Abstract
Tissue engineering provides a new paradigm for periodontal tissue regeneration in which proper stem cells and effective cellular factors are very important. The objective of this study was, for the first time, to investigate the capabilities and advantages of periodontal tissue regeneration using induced pluripotent stem (iPS) cells and enamel matrix derivatives (EMD). In this study the effect of EMD gel on iPS cells in vitro was first determined, and then tissue engineering technique was performed to repair periodontal defects in three groups: silk scaffold only; silk scaffold + EMD; and silk scaffold + EMD + iPS cells. EMD greatly enhanced the mRNA expression of Runx2 but inhibited the mRNA expression of OC and mineralization nodule formation in vitro. Transplantation of iPS cells showed higher expression levels of OC, Osx, and Runx2 genes, both 12 and 24 days postsurgery. At 24 days postsurgery in the iPS cell group, histological analysis showed much more new alveolar bone and cementum formation with regenerated periodontal ligament between them. The results showed the commitment role that EMD contributes in mesenchymal progenitors to early cells in the osteogenic lineage. iPS cells combined with EMD provide a valuable tool for periodontal tissue engineering, by promoting the formation of new cementum, alveolar bone, and normal periodontal ligament.
Periodontal diseases cause significant destruction of alveolar bone, periodontal ligament (PDL), and gingiva, leaving the oral environment exposed and initiating root contamination. Excess bone resorption often leads to tooth loss. Periodontal regeneration is the reproduction and reconstitution of lost or injured parts to restore the form and function of the lost structures. Ideally, regenerated PDL fibers are inserted into the new cementum to connect the root surface and new alveolar bone. Tissue engineering provides a new paradigm based on molecular and cell biology for periodontal regeneration. The regenerative capability of stem cells can be exploited by growing such cells in three-dimensional (3-D) constructs then implanting them into the defect. Through provision of a prefabricated 3-D structure with appropriate instructive messages incorporated, it is possible to overcome the limitations of conventional regenerative technologies (Ivanovski, 2009).
Stem cells are the foundation cells for every organ and tissue in the body, including the periodontium (Thesleff and Tummers, 2003). Stem cells used in tissue engineering may be allogenic, xenogenic, syngeneic, or autologous. Ideally, the cells should be nonimmunogenic, highly proliferative, easy to harvest, and have the ability to differentiate into a variety of cell types with specialized functions (Marler et al., 1998). Autologous stem cells, which can be easily isolated and expanded in vitro, would be an ideal choice. There are two main kinds of stem cells—embryonic stem cells (ESCs) and adult stem cells.
PDL stem cells are the precursor of synthetic cells (e.g., fibroblasts, osteoblasts, cementoblasts) with a multipotent capacity to generate adipocytes, osteoblast-like, and cementoblast-like cells. Tissues similar to cementum and PDL can be formed in vivo, when transplanted into immunocompromised mice (Seo et al., 2004; Nagatomo et al., 2006). Recently, the isolation of these cells from human PDL has presented new opportunities for tissue engineering (Seo et al., 2004). Due to the difficulties of acquiring autogeneic cells, clinical applications are difficult. Bone marrow stromal stem cells (BMSSCs) or mesenchymal stem cells (MSCs) have been reported to form cementum, PDLs, and alveolar bone, in vivo after implantation into periodontal defects in beagles (Hasegawa et al., 2006). This outcome suggests that MSCs may be a potential candidate for periodontal regeneration. The dental follicle is a mesenchymal tissue surrounding the developing tooth germ. During tooth root formation, dental follicle progenitors differentiate into different periodontal components: cementum, PDL, and alveolar bone. Immortalized dental follicle cells can generate PDL-like tissue after in vivo implantation (Yokoi et al., 2007), implying that dental follicular progenitor cells may be a potential stem cells resource for regenerative periodontal tissue engineering.
ESCs, derived from the inner cell mass of blastocysts, are pluripotent stem cells capable of differentiating into almost all kinds of cells of the adult body (Thomson et al., 1998). ESCs possess a higher regenerative capacity than adult stem cells; the latter are generally multipotent stem cells which can form a limited number of cell types corresponding with their tissues of origin. However, research development in both the laboratory and the clinical setting has been hampered by ethical concerns causing difficulties in uncovering these new endeavors.
Induced pluripotent stem (iPS) cells have recently been established by transfecting mouse and human somatic cells with the transcription factors Oct3/4, Sox2, Klf4, and c-Myc (Takahashi and Yamanaka, 2006) or OCT3/4, SOX2, NANOG, and LIN28 (Yu et al., 2007), known to be expressed at high levels in ESCs. iPS cells are identical to natural pluripotent stem cells in many respects: expression of stem cell genes and proteins, chromatin methylation patterns, doubling time, embryoid body formation (EBF), teratoma formation, viable chimera formation, and potency and differentiability. It represents a major breakthrough in stem cell research. It has been shown that iPS cells can be used for regenerating heart muscles (Mauritz et al., 2008), pancreatic beta cells (Furth and Atala, 2009), motor neurons (Dimos et al., 2008), and many other distinct tissues. While obtaining and using ESCs are ethically controversial, iPS cells reprogrammed from somatic cells are an excellent option in regenerative dental medicine, as those cells are easily accessible, autogeneic cells can be acquired individually, and their use does not bring up ethical concerns. Enamel matrix derivatives (EMD) or Emdogain gel (trademark), were first prepared from acid extracts of porcine enamel proteins in 1997 (Hammarström, 1997), contains a protein complex belonging to the amelogenin family. EMD increases alkaline phosphatase (ALP) activity, matrix mineralization in human PDL cells, osteoblasts, and rodent BMSCs (Van der Pauw et al., 2000; Keila et al., 2004). EMD have recently been shown to induce cementoblast differentiation and periodontal regeneration in vivo (Bosshardt et al., 2005), and are also thought to trigger the formation of acellular extrinsic fiber cementum (AEFC). Furthermore, they can regulate mouse and human follicle cell differentiation toward the osteo-cementoblastic phenotype (Hakki et al., 2001; Kémoun et al., 2007).
To further examine the capability and advantages of periodontal tissues regeneration using iPS cells combined with EMD in periodontal tissue engineering, we demonstrated the effect of EMD gel on iPS cells in vitro first, and then performed tissue engineering techniques with iPS cells and EMD in periodontal defects.
Materials and Methods
Human iPS cells culture and differentiation
Human iPS cells were obtained from Wicell Research Institute (Madison, WI). The cell line is iPS (Foreskin)-1-DL-1, which were reprogrammed from male human foreskin fibroblasts by four transfected transcript factors (Oct4, Sox2, Nanog, and Lin28). Cells were cultured on a six-well plate coated with matrigel in mTeSR™ 1 (mTeSR™ 1 basal medium and 20% mTeSR™ 1 supplement, STEMCELL Technologies, Vancouver, BC, Canada). The day after the cell clones confluent was passed, colonies were used to form embryonic body (EB). iPS colonies were treated with 0.5 mg/ml dispase-DMEM/F12 for about 30 min until the colonies dissociated into clumps and departed from the bottom of plate. The colonies were then transferred to nonadherent Petri dishes in EBF (mTeSRTM1) as gently as possible to make the colonies be integral. The EBs formed before they were collected by brief low-speed centrifugation, resuspended in EB media, and plated directly on dishes coated with 0.1% porcine gelatin. After 2 days of culture, most of the cells adhered, the cells were induced in osteogenic media [DMEM/F12 with 20% (v/v) FBS, supplemented with 50 μM ascorbic acid, 10 mM β-glycerophosphate, and 10 nM dexamethasone], and EMD-stimulated media [DMEM/F12 with 20% (v/v) FBS, supplemented with 30 μg/ml EMD (Emdogain® gel, Straumann AG, Basel, Switzerland)]. Cells cultured on basic media (DMEM/F12 with 20% (v/v) FBS) were the control. Media were changed every 2 days.
Total RNA isolation and real-time PCR (RT-PCR)
Total RNA of different cell groups were extracted using a RNeasy Kit (Qiagen, Valencia, CA) following the manufacturer’s instructions at 20 and 30 days after differentiation. One microgram of RNA was reverse transcribed with a SuperScript first-strand synthesis system (Invitrogen, Carlsbad, CA) following the manufacturer’s recommendations and then set up to RT-PCR reactions utilizing iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). Primer sequences were shown in Table 1, and β-actin gene expression was detected for normalization purposes.
TABLE 1.
The sequences of the primers for RT-PCR in vitro experiment
| Primer | Sequence |
|---|---|
| β-Actin | Forward: 5′-ACAGAGCCTCGCCTTTGCC-3′ |
| Reverse: 5′-ACATGCCGGAGCCGTTGTC-3′ | |
| Runx2 | Forward: 5′-CAGTCACCTCAGGCATGTCC-3′ |
| Reverse: 5′-GTGCTGCTGGTCTGGAAGG-3′ | |
| ColI | Forward: 5′-GCAAGGGAGAAAAGGGTGAACC-3′ |
| Reverse: 5′-GTGGCTCCAGCAGGACCAG-3′ | |
| OC | Forward: 5′-CTCCAGGCACCCTTCTTTCC-3′ |
| Reverse: 5′-ATTCCTCTTCTGGAGTTTATTTGGG-3′ |
Alizarin red S staining
EBs were cultured for 7 days in three different media: basic, osteogenic, and EMD-stimulated media, then were digested and cultured in different wells of a six-well plate at 1 × 105/well in their former media for another 21 days. After total 28 days in different media, Alizarin red S staining was performed to analyze the newly formed nodules.
Mouse iPS cell culture and EB formation
Mouse iPS cells were generously provided by Dr. Gustavo Mostoslavsky (Department of Medicine, Boston University School of Medicine, Boston, MA). Cells were cultured on the feeders of MEFs in mouse ESC medium (DMEM supplemented with 15% FBS, L-glutamine, penicillin/streptomycin, nonessential amino acids, β-mercaptoethanol, and 1,000 U/ml leukemia inhibitory factor) as previously described (Sommer et al., 2009). EBs formed in EBF media consisting of DMEM supplemented with 20% (v/v) FBS, 1 mM L-glutamine, 1% nonessential amino acid, and 100 mM β-mercaptoethanol (Sivasubramaniyan et al., 2008).
Scaffold preparation and cell seeding
Apatite-coated silk fibroin scaffolds were prepared as previously described (Jiang et al., 2009). The scaffolds were cut into cubes of approximately 2 × 1.5 × 1 mm3, and then soaked into 70% ethanol for 20 min four times and sterile PBS for 20 min three times. For cell seeding, EBs were collected and dispersed into single cells using trypsin/EDTA (0.25% (w/v) trypsin, 0.02% EDTA) and concentrated to 1 × 10 cells/ml in basal medium. The suspended cells were pipetted onto the 2 × 1.5 × 1 mm3 apatite-coated silk. The construct was incubated overnight to allow for cell attachment in vitro before implantation. In a parallel experiment, the empty scaffold was also soaked in basal medium overnight as control and scaffold groups.
Surgical procedure
Twenty-four, 8-week-old male nude mice (body weight 25–30 g) were used. All experimental procedures were carried out in accordance with the animal experimental guidelines of Tufts University. Under general anesthesia, a periodontal fenestration defect model of rodents was utilized, which were previously described by us and others (Zhao et al., 2004; Tu et al., 2007). An incision was made at the lower border of the mandible bilaterally, followed by the underlying muscle dissection, ensuring the attachment of the oral mucosa on the superior wall to the intraoral keratinized gingival margin.
The bone overlying the first molar, the buccal mandibula, was removed with spur at high speed under PBS irrigation 0.5 mm away from the coronal top. The roots were carefully denuded of PDL, overlying cementum, and superficial dentin under a stereomicroscope. Approximately a 2 × 1.5 mm2 periodontal defect was modified; the surrounding buccal mandibula was removed. The experiments were divided into three groups: (1) scaffold group (just implant silk scaffold); (2) control group (implant silk scaffold and EMD); (3) experimental group (implant silk scaffold with iPS cells and EMD). For the latter two groups, scaffolds were soaked into a 1-mm thick EMD layer for about 20 sec before implantation, and the soft tissues were re-positioned over the implant and sutured with 5.0-USP nylon monofilament.
RT-PCR of newly formed tissue
Groups of 12 mice were sacrificed 12 or 24 days postsurgery. Tissues around the edge of the defect sites were cut from six mandibular samples (three animals) and frozen immediately in liquid nitrogen and kept at −80°C for RT-PCR. Tissues were then be homogenized in TRIzol solution (Invitrogen), followed by the total RNA isolation procedure recommended by the manufacturer. Freshly isolated RNAs were reverse transcribed into cDNAs and then RT-PCR was performed, as we described before (Tu et al., 2008; Xu et al., 2009). The sequences of the primers are shown in Table 2.
TABLE 2.
The sequences of the primers for RT-PCR in vivo experiment
| Primer | Sequence |
|---|---|
| GAPDH | Forward: 5′-AGGTCGGTGTGAACGGATTTG-3′ |
| Reverse: 5′-TGTAGACCATGTAGTTGAGGTCA-3′ | |
| Runx2 | Forward: 5′-GCTCACGTCGCTCATCTTG-3′ |
| Reverse: 5′-CCAACCGAGTCATTTAAGGCT-3′ | |
| BSP | Forward: 5′-CAGGGAGGCAGTGACTCTTC-3′ |
| Reverse: 5′-AGTGTGGAAAGTGTGGCGTT-3′ | |
| OC | Forward: 5′-GCGCTCTGTCTCTCTGACCT-3′ |
| Reverse: 5′-GCCGGAGTCTGTTCACTACC-3′ | |
| Osx | Forward: 5′-ATGGCGTCCTCTCTGCTTG-3′ |
| Reverse: 5′-TGAAAGGTCAGCGTATGGCTT-3′ |
Histomorphometry and micro-computed tomography scanning analyses
Tissues from the original defect area of each group were fixed in 10% formalin at 4°C for 48 h and then scanned by micro-CT (μCT-35, Scanco Medical AG, Bassersdorf, Switzerland) with the resolution of 7 μm. The samples were then decalcified in 10% EDTA for 2 weeks, embedded in paraffin, and serial buccal and lingual sagittal cross-sections were made. Hematoxylin and eosin (H&E) stains were performed by standard methods. Newly formed bone in H&E-stained sections was quantified in four sections with three different defects for each treatment. The H&E stains were examined and photographed with a Nikon Eclipse E600 microscope and Spot Advanced software (Diagnostic Instruments, Sterling Heights, MI). After conventional microscopic examination, computer-assisted histometric measurements of the newly formed bone were obtained using an automated image analysis system. New bone area (mm2) was measured within the boundaries of newly formed bone and new bone formation in each section then expressed as a percentage of the total area of the defect. Furthermore, the boundary of the defects around teeth root was defined and new alveolar bone, PDL, and cementum were observed.
Statistical analysis
Statistically significant differences (P<0.05) between the various groups were measured using ANOVA. All statistical analyses were carried out using a SPSS 11.5 statistical software package (SAS, Cary, NC). All the data were expressed as mean ± standard deviation.
Results
EB formation from human and mouse iPS cells
After 4–5 days of suspension culture for human iPS cells and drop-suspension for 48 h followed by suspending for 3 days from mouse iPS cells, both the EBs exhibited a regular round shape (Fig. 1).
Fig. 1.

A: Free-floating embryoid bodies formed from human iPS cells after 5 days of suspension culture in nonadherent culture dishes (40×). B: Mouse EB formed from mouse iPS cells by drop suspension (40×).
EMD can promote the differentiation of iPS cells to osteogenic cells but inhibit cell maturation and mineralization in a later stage
RT-PCR was used to measure the mRNA expression levels of Coll, OC, and Runx2 of cells cultured in different media after 20 and 30 days. The results showed that the mRNA expression of Runx2, a key transcript factor during osteogenic differentiation, greatly increased in both EMD-stimulated and osteogenic media compared with control media, and EMD-stimulated group showed the highest level after 20 and 30 days culture (Fig. 2A). However, the mRNA expression of OC, a noncollagenous protein thought to play a role in mineralization and calcium ion homeostasis and a biomarker of the fully differentiated osteoblast, decreased sharply with the induction of EMD after 20 and 30 days culture (Fig. 2B). Both in osteogenic and EMD-stimulated media for 20 or 30 days, Coll mRNA displayed the highest expression levels in osteogenic media (Fig. 2C). In addition, Alizarin red S staining showed almost no calcium nodules in EMD-simulated group (Fig. 3).
Fig. 2.

RT-PCR to measure mRNA expression levels of ColI, OC, and Runx2 in different media after being cultured for 20 and 30 days. Expressions of Runx2, both significantly increased in osteogenic media and EMD-stimulated media, that of the latter was the highest (A). However, OC expressions in EMD-stimulated media were the lowest both in 20 and 30 days culture (B). ColI mRNA displayed the highest expression levels in osteogenic media both in 20 and 30 days (C). Levels of OC, ColI, and Runx2 were normalized with those of the loading control β-actin. C, Control media group; O, osteogenic media group; E, EMD-stimulated media group. *Statistically significant difference (P <0.05); **Statistically significant difference (P<0.01).
Fig. 3.

After cultured for 7 + 21 days in six-well plate, there was almost no mineralization nodule in EMD-stimulated media (C). A: Control group; (B) osteogenic media group; (C) EMD-stimulated media group (100×). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Transplantation with iPS cells combined with EMD enhanced new bone regeneration
At 24 days postsurgery, the defect sites in scaffold group were filled with connective tissue and linear new bone formation at the defect area (Fig. 4); in the silk scaffold + EMD control, the defect sites were filled with connective tissue and blocked new bone formation (Fig. 4); while in silk scaffold + EMD + iPS cells experimental group, the defect sites exhibited marked bone formation which almost repaired the whole bone defects with new bone spicules (Fig. 4).
Fig. 4.
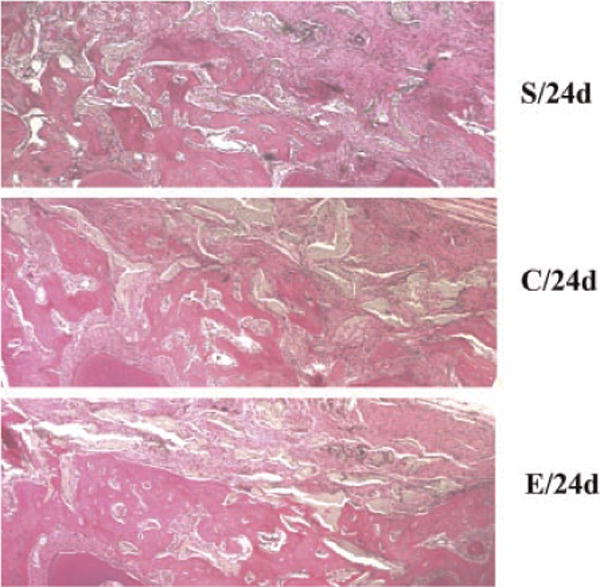
H&E staining sections of mandibular isolated from nude mice of three different groups at 24 days postsurgery. Silk scaffold + EMD + iPS cells experimental group (E/24d) showed increased mandibular bone compared with scaffold only (S/24d) and scaffold + REMD control (C/24d) groups. Photographs were taken at 40× magnification. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The histometric analysis showed new bone area percentages in the scaffold + EMD control similar to those observed in the silk scaffold alone group both 12 and 24 days postsurgery. The new bone area in the silk scaffold + EMD + iPS cells experimental group was greater than those in the other two groups and was significantly different (P< 0.01; Table 3).
TABLE 3.
New bone area percentage (not including residual biomaterial, group means±SD; n=6)
| 12 days | 24 days | |
|---|---|---|
| Silk scaffold | 7.23 ± 0.68 | 39.57 ± 1.58 |
| Silk scaffold + EMD | 8.28 ± 1.06% | 41.25 ±2.14c |
| Silk scaffold + EMD + iPS cells | 15.43 ± 2.43a,b | 58.53 ± 2.67a,b |
Statistically significant difference comparedtosilk scaffold and silk scaffold + EMD + iPS cells group (P< 0.01).
Statistically significant difference compared to silk scaffold + EMD and silk scaffold + EMD + iPS cells group (P < 0.01).
Statistically significant difference compared to silk scaffold and silk scaffold+EMD control group (P< 0.05).
Micro-CT analysis demonstrated that the sample in the EMD + iPS cells experiment group showed more newly formed hard tissue than that in EMD control group in the similar layer (Fig. 5). The defect cubes were cut from the 3-D CT imaging (Efilm Workstation V2.1.2, Merge Healthcare, Hartland, WI) and showed that the density of newly formed tissue in experiment group was higher than that in control group (Fig. 5A,B).
Fig. 5.

More newly formed hard tissue could be seen in EMD + iPS cells group (B) than in EMD group (A). Meanwhile, the defect cubes cut from the 3D CT imaging showed the higher density of newly formed tissue in EMD + iPS cells group (b). D: Defect.
RT-PCR showed that transplanted iPS cells had higher expression levels of OC, Osx, and Runx2
To further determine the effect of iPS transplantation during new bone formation, total RNA was isolated from both scaffold+EMD control and silk scaffold + EMD + iPS cells experimental groups. RT-PCR was performed to measure mRNA expression levels of BSP, OC, Osx, and Runx2. The BSP mRNA expression showed a higher level at 12 days and lower level at 24 days in postsurgery. Meanwhile, other bone markers showed higher expression levels in the experimental group (Fig. 6).
Fig. 6.

Silk scaffold + EMD + iPS cells experimental group showed increased expressions of bone transcription factors-Runx2 (D) and Osx (C) compared to Silk scaffold + EMD control group 24 days post-surgery. Expression of bone marker OC (B) increased 12 and 24 days post-surgery in experimental group, while BSP(A) expression increased 12 days and decreased 24 days post-surgery. Levels of BSP, OC, Runx2, and OSX were normalized with those of the loading control GAPDH. *: Statistically significant difference (P<0.05); **: Statistically significant difference (P< 0.01).
Histomorphometric analysis showed transplantation of iPS cells combined with EMD enhanced the periodontal tissues regeneration
The results of H&E staining showed that the scaffold only group had almost no vertical fibers 24 days after transplantation and the fibers that surrounded the denuded root surface were disordered (Fig. 7A,B); while the extrinsic fibers perpendicular to the denuded root surface could be seen in almost all in the control and experimental EMD-treated groups (Fig. 7C,D).
Fig. 7.

Extrinsic fibers perpendicular to the denuded root surface could be seen at all cases in EMD-treated groups (C,D), while for those in scaffold group, fibers surrounded the denuded root surface were disorder and there were almost no vertical fibers seen 24 days after transplantation (A,B). A,C: 200×; B,D: 400×. ←, New extrinsic fibers. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The silk scaffold + EMD + iPS cell experimental group 24 days postsurgery showed significantly more new alveolar bone formation, new cementum covered almost the whole denuded surface, and the regenerated PDL separated the new bone from new cementum (Fig. 8B). While in control group (Fig. 8A), adequate alveolar bone reconstruction was not yet obtained, cementum regeneration was insufficient, and new PDL separated the new bone from new cementum. The new bone and cementum formation were consistent to some extent.
Fig. 8.

New alveolar bone in silk scaffold + REMD + iPS cells experimental group (B) was more intact and adequate than that in silk scaffold + EMD control group (A). New cementum almost completely covers the denuded root surface in experiment group (B), while in control group, cementum could be seen at the margin of defect where an adequate new alveolar bone could be seen (A). The sites of new alveolar bone and new cementum formation were consistent to some extent. —, Suppositional margins of defect; nAB, new alveolar bone; nPL, new periodontal ligament; →, new cementum. Photographs were taken at 100×. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Discussion
The major component of EMD in the purified acid extract form from pig enamel matrix (>95%) is amelogenins (Amel), whose structure and function are evolutionary well conserved, suggesting a profound role in biomineralization and hard tissue formation. A great number of experimental studies have previously demonstrated that EMD and amelogenins could stimulate growth of multiple mesenchymal cell types including fibroblasts, cementoblasts, and osteoblasts, and enhance the expression of tissue-specific maturation markers of osteoblasts, such as alkaline phosphatase (ALP), collagen, and osteocalcin within osseous tissues (Lyngstadaas et al., 2009). There have also been previous reports that suggest that they could regenerate bone and induce stem cells into osteogenic cells such as mouse bone marrow MSCs (Keila et al., 2004); osteo-cementoblastic phenotype cells such as human PDL and mouse and human follicle cells (Van der Pauw et al., 2000; Hakki et al., 2001; Kémoun et al., 2007) in vitro. Previous studies demonstrated that the osteogenic activity of EMD may be mediated by BMP-like molecules, which could stimulate Cbfa1/Runx2 expression, the phosphorylation of Smad1, and both of these processes can be blocked by noggin (Takayama et al., 2005). While with different BMPs-like effects, EMD could enhance their proliferation but not differentiation of preosteoblasts, but for mature osteoblasts, EMD enhanced their differentiation (Schwartz et al., 2000). EMD is an osteoconductive agent, not osteoinductive. Its ability to regulate cells in the osteogenic lineage depends on the stage of cell differentiation. It can make uncommitted cells with osteoblastic potential and regulated committed osteoblasts (Schwartz et al., 2000). In our study, EMD greatly enhanced the mRNA level of Runx2 but inhibited the expression of OC and mineralization in vitro. Runx2 has an essential role in promoting the differentiation of MSCs into preosteoblasts which do not express osteoblast marker genes (Nakashima et al., 2002). Previous reports showed that EMD could promote the mineralization of stem cells and osteo-cementoblastic phenotype cells. However, other studies reported an inhibitory effect of EMD on the expression of OC (Tokiyasu et al., 2000; Hakki et al., 2001). The discrepancy among results may be due to differences in cell types, culture conditions, methods, the concentration and timing of EMD addition to the culture, or other unknown reasons (Takayama et al., 2005). In our study, EMD greatly enhanced the mRNA expression of Runx2, which has an essential role in differentiation of MSCs into preosteoblasts which do not express osteoblast marker genes (Nakashima et al., 2002); while it inhibited the expression of OC and mineralization in vitro. Therefore, EMD may play a role in the commitment of mesenchymal progenitors to the osteogenic lineage but inhibits their total maturation and mineralization.
The scaffold group without EMD showed fibers around the denuded root surface to have an unorderly arrangement, while in the other two EMD groups, extrinsic fibers were perpendicular to the denuded surface and in an ordered position seen under microscope. Previous histological studies showed that EMD treatment combined with GTR induced the formation of AEFC (Hammarström, 1997; Araújo and Lindhe, 1998). Amelogenin is an intrinsically disordered protein where the molecules can self-assemble into hydrophobic supramolecular monodisperse assemblies, called nanospheres, which are slowly processed by matrix proteases to release active peptides. The amelogenin self-assembly mechanism is controlled by its local environment, changes in temperature, pH, ionic strength, and protein concentration. This ability is most likely what makes these molecules so applicable in the clinic. Meanwhile, EMD has been observed to have a direct cytostatic effect in epithelial cells and osteoclasts, whose stasis is very important in clinical therapy especially in periodontal therapy. For successful regeneration of normal PDL fiber attachment, hard tissues need the exclusion of epithelial cells from the gingiva, giving the advantage to connective tissue cells and a balance between osteoblast and osteoclast activity (Lyngstadaas et al., 2009).
Many researchers have reprogrammed iPS cells by using different kinds of somatic cells and disease-specific cells (Dimos et al., 2008; Park et al., 2008). Human primary keratinocytes were successfully generated into iPS cells making it more efficient and fast when compared with reprogramming human fibroblasts, even from single adult human hairs, which is easily acquired from body. This leaves a big probability of generating a patient-specific or disease-specific human iPS cell line in the future for therapeutic purposes, although the method of reprogramming is still inefficient and many fundamental questions remain unanswered. This type of cell line avoids immunologic rejection and ethical controversy with easier cell resources, which is a great stem cell choice for tissue engineering. To minimize the species and individual difference and imitate the transplantation in clinic therapytothe maximum extent, our in vivo experiment implanted mouse iPS cells into defects of nude mice. In our study, before we relocated the iPS cells into silk scaffold, they were primarily induced into EBs.
Many researchers have revealed that the derivation of osteoblasts from pluripotent ES cells have the capacity to form mineralized tissue both in vitro and in vivo (Buttery et al., 2001; Bielby et al., 2004). It was demonstrated that OC-hESCs could successfully regenerate new bone tissues upon in vivo implantation (Kim et al., 2008). Histological analysis of our in vivo experiment showed that the experimental specimens have a significant amount of new alveolar and mandibular bone, 24 days after iPS cells combined with EMD were transplanted. From the results of the RT-PCR, the expression of BSP, an early osteogenic marker, was higher in 12 days and lower in 24 days than that of the control group. Otherwise, OC, as well as the transcription factors-Runx2 and Osx, were all higher than those in the control group, and all in ascending trend. That suggests more active bone formation activities ongoing in the iPS cell experimental group. EMD may play a role during the procedure by numbers of cells being increased and induced to the earlier cells in the osteogenic lineage. Local factors or primary bone-derived cells may be able to promote iPS cells differentiation into osteoblasts more efficiently and improve its further differentiation. The scaffold we used was apatite-coated silk fibroin scaffolds, highly porous scaffolds which performed the role of a temporary matrix to provide anchorage for dependent cells, biodegradability, and low inflammatory response of silk fibroin, which made silk fibroin scaffold one of the promising scaffolds for osteogenic applications (Jiang et al., 2009). The calcium-phosphate (Ca-P) coatings, on the mineralized silk scaffolds reduced the fibrous encapsulation layer, enhanced direct bone contact and stimulated differentiation of BMSCs along the osteogenic lineage, which made for better bone regeneration.
In our study, adequate new alveolar bone and an almost intact layer of new cementum could be seen in the experimental group, with new normal PDL formed between them, which were not seen in control group. Furthermore, the sites of new alveolar bone and new cementum were corresponding. It has been hypothesized that the type of tissue which predominates in the healing wound determines whether the response is repair or regeneration (Melcher, 1976). Moreover, components of the newly produced cementum matrix provide informational signaling for recruitment, proliferation, and differentiation of periodontal cells and regulate regeneration of cementum. It is possible that cementum components have the potential to participate in the regulation of homeostasis and regeneration of the gingiva, PDL, and alveolar bone tissues (Grzesik and Narayanan, 2002). New cementum formation and restoration of soft tissue attachment to cementum are regarded as the major goal of regenerative periodontal therapy. Our in vivo studies have suggested that transplantation of mouse iPS cells can promote periodontal tissue regeneration. The mechanism by which iPS cells are transplanted with EMD promotes cementum regeneration is still being studied in our laboratory. Previous studies have revealed that the microenvironment and the surrounding tissue provide the nutrients, growth factors, and extracellular matrices to support MCS differentiation (Caplan et al., 1998), as well as host and environment factors can influence transplanted stem cells to differentiate into various connective tissue cells (Liechty et al., 2000). It has also been reported that brain stem cells give rise to hematopoietic cells after engraftment into a hematopoietic system (Bjornson et al., 1999), and bone marrow cells differentiate into epithelial cells after transplantation (Anderson et al., 2001). It seems that when stem cells are removed and transplanted into different locations, they undergo reprogramming of gene expression and cross-lineage boundaries. Local molecules, factors, ECM, cementum components, and ectogenic EMD matrix may participate in the recruitment expansion, and differentiation of cementoblast progenitors, and periodontal defect healing. The direct or indirect interaction between cells and their periodontal environment may be the reasons why iPS cells could promote periodontal regeneration.
In a conclusion, mouse iPS cells combined with EMD can greatly enhance the repair of mouse periodontal defects, by promoting the formation of cementum, alveolar bone, and a normal PDL. But further research should be required on animal model of chronic periodontitis, in which the periodontal condition and environment would be more complex.
Acknowledgments
We would like to acknowledge Dana Murray for her assistance in article preparation. The study was supported by NIH grants DE16710 to J.C.
Literature Cited
- Anderson DJ, Gage FH, Weissman IL. Can stem cells cross lineage boundaries? Nat Med. 2001;7:393–395. doi: 10.1038/86439. [DOI] [PubMed] [Google Scholar]
- Araújo MG, Lindhe J. GTR treatment of degree III furcation defects following application of enamel matrix proteins. An experimental study in dogs. J Clin Periodontol. 1998;25:524–530. doi: 10.1111/j.1600-051x.1998.tb02482.x. [DOI] [PubMed] [Google Scholar]
- Bielby RC, Boccaccini AR, Polak JM, Buttery LD. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng. 2004;10:1518–1525. doi: 10.1089/ten.2004.10.1518. [DOI] [PubMed] [Google Scholar]
- Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: A hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- Bosshardt DD, Sculean A, Windisch P, Pjetursson BE, Lang NP. Effects of enamel matrix proteins on tissue formation along the roots of human teeth. J Periodontal Res. 2005;40:158–167. doi: 10.1111/j.1600-0765.2005.00785.x. [DOI] [PubMed] [Google Scholar]
- Buttery LD, Bourne S, Xynos JD, Wood H, Hughes FJ, Hughes SP, Episkopou V, Polak JM. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng. 2001;7:89–99. doi: 10.1089/107632700300003323. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Reuben D, Haynesworth SE. Cell-based tissue engineering therapies: The influence of whole body physiology. Adv Drug Deliv Rev. 1998;33:3–14. doi: 10.1016/s0169-409x(98)00016-7. [DOI] [PubMed] [Google Scholar]
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Furth ME, Atala A. Stem cell sources to treat diabetes. J Cell Biochem. 2009;106:507–511. doi: 10.1002/jcb.22000. [DOI] [PubMed] [Google Scholar]
- Grzesik WJ, Narayanan AS. Cementum and periodontal wound healing and regeneration. Crit Rev Oral Biol Med. 2002;13:474–484. doi: 10.1177/154411130201300605. [DOI] [PubMed] [Google Scholar]
- Hakki SS, Berry JE, Somerman MJ. The effect of enamel matrix protein derivative on follicle cells in vitro. J Periodontol. 2001;72:679–687. doi: 10.1902/jop.2001.72.5.679. [DOI] [PubMed] [Google Scholar]
- Hammarström L. Enamel matrix, cementum development and regeneration. J Clin Periodontol. 1997;24:658–668. doi: 10.1111/j.1600-051x.1997.tb00247.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa N, Kawaguchi H, Hirachi A, Takeda K, Mizuno N, Nishimura M, Koike C, Tsuji K, Iba H, Kato Y, Kurihara H. Behavior of transplanted bone marrow-derived mesenchymal stem cells in periodontal defects. J Periodontol. 2006;77:1003–1007. doi: 10.1902/jop.2006.050341. [DOI] [PubMed] [Google Scholar]
- Ivanovski S. Periodontal regeneration. Aust Dent J. 2009;54:S118–S128. doi: 10.1111/j.1834-7819.2009.01150.x. [DOI] [PubMed] [Google Scholar]
- Jiang X, Zhao J, Wang S, Sun X, Zhang X, Chen J, Kaplan DL, Zhang Z. Mandibular repair in rats with premineralized silk scaffolds and BMP-2-modified bMSCs. Biomaterials. 2009;30:4522–4532. doi: 10.1016/j.biomaterials.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keila S, Nemcovsky CE, Moses O, Artzi Z, Weinreb M. In vitro effects of enamel matrix proteins on rat bone marrow cells and gingival fibroblasts. J Dent Res. 2004;83:134–138. doi: 10.1177/154405910408300210. [DOI] [PubMed] [Google Scholar]
- Kémoun P, Laurencin-Dalicieux S, Rue J, Farges JC, Gennero I, Conte-Auriol F, Briand-Mesange F, Gadelorge M, Arzate H, Narayanan AS, Brunel G, Salles JP. Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res. 2007;329:283–294. doi: 10.1007/s00441-007-0397-3. [DOI] [PubMed] [Google Scholar]
- Kim S, Kim SS, Lee SH, Eun Ahn S, Gwak SJ, Song JH, Kim BS, Chung HM. In vivo bone formation from human embryonic stem cell-derived osteogenic cells in poly(D,L-lactic-coglycolic acid)/hydroxyapatite composite scaffolds. Biomaterials. 2008;29:1043–1053. doi: 10.1016/j.biomaterials.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- Lyngstadaas SP, Wohlfahrt JC, Brookes SJ, Paine ML, Snead ML, Reseland JE. Enamel matrix proteins; old molecules for new applications. Orthod Craniofac Res. 2009;12:243–253. doi: 10.1111/j.1601-6343.2009.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler JJ, Upton J, Langer R, Vacanti JP. Transplantation of cells in matrices for tissue regeneration. Adv Drug Deliv Rev. 1998;33:165–182. doi: 10.1016/s0169-409x(98)00025-8. [DOI] [PubMed] [Google Scholar]
- Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, Hasenfuss G, Martin U. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–517. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976;47:256–260. doi: 10.1902/jop.1976.47.5.256. [DOI] [PubMed] [Google Scholar]
- Nagatomo K, Komaki M, Sekiya I, Sakaguchi Y, Noguchi K, Oda S, Muneta T, Ishikawa I. Stem cell properties of human periodontal ligament cells. J Periodont Res. 2006;41:303–310. doi: 10.1111/j.1600-0765.2006.00870.x. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz Z, Carnes DL, Jr, Pulliam R, Lohmann CH, Sylvia VL, Liu Y, Dean DD, Cochran DL, Boyan BD. Porcine fetal enamel matrix derivative stimulates proliferation but not differentiation of pre-osteoblastic 2T9 cells, inhibits proliferation and stimulates differentiation of osteoblast-like MG63 cells, and increases proliferation and differentiation of normal human osteoblast NHOst cells. J Periodontol. 2000;71:1287–1296. doi: 10.1902/jop.2000.71.8.1287. [DOI] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- Sivasubramaniyan K, Atluri RR, Sarda K, Arvind M, Balaji V, Deb KD. Endotoxin-induced silencing of mesoderm induction and functional differentiation: Role of HMGB1 in pluripotency and infection. Regen Med. 2008;3:23–31. doi: 10.2217/17460751.3.1.23. [DOI] [PubMed] [Google Scholar]
- Sommer CA, Stadtfeld M, Murphy GJ, Hochedlinger K, Kotton DN, Mostoslavsky G. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells. 2009;27:543–549. doi: 10.1634/stemcells.2008-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cells. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takayama T, Suzuki N, Narukawa M, Tokunaga T, Otsuka K, Ito K. Enamel matrix derivative stimulates core binding factor alpha1/Runt-related transcription factor-2 expression via activation of Smad1 in C2C12 cells. J Periodontol. 2005;76:244–249. doi: 10.1902/jop.2005.76.2.244. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Tummers M. Stem cells and tissue engineering: Prospects for regenerating tissues in dental practice. Med Princ Pract. 2003;12:43–50. doi: 10.1159/000069840. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tokiyasu Y, Takata T, Saygin E, Somerman M. Enamel factors regulate expression of genes associated with cementoblasts. J Periodontol. 2000;71:1829–1839. doi: 10.1902/jop.2000.71.12.1829. [DOI] [PubMed] [Google Scholar]
- Tu Q, Zhang J, James L, Dickson J, Tang J, Yang P, Chen J. Cbfa1/Runx2-deficiency delays bone wound healing and locally delivered Cbfa1/Runx2 promotes bone repair in animal models. Wound Repair Regen. 2007;15:404–412. doi: 10.1111/j.1524-475X.2007.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Q, Zhang J, Paz J, Wade K, Yang P, Chen J. Haploinsufficiency of Runx2 results in bone formation decrease and different BSP expression pattern changes in two transgenic mouse models. J Cell Physiol. 2008;217:40–47. doi: 10.1002/jcp.21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Pauw MT, Van den Bos T, Everts V, Beertsen W. Enamel matrix-derived protein stimulates attachment of periodontal ligament fibroblasts and enhances alkaline phosphatase activity and transforming growth factor beta1 release of periodontal ligament and gingival fibroblasts. J Periodontol. 2000;71:31–43. doi: 10.1902/jop.2000.71.1.31. [DOI] [PubMed] [Google Scholar]
- Xu B, Zhang J, Brewer E, Tu Q, Yu L, Tang J, Krebsbach P, Wieland M, Chen J. Osterix enhances BMSC-associated osseointegration of implants. J Dent Res. 2009;88:1003–1007. doi: 10.1177/0022034509346928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi T, Saito M, Kiyono T, Iseki S, Kosaka K, Nishida E, Tsubakimoto T, Harada H, Eto K, Noguchi T, Teranaka T. Establishment of immortalized dental follicle cells for generating periodontal ligament in vivo. Cell Tissue Res. 2007;327:301–311. doi: 10.1007/s00441-006-0257-6. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhao M, Jin Q, Berry JE, Nociti FH, Jr, Giannobile WV, Somerman MJ. Cementoblast delivery for periodontal tissue engineering. J Periodontol. 2004;75:154–161. doi: 10.1902/jop.2004.75.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]


