Abstract
Human respiratory syncytial virus (hRSV) is the leading cause of respiratory illness in infants and young children around the globe. This pathogen, which was discovered in 1956, continues to cause a huge number of hospitalizations due to respiratory disease and it is considered a health and economic burden worldwide, especially in developing countries. The immune response elicited by hRSV infection leads to lung and systemic inflammation, which results in lung damage but is not efficient at preventing viral replication. Indeed, natural hRSV infection induces a poor immune memory that allows recurrent infections. Here, we review the most recent knowledge about the lifecycle of hRSV, the immune response elicited by this virus and the subsequent pathology induced in response to infection in the airways. Novel findings about the alterations that this virus causes in the central nervous system and potential therapies and vaccines designed to treat or prevent hRSV infection are discussed.
Keywords: hRSV, lung, T helper type 1/type 2 cells, vaccination, viral
Introduction
In 1956 Morris and co-workers isolated a cytopathogenic agent from a colony of chimpanzees at the Walter Reed Army Institute of Research, which presented a respiratory illness characterized by coughing, sneezing and mucopurulent nasal discharge.1,2 The infected animals showed inflammatory damage in the upper respiratory tract and this condition was rapidly spread to other members of the colony, suggesting the presence of a highly infectious pathogen.1 Because the major sign of disease in the affected monkeys was coryza – or nasal inflammation – the pathogen was termed ‘chimpanzee coryza agent’. One year later, Chanock and Finberg3 reported the isolation of a similar agent from two throat swab samples of infants with severe respiratory illness. These viruses were identical to the ‘chimpanzee coryza agent’ reported by Morris, suggesting that this pathogen could infect both chimpanzees and humans.3 The unusual cytopathic effect caused by the virus on HEp-2 cells, characterized by the syncytia formation and giant cells in cultures, led to its current denomination as human respiratory syncytial virus (hRSV).1
Human RSV is now the most important cause of acute lower respiratory tract infections (ALRTI) that include acute bronchitis, bronchiolitis, pneumonia and tracheitis in infants and young children worldwide.4 Data from a recent meta-analysis showed that this pathogen causes up to 33·8 million ALRTI in children under 5 years of age each year, of which around 3·4 million of cases need hospital admission worldwide.5 Further, hRSV infection causes the deaths of 66 000–199 000 children every year in developing countries.5 For these reasons, hRSV is considered a global health burden.
The success of hRSV as a respiratory pathogen is probably explained by its extremely contagious capacity. Estimates suggest that approximately 70% of infants under 1 year of age are infected with this virus, while 100% of 2-year-old children have been infected at least once with hRSV.6,7 Infections in children and adults are recurrent during life and protective immunity against the pathogen is inefficient, despite the production of antibodies after infection.6,8 The inefficient immune response against hRSV is partly due to virulence factors, such as the NS1 and NS2 proteins that interfere with the immune response against this pathogen.8
The severity of hRSV infection is associated with the pre-existence of several risk factors, the most important being age and sex.9 Regarding age, the groups that present severe complications are babies, infants and the elderly.9 In fact, 10–28% of hospitalized infants infected with hRSV are < 6 weeks old, 49–70% below 6 months and 66–100% under 1-year-old.10 The severity of the disease in the elderly has been associated with additional pathological conditions like cardiopulmonary and immunosuppressive diseases.11 Moreover, it has been reported that males are most susceptible to suffer severe ALRTI than females.10 Indeed, male infants are 1·5 times more likely to require hospital admission due to hRSV infection than females.12 Other conditions such as prematurity and congenital diseases have been implicated in the risk for severe hRSV infection.9 Among the most important risk factors are chronic lung disease, cystic fibrosis and congenital heart problems; all these conditions contribute to severe ALRTI and patients need intensive care and mechanical ventilation.9 Further, it has been reported that malnutrition is an important risk factor in developing countries and both smoke exposure and maternal smoking increase the severity of ALRTI due to hRSV infection.9
Despite more than 50 years of intensive research on hRSV pathogenesis, antiviral drugs and treatment against the virus are very limited and no vaccine is currently available to induce long-term protection against hRSV. The study and design of new approaches of prophylactic drugs and vaccines against hRSV is imperative to control the annual outbreaks of the virus and to decrease the high rate of infant hospitalization. To accomplish these aims it would be necessary to understand the virus life cycle and the pathology it causes. Here, we review and describe the most recent findings associated with hRSV infection, pathology and virulence. Also, we discuss strategies developed recently to prevent and treat hRSV infection.
Human RSV virology
Classification
Human respiratory syncytial virus belongs to the Mononegavirales order in the Paramyxoviridae family, and Pneumovirinae subfamily, genus Pneumovirus.13 The Paramyxoviridae family also includes other viruses such as metapneumovirus, and parainfluenza, mumps, measles, Nipah and Hendra viruses.13 Human RSV has two antigenic subgroups, A and B, that shows divergence in sequencing analysis.14 Other members of this genus are bovine RSV, ovine RSV and pneumonia virus of mice.
Human RSV characteristics
Human RSV is an enveloped non-segmented negative sense single-stranded RNA virus. The viral particle consists of a helical nucleocapsid covered by a lipid membrane derived from the infected host cell.15,16 Although hRSV is a spherical particle of 100–350 nm diameter, the virus can also take the form of long filaments. Indeed, a recent study suggests that this can be the most predominant morphology of the virus.16,17 The hRSV genome is 15·2 kb in length comprising 10 genes encoding 11 proteins, as there are two overlapping open reading frames, each of them encoding for an individual protein (M2-1 and M2-2).16 The lipid envelope contains three viral transmembrane glycoproteins: the attachment G protein, the fusion F protein and the small hydrophobic SH protein. Underneath the envelope is the matrix M protein, which is a non-glycosylated protein involved in the assembly of the viral particle.18 As part of the nucleocapsid there are four proteins: nucleoprotein N, the phosphoprotein P, the transcription factor M2-1 and the polymerase L.19 Human RSV expresses two non-structural proteins, named NS1 and NS2,which inhibit the production of type I interferon activity by the host cell.16
Human RSV infective cycle
The transmission of hRSV requires direct contact of secretions from infected individuals.20–23 Small droplets containing hRSV can enter the host through the nose, eyes and upper respiratory tract, which deliver the virus to epithelial cells.8,15,24 Although the main targets of hRSV infection are the airway epithelial cells, this virus can also infect other cell types, such as structural cells of the airway and immune cells.25,26 Human RSV infection in host cells begins with the attachment and entry of the virus through the activity of the G and F glycoproteins, respectively. The RNA of the virus enters the cells upon the fusion of the viral envelope with the cell plasma membrane.25 Once inside the host cell, the transcription of viral genes and viral genome replication are initiated, two processes essential for the infective cycle. While in vitro studies have shown that mRNA and proteins from the virus are detected inside the cell 4–6 hr after infection, expression peaks at 20 hr after infection.25 The transcription leading to mRNA synthesis and the replication of genomes for new viral particles are separate processes, which are modulated by the activity of the M2-2 protein.25
The production and delivery of viral particles start after 12 hr after infection and persist up to 48 hr after viral entry.13,27 Cells infected with hRSV show cytoplasmic inclusion bodies that contain viral RNA and proteins, including N, P, M2-1 and L.27 It has been suggested that inclusion bodies support the RNA synthesis and recent studies showed that these structures can also sequester cellular signalling components to impair the cellular response to infection.27 The structural components of hRSV are mobilized to the plasma membrane for the assembly and budding of viral particles.18 The minimum molecular requirement for viral particle assembly are the F, M, N and P proteins, in addition to the genome and anti-genome.27 The budding of hRSV takes place at the apical membrane in polarized cells. The F protein goes to the apical membrane through the secretory pathway from the endoplasmic reticulum and Golgi, where it is associated with the lipid raft.18 The rest of the hRSV structural proteins and the RNA genome also traffic to the apical membrane from the cytoplasm and from viral inclusion bodies.28 The matrix protein is localized in the nucleus in early stages after infection, but is mostly cytoplasmic in the late phases of infection.28
Immune response elicited by hRSV
Innate immune response to hRSV
Once in the airways, hRSV is recognized by pattern recognition receptors (PRRs) expressed on epithelial and immune cells that induce the secretion of innate cytokines and chemokines. These molecules promote inflammation and the recruitment of eosinophils, neutrophils and monocytes into the lungs, as well as the onset of an anti-viral response. To date, there are three types of PRRs identified, which include toll-like receptors (TLRs), retinoic acid-inducible gene (RIG)-I-like receptors (RLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), all involved in eliciting the immune response against hRSV.29 Several TLRs are activated by hRSV, including TLR2, TLR3, TLR4 and TLR7.25,30–33 As detailed in Fig. 1, TLR2 and TLR4 are expressed in the cell surface and recognize hRSV when associated with the co-receptors TLR6 and CD14, respectively.34 TLR4 interacts with hRSV F protein, leading to nuclear factor-κB (NF-κB) activation and promotes the secretion of the pro-inflammatory cytokines interleukin-6 (IL-6) and IL-8 by epithelial cells. TLR3 is an intracellular receptor that recognizes dsRNA generated during the viral replication. In response to hRSV, TLR3 activates NF-κB and interferon regulatory factor 3 (IRF3) through the adaptor protein TRIF, with the subsequent secretion of interferon-β (IFN-β), CXCL10, CCL12 and CCL5. TLR7 is expressed in the endosomal membrane and recognizes ssRNA. Entry of hRSV into the cytosol is detected by TLR7, which regulates the secretion of IL-12 and IL-23 through signalling via MyD88.29
Figure 1.
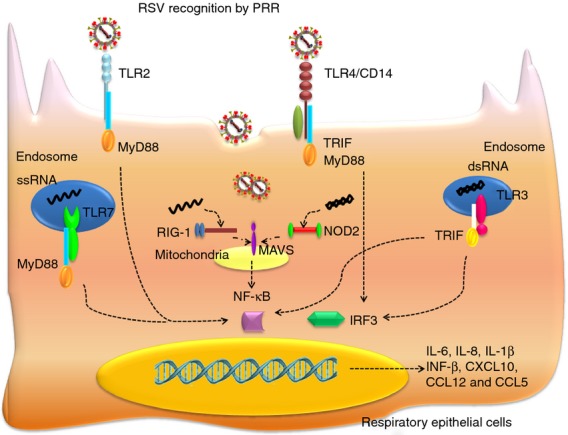
Human respriatory syncytial virus (hRSV) recognition by airways epithelial cells: upon infection, different hRSV components activate pattern recognition receptors (PRRs). Toll-like receptors (TLRs) are crucial in the hRSV recognition and the triggering of innate immune response against this pathogen; TLR2 and TLR4, expressed in the cell surface, and TLR3 and TLR7, present in cytoplasmatic endosomes, are activated in response to hRSV and promote the secretion of pro-inflammatory cytokines, such as interleukin-8 (IL-8), IL-1β and IL-6 through the nuclear factor-κB (NF-κB) and interferon regulatory factor 3 (IRF3) pathway. Also retinoic acid-inducible gene (RIG-1) and nucleotide-binding oligomerization domain (NOD2), which belong to RIG-I-like receptors (RLRs) and NOD-1-like receptors (NLRs), respectively, participate in the recognition of viral RNA associated with mitochondrial anti-viral signalling (MAVS) in the mitochondrial membrane in the cytoplasm leading to activation of NF-κB and IRF3.
In addition, RIG-1 is a cytosolic RLR (that belongs to the RNA helicase family) that detects intracellular viral RNAs.29 Upon hRSV infection, RIG-1 is activated by the 5′ triphosphate structure of viral RNA, which activates the NF-κB and IRF3 pathways using the mitochondrial anti-viral signalling (MAVS) adaptor localized in the mitochondrial membrane, inducing the expression of IFN-β, IP-10 and CCL5 in the airway epithelium.29 Furthermore, NOD2 is an NLR that belongs to the large cytosolic receptor family. NOD2 is activated by hRSV ssRNA and then is translocated to the mitochondria, where it interacts with MAVS to induce activation of both IRF3 and NF-κB.29 Recognition of RSV though PRR is schematized in Fig. 1.
Among the pro-inflammatory cytokines described below, IL-8 is a key molecule produced by epithelial cells and macrophages during the early response to hRSV and works as a chemoattractant in the recruitment of neutrophils, which infiltrate the site of infection.35 Another important molecule of the innate response against hRSV infection is IL-1β, a pro-inflammatory cytokine involved in the antiviral response. First, hRSV stimulates PRR to induce the expression of pro-IL-1β (IL-1β precursor) and inflammasome components, trigged by TLR2/MyD88 that activates the NF-κB pathway.33,35 Second, the assembly of the inflammasome complex takes place and caspase-1 cleaves pro-IL-1β into IL-1β in response to the production of reactive oxygen species, cellular potassium efflux, or cathepsin leakage into the cytosol after lysosomal disintegration.34,36
The NF-κB pathway is important for the activation of an innate response against hRSV, not only for the cytokine response, but also for the formation of tight junctions between nasal epithelial cells.37 Infection with hRSV induces the up-regulation of genes encoding structural components of tight junctions, including claudin-2, -4, -7, -9, -14, -19, occludin, ZO-2, cingulin and MAG-1, mediated by the protein kinase Cδ signalling.37 This phenomenon seems to be beneficial for the replication of the virus, because inhibition of NF-κB and protein kinase Cδ activation leads to an impairment of viral replication and formation of virus filaments.37 In addition, the induction of tight junctions could increase the cell polarity necessary for viral budding.13
Adaptive immune response against hRSV
Human RSV infection has been associated with an inefficient adaptive immune response, characterized by an excessive T helper type 2 (Th2) and a deficient antiviral Th1 response.15,36,38 The Th1 responses usually involve the production of IFN-γ, IL-2 and tumour necrosis factor-α, whereas IL-4, IL-5, IL-10 and IL-13 secretion characterize Th2 responses. Further, a Th17 response has been associated with hRSV pathogenesis because it contributes to the development of asthma in infected children.15,39 Studies using an in vitro model comprising both human airway epithelial cells (A549 cells) and human immune cells (peripheral blood mononuclear cells) have shown that hRSV infection induces the production of IFN-γ,IL-4 and IL-17, suggesting that the three subsets (Th1, Th2 and Th17) can be activated upon viral infection.40 Assays performed with peripheral blood mononuclear cells co-cultured with hRSV-infected A549 cells have also shown a Th2 and Th17 differentiation and the suppression of the generation of regulatory T cells.8,41 Indeed, as shown in Fig. 2, epithelial cells infected with hRSV expressed MHC-II and the co-stimulatory molecules CD80 and CD86, suggesting that these cells can directly activate naive CD4+ T cells during hRSV infection, promoting a Th2 and Th17 differentiation and suppressing the differentiation of regulatory T cells.8,25,36,42,43 Studies from hRSV infection in mice demonstrated a Th1 response with production of IFN-γ, IL-2 and IgG2a followed by the production of cytotoxic T lymphocytes.13 Also, studies using murine models have shown that the vaccination with different hRSV proteins and peptides followed by hRSV challenge allows the modulation of T-cell responses and disease severity. The immunization with recombinant vaccinia viruses expressing F protein induced a Th1 CD4+ T-cell response and a strong cytotoxic lymphocyte response, leading to a secondary hRSV disease with polymorphonuclear cell efflux. Immunizing mice with hRSV G protein promoted a Th2 CD4+ T-cell response and eosinophilic infiltration in lungs after subsequent infection with hRSV. In humans, production of both Th1 or Th2 cytokines has been detected in blood, nasopharyngeal aspirates and bronchoalveolar lavage taken from infants with hRSV disease.
Figure 2.
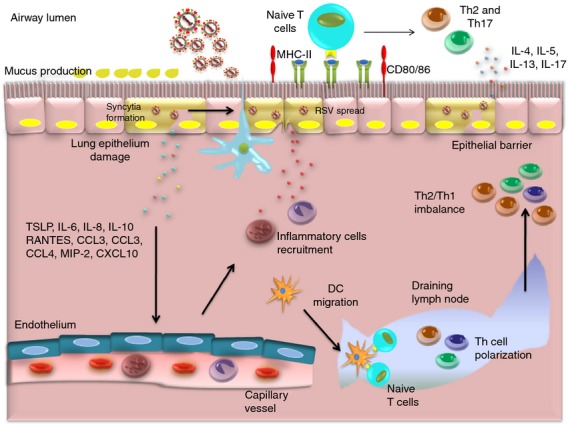
Inflammatory environment after human respiratory syncytial virus (hRSV) infection. Human RSV infection has been associated with an inefficient immunity characterized by a T helper type 2 (Th2) and Th17 polarized response and a weak Th1 response. Human RSV enters lung epithelial cells and induces the secretion of cytokines and chemokines such as interleukin-6 (IL-6), IL-8, RANTES, CCL3, MIP-2, CXCL10 to promote the recruitment of neutrophils infiltrating the infection site. Also, resident dendritic cells (DCs) migrate to draining lymph nodes to activate T-cell responses against hRSV characterized by an exacerbated Th2 and Th17 response. Indeed, epithelial cells expressed MHC-II, the co-stimulatory molecules CD80 and CD86 that can activate naive T cells to Th2 and Th17 polarization after hRSV infection. The pro-inflammatory cytokines and effector cells in the environment cause damage in the epithelium, which causes the characteristic pathology of hRSV.
Antibody responses also play an important role in hRSV infection, preventing the occurrence of re-infection by neutralizing or opsonizing extracellular viral particles. However, hRSV fails to induce a long-lasting antibody response. G and F glycoproteins are the major antigens of hRSV-specific neutralizing antibodies. IgA and IgG are secreted during hRSV infection and confer protection in the upper and lower respiratory tract.44 In humans, IgA and IgG titres decreased quickly after acute hRSV infection, especially in young children.45 The declining of antibody titres is thought to contribute to re-infection with hRSV and is also correlated with an increased susceptibility to hRSV infection in the elderly. Young children have an immature immune system and combined with the presence of maternal antibodies develop poor antibody responses against hRSV.45 Indeed, neutralizing hRSV-specific antibodies are detected only in 50–75% of children younger than 6 months of age. Hence, hRSV infection induces a deficient antibody response that fails to produce long-term protection against the pathogen and results in re-infections throughout life.45 The stimulation of primary antibody responses against hRSV occurs mostly in the lymph nodes draining the respiratory tract. In those tissues, virus-specific extrafollicular and marginal zone B cells found viral components and hRSV antigens, to initiate the engagement of their surface immunoglobulin B-cell receptor. Simultaneously, naive CD4+ T cells interact with dendritic cells (DCs) that have migrated from the airways to lymph nodes and become activated through the assembly of an immunological synapse. In this step the presence of co-stimulatory molecules (e.g. inducible co-stimulatory molecule) and the secretion of inflammatory cytokines (e.g. IL-6) is critical for differentiation of hRSV-specific T follicular helper cells. Hence, T follicular helper cells contribute to the differentiation of extrafollicular B cells into antibody-secreting plasma cells by promoting germinal center formation and affinity maturation.
Epithelial cells also participate in the adaptive immune response elicited by hRSV infection through the secretion of thymic stromal lymphopoietin, a cytokine that promotes the activation of T cells.46 A recent study that used primary rat airway epithelial cells infected with hRSV and co-cultivated with DCs, showed that these latter cells displayed increased expression of MHC-II and CD86 on their surface.47,48 Blockade of thymic stromal lymphopoietin in this system decreased significantly the expression of both maturation markers.47 It has also been described how DCs infected with hRSV up-regulate the expression of molecules that promote Th2 polarization as represented in Fig. 2,36,49 such as thymus- and activation-regulation chemokine and OX40 ligand.47 These data suggest that epithelial cells infected with hRSV contribute to the nature of T-cell differentiation through the modulation of DCs.
Pathogenesis in the respiratory tract
The respiratory disease caused by hRSV begins with viral replication in the nasopharynx.50 The spread from the upper respiratory tract to the lower respiratory tract takes place possibly through the direct spread along the respiratory epithelium and/or the aspiration of nasopharyngeal secretions.13 Spreading from cell to cell is also common for hRSV by means of the induction of cell fusion and syncytia formation (Fig. 2). Another mechanism proposed to explain the spread of hRSV in lungs is the infection of macrophages that migrate to the lower respiratory tract. Evidence supporting this mechanism consists of the detection of infected alveolar macrophages in vivo and the infection of monocyte-derived macrophages in vitro.51
During the first days of hRSV infection, patients show mild compromise of the upper respiratory tract, presenting signs such as cough and low-grade fever. The signs of disease in the lower respiratory tract include tachypnoea, wheezing, dyspnoea and retractions of the chest wall.50,52 During hRSV bronchiolitis, the ciliated epithelial cells are destroyed and in severe cases an extensive bronchiolar epithelial necrosis is observed. Severe cases of hRSV infection included peribronchiolar mononuclear cell infiltrates accompanied by submucosal oedema and bronchorrhoea. This phenomenon leads to bronchiolar obstruction with irregular atelectasis and areas of compensatory emphysema. Also, pneumonitis can occur when the alveoli become filled with fluid. In cases of milder bronchiolitis, the infection affects mostly lower airways, with peribronchiolar and interstitial inflammation.
Damage to central nervous system as a novel effect of RSV infection
In addition to the multiple deleterious effects of hRSV in the airways, during the last decade several reports have provided evidence for an association between hRSV infection and alterations in other tissues, such as the heart, liver and brain. Infection by this virus has been shown to produce different clinical manifestations, such as cardiopathy, hepatitis and encephalitis.53,54 Infection of the central nervous system (CNS) by hRSV has been supported by the presence of viral RNA in human cerebrospinal fluid,53 which correlates with neurological symptoms including seizures, central apnoea, lethargy, feeding or swallowing difficulties, abnormalities of muscle tone, strabismus, abnormalities of the cerebrospinal fluid and encephalopathy.54 Our group evaluated whether the CNS of mice and rats challenged with hRSV can be reached by this virus after intranasal infection.55 The presence of hRSV was corroborated in brain tissues using immunofluorescence and real-time PCR assays, which showed hRSV proteins and nucleic acids in several zones of the brain, supporting the notion that hRSV infection reaches the CNS.55 Entrance of hRSV to the CNS was dependent on the blood–brain barrier, because the blockade of CD49d by a monoclonal antibody that targets integrin α4 and impairs leucocyte extravasation through the blood–brain barrier decreased viral loads in the brain but not in the lungs.55 As a result of hRSV infection, impairment in cognition was revealed in rodents submitted to water-maze as a spatial learning test and to marble burying as a behavioural test.55 These alterations were correlated with electrophysiological studies that showed an impairment in the induction of long-term potentiation in stratum radiatum at the hippocampus area.55 Together, these observations support the previously described notion that hRSV has the ability to infect CNS tissues in a disseminated pattern and that this virus is capable of disrupting cognitive functions by altering the synaptic plasticity of the infected brain tissue.55
Drug design to treat RSV infection
Human RSV is considered an important health burden affecting mainly children and the elderly. Unfortunately, currently available treatments for infections by this pathogen are limited and it is not possible to use them broadly because of their high cost. However, there are many efforts invested in the design of new drugs to control the symptoms and unwanted effects caused by hRSV infection. The knowledge of the life cycle of hRSV and the pathology induced in the infected host is essential for the design of drugs with curative or prophylactic purposes. Along these lines, the most relevant processes in the life cycle of hRSV are replication, transcription and fusion, which are potential targets for antiviral drugs.56 Table 1 summarizes the antiviral drugs designed up to date against hRSV infection.
Table 1.
Therapeutic approaches to treat respiratory syncytial virus (RSV) infection (Source Clinical trials.gov)
| Name | Mechanism | Remarks |
|---|---|---|
| Ribavirin | Inhibition of RSV replication | High toxicity |
| Difficult administration | ||
| FDA approved | ||
| RSV604 | Inhibition of RSV replication | Safety, Tolerability and Pharmacokinetic |
| Study in Healthy Subjects complete | ||
| Phase I, complete; Phase II, in progress | ||
| 17-DMAG | Inhibition of the heat-shock protein hsp 90 | Impairs the RSV replication |
| Antitumoral drug | ||
| No clinical trial | ||
| HR121 and HR212 | Fusion inhibitor peptides | IC50 of 4·13 and 0·95 μm, respectively |
| No clinical trial | ||
| JNJ2408068 and BMS-433771 | Chemical compounds that prevent the fusion process | Phase I/II, discontinued |
| RFI-641 | Blocks viral F protein-mediated fusion and cell syncytium formation | Anti-RSV agent with potent in vitro and in vivo activity |
| Phase I/II, discontinued | ||
| ALN-RSV01 | Small interfering RNA specific against NS1 | Phase 2b Study of ALN-RSV01 in Lung Transplant Patients Infected with Respiratory Syncytial Virus complete |
Ribavirin is an antiviral drug that interferes with the replication of DNA and RNA viruses. This drug was the first antiviral drug approved for the treatment of hRSV infection in humans.57 Even though ribavirin is effective against hRSV when tested in vitro and in animals models, the clinical use of this molecule is currently very limited because of poor efficiency and difficult administration (nasal by aerosol), in addition to a potential elevated risk of tissue toxicity.56
Another therapeutic strategy has focused on the inhibition of hRSV replication by using drugs, such as RSV604. RSV604 is a benzodiazepine that affects the replication and promotes the positive selection of hRSV variants with mutations in the gene encoding the N protein. A phase 1 trial has been completed for RSV604 and a phase II trial is currently in progress, showing positive results as an antiviral drug for hRSV.58 Another promising antiviral drug is a derivative of the antibiotic geldanamycin, named 17AAG and 17DMAG, used commonly against cancer.59 These compounds inhibit the heat-shock protein hsp 90, which plays an important role in the replication of hRSV and is also efficient against other respiratory viruses; however, to date no clinical trials aim to use this drug for hRSV treatment are in progress.59
Another class of antiviral drugs are inhibitors of the fusion process. These molecules are synthetic compounds that block the fusion of the virus with the host cells, avoiding the entry of hRSV.56 Fusion inhibitors that target hRSV have been designed to bind the conserved region of the F protein. For instance, the peptide T-118 blocks the fusion activity of the F hRSV protein and it has been shown to be effective as an antiviral drug to prevent hRSV infection.56 There are other peptides similar to T-118, namely HR121 and HR212, which differ in effectiveness. Although the peptides described above have shown high anti-hRSV activity in in vitro assays, none of them has been reported in clinical trials, probably because of the lack of oral availability, high cost of production and relatively low half-life in the circulation.60 A similar pharmacological approach consisted of the peptide Rho-A, which inhibits the syncytia formation that is characteristic of hRSV infection. RhoA is a small GTPase that is involved in the fusion process and the inhibitor of this protein has been tested in HEp-2 cells and mice, with promising results.56,61
Besides peptides that inhibit hRSV fusion, there are several other chemical compounds that impair the fusion process. The benzimidazole JNJ2408068 has shown a high antiviral activity, 100 000 times higher than ribavirin and acts by preventing virus fusion and syncytia formation.62 Similarly, another synthetic compound is the antiviral BMS-433771,63,64 a benzotriazole derivative that interacts with the F protein and alters the conformation of this protein. RFI-641, a biphenyl triazine, is another drug that has shown the most potent anti-hRSV activity in vitro and in vivo.65 BMS-433771 and RFI-641 have been evaluated in clinical trials, but all of these trials were discontinued, mainly because of the disadvantageous pharmaceutical properties of the compounds.60
Nanotechnology has brought new options for hRSV treatment and prophylaxis, using the anti-microbial activity of metals, such as silver and gold.66 Although due to their toxicity, the clinical use of these metals in humans seems unfeasible, the development of silver or gold nanoparticles combined with polyvinylpyrrolidone have been shown to efficiently inhibit hRSV replication, showing low toxicity in cell lines. Further, gold nanoparticles fused with inhibitor peptides displayed a high inhibitory capacity against hRSV.66 Human RSV F protein nanoparticle vaccines have recently initiated clinical and preclinical studies to evaluate safety.67
Another interesting therapeutic approach is the use of interference RNA that targets different steps during the hRSV infective cycle. The small interfering RNA (siRNA) strategy was initially used to target the expression of NS268 and the P69 proteins, the latter showing an efficient capacity to protect mice against hRSV infection. This approach was also used to target the F gene, showing inhibition of hRSV infection.70 Nanotechnology has also been applied in combination with the siRNA approach to target the NS1 gene, resulting in the increase of IFN-β production by DCs and stimulated the Th1 differentiation of CD4+ cells.71 Such a strategy protected mice against RSV infection, because treated mice showed decreased viral loads in lungs and reduced inflammation in this tissue. A new siRNA specific against NS1(ALN-RSV01) showed high antiviral activity that impaired nucleocapsid expression.72 Studies in mice reported that administration of this molecule reduces RSV titres in the lungs.73 This antiviral drug has also been evaluated in human clinical trials, demonstrating their safety and tolerance in healthy adults.72 In addition, the effectiveness of ALN-RSV01 against hRSV infection was evaluated in humans, with a 44% reduction of hRSV infection without adverse effects74 and the phase IIb clinical trial has concluded. Further, this drug has been tested in lung transplant patients, where it has demonstrated safety and effectiveness.74
Another strategy to combat the disease caused by hRSV is to target the harmful immune response elicited by hRSV infection. The exacerbated Th2 response associated with the hRSV bronchiolitis is characterized by high production of IL-4. Along these lines, a study generated an antisense oligomer to promote local silencing of il4 gene expression, which was delivered intranasally.75 This approach was evaluated in neonatal murine models, showing a reduction of Th2 response and decreasing the airway damage caused by hRSV.75
To improve the specificity of siRNA technology as an antiviral approach for hRSV, the use of phosphorodiamidatemorpholino oligomers (PMOs) has been proposed. PMOs consist of oligomers in which the nucleobases are covalently attached to the morpholine ring, replacing the deoxyribose sugar while the phosphodiester bond is replaced by the phosphorodiamidate linkage.76 This strategy gives specificity, stability and target delivery.77 The function of PMOs is blocking the interaction of proteins with the target RNA. This method has been applied against the L gene of hRSV to impair infection in cell lines and in animal models.76
Novel strategies to induce protective RSV immunity
Passive immunization
There are limited options of prophylaxis and currently no vaccines are available to prevent hRSV infection (Table 2). Current clinical approaches to control hRSV infection comprise passive immunization with neutralizing antibodies against F and G proteins, which has been successful at decreasing the symptoms of hRSV infection. Further, these strategies can reduce the severe detrimental effects caused by hRSV infection in patients with risk factors, who can develop serious illness.78,79 A humanized monoclonal antibody that prevents hRSV fusion to the host cells by the neutralizing F protein (palivizumab or Synagis®; MedImmune, Gaithersburg, MD), is the most efficient and used antibody to prevent severe cases of hRSV disease.80,81 Motavizumab is another humanized antibody that binds the fusion protein after attachment to the host cell, but before starting the transcription of the viral genome.82,83 Neither monoclonal antibody completely prevents viral entry to the host cell but they decrease the viral replication and prevent hRSV infection. Despite the effectiveness of palivizumab in the treatment of hRSV infection, the use of this drug is seriously limited due to high costs and is restricted to patients with high risk of severe bronchiolitis associated with congenital diseases and preterm birth.84
Table 2.
Prevention of respiratory syncytial virus (RSV), passive immunization and new vaccines. Source Clinical trials.gov
| Name | Mechanism | Remarks |
|---|---|---|
| Palivizumab (Synagis®) | Humanized monoclonal antibody that prevents | The most efficient and used antibody to cure or treat severe cases of RSV disease FDA approved |
| RSV fusion to the host cells (passive immunization) | ||
| Motavizumab Medi-524 | Humanized antibody that binds to fusion protein after attachment to the host cell (passive immunization) | Safety, Tolerability and Immunogenicity of MEDI-524 After Dosing for a Second Season complete Phase 1-III, complete |
| DNA vaccines | RSV G protein construct | Induces neutralizing antibodies and balances the pulmonary T helper type 1 (Th1)/Th2 cytokines |
| NDV-F | Newcastle disease virus (NDV) vector plus F protein | Induces a high interferon-β response |
| rBCG-RSV | Bacillus Calmette–Guérin (BCG attenuated Mycobacterium bovis) modified to express RSV N and M2 RSV proteins | Induces a Th1 immune response specific against RSV |
| RSV Fusion protein particle vaccine | Trimers Form F protein Nanoparticles (Nanovax) | Safety study complete |
Novel vaccine approaches for hRSV
The development of an efficient vaccine against hRSV requires that the formulation promotes protective and efficient immunity against the virus, without adverse effects. Human RSV proteins are immunogenic and are good candidates to design vaccines, but it is important to consider that some hRSV proteins or peptides can negatively modulate the host immune response.85 Further, an efficient vaccine candidate has to prevent the Th2 immune response and needs to promote viral clearance before the development of the disease.85
Vectors comprising hRSV genes or parts of the genome of this virus have been used as DNA vaccines, in combination with adjuvants that promote Th1 immunity. An example of this approach is an hRSV-G construct that induces neutralizing antibodies to balance the production of pulmonary Th1/Th2 cytokines during hRSV infection.86 The gene coding for the F protein has also been used as a DNA vaccine, an example of this approach is the insert of the F gene into the Newcastle disease virus vector (NDV-F). Immunization with this vaccine induces high levels of IFN-β.87
Another approach of the DNA vaccine was a strategy designed as an immunization methodology including a mucosal adjuvant,88 consisting of two F gene fragments, DRF-412 and DRF-412P, which were cloned into the phCMV1 vaccine vector. Immunization with this recombinant formulation induced neutralizing antibody responses (IgG, IgG1, IgG2a and IgG2b) and a mix of Th1/Th2 cytokine responses in mice.88
Attenuated bacterial vectors expressing hRSV proteins are another interesting strategy to induce protection against hRSV and induce Th1 immunity. Recently, a recombinant bacillus Calmette–Guérin bacteria (BCG-attenuated Mycobacterium bovis) modified to express N and M2-1 proteins from hRSV (rBCG-RSV) was shown to induce protective hRSV immunity in animal models.55,77,89,90 This vaccine was able to induce a Th1 immune response against hRSV, characterized by the presence of T cells secreting IFN-γ and a significant decrease of lung damage and inflammation after infection.89,90 Further, the immunization with rBCG-RSV prevented viral replication in the lungs of infected animals.55,89,90 One important feature shown by this vaccine was the ability to prevent the CNS alterations caused by hRSV.55 The BCG-based vaccine prevented the cognitive and behavioural impairment observed in hRSV-infected mice and rats.55 These data suggest that rBCG-RSV vaccination induces a specific T-cell response that protects against hRSV infection and prevents the spread of the virus to the CNS. BCG vaccination has been used worldwide as a vaccine against tuberculosis in newborns, hence the safety of this vaccine candidate might lead to an efficient and reachable vaccine against hRSV.
Using bacteria as a delivery system of plasmid-expressing viral antigens is also an efficient strategy that allows activation of the natural immune response. This system activates the innate immunity of the host through TLRs and redirects the immune response to the efficient clearance of the pathogen. This is the case of an attenuated Salmonella typhimurium strain SL7207 containing a plasmid encoding the F hRSV protein. This live attenuated vaccine was administered orally to mice and induced an efficient humoral and cellular response, as well as mucosal immunity.91 Attenuated viruses have also been used as vaccines, which consist of the replacement of structural genes with hRSV genes. This method was applied with the Venezuelan equine encephalitis virus and immunization with this prototype vaccine confers protection against RSV and induces a balanced Th1/Th2 immune response.92
The use of subunit vaccines has also been evaluated to prevent hRSV infection. Human RSV F was the most accepted subunit vaccine because this is a conserved protein in the paramyxoviridae family. The rF255 is a region of F protein that has been cloned into a vector containing the gene encoding ctxA2 B, which encodes the cholera toxin and induces a Th1 response in mice.56 Further, another strategy of subunit vaccine is a multivalent recombinant protein that mixes the F, M2 and G proteins cloned in a bacterial pET32a(+) vector, named rFM2G. Immunization with this prototype vaccine promotes an increase of IgG titres, reducing illness in infected mice.93
Recent studies of the structure of hRSV proteins have allowed a new candidate vaccine to be designed based on the different conformations adopted by the F protein. The hRSV F protein displays conformational changes during hRSV cell attachment, forming two states; a metastable pre-fusion and a stable post-fusion form.94 The conformation and reactivity of the different variants of the F protein neutralizing antibodies were analysed and these studies suggest that in the metastable pre-fusion form of F protein there are more relevant exposed epitopes than in the stable post-fusion form. Supporting this notion, it has been described that the pre-fusion epitopes induce a strong anti-F neutralizing humoral response.94 An example of this strategy is the vaccine designed based on a recombinant prefusion-like form of the F protein bound to bacterium-like particles derived from the food-grade bacterium Lactococcus lactis.94
Recently, the idea of maternal immunization to prevent hRSV infection in young infants has become the focus of research efforts leading to an hRSV vaccine. This strategy aims to increase the serum-neutralizing antibody levels against hRSV during the second or third trimester of pregnancy to transfer these antibodies through the placenta to the fetus.95 In addition, it is expected that passive immunization continues during the breastfeeding period, protecting the infant from early and recurrent infections. Maternal antibodies can confer effective hRSV protection in young infants.96,97However, the major concern of this strategy is whether the maternal antibody transfer is enough to induce protective immunity without the need for infant vaccination.95 The first clinical trials in pregnant women showed reduced antibody responses possibly due to maternal immunosuppression in the pregnancy third trimester. These data suggest that maternal immunization could be more effective in the second trimester of pregnancy.95 Vaccines used to immunize pregnant women must be safe for the mother and the fetus, as has been shown by the influenza vaccine experience, which opens the possibility of maternal immunization for hRSV using attenuated viral or bacterial vectors.98
Concluding remarks
Since the isolation of hRSV more than 50 years ago, several groups have tried to explain the mechanism implicated in the respiratory disease caused by this pathogen. They established the consensus that hRSV induces a detrimental inflammation in the airways, characterized by an exacerbated Th2 response, the result of which is not efficient for viral clearance, promoting destruction of ciliated epithelial cells and peribronchiolar cell infiltrates. In addition to the airway damage caused by hRSV infection, our group has also described that hRSV infection leads to alterations in cognition and behaviour associated with the presence of mRNA and viral protein in the brain tissue of infected animals. There are several strategies in course to develop new prophylactic drugs and vaccines based on inhibition of different processes of the viral life cycle, such as the fusion and replication. An efficient vaccine candidate has to promote the differentiation of T cells in an appropriate antiviral response to elicit the viral clearance. Until now, our knowledge was insufficient to understand the complete picture of hRSV infection but progress is promising an effective and safe vaccine available for the population most affected by this pathogen.
Acknowledgments
This work was supported by grants FONDECYT no 1070352, FONDECYT no 1050979, FONDECYT no 1040349, FONDECYT no 1100926, FONDECYT no 1110397, FONDECYT no 1100971, FONDECYT no 1110604, FONDECYT no 1130996, CONICYT Proyecto de Inserción de Capital HumanoAvanzado en la Academia no 791100015 and Millennium Institute on Immunology and Immunotherapy (P09-016-F), Grant from La Région Pays De La Loire through the ‘Chaird'excellence program’, Grant ‘NouvellesEquipes-nouvellesthématiques'from the La Région Pays De La Loire, INSERM CDD grant.
Disclosures
The authors declare no financial or commercial conflict of interest.
References
- Blount RE, Jr, Morris JA, Savage RE. Recovery of cytopathogenic agent from chimpanzees with coryza. Proc Soc Exp Biol Med. 1956;92:544–9. doi: 10.3181/00379727-92-22538. [DOI] [PubMed] [Google Scholar]
- Beem M, Wright FH, Hamre D, Egerer R, Oehme M. Association of the chimpanzee coryza agent with acute respiratory disease in children. N Engl J Med. 1960;263:523–30. doi: 10.1056/NEJM196009152631101. [DOI] [PubMed] [Google Scholar]
- Chanock R, Finberg L. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). II. Epidemiologic aspects of infection in infants and young children. Am J Hyg. 1957;66:291–300. doi: 10.1093/oxfordjournals.aje.a119902. [DOI] [PubMed] [Google Scholar]
- Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair H, Brooks WA, Katz M, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2010;378:1917–30. doi: 10.1016/S0140-6736(11)61051-9. [DOI] [PubMed] [Google Scholar]
- Baker KA, Ryan ME. RSV infection in infants and young children. What's new in diagnosis, treatment, and prevention? Postgrad Med. 1999;106:97–9. doi: 10.3810/pgm.1999.12.803. , 103–4, 7–8 passim. [DOI] [PubMed] [Google Scholar]
- Henderson FW, Collier AM, Clyde WA, Jr, Denny FW. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979;300:530–4. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- Gonzalez PA, Bueno SM, Carreno LJ, Riedel CA, Kalergis AM. Respiratory syncytial virus infection and immunity. Rev Med Virol. 2012;22:230–44. doi: 10.1002/rmv.1704. [DOI] [PubMed] [Google Scholar]
- Aujard Y, Fauroux B. Risk factors for severe respiratory syncytial virus infection in infants. Respir Med. 2002;96(Suppl. B):S9–14. [PubMed] [Google Scholar]
- Hall CB, Simoes EA, Anderson LJ. Clinical and epidemiologic features of respiratory syncytial virus. Curr Top Microbiol Immunol. 372:39–57. doi: 10.1007/978-3-642-38919-1_2. [DOI] [PubMed] [Google Scholar]
- Falsey AR. Respiratory syncytial virus infection in adults. Semin Respir Crit Care Med. 2007;28:171–81. doi: 10.1055/s-2007-976489. [DOI] [PubMed] [Google Scholar]
- Respiratory syncytial virus infection: admissions to hospital in industrial, urban, and rural areas. Report to the Medical Research Council Subcommittee on Respiratory Syncytial Virus Vaccines. Br Med J. 1978;2:796–8. doi: 10.1136/bmj.2.6140.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 2011;162:80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Coenjaerts FE, Houspie L, Viveen MC, van Bleek GM, Wiertz EJ, Martin DP, Lemey P. The comparative genomics of human respiratory syncytial virus subgroups A and B: genetic variability and molecular evolutionary dynamics. J Virol. 87:8213–26. doi: 10.1128/JVI.03278-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez PA, Carreno LJ, Bueno SM, Riedel CA, Kalergis AM. Understanding respiratory syncytial virus infection to improve treatment and immunity. Curr Mol Med. 2013;13:1122–39. doi: 10.2174/1566524011313070007. [DOI] [PubMed] [Google Scholar]
- Rodriguez R, Ramilo O. Respiratory syncytial virus: how, why and what to do. J Infect. 2014;68(Suppl. 1):S115–8. doi: 10.1016/j.jinf.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Shaikh FY, Utley TJ, Craven RE, Rogers MC, Lapierre LA, Goldenring JR, Crowe JE., Jr Respiratory syncytial virus assembles into structured filamentous virion particles independently of host cytoskeleton and related proteins. PLoS One. 2012;7:e40826. doi: 10.1371/journal.pone.0040826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batonick M, Wertz GW. Requirements for human respiratory syncytial virus glycoproteins in assembly and egress from infected cells. Adv Virol. 2011;2011 doi: 10.1155/2011/343408. : Article ID: 343408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno SM, Gonzalez PA, Pacheco R, et al. Host immunity during RSV pathogenesis. Int Immunopharmacol. 2008;8:1320–9. doi: 10.1016/j.intimp.2008.03.012. [DOI] [PubMed] [Google Scholar]
- White LJ, Mandl JN, Gomes MG, et al. Understanding the transmission dynamics of respiratory syncytial virus using multiple time series and nested models. Math Biosci. 2007;209:222–39. doi: 10.1016/j.mbs.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CB, Douglas RG, Jr, Schnabel KC, Geiman JM. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect Immun. 1981;33:779–83. doi: 10.1128/iai.33.3.779-783.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G, Fratini M, Della Libera S, Iaconelli M, Muscillo M. Viral infections acquired indoors through airborne, droplet or contact transmission. Ann Ist Super Sanita. 2013;49:124–32. doi: 10.4415/ANN_13_02_03. [DOI] [PubMed] [Google Scholar]
- Gralton J, Tovey ER, McLaws ML, Rawlinson WD. Respiratory virus RNA is detectable in airborne and droplet particles. J Med Virol. 2013;85:2151–9. doi: 10.1002/jmv.23698. [DOI] [PubMed] [Google Scholar]
- Imaz MS, Sequeira MD, Videla C, Veronessi I, Cociglio R, Zerbini E, Carballal G. Clinical and epidemiologic characteristics of respiratory syncytial virus subgroups A and B infections in Santa Fe, Argentina. J Med Virol. 2000;61:76–80. doi: 10.1002/(sici)1096-9071(200005)61:1<76::aid-jmv12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Lotz MT, Peebles RS., Jr Mechanisms of respiratory syncytial virus modulation of airway immune responses. Curr Allergy Asthma Rep. 2012;12:380–7. doi: 10.1007/s11882-012-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez RS, Mora JE, Cortes CM, Riedel CA, Ferres M, Bueno SM, Kalergis AM. Respiratory syncytial virus detection in cells and clinical samples by using three new monoclonal antibodies. J Med Virol. 2013;86:1256–66. doi: 10.1002/jmv.23807. [DOI] [PubMed] [Google Scholar]
- Lindquist ME, Mainou BA, Dermody TS, Crowe JE., Jr Activation of protein kinase R is required for induction of stress granules by respiratory syncytial virus but dispensable for viral replication. Virology. 2011;413:103–10. doi: 10.1016/j.virol.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghildyal R, Ho A, Dias M, Soegiyono L, Bardin PG, Tran KC, Teng MN, Jans DA. The respiratory syncytial virus matrix protein possesses a Crm1-mediated nuclear export mechanism. J Virol. 2009;83:5353–62. doi: 10.1128/JVI.02374-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng R, Cui Y, Hai Y, Liu Y. Pattern recognition receptors for respiratory syncytial virus infection and design of vaccines. Virus Res. 2011;167:138–45. doi: 10.1016/j.virusres.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Satkunanathan S, Kumar N, Bajorek M, Purbhoo MA, Culley FJ. Respiratory syncytial virus infection, TLR3 ligands, and proinflammatory cytokines induce CD161 ligand LLT1 expression on the respiratory epithelium. J Virol. 2014;88:2366–73. doi: 10.1128/JVI.02789-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski MR, Bowen GN, Cerny AM, Anderson LJ, Haynes LM, Tripp RA, Kurt-Jones EA, Finberg RW. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J Virol. 2009;83:1492–500. doi: 10.1128/JVI.00671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes LM, Moore DD, Kurt-Jones EA, Finberg RW, Anderson LJ, Tripp RA. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75:10730–7. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedimonte G. Pathophysiological mechanisms for the respiratory syncytial virus-reactive airway disease link. Respir Res. 2002;3(Suppl. 1):S21–5. doi: 10.1186/rr185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia J, Sabbah A, Mgbemena V, et al. TLR2/MyD88/NF-κB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS One. 2012;7:e29695. doi: 10.1371/journal.pone.0029695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S, Watkiss ER. Pathogenesis of respiratory syncytial virus. Curr Opin Virol. 2012;2:300–5. doi: 10.1016/j.coviro.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Gonzalez PA, Prado CE, Leiva ED, Carreno LJ, Bueno SM, Riedel CA, Kalergis AM. Respiratory syncytial virus impairs T cell activation by preventing synapse assembly with dendritic cells. Proc Natl Acad Sci USA. 2008;105:14999–5004. doi: 10.1073/pnas.0802555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi H, Kojima T, Hirakawa S, et al. Respiratory syncytial virus infection and the tight junctions of nasal epithelial cells. Adv Otorhinolaryngol. 2011;72:153–6. doi: 10.1159/000324777. [DOI] [PubMed] [Google Scholar]
- Gonzalez PA, Bueno SM, Riedel CA, Kalergis AM. Impairment of T cell immunity by the respiratory syncytial virus: targeting virulence mechanisms for therapy and prophylaxis. Curr Med Chem. 2009;16:4609–25. doi: 10.2174/092986709789760724. [DOI] [PubMed] [Google Scholar]
- Bueno SM, Gonzalez PA, Riedel CA, Carreno LJ, Vasquez AE, Kalergis AM. Local cytokine response upon respiratory syncytial virus infection. Immunol Lett. 2011;136:122–9. doi: 10.1016/j.imlet.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Chirkova T, Boyoglu-Barnum S, Gaston KA, Malik FM, Trau SP, Oomens AG, Anderson LJ. Respiratory syncytial virus G protein CX3C motif impairs human airway epithelial and immune cell responses. J Virol. 2013;87:13466–79. doi: 10.1128/JVI.01741-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bera MM, Lu B, Martin TR, Cui S, Rhein LM, Gerard C, Gerard NP. Th17 cytokines are critical for respiratory syncytial virus-associated airway hyperreponsiveness through regulation by complement C3a and tachykinins. J Immunol. 2011;187:4245–55. doi: 10.4049/jimmunol.1101789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Su Z, Schwarze J. Healthy but not RSV-infected lung epithelial cells profoundly inhibit T cell activation. Thorax. 2009;64:283–90. doi: 10.1136/thx.2007.094870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Hu CP, Feng JT, Xia Q. Activation of lymphocytes induced by bronchial epithelial cells with prolonged RSV infection. PLoS One. 2011;6:e27113. doi: 10.1371/journal.pone.0027113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh EE, Falsey AR. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis. 2004;190:373–8. doi: 10.1086/421524. [DOI] [PubMed] [Google Scholar]
- Singleton R, Etchart N, Hou S, Hyland L. Inability to evoke a long-lasting protective immune response to respiratory syncytial virus infection in mice correlates with ineffective nasal antibody responses. J Virol. 2003;77:11303–11. doi: 10.1128/JVI.77.21.11303-11311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Headley MB, Loo YM, Berlin A, Gale M, Jr, Debley JS, Lukacs NW, Ziegler SF. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J Allergy Clin Immunol. 2012;130:1187–96. doi: 10.1016/j.jaci.2012.07.031. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay MK, Gonzalez PA, Leon MA, Cespedes PF, Bueno SM, Riedel CA, Kalergis AM. Advances in understanding respiratory syncytial virus infection in airway epithelial cells and consequential effects on the immune response. Microbes Infect. 2013;15:230–42. doi: 10.1016/j.micinf.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Gonzalez PA, Carreno LJ, Figueroa CA, Kalergis AM. Modulation of immunological synapse by membrane-bound and soluble ligands. Cytokine Growth Factor Rev. 2007;18:19–31. doi: 10.1016/j.cytogfr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Carreno LJ, Gonzalez PA, Bueno SM, Riedel CA, Kalergis AM. Modulation of the dendritic cell–T-cell synapse to promote pathogen immunity and prevent autoimmunity. Immunotherapy. 2011;3:6–11. doi: 10.2217/imt.11.38. [DOI] [PubMed] [Google Scholar]
- Domachowske JB, Rosenberg HF. Respiratory syncytial virus infection: immune response, immunopathogenesis, and treatment. Clin Microbiol Rev. 1999;12:298–309. doi: 10.1128/cmr.12.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogra PL. Respiratory syncytial virus: the virus, the disease and the immune response. Paediatr Respir Rev. 2004;5(Suppl. A):S119–26. doi: 10.1016/S1526-0542(04)90023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FD. Respiratory syncytial virus bronchiolitis and the pathogenesis of childhood asthma. Pediatr Infect Dis J. 2003;22:S76–82. doi: 10.1097/01.inf.0000053889.39392.a7. [DOI] [PubMed] [Google Scholar]
- Eisenhut M. Extrapulmonary manifestations of severe RSV bronchiolitis. Lancet. 2006;368:988. doi: 10.1016/S0140-6736(06)69409-9. [DOI] [PubMed] [Google Scholar]
- Eisenhut M. Cerebral involvement in respiratory syncytial virus disease. Brain Dev. 2007;29:454. doi: 10.1016/j.braindev.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Espinoza JA, Bohmwald K, Cespedes PF, et al. Impaired learning resulting from respiratory syncytial virus infection. Proc Natl Acad Sci USA. 2013;110:9112–7. doi: 10.1073/pnas.1217508110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawage SS, Tiwari PM, Pillai S, Dennis V, Singh SR. Recent advances in diagnosis, prevention, and treatment of human respiratory syncytial virus. Adv Virol. 2013;2013:595768. doi: 10.1155/2013/595768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez WJ, Parrott RH. Ribavirin aerosol treatment of serious respiratory syncytial virus infection in infants. Infect Dis Clin North Am. 1987;1:425–39. [PubMed] [Google Scholar]
- Chapman J, Abbott E, Alber DG, et al. RSV604, a novel inhibitor of respiratory syncytial virus replication. Antimicrob Agents Chemother. 2007;51:3346–53. doi: 10.1128/AAC.00211-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller R, Andino R, Frydman J. Hsp90 inhibitors exhibit resistance-free antiviral activity against respiratory syncytial virus. PLoS One. 2013;8:e56762. doi: 10.1371/journal.pone.0056762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Pan Y, Jiang S, Lu L. Respiratory syncytial virus entry inhibitors targeting the F protein. Viruses. 2013;5:211–25. doi: 10.3390/v5010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budge PJ, Lebowitz J, Graham BS. Antiviral activity of RhoA-derived peptides against respiratory syncytial virus is dependent on formation of peptide dimers. Antimicrob Agents Chemother. 2003;47:3470–7. doi: 10.1128/AAC.47.11.3470-3477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde PR, Chetty SN, Jewell AM, Boivin G, Piedra PA. Comparison of the inhibition of human metapneumovirus and respiratory syncytial virus by ribavirin and immune serum globulin in vitro. Antiviral Res. 2003;60:51–9. doi: 10.1016/s0166-3542(03)00153-0. [DOI] [PubMed] [Google Scholar]
- Cianci C, Meanwell N, Krystal M. Antiviral activity and molecular mechanism of an orally active respiratory syncytial virus fusion inhibitor. J Antimicrob Chemother. 2005;55:289–92. doi: 10.1093/jac/dkh558. [DOI] [PubMed] [Google Scholar]
- Cianci C, Yu KL, Combrink K, et al. Orally active fusion inhibitor of respiratory syncytial virus. Antimicrob Agents Chemother. 2004;48:413–22. doi: 10.1128/AAC.48.2.413-422.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley CC, Weiss WJ, Gazumyan A, et al. RFI-641, a potent respiratory syncytial virus inhibitor. Antimicrob Agents Chemother. 2002;46:841–7. doi: 10.1128/AAC.46.3.841-847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JW, Thornburg NJ, Blum DL, Kuhn SJ, Wright DW, Crowe JE., Jr Gold nanorod vaccine for respiratory syncytial virus. Nanotechnology. 2013;24:295102. doi: 10.1088/0957-4484/24/29/295102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Kim Y, Lee S, Yi H, Kang SM, Bozja J, Moore ML, Compans RW. Viruslike particle vaccine induces protection against respiratory syncytial virus infection in mice. J Infect Dis. 2011;204:987–95. doi: 10.1093/infdis/jir474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jairath S, Vargas PB, Hamlin HA, Field AK, Kilkuskie RE. Inhibition of respiratory syncytial virus replication by antisense oligodeoxyribonucleotides. Antiviral Res. 1997;33:201–13. doi: 10.1016/s0166-3542(96)01015-7. [DOI] [PubMed] [Google Scholar]
- Bitko V, Musiyenko A, Shulyayeva O, Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nat Med. 2005;11:50–5. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- Vig K, Lewis N, Moore EG, Pillai S, Dennis VA, Singh SR. Secondary RNA structure and its role in RNA interference to silence the respiratory syncytial virus fusion protein gene. Mol Biotechnol. 2009;43:200–11. doi: 10.1007/s12033-009-9190-8. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yang H, Kong X, et al. Inhibition of respiratory syncytial virus infection with intranasal siRNA nanoparticles targeting the viral NS1 gene. Nat Med. 2005;11:56–62. doi: 10.1038/nm1174. [DOI] [PubMed] [Google Scholar]
- DeVincenzo J, Cehelsky JE, Alvarez R, et al. Evaluation of the safety, tolerability and pharmacokinetics of ALN-RSV01, a novel RNAi antiviral therapeutic directed against respiratory syncytial virus (RSV) Antiviral Res. 2008;77:225–31. doi: 10.1016/j.antiviral.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Alvarez R, Elbashir S, Borland T, et al. RNA interference-mediated silencing of the respiratory syncytial virus nucleocapsid defines a potent antiviral strategy. Antimicrob Agents Chemother. 2009;53:3952–62. doi: 10.1128/AAC.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora MR, Budev M, Rolfe M, et al. RNA interference therapy in lung transplant patients infected with respiratory syncytial virus. Am J Respir Crit Care Med. 2011;183:531–8. doi: 10.1164/rccm.201003-0422OC. [DOI] [PubMed] [Google Scholar]
- Ripple MJ, You D, Honnegowda S, Giaimo JD, Sewell AB, Becnel DM, Cormier SA. Immunomodulation with IL-4Rα antisense oligonucleotide prevents respiratory syncytial virus-mediated pulmonary disease. J Immunol. 2010;185:4804–11. doi: 10.4049/jimmunol.1000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SH, Stein DA, Guerrero-Plata A, et al. Inhibition of respiratory syncytial virus infections with morpholino oligomers in cell cultures and in mice. Mol Ther. 2008;16:1120–8. doi: 10.1038/mt.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti JF, Meyer C, Doublet F, et al. Selection of a respiratory syncytial virus fusion inhibitor clinical candidate. 2. Discovery of a morpholinopropylaminobenzimidazole derivative (TMC353121) J Med Chem. 2008;51:875–96. doi: 10.1021/jm701284j. [DOI] [PubMed] [Google Scholar]
- Monoclonal antibody approved for RSV disease prevention. Am J Health Syst Pharm. 1998;55:1654. doi: 10.1093/ajhp/55.16.1654. , 6. [DOI] [PubMed] [Google Scholar]
- Welliver RC. Respiratory syncytial virus immunoglobulin and monoclonal antibodies in the prevention and treatment of respiratory syncytial virus infection. Semin Perinatol. 1998;22:87–95. doi: 10.1016/s0146-0005(98)80010-4. [DOI] [PubMed] [Google Scholar]
- Palivizumab (Synagis) for prevention of RSV infection. Med Lett Drugs Ther. 1999;41:3–4. [PubMed] [Google Scholar]
- Fisher RG, Johnson JE, Dillon SB, Parker RA, Graham BS. Prophylaxis with respiratory syncytial virus F-specific humanized monoclonal antibody delays and moderately suppresses the native antibody response but does not impair immunity to late rechallenge. J Infect Dis. 1999;180:708–13. doi: 10.1086/314965. [DOI] [PubMed] [Google Scholar]
- Wu H, Pfarr DS, Losonsky GA, Kiener PA. Immunoprophylaxis of RSV infection: advancing from RSV-IGIV to palivizumab and motavizumab. Curr Top Microbiol Immunol. 2008;317:103–23. doi: 10.1007/978-3-540-72146-8_4. [DOI] [PubMed] [Google Scholar]
- Mejias A, Chavez-Bueno S, Raynor MB, Connolly J, Kiener PA, Jafri HS, Ramilo O. Motavizumab, a neutralizing anti-Respiratory Syncytial Virus (RSV) monoclonal antibody significantly modifies the local and systemic cytokine responses induced by RSV in the mouse model. Virol J. 2007;4:109. doi: 10.1186/1743-422X-4-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejias A, Chavez-Bueno S, Rios AM, et al. Comparative effects of two neutralizing anti-respiratory syncytial virus (RSV) monoclonal antibodies in the RSV murine model: time versus potency. Antimicrob Agents Chemother. 2005;49:4700–7. doi: 10.1128/AAC.49.11.4700-4707.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenney W. RSV disease–is there a role for prevention? Respir Med. 2001;95:170–2. doi: 10.1053/rmed.2000.1018. [DOI] [PubMed] [Google Scholar]
- Li X, Sambhara S, Li CX, et al. Plasmid DNA encoding the respiratory syncytial virus G protein is a promising vaccine candidate. Virology. 2000;269:54–65. doi: 10.1006/viro.2000.0186. [DOI] [PubMed] [Google Scholar]
- Martinez-Sobrido L, Gitiban N, Fernandez-Sesma A, et al. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. J Virol. 2006;80:1130–9. doi: 10.1128/JVI.80.3.1130-1139.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Dennis VA, Pillai SR, Singh SR. RSV fusion (F) protein DNA vaccine provides partial protection against viral infection. Virus Res. 2009;145:39–47. doi: 10.1016/j.virusres.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cautivo KM, Bueno SM, Cortes CM, Wozniak A, Riedel CA, Kalergis AM. Efficient lung recruitment of respiratory syncytial virus-specific Th1 cells induced by recombinant bacillus Calmette–Guérin promotes virus clearance and protects from infection. J Immunol. 2010;185:7633–45. doi: 10.4049/jimmunol.0903452. [DOI] [PubMed] [Google Scholar]
- Bueno SM, Gonzalez PA, Cautivo KM, et al. Protective T cell immunity against respiratory syncytial virus is efficiently induced by recombinant BCG. Proc Natl Acad Sci USA. 2008;105:20822–7. doi: 10.1073/pnas.0806244105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, He JS, Zhang M, et al. Oral respiratory syncytial virus (RSV) DNA vaccine expressing RSV F protein delivered by attenuated Salmonella typhimurium. Hum Gene Ther. 2007;18:746–52. doi: 10.1089/hum.2007.053. [DOI] [PubMed] [Google Scholar]
- Mok H, Lee S, Utley TJ, et al. Venezuelan equine encephalitis virus replicon particles encoding respiratory syncytial virus surface glycoproteins induce protective mucosal responses in mice and cotton rats. J Virol. 2007;81:13710–22. doi: 10.1128/JVI.01351-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarayan P, Qin H, Pillai S, et al. Expression and characterization of a multivalent human respiratory syncytial virus protein. Mol Biol. 2010;44:477–87. [PubMed] [Google Scholar]
- Rigter A, Widjaja I, Versantvoort H, et al. A protective and safe intranasal RSV vaccine based on a recombinant prefusion-like form of the F protein bound to bacterium-like particles. PLoS One. 2013;8:e71072. doi: 10.1371/journal.pone.0071072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaijk P, Luytjes W, Rots NY. Vaccination against RSV: is maternal vaccination a good alternative to other approaches? Hum Vaccin Immunother. 2013;9:1263–7. doi: 10.4161/hv.24096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz FM, Piedra PA, Glezen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine. 2003;21:3465–7. doi: 10.1016/s0264-410x(03)00352-9. [DOI] [PubMed] [Google Scholar]
- Englund JA. Passive protection against respiratory syncytial virus disease in infants: the role of maternal antibody. Pediatr Infect Dis J. 1994;13:449–53. doi: 10.1097/00006454-199405000-00037. [DOI] [PubMed] [Google Scholar]
- Blanchard-Rohner G, Meier S, Bel M, Combescure C, Othenin-Girard V, Swali RA, Martinez de Tejada B, Siegrist CA. Influenza vaccination given at least 2 weeks before delivery to pregnant women facilitates transmission of seroprotective influenza-specific antibodies to the newborn. Pediatr Infect Dis J. 2013;32:1374–80. doi: 10.1097/01.inf.0000437066.40840.c4. [DOI] [PubMed] [Google Scholar]


