Abstract
Objective(s):
This study was carried out to investigate the effects of COX-2 selective inhibitor (Celecoxib) or non-selective COX inhibitor (Ibuprofen) on gastrointestinal motility.
Materials and Methods:
The rats were randomly divided into five groups including: intact, sham, traumatic brain injury (TBI) group (intact rats under TBI), Celecoxib group (10 mg/kg), Ibuprofen group (10 mg/kg). Rats of the treatment groups received gavages at 1 hr before the TBI induction. The TBI was moderate and diffused using the Marmarou method. The gastric emptying and small intestine transit were measured by phenol red method.
Results:
The gastric emptying didn’t change following TBI induction compared to intact group. The consumption of ibuprofen or celecoxib didn’t have any effect on gastric emptying compared to sham group. TBI induction didn’t have any effect on the intestinal transit. Also, there was no significant difference between ibuprofen or celecoxib consumption vs. sham group (P>0.05).
Conclusion:
The COX-2 selective inhibitor (celecoxib) or non-selective COX inhibitor (ibuprofen) have no effects on gastric or small bowel transit. Further work is necessary to investigate the effects of non-selective COX inhibitors and their impact on gastrointestinal motility disorders.
Keywords: Brain injury, Celecoxib, Gastric emptying, Ibuprofen
Introduction
Acute brain injury is a major cause of morbidity and mortality which correlates partly to the initial cerebral insult (1). Following traumatic brain injury (TBI), the function of some organs is impaired, contributing to a worse outcome. Systemic inflammatory response syndrome (SIRS) is commonly encountered following acute brain injury. The gastrointestinal tract is frequently affected within SIRS. Distorted motility, gastrointestinal bleeding, splanchnic ischemia, gut hyperpermeability and bacterial translocation have been described as the acute brain injury consequences (2).
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most frequently used drugs in inflammatory diseases, since they are helpful in management of the pain, fever, redness, and edema arising as a result of inflammatory mediator liberation (3). Studies have shown that both therapeutic and side effects of NSAIDs are dependent on cyclooxygenase (COX) inhibition (4). It has been proposed that COX-2 inhibition is responsible for the therapeutic effects of NSAIDs, while COX-1 inhibition causes the gastrointestinal and renal side effects (5). In spite of, similar amino acid sequence and catalytic activity of two COX isoforms, they were demonstrated to have different roles. These isoforms were named ‘constitutive’COX-1 and ‘inducible’ COX-2 (6).
COX-1 catalyzes the construction of cyto-protective prostaglandins (PGs) in thrombocytes, vascular endothelium, stomach mucosa, kidneys, pancreas, Langerhans islets, and brain. COX-1 products (prostaglandins (PGI2 and PGE2)), keeps integrity of gastrointestinal tract by reducing gastric acid secretion, increasing the thickness of mucus layer, stimulating bicarbonate secretion, and enhancing mucosal blood flow (7, 8). Drugs, which inhibit COX-1 more than COX-2, such as indomethacin, naproxen, and ibuprofen, cause more severe damage to the gastric tissues. As a result of studies focused on reduction of the adverse effects of NSAIDs, selective COX-2 inhibitors, such as celecoxib and rofecoxib, have been developed (9).
Selective inhibitors of COX-2 are drugs in which therapeutic effects are as strong as conventional NSAIDs but would lead to fewer side effects (10). They possess analgesic, antipyretic and anti-inflammatory effects as potent as traditional antiinflammatory drugs. Celecoxib and rofecoxib inhibit COX-2 more than COX-1 (11). It has been reported that these drugs do not induce any damage to the stomach tissue (12).
While the effects of selective and non-selective COX inhibitors on gastric mucosal integrity are the subject of continued investigation, there is little information about the specific effect of COX-1 and COX-2 inhibitors on gastrointestinal motility following traumatic brain injury. In the present study, we evaluated the effects of COX-2 selective inhibitor (celecoxib) or non-selective COX inhibitor (ibuprofen) on gastrointestinal motility, using gastric emptying and intestinal transit model after the induction of traumatic brain injury.
Materials and Methods
Animals
Male Wistar rats (250 to 300 g) were purchased from animal center of Kerman University of Medical Sciences, Iran. The rats were housed in temperature and humidity controlled animal quarters with a 12 hr light/dark cycle. All procedures were approved by the Institutional Animal Care Committee of Kerman University and were in accordance with the guidelines of the National Institutes of Health on the care and use of animals surgery.
Experimental groups
The rats were randomly divided into five groups (7 rats in each group): 1) intact group: the animals that were not given any drugs; 2) sham group: sham operated rats, but without actual induction of TBI; 3)TBI group: intact rats injured using the traumatic brain injury device; 4) celecoxib group: gavaged with celecoxib (10 mg/kg) at 1 hr before the TBI induction (13); 5) iboprofen group: gavaged with ibuprofen (10 mg/kg) at 1 hr before the TBI induction (14).
Induction of TBI
All animals were intubated before the TBI induction. The TBI was moderate and diffused using the Marmarou method. The process of the TBI induction (made by department of Physiology, Kerman University of Medical Sciences) was as follows: a 250 g weight was dropped from a 2 meter height on the head of the anesthetized rat (with halothane in an mixture of 70% N2O / 30% O2 gas) when a metal disc (stainless steel) 10 mm in diameter and 3mm thick is attached on the animals skull. After the induction of trauma, the rats were immediately connected to animals’ respiratory pump (TSA animal respiratory compact, Germany). Endotracheal tube was removed after restoration of spontaneous breathing. After recovery, the rats were placed in individual cages (15).
Phenol red meal
Phenol red indicator (25 mg) was dissolved in 50 ml of distilled water and filtered. The filtrate was heated to 70°C and methylcellulose (0.75 g) was added to it with continuous stirring. Then, the mixture was cooled to 37°C (16).
Gastric emptying (GE)
The standard method of phenol red marker meal accomplished by earlier workers, was used. Rats were deprived of food for 24 hr prior to experimentation but had free access to water, and 0.5 ml of phenol red meal was administered by the aid of the oral feeding syringe. Animals were killed by cervical dislocation under intravenous thiopentone sodium anesthesia 15 min after the administration of the meal. Abdomen was opened and stomach was dissected out after careful ligation at the cardiac and pyloric ends, and was washed with normal saline. The stomach was homogenized with 25 ml of 0.1 N NaOH. Then, 0.5 ml of trichloroacetic acid (20 % w/v) was added to 5 ml homogenate and centrifuged at 3000 rpm for 20 min. One ml of supernatant mixed with 4 ml of 0.5 N NaOH. The absorbance of acquired pink colored liquid was measured using spectrophotometer at 560 nm. This correlates with the concentration of phenol red in the stomach, which in turn depends upon the gastric emptying. The percentage gastric emptying is derived as (1-X/Y) 100 where, X is absorbance of phenol red recovered from the stomach of animals sacrificed 15 min after test meal. Y is mean (n=5) absorbance of phenol red recovered from the stomachs of control animals (killed at 0 min following test meal) (16).
The small intestine transit
The small intestine was dissected out from the pylorus and ileocaecal junction and the point to which meal had traversed was secured with thread to avoid change in the length of the transit due to handling. The total length of the small intestine and distance traveled by phenol red meal was measured. The small intestinal transit (SIT) was calculated considering the distance traveled by phenol red meal divided by total length of the small intestine, multiplied by 100. Small intestinal transit was expressed as mean ± SEM. The observer was blinded for the drugs administered to the mice (16).
Statistical analysis
SPSS 11.5 was used for the statistical analysis. Each parameter was expressed as mean ± SEM, and compared using one-way analysis of variance (ANOVA), followed by least significant difference (LSD) test. The level of significance was P<0.05.
Results
Gastric emptying
As shown in Figure 1, the gastric emptying didn’t change in TBI group (95.42 ± 1.2) compared to intact group (95 ± 0.61). Also, the consumption of iboprophen (94 ± 1) or celecoxib (95 ± 1.2) didn’t have any effect on gastric emptying compared to sham group.
Figure 1.
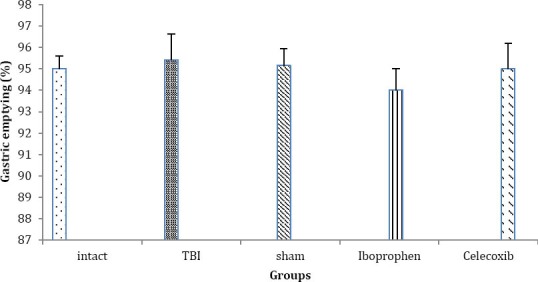
Gastric emptying in different groups (n= 7 in each group) after traumatic brain injury. Data are presented as mean±SEM. Abbreviation: TBI: Traumatic brain injury
Intestinal transit
As shown in Table 1, TBI induction didn’t have any effect on the intestinal transit. Also, there is no significant difference between ibuprofen or celecoxib consumption compared to sham group (P> 0.05).
Table 1.
Small intestinal transit in different groups (n= 7 in each group) after traumatic brain injury. Data are presented as mean ± SEM. Abbreviation: TBI: Traumatic brain injury
| Treatment (mg/kg) | Small intestine transit (%) | P-Value |
|---|---|---|
| Intact | 85.42± 2.10 | P> 0.05 |
| TBI | 87.85± 1.31 | P> 0.05 |
| Sham | 86.42± 0.71 | P> 0.05 |
| Ibuprofen (10) P.O. | 85.57± 0.54 | P> 0.05 |
| Celecoxib (10) P.O. | 85.71± 0.8 | P> 0.05 |
Discussion
TBI induces changes in some organs far from the initial injury site. This study describes a model of TBI in rat and the effects of a COX-2 selective inhibitor (celecoxib) or non-selective COX inhibitor (ibuprofen) on gastrointestinal motility.
In the present study it was found that gastric and intestinal transit didn’t change following brain trauma. In consistent with our results, Melro et al reported that gastric emptying did not change following mild to moderate ischemic brain injury (17). However, the presence of vomiting, abdominal distension, and increased gastric remnants after neurological trauma suggests abnormal gastric movements in some studies. It has been observed that gastric movements were inhibited during the first min by bilateral carotid artery ligation and cerebral ischemia (18). Also, increased intracranial pressure caused reversible inhibition of duodenal and gastric motor function immediately (19). Possible reasons for this controversy could be explained by ischemic brain injury type, time, and method of gastric motility measurement.
The current study also showed that gastric motility and intestinal transit didn’t change following treatment with ibuprofen or celecoxib. Arachidonic acid is released from cell membranes following TBI and converted to prostaglandins by cyclooxygenases (20). Cyclooxygenase-2 (COX-2) is a primary inflammatory mediator that converts arachidonic acid from damaged membranes into vasoactive prostaglandins, producing reactive oxygen species in the process (21). Also, COX-1 and COX-2 are expressed in the gastrointestinal neuromuscular tissue, and that the mechanical activities of gastrointestinal muscles are modulated by prostanoids originating from both isoforms (22).
In agreement with our results, Bouras et al showed that COX-2 inhibitors, celecoxib or rofecoxib, had no effect on gastric emptying ofliquids and solids in humans (23). Tanaka et al showed that a selective COX-1 inhibitor (SC-560) caused marked gastric and intestinal hypermotility, whereas a selective COX-2 inhibitor (rofecoxib) had no effect on basal gastric or intestinal motor activities (24). Santoz et al showed that pharmacologically selective COX-2 inhibitor delayed gastric emptying but not intestinal transit, and inhibition of COX-1 delayed intestinal transit but not gastric emptying of liquids in awake rats (25). However, Porcher et al showed that both GR253035X (COX-2 selective inhibitor) and indomethacin (non selective COX inhibitor) reduced the fundal tone, and increased antral phasic contraction in the mouse stomach (22). Takeuchiet et al showed that SC-560, a COX-1 selective inhibitor, enhanced both amplitude and frequency of intestinal contractions in rats (26).
In general, the results with COX-2 inhibitors were somewhat unexpected, as previous data with prostaglandins and non-selective COX inhibitors showed motor effects in vivo. In animal studies of myoelectrical activity and gastric emptying, prostaglandins have variable effects on the upper gut (22). Although some studies suggest prostaglandins accelerate emptying of liquids from the human stomach, many studies have shown either no effect or a delay in gastric emptying in humans secondary to prostaglandins (27).
In the current study, one possible explanation for the lack of the effect of the studied COX-inhibitors is inadequate dosing of drugs. The lack of the effect could also be secondary to the class of the COX inhibitors studied, based on the difference in expression of the COX isoforms (28). It is also possible that if we could use heavier weight, a significant inflammation might be induced. In this condition, we can assess the treatment groups more careful.
Conclusion
In summary, by the applied dosages, the COX-2 selective inhibitor (celecoxib) or non-selective COX inhibitor (ibuprofen) had no effects on gastric or small bowel transit. Further works are needed to investigate the effects of non-selective COX inhibitors and their impact on gastrointestinal motility in diseases associated with inflammation.
Acknowledgment
The present study was financially supported by Physiology Research Center of Kerman University of Medical Sciences, Kerman, Iran. The authors declare that they have no conflict of interests.
References
- 1.Wang KK, Larner SF, Robinson G, Hayes RL. Neuroprotection targets after traumatic brain injury. Curr Opin Neurol. 2006;19:514–519. doi: 10.1097/WCO.0b013e3280102b10. [DOI] [PubMed] [Google Scholar]
- 2.Hernández G, Hasbun P, Velasco N, Wainstein C, Bugedo G, Bruhn A, et al. Splanchnic ischemia and gut permeability after acute brain injury secondary to intracranial hemorrhage. Neurocrit Care. 2007;7:40–44. doi: 10.1007/s12028-007-0026-8. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira SH. Peripheral analgesic sites of action of anti-inflammatory drugs. Int J Clin Pract. 2002;128:2–10. [PubMed] [Google Scholar]
- 4.Warner TD, Giluliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroidal drug selectivities for cyclooxygenase-1 rather than cyclooxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci USA. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patrignani P. Nonsteroidal anti-inflammatory drugs, COX-2 and colorectal cancer. Toxicol Lett. 2000;112-113:493–498. doi: 10.1016/s0378-4274(99)00210-6. [DOI] [PubMed] [Google Scholar]
- 6.Maricic N, Ehrlich K, Gretzer B, Schuligoi R, Respondek M, Peskar BM. Selective cyclooxygenase-2 inhibitors aggravate ischemia-reperfusion injury in the rat stomach. Br J Pharmacol. 1999;128:1659–1666. doi: 10.1038/sj.bjp.0702966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkey CJ. Nonsteroidal anti-inflammatory drug gastropathy. Gastroenterology. 2000;119:521–535. doi: 10.1053/gast.2000.9561. [DOI] [PubMed] [Google Scholar]
- 8.Tegeder I, Neupert W, Guhring H, Geisslinger G. Effects of selective and unselective cyclooxygenase inhibitors on prostanoid release from various rat organs. J Pharmacol Exp Ther. 2000;292:1161–1168. [PubMed] [Google Scholar]
- 9.Laudanno OM, Cesolari JA, Esnarriaga J, San MP, Bedini OA. In vivo selectivity of nonsteroidal anti-inflammatory drugs and gastrointestinal ulcers in rats. Dig Dis Sci. 2000;45:1359–1365. doi: 10.1023/a:1005508120776. [DOI] [PubMed] [Google Scholar]
- 10.Simon LS, Milis JA. Non-steroidal antiinflammatory drugs. N Engl J Med. 1980;302:12371243. doi: 10.1056/NEJM198005293022206. [DOI] [PubMed] [Google Scholar]
- 11.Kayaalp O. Medicinal Pharmacology from Rational Cure Aspect (in Turkish) Feryal Press. 2000:p.1026. [Google Scholar]
- 12.Buttgereit F, Burmester G, Simon LS. Gastrointestinal toxic side effects of non-steroidal anti-inflammatory drugs and cyclooxygenase-2- specific inhibitors. Am J Med. 2001;110:135–195. doi: 10.1016/s0002-9343(00)00728-2. [DOI] [PubMed] [Google Scholar]
- 13.Fabricio AS, Veiga FH, Cristofoletti R, Navarra P, Souza GE. The effects of selective and nonselective cyclooxygenase inhibitors on endothelin-1-induced fever in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R671–677. doi: 10.1152/ajpregu.00532.2004. [DOI] [PubMed] [Google Scholar]
- 14.Dunn AJ, Chuluyan HE. The role of cyclooxygenase and lipoxygenase in the interleukin-1 induced activation of the HPA axis: dependence on the route of injection. Life Sci. 1992;51:219–225. doi: 10.1016/0024-3205(92)90078-4. [DOI] [PubMed] [Google Scholar]
- 15.Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. J Neurosurg. 1994;80:291–300. doi: 10.3171/jns.1994.80.2.0291. [DOI] [PubMed] [Google Scholar]
- 16.Suchitra AD, Dkhar SA, Shewade DG, Shashindran CH. Relative efficacy of some prokinetic drugs in morphine-induced gastrointestinal transit delay in mice. World J Gastroenterol. 2003;9:779–783. doi: 10.3748/wjg.v9.i4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melro APC, Collares EF, Silva JMB. Effect of an isolated mild to moderate ischemic brain injury in the gastric emptying of liquids in rats. Acta Cir Bras. 2008;23:486. doi: 10.1590/s0102-86502008000600003. [DOI] [PubMed] [Google Scholar]
- 18.Lin-jie W, Li X, Yi-geng S, Hong-bin A. Effects of ligation of bilateral common carotid arteries on the gastric motilityin rats. J Biomed Engin Res. 2007;1 [Google Scholar]
- 19.Garrick T, Mulvihill S, Buack S, Maeda-Hagiwara M, Tache Y. “Intracerebroventricular pressure inhibits gastric antral and duodenal contractility but not acid secretion in conscious rabbits”. Gastroenterology. 1988;95:26. doi: 10.1016/0016-5085(88)90286-7. [DOI] [PubMed] [Google Scholar]
- 20.Dhillon HS, Donaldson D, Dempsey RJ, Prasad MR. Regional levels of free fatty acids and Evans blue extravasation after experimental brain injury. J Neurotrauma. 1994;11:405–415. doi: 10.1089/neu.1994.11.405. [DOI] [PubMed] [Google Scholar]
- 21.Kontos HA, Weiep EP, Povlishock JT, Dietrich WD, Magiera CJ, Ellis EF. Cerebral arteriolar damage by arachidonic acid and prostaglandin G2. Science. 1980;209:1242–1245. doi: 10.1126/science.7403881. [DOI] [PubMed] [Google Scholar]
- 22.Porcher C, Horowitz B, Bayguinov O, Ward SM, Sanders KM. Constitutive expression and function of cyclooxygenase-2 in murine gastric muscles. Gastroenterology. 2002;122:1442–1454. doi: 10.1053/gast.2002.33065. [DOI] [PubMed] [Google Scholar]
- 23.Bouras EP, Burton DD, Camilleri M, Stephens DA, Thomforde GM. Effect of cyclooxygenase-2 inhibitors on gastric emptying and small intestinal transit in humans. Neurogastroenterol Motil. 2004;16:729–735. doi: 10.1111/j.1365-2982.2004.00547.x. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka A, Hase S, Miyazawa T, Ohno R, Takeuchi K. Role of cyclooxygenase (COX)-1 and COX-2 inhibition in nonsteroidalantiinflammatory drug-induced intestinal damage in rats: relation to various pathogenic events. J Pharmacol Exp Ther. 2002;303:1248–1254. doi: 10.1124/jpet.102.041715. [DOI] [PubMed] [Google Scholar]
- 25.Santos CL, Medeiros BA, Palheta-Junior RC, Macedo GM, Nobre-e-Souza MA, Troncon LE, Santos AA, Souza MH. Cyclooxygenase-2 inhibition increases gastric tone and delays gastric emptying in rats. Neurogastroenterol Motil. 2007;19:225–232. doi: 10.1111/j.1365-2982.2007.00913.x. [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi K, Tanaka A, Hayashi Y, Kubo Y. Functional mechanism underlying COX-2 expression following administration of indomethacin in rat stomachs: importance of gastric hypermotility. Dig Dis Sci. 2004;49:180–187. doi: 10.1023/b:ddas.0000017436.05273.fd. [DOI] [PubMed] [Google Scholar]
- 27.Penston JG, Wormsley KG. The effect of prostaglandins on gastric emptying. Scand J Gastroenterol. 1989;164:127–132. doi: 10.3109/00365528909091200. [DOI] [PubMed] [Google Scholar]
- 28.Shahbazian A, Schuligoi R, Heinemann A, Peskar BA, Holzer P. Disturbance of peristalsis in the guinea-pig isolated small intestine by indomethacin, but not cyclo-oxygenase isoform-selective inhibitors. Br J Pharmacol. 2001;132:1299–1309. doi: 10.1038/sj.bjp.0703940. [DOI] [PMC free article] [PubMed] [Google Scholar]


