Abstract
A growing body of evidence suggests that inflammatory cytokines have a dualistic role in immunity. In this study, we sought to determine the direct effects of interferon-γ (IFN-γ) on the differentiation and maturation of human peripheral blood monocyte-derived dendritic cells (moDC). Here, we report that following differentiation of monocytes into moDC with granulocyte–macrophage colony-stimulating factor and interleukin-4, IFN-γ induces moDC maturation and up-regulates the co-stimulatory markers CD80/CD86/CD95 and MHC Class I, enabling moDC to effectively generate antigen-specific CD4+ and CD8+ T-cell responses for multiple viral and tumour antigens. Early exposure of monocytes to high concentrations of IFN-γ during differentiation promotes the formation of macrophages. However, under low concentrations of IFN-γ, monocytes continue to differentiate into dendritic cells possessing a unique gene-expression profile, resulting in impairments in subsequent maturation by IFN-γ or lipopolysaccharide and an inability to generate effective antigen-specific CD4+ and CD8+ T-cell responses. These findings demonstrate that IFN-γ imparts differential programmes on moDC that shape the antigen-specific T-cell responses they induce. Timing and intensity of exposure to IFN-γ can therefore determine the functional capacity of moDC.
Keywords: antigen presentation, dendritic cells, inflammation, interferon-γ, T lymphocytes
Introduction
The inflammatory response is a complex and coordinated event with intricate checkpoints, balances and self-regulation that evolved to control the deleterious effects of an unchecked immune response.1–5 Interferon-γ (IFN-γ), a type II interferon, is a critical endogenous mediator of inflammation and plays a key role in bridging adaptive and acquired immune responses.6–11 In the five decades since IFN-γ was first characterized, a plethora of data has defined the biological activity of this pleiotropic molecule.6,12 Interferon-γ plays a dualistic role in several immune scenarios: It can either augment or suppress cancer, autoimmunity and various other pathological conditions such as graft-versus-host disease following allogeneic transplantation.11,13–21
Key functions of IFN-γ in immunity are the differentiation and activation of macrophages from monocytes, maturation of dendritic cells (DC), and the induction of T-cell differentation to T helper type 1 (Th1) effector T cells.22–27 The pathway of activation, dependent on downstream signalling through the IFN-γ receptor is independent from more potent pathogen-dependent inducers of innate immunity such as lipopolysaccharide (LPS) controlled Toll-like receptor-4 signalling. Ligands for various Toll-like receptors (expressed on DC and macrophages) are abundantly present in foreign pathogens such as bacteria, viruses, fungi or parasites. Historical immunological studies show that Toll-like receptor agonists induce strong activation of innate immunity, as witnessed by changes in the secretion of pro-inflammatory cytokines such as interleukin-12 (IL-12), tumour necrosis factor-α (TNF-α) and IL-6, co-stimulatory molecules and the ability to process and present antigen. In the absence of foreign pathogen-mediated signalling, IFN-γ can still induce a degree of activation in DC and macrophages, albeit at lower levels and through completely independent mechanisms compared with Toll-like receptors. This phenomenon can be viewed in terms of an immunological response to sterile inflammation.
In the generation of cytotoxic T cells for adoptive cell therapy, LPS is commonly used to mature DC for T-cell expansion but some protocols also use IFN-γ in combination with LPS for DC maturation.28–32 Under alternate conditions, IFN-γ can paradoxically tolerize monocyte-derived DC (moDC).14,33 Here, we explore possible mechanisms for this opposing functionality and show that IFN-γ incorporated during maturation of moDC increases co-stimulatory molecule expression, and generates moDC competent to induce the proliferation of multiple viral and tumour antigen-specific cytotoxic T cells. Conversely, IFN-γ introduced together with granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4 during moDC differentiation generated macrophages at high concentrations and dysfunctional moDC possessing a unique gene expression profile at low concentrations. Later addition of IFN-γ or LPS failed to induce maturation of moDC. This study further defines the optimum dose and timing of IFN-γ for generating moDC that either enhance or dampen T-cell-specific immune responses.
Materials and methods
Cytokines
Recombinant human IFN-γ1B (ACTIMMUNE) was purchased from Vidara Therapeutics, Roswell, GA. Recombinant human IL-2 was purchased from Novartis (East Hanover, NJ). Recombinant human GM-CSF, IL-4 and IL-7 were purchased from Peprotech (Rocky Hill, NJ).
Isolation, growth and differentiation of human peripheral blood monocytes into dendritic cells
Elutriated monocytes and lymphocytes were prepared from healthy donors, aged 20–80 years, from the Department of Transfusion Medicine at the National Institutes of Health. All blood-product acquisitions and ethics approval was granted by the Institutional Review Board in accordance with the declaration of Helsinki. Elutriated monocytes were plated onto 24-well plates and differentiated into moDC with RPMI-1640 (+ glutamine; Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal calf serum, 0·5% HEPES, 1% penicillin/streptomycin, and 0·01% gentamycin, in the presence of GM-CSF (800 IU/ml), IL-4 (1000 IU/ml) and various concentrations of IFN-γ (0, 1, 10, 100, 1000, 10 000 IU) for 5–7 days before analysis or further studies. Monocyte purity from elutriation was between 80 and 85% (with admixed lymphocyte/natural killer cells) but after the addition of GM-CSF and IL-4 for 2 days, > 99% of cells were monocyte-derived CD33-positive cells.
Flow cytometry-based phenotypic and cytokine analysis
For phenotypic analysis of monocytes, macrophages, DC and T cells, fluorescently labelled antibodies for human CD4 (V500/RPA-T4), CD8 (allophycocyanin-Cy7/SK1), CD11b [phycoerythrin (PE)-Cy5/ICRF44], CD11c (FITC, R2), CD14 (PE, ME52), CD33 (V450, WM53), CD80 (FITC, L307.4), CD83 (AF-488/HB15e), CD86 (PE-Cy5/233.1), CD95 (PE/PE-Cy5), CD107a (FITC), HLA-DR (allophycocyanin-Cy7/L243), HLA-ABC (PE-Cy5/G46-2.6), and IFN-γR1 (PE/GIR-94) were purchased from BD Biosciences (San Jose, CA). Antibodies for CD3 (Brilliant Violet/OKT3), CD68 (FITC/Y1/82A) and CD163 (PE/RM3/1) were purchased from Biolegend, San Diego, CA. Intracellular fixation and permeabilization were performed using the intracellular kits manufactured by BD Biosciences. Fluorescently labelled antibodies for Foxp3 and Helios were purchased from BD Biosciences and antibodies for IFN-γ (PE/45-B3), TNF-α (PE-Cy7/MAb/1), and IL-2 (PerCP/MQ1-17H12) were purchased from eBioscience (San Diego, CA). All flow cytometry data were obtained using the FACS FORTESSA (BD Biosciences) and analysed using flowjo Version 9.4.10 software (TreeStar, Ashland, OR).
Generation of antigen-specific lymphocyte responses
To measure the functional capacity of moDC, autologous elutriated lymphocytes were co-cultured overnight with the various moDC pulsed with pools of overlapping peptides34 for the cytomegalovirus (CMV) proteins IE and pp65 (JPT Peptide Technologies, Berlin, Germany) in the presence of golgiplug (1 μl/ml) and golgistop (0·7 μl/ml). CMV responses were measured in patients with documented CMV seropositivity. To generate tumour-antigen-specific T-cell responses, moDC were differentiated with GM-CSF and IL-4 for 5 days and matured with 1000 IU/ml of IFN-γ for 48 hr and pulsed with pools of overlapping peptides for WT1, PR3, AK1 and PRAME or a mix of all four peptide pools. These moDC were co-cultured with autologous elutriated lymphocytes in 96-well plates at a four T cells to one DC ratio and grown for 1 week in IL-4 (1000 IU/ml) and IL-7 (10 ng/ml). T cells were then stimulated again with peptide-pulsed irradiated autologous monocytes and supplemented with 10 IU of IL-2 in addition to IL-4 and IL-7 in 24-well plates.
Whole transcriptome expression analysis
Human moDC from four healthy donors were differentiated with either GM-CSF and IL-4 or GM-CSF, IL-4, and 1 IU of IFN-γ for 6 days and were resuspended into RLT buffer (Qiagen, Hilden, Germany). Total RNA was isolated using the RNeasy kit (Qiagen) and was amplified using the Ambion WT expression kit (Invitrogen) according to the manual. Fragmented single-stranded sense DNA were terminally labelled and hybridized to the Human GeneChip 1.0 ST array (Affymetrix, Santa Clara, CA) and stained on a Genechip Fluidics Station 450 (Affymetrix), all according to the respective manufacturers' instructions. Arrays were then scanned on a GeneChip Scanner 3000 7G (Affymetrix). After Robust Multichip Average normalization, differentially expressed genes were identified by one-way repeated measures analysis of variance (P < 0·01) corrected by Benjamini–Hochberg's False Discovery Rate method (P < 0·05) and this gene list was further filtered for between-group α levels of P < 0·01, P < 0·005 and P < 0·001 for genes differentially expressed from moDC differentiated with GM-CSF and IL-4 or GM-CSF, IL-4 and 1 IU of IFN-γ. The statistically significant gene expression changes, defined by a P-value cut-off of 0·01 were analysed using Ingenuity Pathways Analysis (Ingenuity Systems, Redwood City, CA). This software identified 461 genes that were differentially expressed between the two groups. The accession number for the micro-array data is GSE47621 and the URL is: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE47621.
Light microscopy
To evaluate the morphology of peripheral blood monocytes differentiated under various conditions, images for cells in culture were captured using an AMG Evos core light microscope (Advanced Microscopy Group, Bothell, WA).
Statistical analysis
All data are represented as a mean ± standard error of the mean (SEM). One-way analysis of variance and two-tailed Student's t-tests were used to test for significant differences. P < 0·05 was considered significant.
Results
IFN-γ induces maturation of human moDC
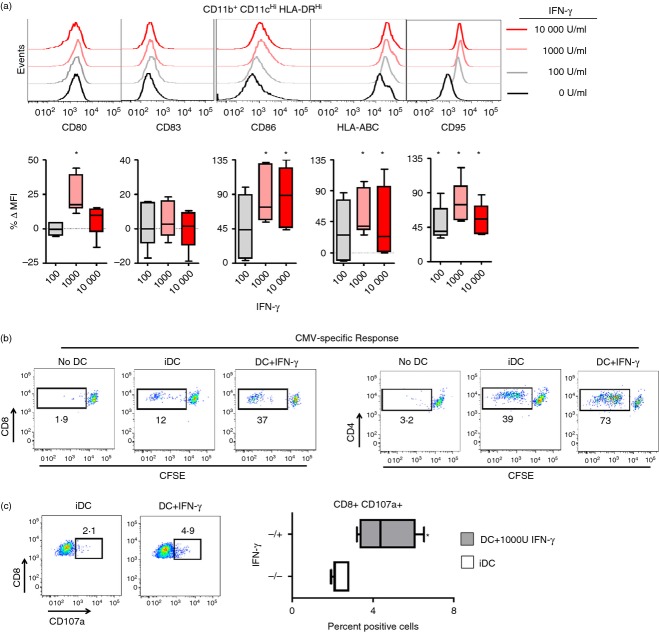
Peripheral blood monocytes were differentiated into immature dendritic cells (iDC) with GM-CSF and IL-4 for 5–7 days. Following iDC formation, we added increasing concentrations of IFN-γ for 48 hr and observed a significant increase in the expression of CD80, CD86, CD95 and HLA-ABC (Fig. 1a). Interestingly, the optimal maturation dose appeared to be 1000 U/ml, with higher concentrations conferring detrimental effects with regard to CD80 and CD95 expression (Fig. 1a).
Figure 1.
Interferon-γ (IFN-γ) induces the maturation of human monocyte-derived dendritic cells (moDC). (a) Flow cytometric analysis of CD80, CD83, CD86, HLA-ABC and CD95 expression for moDC matured for 48 hr with 0, 100, 1000 or 10 000 IU of IFN-γ (top panel). The percentage change in mean fluorescence intensity (MFI) following IFN-γ maturation compared with immature DC (iDC) exposed to 0 IU IFN-γ from samples of three separate healthy donors (bottom panel). *P < 0·05 compared with baseline MFI expression of iDC. All plots are gated on live (vivid−) CD11b+ CD11cHi HLA-DRHi cells. (b) Autologous lymphocytes were labelled with CFSE, co-cultured with iDC or IFN-γ matured moDC pulsed with overlapping peptides for the cytomegalovirus (CMV) antigens IE and pp65 and examined for CFSE dilution (c) Expression of CD107a for autologous CD8+ T cells following overnight co-culture with moDC from CMV-seropositive patients pulsed with peptide pools for CMV antigens IE and pp65 (left panel). Quantification of the percentage of CD8+ CD107a+ T cells for similar treatments (right panel). All plots gated on live vivid−, CD3+ cells.
Generation of virus-specific CD8+ T cells with IFN-γ matured moDC
We next sought to determine the ability of IFN-γ matured moDC to stimulate the proliferation and function of CD8+ and CD4+ T cells. Interferon-γ-matured moDC or iDC were pulsed with multi-epitope-spanning overlapping peptides for CMV antigens pp65 and IE-1, and used to stimulate autologous lymphocytes. Lymphocytes were labelled with carboxyfluorescein succinimidyl ester (CFSE) fluorescent dye, and co-cultured with peptide-pulsed standard iDC or IFN-γ matured moDC. Both CD8+ and CD4+ T cells co-cultured with IFN-γ matured moDC exhibited an increase in CFSE dilution, indicating that the maturation of moDC with IFN-γ improved the ability of moDC to stimulate T-cell proliferation (Fig. 1b). We also measured the ability of IFN-γ-matured moDC to improve the function of co-cultured CD8+ T lymphocytes by examining the expression of CD107a (a surrogate marker for lytic degranulation) on CD8+ T cells. We witnessed a significant increase in the percentage of CD8+ CD107a+ cells with peptide-pulsed IFN-γ matured moDC compared with iDC (Fig. 1c). Hence, IFN-γ directly induces moDC maturation, enabling for the generation of CMV-specific cytotoxic CD8+ T-cell effectors.
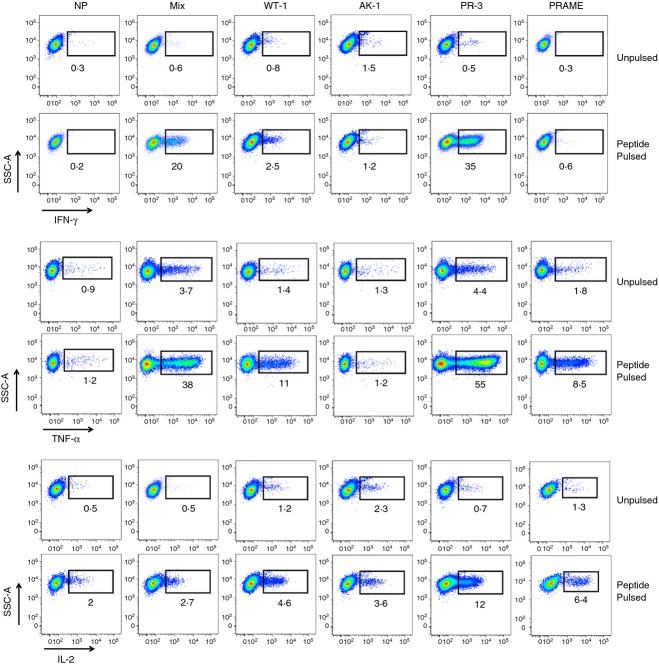
Generation of multi-tumour antigen-specific CD4+ T cells with IFN-γ matured moDC
Interferon-γ matured moDC were pulsed with peptide mixes for overlapping epitopes for Wilm's tumour 1 (WT1), Aurora kinase-1 (AK1), Proteinase-3 (PR-3), and Preferentially Expressed Antigen in Melanoma (PRAME) and co-cultured with autologous lymphocytes for 7 days in the presence of IL-4 and IL-7.35 Additional groups included moDC pulsed with a mix of all four peptide pools (Mix) and moDC not pulsed with any peptides (NP). Following a second stimulation and the addition of low doses of IL-2 (10 IU/ml) to induce T-cell growth for seven additional days, large numbers of tumour antigen-specific CD4+ T cells were generated. A significant percentage of T cells secreted IFN-γ, TNF-α and IL-2 upon re-stimulation with peptide, indicating the ability to generate tumour antigen-specific T cells using IFN-γ-stimulated moDC (Fig. 2).
Figure 2.
Generation of multi-tumour antigen-specific CD4+ T cells with interferon-γ (IFN-γ) matured dendritic cells (DC). Representative intracellular flow cytometry plots for IFN-γ, tumour necrosis factor-α (TNF-α) and interleukin-2 (IL-2) secretion by CD4+ T cells following the generation of large numbers of antigen-specific T cells specific for WT1, PR3, AK1, and PRAME. Autologous lymphocytes were primed with IFN-γ-matured monocyte-derived DC (moDC) pulsed with the various overlapping peptide pools individually or all together (Mix) and grown for 2 weeks in culture media supplemented with IL-4 1000 IU/ml, IL-7 (10 ng/ml) and IL-2 (10 IU/ml). NP = T cells grown with moDC with no peptides. All plots gated on live vivid−, CD3+, and CD4+ T cells. Data are representative of five healthy donors.
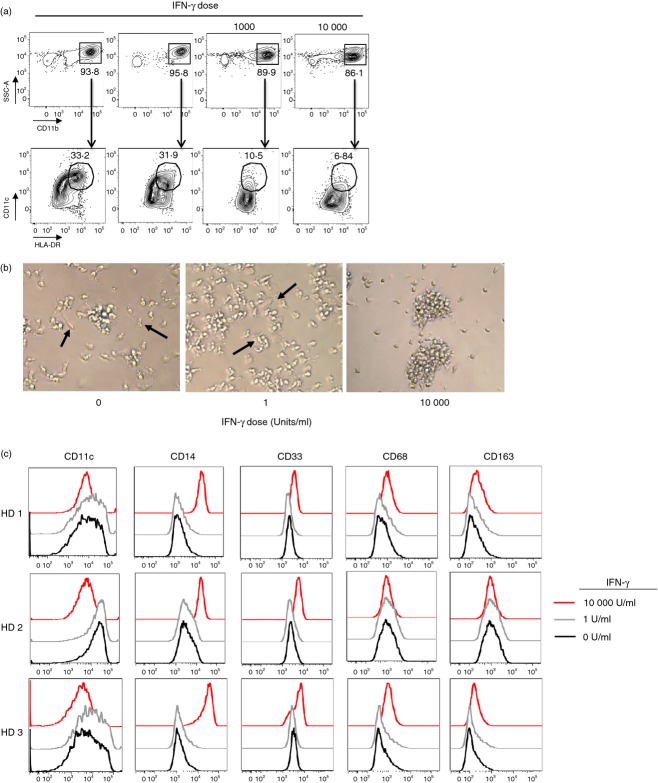
IFN-γ modifies monocyte differentiation
To assess the effects of IFN-γ on the differentiation of monocytes, we cultured human peripheral blood monocytes with GM-CSF, IL-4 and varying concentrations of IFN-γ and observed a loss of DC populations (CD11b+ CD11c+ ClassIIHi) under high concentrations of IFN-γ (Fig. 3a). At higher concentrations of IFN-γ (≥ 1000 IU; IFN-γHi), we witnessed a change in cell morphology (Fig. 3b) with an increase in the expression of CD14, CD33, CD68 and CD163 and loss of CD11c expression (Fig. 3c). Hence, higher concentrations of IFN-γ appeared to deviate monocyte differentiation towards macrophages despite the presence of GM-CSF and IL-4. However, at low concentrations of IFN-γ (1 IU; IFN-γLow), differentiation of monocytes into moDC based on dendritic cell morphology (Fig. 3b) and phenotypic markers (Fig. 3c) was unchanged.
Figure 3.
High concentrations of interferon-γ (IFN-γ) during monocyte differentiation with interleukin-4 (IL-4) and granulocyte–macrophage colony-stimulating factor (GM-CSF) skew lineage commitment towards macrophages. (a) Generation of CD11b+ CD11cHi HLA-DRHi DC populations following differentiation of peripheral blood monocytes for 5–7 days with GM-CSF, IL-4 and IFN-γ (0, 1, 1000 or 10 000 IU). (b) Representative light microscopy images evaluating the morphology of cells treated as in (a). All pictures taken with the AMG Evos core light microscope, 40×. (c) Phenotypic flow cytometric analysis for monocyte, DC and macrophage lineage-specific markers (CD11c, CD14, CD33, CD68, and CD163) following monocyte differentiation with GM-CSF, IL-4 and IFN-γ (0, 1, or 10 000 IU). All plots gated on live vivid− cells. HD = Healthy donor.
During monocyte differentiation, IFN-γLow imparts a unique gene expression profile
We performed whole transcriptome analysis on monocytes differentiated in the presence of GM-CSF and IL-4 or GM-CSF, IL-4 and IFN-γLow in four healthy individuals (Fig. 4a). An unbiased analysis revealed a unique gene expression profile for monocytes differentiated in GM-CSF + IL-4 compared with GM-CSF, IL-4 and IFN-γLow (Fig. 4b). Using a P-value cut-off of 0·001, 32 transcripts were differentially expressed (see Supporting information, Fig. S1) while at a P-value cut-off of 0·01, 461 genes were differentially expressed. Using Ingenuity-based software, we found differential expression in a cluster of transcriptional factors involved in cellular growth, proliferation and differentiation. The importance of these genes in the differentiation and function of human peripheral blood monocytes is yet to be determined (Fig. 4c). Taken together, whole transcriptome analysis indicated that despite no changes in phenotype and morphology, moDC cultured in IFN-γLow developed a distinct molecular and transcriptional signature, indicating possible functional differences.
Figure 4.
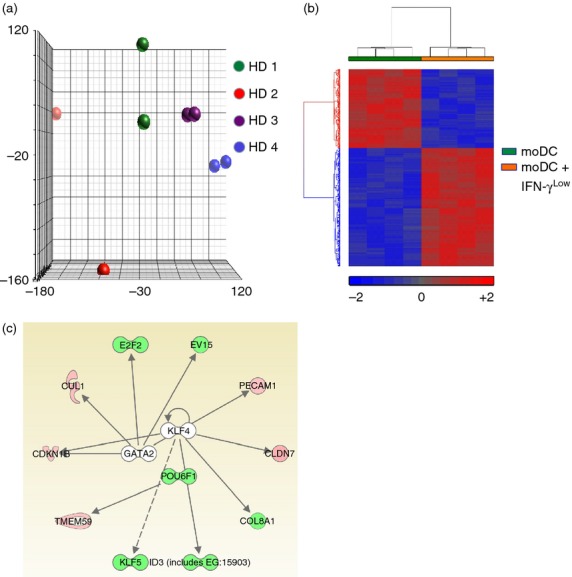
Dendritic cells (DC) differentiated in the presence of low concentrations of interferon-γ (IFN-γLow) possess a distinct molecular signature. (a) Principal component analysis (PCA) of the transcriptome of peripheral blood monocytes from four healthy donors differentiated into DC with either granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) or GM-CSF, IL-4 and IFN-γLow reveals the heterogeneity of donors (indicated by colour) and the global transcriptome changes (distance between the same colour dots). (b) Dendrogram of whole transciptome analysis displaying 461 genes differentially expressed with a P-value cut-off of 0·01 for moDCs differentiated with GM-CSF and IL-4 or GM-CSF, IL-4 and IFN-γLow. (c) Differential expression of transcriptional networks involving POU6F1, E2F2 and KLF5 following exposure to low levels of IFN-γ during monocyte differentiation into DC. Red = up-regulated genes; Green = down-regulated genes.
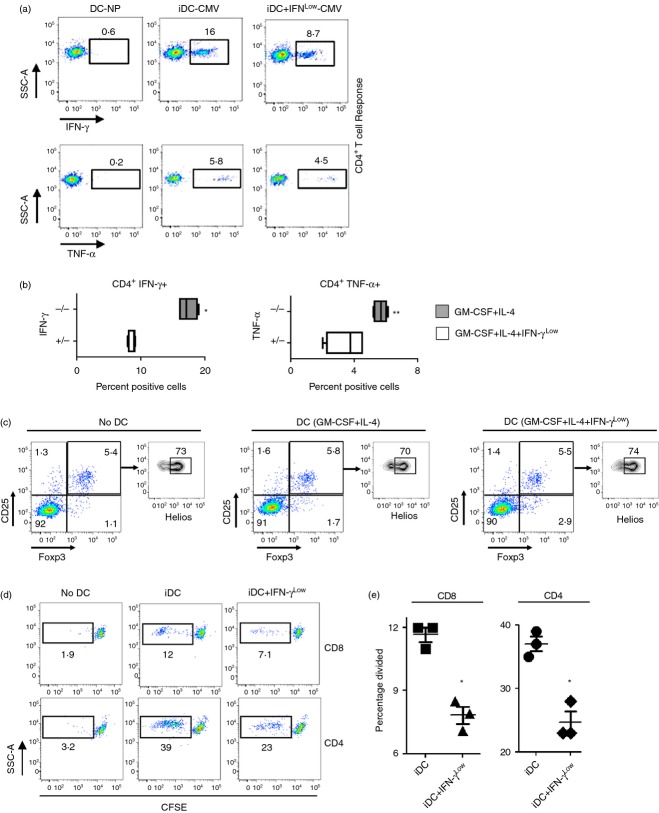
Exposure to IFN-γLow during differentiation further impairs the ability of immature DC to generate T-cell responses
To assess the functional significance of exposure to low concentrations of IFN-γ, we harvested iDC grown with or without IFN-γLow, pulsed them with overlapping peptide-epitope libraries for the CMV antigens pp65 and IE-1, and compared the ability of the two moDC types to stimulate autologous antigen-specific T-cell responses in CMV-seropositive individuals. Immature moDC differentiated with IFN-γLow showed a significant decrease in their ability to stimulate antigen-specific CD4+ T cells producing IFN-γ and TNF-α (Fig. 5a,b). To explore the effect of iDC for the induction of CD4+, CD25+, Foxp3+ T regulatory cells, we co-cultured autologous lymphocytes with moDC differentiated with or without IFN-γLow and compared the frequency of CD3+, CD4+, CD25+, Foxp3+, Helios+/− T cells to control lymphocytes not co-cultured with moDC.36 No differences were found in the percentage of induced or naturally occurring regulatory T cells between the groups (Fig. 5c),36 indicating that moDC specifically impacted CD4+ T-cell memory recall responses and did not induce regulatory T cell differentiation (Fig. 5c).
Figure 5.
Monocyte-derived dendritic cells (moDC) exposed to low concentrations of interferon-γ (IFN-γLow) during differentiation show decreased antigen-specific T-cell responsiveness. (a) Representative flow cytometry plots depicting cytomegalovirus-specific autologous CD4+ T-cell responses by intracellular staining for IFN-γ and tumour necrosis factor-α (TNF-α) following priming with IE and pp65 peptide pool pulsed DC differentiated with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) or GM-CSF, IL-4 and 1 U IFN-γ. DC-NP represents co-cultures with DCs not pulsed with peptide. All plots gated on live vivid−, CD3+, CD4+ cells. (b) Quantification of the percentage of live vivid−, CD3+, CD4+, IFN- γ+ and live vivid−, CD3+, CD4+, TNF-α+ cells from co-cultures similar to (a). *P < 0·05, **P < 0·10 compared with co-cultures with moDC grown with GM-CSF, IL-4 and IFN-γLow (+/−). Data are representative of three separate experiments in different healthy donors. (c) Autologous peripheral blood lymphocytes were co-cultured with moDC differentiated with GM-CSF and IL-4 or GM-CSF, IL-4 and IFN-γLow and examined for differences in induced, vivid−, CD3+, CD4+, CD25+, Foxp3+, and Helios−, or naturally occurring, vivid−, CD3+, CD4+, CD25+, Foxp3+, Helios+ T regulatory cells. No DC = freshly thawed lymphocytes without DC co-culture. Data are representative of three separate experiments in different healthy donors. (d) Autologous lymphocytes were labelled with CFSE, co-cultured with moDC described above (c), and examined for CFSE dilution (left panel). (e) Quantification of the percentage of CD4+ and CD8+ T cells that have divided based on CFSE dilution from (d) left panel. *P < 0·05 compared with co-cutlures with immature DC (iDC), two-tailed Student's t-test.
We next assessed whether IFN-γ impaired the ability of iDC to induce T-cell proliferation. Lymphocytes were labelled with CFSE fluorescent dye, and co-cultured with standard iDC or IFN-γLow iDC. Both CD8+ and CD4+ T cells co-cultured with IFN-γLow iDC exhibited significantly reduced CFSE dilution, indicating that IFN-γLow iDC had an impaired ability to induce T-cell proliferation (Fig. 5d). Hence, IFN-γLow iDC have a reduced ability to generate antigen-specific T-cell responses.
IFN-γLow iDC are unresponsive to subsequent maturation by IFN-γ or LPS
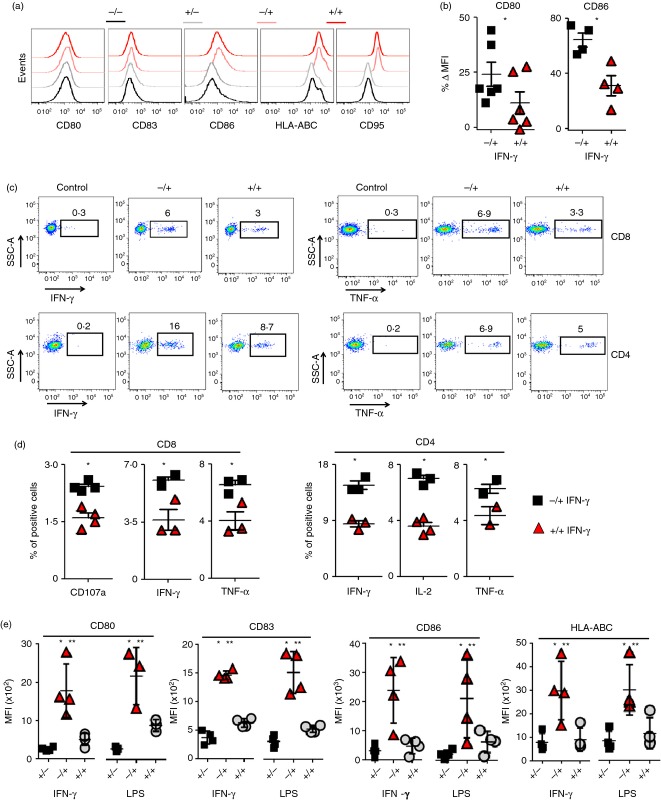
To assess the effect of differential dosing and timing of IFN-γ, moDC were grown under four conditions: (i) differentiation with GM-CSF/IL-4, no IFN-γ maturation (−/−); (ii) differentiation with GM-CSF/IL-4/IFN-γLow, no IFN-γ maturation (+/−); (iii) differentiation with GM-CSF/IL-4 with 1000 IU IFN-γ maturation (−/+); (iv) differentiation with GM-CSF/IL-4/IFN-γLow with 1000 IU IFN-γ maturation (+/+). Critically, moDC differentiated in the presence of low concentrations of IFN-γ and subsequently matured with higher doses of IFN-γ failed to up-regulate expression of CD80 and CD86 to the same degree as IFN-γ matured moDC that were differentiated without IFN-γ (Fig. 6a). The differences in the change in mean fluorescence intensity expression for CD80 and CD86 were statistically significant (Fig. 6b). Next, we tested whether moDC differentiated in the presence of IFN-γLow had reduced ability to generate antigen-specific T cells. Monocyte-derived DC generated in the four culture conditions described above were pulsed with overlapping peptide-epitope libraries for pp65 and IE-1. Exposure of moDC differentiated with IFN-γLow to 1000 IU IFN-γ at maturation did not rescue the ability of IFN-γLow moDC to induce a robust CMV-specific IFN-γ and TNF-α CD8+ or CD4+ T-cell response (Fig. 6c). Compared with IFN-γ matured moDC with no prior exposure to IFN-γ, the IFN-γLow moDC also had a statistically significant decrease in the percentage of CD8+ CD107a+, CD8+ IFN-γ+, CD8+ TNF-α+, CD4+ IFN-γ+, CD4+ TNF-α+ and CD4+ IL-2+ cells (Fig. 6d).
Figure 6.
Monocyte-derived dendritic cells (moDC) differentiated in the presence of low concentrations of interferon-γ (IFN-γLow) are resistant to subsequent IFN-γ or lipopolysaccharide (LPS) -induced maturation. (a) Representative histograms for expression of CD80, CD83, CD86, CD95 and HLA-ABC for moDCs differentiated with granulocyte–macrophage colony-stimulating factor (GM-CSF)/interleukin-4 (IL-4) (−/−), GM-CSF/IL-4/1 IU IFN-γ (+/−), GM-CSF/IL-4 followed by maturation with 1000 IU IFN-γ (−/+), and GM-CSF/IL-4/1 IU IFN-γ followed by maturation with 1000 IU IFN-γ (+/+). All plots are gated on vivid– CD11b+, CD11cHi, HLA-DRHi cells. (b) Quantification of the percentage change in MFI for CD80 and CD86 expression between the (−/+) and (+/+) groups compared with the (−/−) group. Data are representative of three donors. *P < 0·05, two-tailed Student's t-test. (c) Representative flow cytometry plots for cytomegalovirus-specific CD8+ and CD4+ T-cell responses measured by intracellular staining for IFN-γ and TNF-α following priming with (−/+) and (+/+) moDC pulsed with overlapping peptides for IE and pp65. (d) Quantification of the percentage of CD8+ CD107a+, CD8+ IFN-γ+, CD8+ TNF-α+, CD4+ IFN-γ+, CD4+ IL-2+, and CD4+ TNF-α+ cells between lymphocytes co-cultured with moDC from (c). (e) The MFI for CD80, CD83, CD86, CD95 and HLA-ABC following moDC differentiated with GM-CSF/IL-4 (−/) or GM-CSF/IL-4/1 IU IFN-γ (+/), followed by maturation with 1000 IU IFN-γ or 100 mg/ml LPS (/+). All plots gated on live vivid– CD3+ cells. *, **P < 0·05, two-tailed Student's t-test. Data are representative of three to four healthy donor patients (n = 3 or n = 4).
Lipopolysaccharide is known to stimulate moDC to a greater capacity than IFN-γ. Although we did not see any significant differences in the increase of co-stimulatory molecules when comparing LPS to IFN-γ maturation, we did observe an increase in the secretion of IL-12p70 and TNF-α in LPS-matured DC (see Supporting information, Fig. S2). Additionally, allogeneic mixed lymphocyte reaction assays showed that LPS-matured DC induced higher levels of T-cell proliferation compared with IFN-γ matured DC (see Supporting information, Fig. S3). In contrast, we did not observe significant differences in the expression of co-stimulatory molecules such as CD80, CD83, CD86, HLA-ABC or HLA-DR between LPS- or IFN-γ-matured DC (see Supporting information, Fig. S4).
We next assessed if subsequent exposure to LPS could mature IFN-γLow differentiated moDC. Surprisingly, moDC differentiated in the presence of IFN-γLow had a statistically significant decrease in the up-regulation of co-stimulatory molecules CD80, CD83, CD86 and HLA-ABC compared with standard moDC following maturation with either higher doses of IFN-γ or LPS (Fig. 6e). Hence, differentiating human peripheral blood monocytes with GM-CSF, IL-4 and IFN-γLow generates dysfunctional moDC incapable of subsequent maturation with IFN-γ or LPS. In contrast, monocytes differentiated with GM-CSF and IL-4 alone, retain their ability to be matured by IFN-γ and induce robust CD8+ and CD4+ T-cell responses to multiple viral and tumour antigens (Fig. 7).
Figure 7.
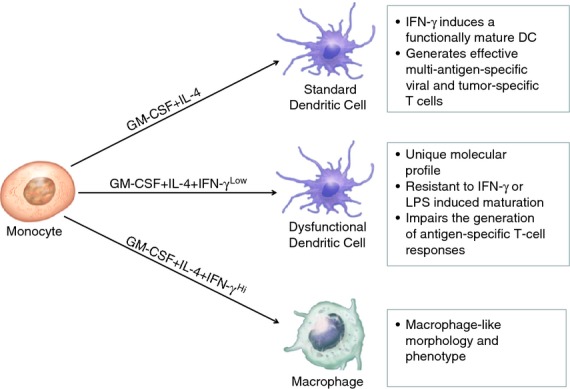
Representative diagram for the differential programming of human peripheral blood monocytes by interferon-γ (IFN-γ). Dendritic cells developed under standard conditions with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) are capable of being matured by IFN-γ into efficient stimulators of antigen-specific CD8+ and CD4+ T cells. In contrast, monocytes exposed to low concentrations of IFN-γ during differentiation generate dendritic cells that induce a decrease in antigen-specific T-cell responsiveness and have impaired maturation upon a re-challenge with IFN-γ or LPS.
Discussion
Two seminal discoveries, first by Ralph Steinman in identifying DC as a unique subset of mononuclear cells,37,38 and then the discovery by Federica Sallusto and Antonio Lanzavecchia that GM-CSF and IL-4 differentiate monocytes into moDC, paved the way for a better understanding of the biology of monocytes and moDC.39 The knowledge gained from these fundamental studies is now enabling scientists to develop cellular products that have the potential to treat a wide variety of human diseases such as cancer, infectious diseases and autoimmunity.32,40–44
In this study, we demonstrate that IFN-γ, an important mediator of sterile inflammation, possesses a dualistic role in shaping the function of human peripheral blood monocyte-derived DC. In the presence of GM-CSF and IL-4, high concentrations of IFN-γ skew the in vitro differentiation of monocytes into macrophages, but under lower concentrations, monocytes continue down their lineage path into DC. Monocyte-derived dendritic cells differentiated in the presence of low levels of IFN-γ possess a unique gene expression profile and induce significant antigen-specific T-cell hyporesponsiveness compared with standard immature moDC, despite not possessing any differences in surface co-stimulatory molecules or differences in cytokine profiles based on microarray expression data. However, significant differences are seen in the expression of genes implicated in the differentiation of haematopoietic cells such as POU6F1, CDKN1B and KLF5. Additionally, these genes are linked to important regulators of cellular differentiation such as KLF4 and GATA-2. Immature DC are known to be tolerogenic, but here, we show that the addition of low concentrations of IFN-γ during differentiation induces moDC to impair T-cell function to an even greater degree than immature moDC. Furthermore, this dysfunction is programmatically incorporated in moDC, as subsequent maturation by higher doses of IFN-γ or LPS fail to induce maturation. Taken together, these findings imply that low concentrations of IFN-γ induce an altered differentiation state for moDC, although further studies will be needed to develop this concept. In the translational setting, it is conceivable that the addition of low doses of IFN-γ to culture conditions during moDC differentiation may aid investigators devising strategies to generate moDC intended for clinical use in transplantation tolerance, graft-versus-host disease, or autoimmunity.45
In contrast, following lineage commitment with GM-CSF and IL-4, IFN-γ efficiently matures moDC. The addition of IFN-γ to fully differentiated moDC up-regulates important co-stimulatory molecules and induces moDC to potently stimulate the division and function of T cells. Furthermore, the maturation of moDC with IFN-γ enables the generation of effective CD8+ T-cell and CD4+ T-cell responses to multiple viral and tumour antigens. Taken together, these findings consolidate previous conflicting reports on the inhibitory or stimulatory roles of IFN-γ and suggest that the timing and intensity of direct exposure to IFN-γ determine whether DC inhibit or stimulate T-cell responses.
It is well known that pathogen-derived stimuli such as LPS are potent stimulators of innate immunity. However, many disease conditions such as autoimmunity, hereditary inflammatory conditions, inflammatory bowel disease, vasculitis, graft-versus-host disease and cancer trigger various degrees of chronic inflammation and IFN-γ expression in the absence of pathogen-induced signals. The immunological responses under these conditions are probably fundamentally different from those triggered by infectious stimuli. Here, we show that IFN-γ, as a sole physiological mediator, can both augment and dampen DC function depending on the nature and context of exposure. These findings may help to explain some of the varying literature on the immunological impact of IFN-γ. Classic studies show the importance of IFN-γ for immunosurveillance against cancer and the critical requirement for IFN-γ to eliminate established tumours. Nevertheless, others suggest that IFN-γ contributes to chronic inflammation within tumours and is critical for carcinogenesis. One possibility, although speculative, is that during tumour growth, low or chronic levels of IFN-γ may be a mechanism of immune escape and dampens the ability of innate immune cells within tumours [DC, myeloid-derived suppressor cells (MDSC), and macrophages] to present tumour antigens to infiltrating antigen-specific T cells.
The importance of IFN-γ for antigen presentation and cross-presentation by DC, MDSC, and macrophages is demonstrated in tumour models.20 Here, we show that DC matured with high doses of IFN-γ following standard differentiation up-regulate co-stimulatory molecules and improve the ability of DC to stimulate antigen-dependent proliferation and function of T cells. On the other hand, we also show that if DC are differentiated in the presence of low levels of IFN-γ, the ability of IFN-γ or LPS to enhance the functionality of DC and induce T-cell activity and proliferation is considerably dampened.
With regard to the translational implications of this study, recent successes in cellular therapies for cancer have highlighted the importance of generating functionally activated moDC and antigen-specific T cells for clinical trials.46–57 Our study confirms that the addition of IFN-γ to a fully differentiated moDC promotes the generation of large numbers of endogenously circulating T cells specific for a wide variety of viral and tumour antigens for adoptive transfer. However, our findings that moDC generated in the presence of low concentrations of IFN-γ can impair cytotoxic T lymphocyte generation is of importance because it implies that unplanned introduction of small amounts of IFN-γ from, for example, IFN-γ-producing cell contamination, can pervert the generation of effective antigen-specific cytotoxic T lymphocytes for adoptive immunotherapy. Neutralizing antibodies against IFN-γ in cell cultures of differentiating DC may significantly improve the quality of moDC for cell production.
In summary, this study adds to the growing body of evidence that suggests that inflammatory cytokines possess the ability to both aid and impede antigen-specific immune responses. The quality of cross-talk between the innate and acquired arms of the immune system probably depends on the context of the inflammatory response. Here, we describe the critical effects of the timing and intensity of exposure to IFN-γ in shaping the ability of moDC to either impair or stimulate antigen-specific T-cell responses. Further in vivo studies will be needed to carefully decipher the importance of these findings within local tissue microenvironments.57
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Heart Lung and Blood Institute.
Disclosures
The authors have no conflicting financial interests.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Dendritic cells differentiated in the presence of low concentrations of IFN-γ possess a distinct molecular signature.
Figure S2. Human monocyte-derived dendritic cells differentiated with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) were matured with prostaglandin E2 (PGE2), tumour necrosis factor-α (TNF-α), PGE2 plus TNF-α, soluble CD40 ligand (sCD40L), lipopolysaccharide (LPS), interferon-γ (IFN-γ) or IFN-γ + LPS.
Figure S3. Human monocyte-derived dendritic cells were matured with varying regimens and co-cultured with allogeneic T cells.
Figure S4. Human monocyte-derived dendritic cells were matured with either 1000 U of interferon-γ (IFN-γ) or 100 mg/ml of lipopolysaccharide (LPS) for 48 hr and assessed for the expression of co-stimulatory markers by flow cytometry.
References
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Germain RN. Maintaining system homeostasis: the third law of Newtonian immunology. Nat Immunol. 2012;13:902–6. doi: 10.1038/ni.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol. 2011;11:221–30. doi: 10.1038/nri2940. [DOI] [PubMed] [Google Scholar]
- O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Young HA. Unraveling the pros and cons of interferon-γ gene regulation. Immunity. 2006;24:506–7. doi: 10.1016/j.immuni.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Young HA, Bream JH. IFN-γ: recent advances in understanding regulation of expression, biological functions, and clinical applications. Curr Top Microbiol Immunol. 2007;316:97–117. doi: 10.1007/978-3-540-71329-6_6. [DOI] [PubMed] [Google Scholar]
- Farrar MA, Schreiber RD. The molecular cell biology of interferon-γ and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–95. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–61. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock EF. Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin. Science. 1965;149:310–1. [PubMed] [Google Scholar]
- Pan J, Zhang M, Wang J, et al. Interferon-γ is an autocrine mediator for dendritic cell maturation. Immunol Lett. 2004;94:141–51. doi: 10.1016/j.imlet.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Rojas D, Krishnan R. IFN-γ generates maturation-arrested dendritic cells that induce T cell hyporesponsiveness independent of Foxp3+ T-regulatory cell generation. Immunol Lett. 2010;132:31–7. doi: 10.1016/j.imlet.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Zaidi MR, Merlino G. The two faces of interferon-γ in cancer. Clin Cancer Res. 2011;17:6118–24. doi: 10.1158/1078-0432.CCR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon γ: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–50. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo M, Nagata Y, Sato E, et al. IFN-γ enables cross-presentation of exogenous protein antigen in human Langerhans cells by potentiating maturation. Proc Natl Acad Sci U S A. 2004;101:14467–72. doi: 10.1073/pnas.0405947101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eljaafari A, Li YP, Miossec P. IFN-gamma, as secreted during an alloresponse, induces differentiation of monocytes into tolerogenic dendritic cells, resulting in FoxP3+ regulatory T cell promotion. J Immunol. 2009;183:2932–45. doi: 10.4049/jimmunol.0804352. [DOI] [PubMed] [Google Scholar]
- Rojas-Canales D, Krishnan R, Jessup CF, Coates PT. Early exposure of interferon-γ inhibits signal transducer and activator of transcription-6 signalling and nuclear factor αβγκB activation in a short-term monocyte-derived dendritic cell culture promoting ‘FAST’ regulatory dendritic cells. Clin Exp Immunol. 2012;167:447–58. doi: 10.1111/j.1365-2249.2011.04537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkar SP, Goldszmid RS, Muranski P, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011;121:4746–57. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli RT, Wysocka M, Hayashi S, et al. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–43. [PubMed] [Google Scholar]
- Bradley LM, Dalton DK, Croft M. A direct role for IFN-γ in regulation of Th1 cell development. J Immunol. 1996;157:1350–8. [PubMed] [Google Scholar]
- Delneste Y, Charbonnier P, Herbault N, et al. Interferon-γ switches monocyte differentiation from dendritic cells to macrophages. Blood. 2003;101:143–50. doi: 10.1182/blood-2002-04-1164. [DOI] [PubMed] [Google Scholar]
- Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–44. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–58. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev. 2008;222:129–44. doi: 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiello L, Sabatino M, Jin P, et al. Monocyte-derived DC maturation strategies and related pathways: a transcriptional view. Cancer Immunol Immunother. 2011;60:457–66. doi: 10.1007/s00262-010-0954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han TH, Jin P, Ren J, Slezak S, Marincola FM, Stroncek DF. Evaluation of 3 clinical dendritic cell maturation protocols containing lipopolysaccharide and interferon-γ. J Immunother. 2009;32:399–407. doi: 10.1097/CJI.0b013e31819e1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vopenkova K, Mollova K, Buresova I, Michalek J. Complex evaluation of human monocyte-derived dendritic cells for cancer immunotherapy. J Cell Mol Med. 2012;16:2827–37. doi: 10.1111/j.1582-4934.2012.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldszmid RS, Caspar P, Rivollier A, et al. NK cell-derived interferon-γ orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity. 2012;36:1047–59. doi: 10.1016/j.immuni.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Garra A, Trinchieri G. Are dendritic cells afraid of commitment? Nat Immunol. 2004;5:1206–8. doi: 10.1038/ni1204-1206. [DOI] [PubMed] [Google Scholar]
- Rojas-Canales D, Krishnan R, Jessup CF, Coates PT. Early exposure of interferon-γ inhibits signal transducer and activator of transcription-6 signalling and nuclear factor κB activation in a short-term monocyte-derived dendritic cell culture promoting ‘FAST’ regulatory dendritic cells. Clin Exp Immunol. 2012;167:447–58. doi: 10.1111/j.1365-2249.2011.04537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Celis E. Use of two predictive algorithms of the world wide web for the identification of tumor-reactive T-cell epitopes. Cancer Res. 2000;60:5223–7. [PubMed] [Google Scholar]
- Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. 2011;11:330–42. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM, Korty PE, Tran DQ, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–41. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Nussenzweig MC. Dendritic cells: features and functions. Immunol Rev. 1980;53:127–47. doi: 10.1111/j.1600-065x.1980.tb01042.x. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar MV, Krasovsky J, Steinman RM, Bhardwaj N. Mature dendritic cells boost functionally superior CD8+ T-cell in humans without foreign helper epitopes. J Clin Invest. 2000;105:R9–14. doi: 10.1172/JCI9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–8. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esche C, Shurin MR, Lotze MT. The use of dendritic cells for cancer vaccination. Curr Opin Mol Ther. 1999;1:72–81. [PubMed] [Google Scholar]
- Bluestone JA, Thomson AW, Shevach EM, Weiner HL. What does the future hold for cell-based tolerogenic therapy? Nat Rev Immunol. 2007;7:650–4. doi: 10.1038/nri2137. [DOI] [PubMed] [Google Scholar]
- Tran DQ, Shevach EM. Therapeutic potential of FOXP3(+) regulatory T cells and their interactions with dendritic cells. Hum Immunol. 2009;70:294–9. doi: 10.1016/j.humimm.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12:443–58. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–6. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- Melenhorst JJ, Barrett AJ. Tumor vaccines and beyond. Cytotherapy. 2011;13:8–18. doi: 10.3109/14653249.2010.530649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulos CM, Suhoski MM, Plesa G, et al. Adoptive immunotherapy: good habits instilled at youth have long-term benefits. Immunol Res. 2008;42:182–96. doi: 10.1007/s12026-008-8070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–81. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- Brenner MK, Heslop HE. Adoptive T cell therapy of cancer. Curr Opin Immunol. 2010;22:251–7. doi: 10.1016/j.coi.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatchez C, Radvanyi LG, Hwu P. Advances in the treatment of metastatic melanoma: adoptive T-cell therapy. Semin Oncol. 2012;39:215–26. doi: 10.1053/j.seminoncol.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria S, Pikarsky E, Karin M, et al. Cancer and inflammation: promise for biologic therapy. J Immunother. 2010;33:335–51. doi: 10.1097/CJI.0b013e3181d32e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol. 2011;29:4828–36. doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkar SP, Muranski P, Kaiser A, et al. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res. 2010;70:6725–34. doi: 10.1158/0008-5472.CAN-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkar SP, Restifo NP. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res. 2012;72:3125–30. doi: 10.1158/0008-5472.CAN-11-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Dendritic cells differentiated in the presence of low concentrations of IFN-γ possess a distinct molecular signature.
Figure S2. Human monocyte-derived dendritic cells differentiated with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) were matured with prostaglandin E2 (PGE2), tumour necrosis factor-α (TNF-α), PGE2 plus TNF-α, soluble CD40 ligand (sCD40L), lipopolysaccharide (LPS), interferon-γ (IFN-γ) or IFN-γ + LPS.
Figure S3. Human monocyte-derived dendritic cells were matured with varying regimens and co-cultured with allogeneic T cells.
Figure S4. Human monocyte-derived dendritic cells were matured with either 1000 U of interferon-γ (IFN-γ) or 100 mg/ml of lipopolysaccharide (LPS) for 48 hr and assessed for the expression of co-stimulatory markers by flow cytometry.