, Iran. The reported results were part of MSc student thesis.
Footnotes
Conflict of interest The authors declare no conflict of interest. The author alone is responsible for the contents and authoring of the paper.
References
- 1.Correa DD, Ahles TA. Neurocognitive changes in cancer survivors. Cancer J. 2008;14:396–400. doi: 10.1097/PPO.0b013e31818d8769. [DOI] [PubMed] [Google Scholar]
- 2.Liang GW, Lu WL, Wu JW, Zhao JH, Hong HY, Long C, et al. Enhanced therapeutic effects on the multi-drug resistant human leukemia cells in vitro and xenograft in mice using the stealthy liposomal vincristine plus quinacrine. Fundam Clin Pharmacol. 2008;22:429–437. doi: 10.1111/j.1472-8206.2008.00613.x. [DOI] [PubMed] [Google Scholar]
- 3.Tin SNW, Martin-Duverneuil N, Idbaih A, Garel C, Ribeiro M, Parker JL, et al. Efficacy of vinblastine in central nervous system Langerhans cell histiocytosis: a nationwide retrospective study. Orphanet J Rare Dis. 2011;6:83. doi: 10.1186/1750-1172-6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shabani M, Larizadeh MH, Parsania S, Asadi-shekaari M, Shahrokhi N. Profound destructive effects of adolescent exposure to vincristine accompanied with some sex differences in motor and memory performance. Can J Physiol Pharmacol. 2012;90:379–386. doi: 10.1139/y11-132. [DOI] [PubMed] [Google Scholar]
- 5.Steward O, Goldschmidt RB, Sutula T., IV Neurotoxicity of colchicine and other tubulin-binding agents: A selective vulnerability of certain neurons to the disruption of microtubules. Life Sci. 1984;35:4351. doi: 10.1016/0024-3205(84)90150-4. [DOI] [PubMed] [Google Scholar]
- 6.Kruczynski A, Hill BT. Vinflunine, the latest Vinca alkaloid in clinical development: : A review of its preclinical anticancer properties. Crit Rev Oncol Hematol. 2001;40:159–173. doi: 10.1016/s1040-8428(01)00183-4. [DOI] [PubMed] [Google Scholar]
- 7.Kruczynski A, Barret JM, Etiévant C, Colpaert F, Fahy J, Hill BT. Antimitotic and tubulin-interacting properties of vinflunine, a novel fluorinated Vinca alkaloid. Biochem Pharm. 1998;55:635–648. doi: 10.1016/s0006-2952(97)00505-4. [DOI] [PubMed] [Google Scholar]
- 8.Suman G, Jamil K. Application of human lymphocytes for evaluating toxicity of anti-cancer drugs. Int J Pharmacol. 2006;2:374–381. [Google Scholar]
- 9.Martinez FJ, Zeng GQ, Pineyro A, Garza-Ocañas L, Tomei LD, Umansky SR. Apoptosis induction and cell cycle perturbation in established cell lines by peroxysomicine A1 (T-514) 1 Drug Chem Toxicol. 2001;24:287–299. doi: 10.1081/dct-100103725. [DOI] [PubMed] [Google Scholar]
- 10.Seigers R, Schagen SB, Beerling W, Boogerd W, van Tellingen O, van Dam FSAM, et al. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav Brain Res. 2008;186:168–175. doi: 10.1016/j.bbr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Shabani M, Larizadeh Mh, Parsania S, Hajali V, Shojaei A. Evaluation of destructive effects of exposure to cisplatin during developmental stage: No profound evidence for sex differences in impaired motor and memory performance. Int J Neurosci. 2012;22:439–448. doi: 10.3109/00207454.2012.673515. [DOI] [PubMed] [Google Scholar]
- 12.Argyriou A, Polychronopoulos P, Koutras A, Xiros N, Petsas T, Argyriou K, et al. Clinical and electrophysiological features of peripheral neuropathy induced by administration of cisplatin plus paclitaxel based chemotherapy. Eur J Cancer Care. 2007;16:231–237. doi: 10.1111/j.1365-2354.2006.00718.x. [DOI] [PubMed] [Google Scholar]
- 13.Yalcin S NG, Orhan B, Zeybek D, Müftüoğlu S, Sarer B, Yildirim BA, et al. Protective effect of amifostine against cisplatin-induced motor neuropathy in rat. Med Oncol. 2003;20:175–180. doi: 10.1385/MO:20:2:175. [DOI] [PubMed] [Google Scholar]
- 14.Van Wijk N, Rijntjes E, Van De Heijning B. Perinatal and chronic hypothyroidism impair behavioural development in male and female rats. Exp Physiol. 2008;93:1199–1209. doi: 10.1113/expphysiol.2008.042416. [DOI] [PubMed] [Google Scholar]
- 15.Haghani M, Shabani M, Moazzami K. Maternal mobile phone exposure adversely affects the electrophysiological properties of Purkinje neurons in rat offspring. Neuroscience. 2013;250:588–598. doi: 10.1016/j.neuroscience.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 16.Shabani M, Haghani M, Shaibani V, Janahmadi M. Maternal exposure to the CB1 cannabinoid agonist WIN 55212-2 produces robust changes in motor function and intrinsic electrophysiological properties of cerebellar Purkinje neurones in rat offspring. Neuroscience. 2011;172:139–152. doi: 10.1016/j.neuroscience.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 17.Golchin L, Vahidi A, Shabani M. Hippocampus and cerebellum function following imipenem treatment in male and female rats: evaluation of sex differences during developmental stage. Pak J Biol Sci. 2013;16:151–159. doi: 10.3923/pjbs.2013.151.159. [DOI] [PubMed] [Google Scholar]
- 18.Razavinasab M, Shamsizadeh A, Shabani M, Nazeri M, Allahtavakoli M, Asadi-Shekaari M. Pharmacological blockade of TRPV1 receptors modulates the effects of 6-OHDA on motor and cognitive functions in a rat model of Parkinson's disease. Fundam Clin Pharmacol. 2013;27:632–640. doi: 10.1111/fcp.12015. [DOI] [PubMed] [Google Scholar]
- 19.de Souza Lisboa SF, Gonçalves G, Komatsu F, Salci Queiroz CA, Aparecido Almeida A, Gastaldello Moreira E. Developmental lead exposure induces depressive-like behavior in female rats. Drug Chem Toxicol. 2005;28:67–77. doi: 10.1081/dct-39696. [DOI] [PubMed] [Google Scholar]
- 20.Shojaei A, Shabani M, Pilevarian A, Parsania S, Razavinasab M. Effect of acute administration of Cisplatin on memory, motor learning, balance and explorative behaviours in Rats. Physiol Pharmacol. 2012;16:121–135. [Google Scholar]
- 21.Frick KM, Stillner ET, Berger-Sweeney J. Mice are not little rats: species differences in a one-day water maze task. Neuroreport. 2000;11:3461. doi: 10.1097/00001756-200011090-00013. [DOI] [PubMed] [Google Scholar]
- 22.Azimi L, Pourmotabbed A, Ghadami MR, Nedaei SE, Pourmotabbed T. Effects of peripheral and Intra-hippocampal administration of sodium salicylate on spatial learning and memory of rats. Iran J Basic Med Sci. 2012;15:709. [PMC free article] [PubMed] [Google Scholar]
- 23.Movassaghi S, Sharifi ZN, Soleimani M, Joghataii MT, Hashemi M, Shafaroodi H, et al. Effect of pentoxifylline on ischemia-induced brain damage and spatial memory impairment in rat. Iran J Basic Med Sci. 2012;15:1083–1090. [PMC free article] [PubMed] [Google Scholar]
- 24.Wright M, Garès M, Verdier-Pinard P, Moisand A, Berlion M, Legrand JJ, et al. Differential in vitro action of S-12363, a new vinblastine derivative, and of its epimer on microtubule proteins. Cancer Chemother Pharmacol. 1991;28:434–440. doi: 10.1007/BF00685819. [DOI] [PubMed] [Google Scholar]
- 25.Gozdz A, Habas A, Jaworski J, Zielinska M, Albrecht J, Chlystun M, et al. Role of N-methyl-D-aspartate receptors in the neuroprotective activation of extracellular signal-regulated kinase 1/2 by cisplatin. J Biol Chem. 2003;278:43663–43671. doi: 10.1074/jbc.M301554200. [DOI] [PubMed] [Google Scholar]
- 26.Park DS, Morris EJ, Stefanis L, Troy CM, Shelanski ML, Geller HM, et al. Multiple pathways of neuronal death induced by DNA-damaging agents, NGF deprivation, and oxidative stress. J Neurosci. 1998;18:830–840. doi: 10.1523/JNEUROSCI.18-03-00830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Souid A-K, Tacka KA, Galvan KA, Penefsky HS. Immediate effects of anticancer drugs on mitochondrial oxygen consumption. Biochem Pharmacol. 2003;66:977–987. doi: 10.1016/s0006-2952(03)00418-0. [DOI] [PubMed] [Google Scholar]
- 28.Zhou B-BS, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 29.Goldschmidt R, Steward O. Comparison of the neurotoxic effects of colchicine, the vinca alkaloids, and other microtubule poisons. Brain Res. 1989;486:133–140. doi: 10.1016/0006-8993(89)91285-7. [DOI] [PubMed] [Google Scholar]
- 30.Shabani M, Haghani M, Sheibani V, Janahmadi M. Changes in motor and learning behaviors of rats prenatally exposed to WIN 55212-2, a cannabinoid receptor agonist. Physiol Pharmacol. 2009;13:120–129. [Google Scholar]
- 31.Razavinasab M, Shamsizadeh A, Shabani M, Nazeri M, Allahtavakoli M, Asadi-Shekaari M, et al. Pharmacological blockade of TRPV1 receptors modulates the effects of 6-OHDA on motor and cognitive functions in a rat model of Parkinson's disease. Fundam Clin Pharmacol. 2012;27:632–640. doi: 10.1111/fcp.12015. [DOI] [PubMed] [Google Scholar]
- 32.Joseph EK, Levine JD. Sexual dimorphism for protein kinase c signaling in a rat model of vincristine-induced painful peripheral neuropathy. Neuroscience. 2003;119:831–838. doi: 10.1016/s0306-4522(03)00203-3. [DOI] [PubMed] [Google Scholar]
- 33.Snell RS. Lippincott Williams & Wilkins; 2009. Clinical neuroanatomy. [Google Scholar]
- 34.Wang J, Huang Y. Pharmacogenomics of sex difference in chemotherapeutic toxicity. Curr Drug Discov Technol. 2007;4:59–68. doi: 10.2174/157016307781115485. [DOI] [PubMed] [Google Scholar]
- 35.Allen JS, Damasio H, Grabowski TJ, Bruss J, Zhang W. Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. Neuroimage. 2003;18:880–894. doi: 10.1016/s1053-8119(03)00034-x. [DOI] [PubMed] [Google Scholar]
- 36.Madeira MD, Lieberman AR. Sexual dimorphism in the mammalian limbic system. Prog Neurobiol. 1995;45:275–333. doi: 10.1016/0301-0082(94)00052-j. [DOI] [PubMed] [Google Scholar]
- 37.Reza E, Nezhad H, Kamyab S, Center B, Center SO. Consequences of Ischemic Preconditioning of Kidney: Comparing between male and female rats. Iran J Basic Med Sci. 2012;15:1148–1153. [PMC free article] [PubMed] [Google Scholar]
- 38.Turner BB, Weaver DA. Sexual dimorphism of glucocorticoid binding in rat brain. Brain Res. 1985;343:16–23. doi: 10.1016/0006-8993(85)91153-9. [DOI] [PubMed] [Google Scholar]
- 39.Valencia-Sánchez A, Esparza-Avalos N, Cruz M, Ortega-Corona B. Amine neurotransmitter levels in male and female rats through developmental periods. Arch Androl. 1997;39:79–83. doi: 10.3109/01485019708987905. [DOI] [PubMed] [Google Scholar]
- 40.Waber DP, Gioia G, Paccia J, Sherman B, Dinklage D, Sollee N, et al. Sex differences in cognitive processing in children treated with CNS prophylaxis for acute lymphoblastic leukemia. J Pediatr Psychol. 1990;15:105–122. doi: 10.1093/jpepsy/15.1.105. [DOI] [PubMed] [Google Scholar]


