Abstract
Exposure to prenatal stress (PNS) has been shown to induce a set of psychological and behavioral changes in developing offspring. We used the rodent model to investigate whether PNS produces changes in the ability of the pup to express social motivation. We used a set of behavioral tasks including monitoring ultrasonic vocalizations after isolation, a conditioned place preference, and a novel and familiar odor approach test. Pregnant Long–Evans rats were exposed to an unpredictable, variable stressor twice daily during the third week of gestation. Isolation vocalizations were assessed on postnatal day (PND) 10. Pup affinity for the dam was evaluated on PND 15. Typically, pups display a selective preference for an odor-paired environment only after the odor has been associated with the dam. This previous association produces a positive conditioned stimulus (CS). Normally, pups exposed to a neutral CS (odor paired with cotton balls) do not form this place preference. Results indicate that PNS exposed pups had significantly increased distress vocalizations and an equal preference for the positive and neutral conditioned stimuli. This type of alteration in forming early preferences could be detrimental because of decreases in the specificity of social learning and an impaired responsiveness in social relationships.
Keywords: Bonding, isolation vocalization, odor preference, prenatal stress, sex differences, social behavior
Introduction
Previous researchers have shown that prenatal adverse experiences can have long lasting effects on the behavioral, cognitive, and neuroendocrinological development in the offspring. For example, children who have been exposed to prenatal stress (PNS) have been observed to have a delay in speech production onset and in walking (Meijer 1985). In humans, exposure to PNS can also alter the susceptibility to various diseases and disorders in infancy through adulthood (Barker 1998). PNS has been implicated in the etiology of schizophrenia (Brixey et al. 1993), Tourette’s syndrome (Leckman et al. 1990), depression (Watson et al. 1999; O’Connor et al. 2003), attention deficit-hyperactivity disorder (Clements 1992) and autism (Beversdorf et al. 2005).
The present work is novel in that we examined the effects of PNS on very early social abilities in the rat model. We used a mild variable stress paradigm including saline injections, restraint, random handling, and placement into a novel cage during the final week of gestation (gestational days 14–20). This mild stress regimen has been demonstrated in rats to produce disruption of the HPA axis, increased fearfulness, and changes in amygdala morphology (Ward et al. 2000; Salm et al. 2004; Kraszpulski et al. 2006). Kraszpulski et al. (2006) measured the various nuclei that compose the rat amygdala for nuclear volumes and lengths and numbers of neurons and glia in offspring after exposure to a prenatal variable mild stress paradigm. They reported a retardation of amygdala growth as early as PND 7, yet no difference in total brain weight was found (Kraszpulski et al. 2006). In previous work, Salm et al. (2004) found an increase in size of the lateral amygdala nucleus of the rat. The differences observed in the lateral amygdala were an almost 30% increase in volume, as well as an increase in both neuronal cell density and quantity compared to control groups (Salm et al. 2004). Although there is dramatic altering of the developmental trajectory of the amygdala in the PNS rat compared to control rats, the PNS regimen does not cause termination of the pregnancy, infanticide, or drastic weight loss in the dam (Salm et al. 2004).
Isolation calls, sometimes referred to as distress calls or vocalizations, are ultrasonic vocalizations (USVs) emitted by infant rat pups in response to social isolation (Sales and Pye 1974). It has been suggested that these USVs are emitted to attract the attention of the dam, thus prompting retrieval into the nest (Allin and Banks 1972; Smotherman et al. 1974; Brewster and Leon 1980; Blumberg and Alberts 1990; Brunelli et al. 1994). Empirical research has shown that administration of anxiolytic drugs reduces the frequency of USVs while anxiogenic drugs increase USVs (Insel et al. 1986; Insel and Harbaugh 1989). Here we measured isolation calls to estimate the amount of distress experienced by the infantile rat and to infer its motivation or desire to be retrieved.
To measure the pup’s affinity for its mother, we used a conditioned odor preference (COP) paradigm. This paradigm is similar to a conditioned place preference task, but the animals are conditioned to associate a unique scent with the dam (Nelson and Panksepp 1996). This paradigm has been successfully used in other studies (Nelson and Panksepp 1996; Cromwell et al. 2007; Harmon et al. 2008), in that typically rat pups conditioned with the positive CS (scent + dam) show a preference for the maternally-associated odor side of the chamber, and conversely, pups exposed to a neutral stimulus (scent + cotton balls) do not show a place preference.
Since PNS-exposed pups show an increased corticosterone response to a stressor (Henry et al. 1994; Barbazanges et al. 1996; Takahashi et al. 1998; Koehl et al. 1999; Szuran et al. 2000), indicating an increase in fearfulness and anxiety to stressful stimuli, we hypothesized that the social isolation would elicit more anxiety and distress compared to controls, measured by an increase in isolation calls. In addition, we predicted that the PNS-exposed pups would be significantly more motivated to interact with the dam, and therefore spend significantly more time in the maternally-conditioned odor portion of the COP apparatus compared to the control pups. Several lines of research have shown that increases in HPA activity lead to enhanced or stronger place preference (Dai et al. 2006; Grakalic et al. 2006). Yang et al. (2006) demonstrated that PNS enhanced conditioned place preference to morphine (Yang et al. 2006). Similarly, we hypothesized that rat pups that experienced PNS would show enhanced conditioned place preference to the positive CS.
Methods
Subjects
Fourteen female Long–Evans rats were paired with male Long–Evans rats for breeding at Bowling Green State University. Three females were group housed with one male. Females were checked daily by vaginal smear for the presence of spermatozoa. Presence of spermatozoa indicated gestational day 1 and the females were removed from group housing. Females were housed singly in clear plastic cages (65 × 24 × 15 cm) with food (Harlan Teklad Rat Chow #8604) and tap water ad libitum. Corn cob chips were provided for bedding. All subjects were maintained on a day–night cycle of 12 h:12 h light/dark cycle (lights on at 07:00 h); room temperature was kept at 22°C and humidity was controlled at 40–50%. The Institutional Animal Care and Use Committee (IACUC) at Bowling Green State University approved all procedures (IACUC Approval #04-014) and all procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
The pregnant females were randomly placed into one of two experimental groups, PNS (n = 7) or controls (n = 7). Rats in the control group were housed individually and left undisturbed except for routine handling by animal facility staff. From gestational day 14, the pregnant females in the PNS group were exposed to seven continuous days of mild gestational stress twice a day. Gestational stressors consisted of a saline injection (0.9% NaCl, 0.1 ml, i.m.), handling and placement into a novel environment (removal from the home cage to a fresh cage)for ten minutes, and placement into a Plexiglas restraining chamber (10.8 × 7.0 × 4.5 cm.) under bright light for ten minutes. A sample PNS regimen is illustrated in Table I. Prenatal stressors were applied randomly and at various times to prevent habituation. The first stressor was applied during the AM hours (0700–1200 h) and a second stressor during the PM hours (1300–1900 h).
Table I.
Example of prenatal stress regimen.
| Gestational day
|
||||||||
|---|---|---|---|---|---|---|---|---|
| 14 | 15 | 16 | 17 | 18 | 19 | 20 | ||
| Female 1 | AM | Restraint | Injection | Restraint | Novel cage | Novel cage | Restraint | Injection |
| PM | Novel cage | Injection | Injection | Restraint | Novel cage | Novel cage | Restraint | |
| Female 2 | AM | Restraint | Injection | Restraint | Novel cage | Restraint | Restraint | Injection |
| PM | Restraint | Injection | Restraint | Novel cage | Novel cage | Restraint | Injection | |
| Female 3 | AM | Injection | Restraint | Restraint | Injection | Novel cage | Novel cage | Novel cage |
| PM | Restraint | Injection | Restraint | Novel cage | Novel cage | Restraint | Injection | |
Each dam received a random stressor during the AM hours (0700–1200 h) and a second stressor during the PM hours (1300–1900 h). Selection of stressors was random and varied between dams. Gestational stressors consisted of a saline injection (0.9% NaCl, 0.1 ml, i.m.), handling and placement into a novel environment for 10 min, and placement into a plexiglas restraining chamber under bright light for 10 min.
Rats were checked daily at 08:00 h for the presence of pups and the day the neonates were first sighted was designated PND 1. Litters were culled to 10 pups within 48 h of parturition. Three males and three females were randomly selected from each litter for behavioral testing yielding 42 PNS pups (Males = 21 and Females = 21) and 39 Control pups (Males = 21 and Females = 18). Litter sizes did not significantly vary in number of pups (Mean ± SEM, Control 9.29 ± 0.57 and PNS 9.71 ± 0.18).
Isolation vocalizations
The isolation testing chamber, which was located in a testing room separate from the housing room, consisted of a 500 ml glass beaker with an ultrasonic microphone suspended approximately 12 cm above the base of the beaker. USVs were recorded using a high frequency bat detector, Pettersson D230 ultrasound detector (Uppsala, Sweden) that digitally records at 196 kHz, and USVs were analyzed offline via a sonogram (Avisoft Bioacoustics, Berlin, Germany). The pups were habituated to the testing chamber for 1 min on PND 9. On PND 10, the pups were removed from the colony room and placed individually in the isolation testing apparatus for 2 min with USVs being recorded. There were no other animals present in the testing room during the testing session. After testing, pups were transported back to the colony room and were kept in a holding chamber maintained at approximately 29°C until all testing was complete. Upon completion of testing, all pups were returned to their home cages. Pups were tested during the light phase of a 12 h:12 h light/dark cycle. Data were manually scored offline for a total number of 40 kHz isolation vocalizations.
Conditioned odor preference
The condition odor preference testing apparatus consisted of a Plexiglas chamber (5 × 18 × 7.6 cm), which was divided crosswise by visual marker into three equal sections of 6 cm. The apparatus was located in a testing room with fluorescent illumination, separate from the colony room. The chamber, which had metal bars across the floor, rested on two equally spaced glass jars (7.6 cm in height).
Pups were removed from their home cage in the colony room and were habituated to the COP chamber on PND 13 for 1 min. Conditioning began on PND 14. Pups from each litter from each condition (Control and PNS) were divided into two groups. One group was exposed to a positive conditioned stimulus (+ CS) (lemon scent placed on dam) while the second group was exposed to a neutral CS (lemon scent placed on cotton balls). For the rest of the description of the study, we will refer to these two stimuli as either the positive CS or the neutral CS. This led to four total conditioning groups: (1) PNS pups with neutral CS exposure; (2) PNS pups with positive CS exposure; (3) control pups with neutral CS exposure; and (4) control pups with positive CS exposure. Within each litter pups were exposed to a CS, but half the littermates received the positive stimulus, while the other half of the littermates experienced the neutral stimulus.
Conditioning was conducted three times during PND 14 with three hours of maternal deprivation preceding each 30 min conditioning session. Both conditioning and maternal deprivation times were performed in an experiment room other than the testing room or the colony room. Pups conditioned with the neutral CS were removed from their home cage and were placed, as a litter, in a novel cage (23.5 × 21 × 20.3 cm) with four cotton balls in each corner saturated with 0.25 ml of scented extract on each cotton ball. The groups conditioned with the positive CS were removed from their home cage and were placed, as a litter, in an identical novel cage with their dam that had been saturated with 1 ml of scented extract on her ventral surface 1 min prior to being placed in the novel conditioning cage. All animals remained in the conditioning cages for 30 min. Following the third conditioning session, the dam was bathed with non-scented soap and water to completely remove the scent from her ventral surface and she was returned, along with the pups, to the home cage.
Testing began the day immediately following the conditioning day (PND 15). Testing was preceded by 3 h of maternal deprivation with the pups housed as a litter. Each testing session consisted of placing one pup in the middle of the testing apparatus always facing to the left and allowed the pup free access to the odor preference apparatus for 5 min. The side containing the CS was counterbalanced across trials. Trials were recorded via a commercially available DVD recorder and camera and were analyzed offline. A trained experimenter, blind to testing conditions, used a computer-based behavioral scoring system to accurately code compartment entries and duration via joystick control.
Familiar and novel odor approach
After a pup was tested in the COP apparatus on PND 15, the pup was tested on both a familiar and a novel odor approach task. In this task, the pup was placed at the “start” end of one of two Plexiglas-covered alleyways (6.4 × 36.8 × 5.1 cm) with its head facing the opposing “goal” end. In the “goal” end of the alleyway, a jar was placed with the open top facing towards the pup, the jar contained one cotton ball sprayed with 1 ml of the CS. For the novel odor approach, a cotton ball was saturated with 1 ml of a novel odor (peppermint extract), placed into a separate open-ended jar at the “goal” end of the alleyway. Five trials were conducted for each stimulus, using a different apparatus for each stimulus, with the presentation of each stimulus counter-balanced among pups.
For each trial, the approach latency was recorded. A pup was considered to have reached the “goal” end when its nose crossed the plane of the “goal” indicated by a drawn line. If a pup did not reach the goal within 60 s, 60 s was recorded as the approach latency and the trial was terminated. After all 10 trials were conducted, the pup was returned to the home cage.
Statistical analysis
For each dependent measure for the different behavioral paradigms, we performed a different analysis of variance (ANOVA). For each ANOVA, the independent factors were treatment group (PNS and control group) and sex (male and female). For COP data, an additional independent factor was used to account for the conditioning history ( + CS or Neutral CS). Dependent measures for general litter characteristics included average litter size, average pup weight on PND 10 and average pup weight on PND 15. For isolation vocalizations, the dependent variable was number of isolation vocalizations (40 kHz). For COP testing, dependent measures included total number of line crosses and a within subject variable for the amount of time spent in each compartment (CS or Neutral). For familiar and novel odor approach, a dependent factor for latency to approach the “goal” was used for evaluating performance across the five different trials. A final ANOVA was used to compare the mean latency to approach the familiar odor versus the novel odor. An approach latency was computed for each animal as the mean latency across the five trials for the familiar or novel scent. Data are given as mean ±SEM. The threshold significance level for including the results reported in the text was p < 0.05. When this threshold criterion was met, post hoc t-tests were completed using a Bonferroni correction for multiple comparisons. Data analysis was conducted with SPSS Software 15.0 and Microsoft Excel 2007.
Results
Litter statistics
Fourteen dams (seven dams in the control group and seven dams in the PNS Group) successfully gave birth to litters. Two control dams gave birth to fewer than 10 pups (one of the control dams gave birth to nine pups and one control dam gave birth to six pups). All PNS dams gave birth to more than 10 pups except for two dams which gave birth to nine pups each. For statistical analysis and to control for potential litter effects, three males and three females were randomly selected from each litter for behavioral testing. A total of 39 control pups (21 males and 18 females) and 42 PNS pups were studied (21 males and 21 females). There was a significant main effect for pup weight between the PNS and control groups at PND 10 (F(1, 77) = 14.7, p < 0.001; Controls: 22.8 ± 0.4 g, PNS: 21.2 ± 0.2 g) and also at PND 15 (F(1,66) = 23.34, p < 0.001; Controls: 31.7 ± 0.6 g, PNS: 28.6 ± 0.2 g).
Isolation vocalizations
The results of a univariate ANOVA revealed that exposure to PNS had a significant effect on the number of isolation vocalizations emitted by the rat pup (F(1,77) = 8.94, p < 0.01). PNS-exposed pups showed significantly more isolation calls compared to control pups (Controls: 131.7 ± 17.1 vocalizations; PNS: 201.8 ± 16.5 vocalizations). There was no main effect for sex and no interactions were found.
Conditioned odor preference
For the COP testing, two dams and their pups were removed from testing due to procedural problems with these subjects (e.g. inadequate conditioning). A subsample of the pups were tested on COP (Control N = 34; PNS N = 36). There was a main effect for place preference (F(1,66) = 28.15, p < 0.001). All pups showed a preference for the portion of the testing chamber directly over the CS scent in the apparatus. Additionally, there was an interesting main effect for sex (F(1,66) = 4.68, p < 0.05) indicating that females spent less time in the no stimulus chamber than did the male conspecifics. There were no other main effects or interactions found from the analysis.
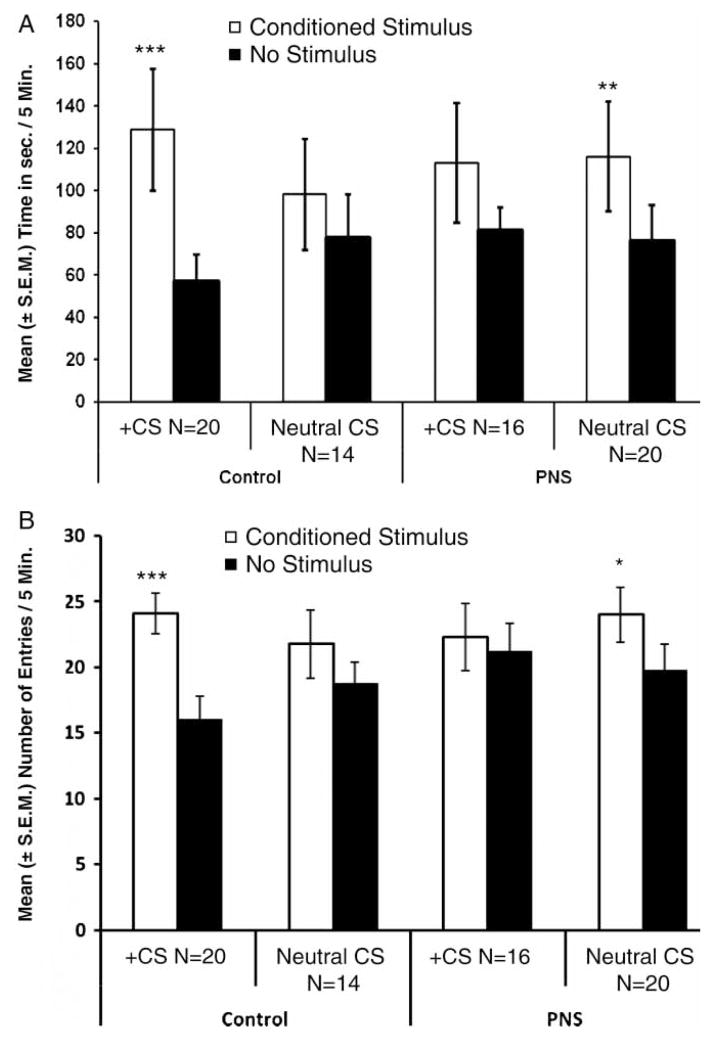
The PNS-exposed pups spent more time above the CS portion of the apparatus (F(1,32) = 7.40, p < 0.05; Figure 1(A)). In contrast to the control pups, no main effect for conditioning history (+, lemon-scented mother; or neutral, lemon-scented cotton ball) was obtained for the PNS subsample. This indicates that these pups were not showing a preference dependent upon previous exposure to the dam. There was also no main sex effect. There were no additional interactions between preference, conditioning history, or sex. A key finding for PNS pups was that they failed to show main effects or interaction effects related to conditioning history. As indicated in Figure 1(A), the PNS pups showed a strong place preference for the CS, but this preference was not dependent upon the conditioning history. In conclusion, PNS pups showed an alteration in COP behavior by not differentiating between + CS and neutral CS conditioning.
Figure 1.
COP for control and PNS rat pups. (A) Time spent by the pups conditioned with + CS (lemon-scented mother) or with the neutral CS (lemon-scented cotton ball) in the two portions of the COP apparatus (conditioned-lemon scent; no stimulus-no scent). (B) Number of entries made by pups conditioned with + CS or with the neutral CS into the two portions of the COP apparatus. N = 34 control pups, and N = 36 PNS pups. Data are group means + SEM. Post hoc pairwise comparisons were conducted within subjects between CS time/entries and neutral time/entries. *p < 0.05, **p < 0.01, and ***p < 0.001.
An analysis of exclusively control pups led to an expected main effect for place preference (F(1,30) = 22.4, p < 0.001); however, no additional main effects were found. There was an archetypal significant interaction effect between place preference and conditioning history (F(1,30) = 6.83, p < 0.05). This means that pups conditioned with the + CS showed a significantly stronger place preference for the CS compared to pups that received exposure to the neutral CS. This was supported by pairwise comparisons (t-tests), which revealed a significant place preference for the CS portion of the apparatus only for pups that had been paired with the + CS ( + CS: t(19) = 5.8, p < 0.001; Neutral CS: t(13) = 1.5, p = 0.16; Figure 1(A)). This indicates that control pups conditioned with + CS showed the characteristic stronger preference for the CS portion, which we interpret as a stronger motivation to interact with the dam. This effect for control pups has been shown before and is what we predicted (Nelson and Panksepp 1996; Cromwell et al. 2007; Harmon, et al. 2008). There were no additional significant interactions.
Analysis of the data for entries into each compartment showed a main effect for entries into either compartment (F(1,62) = 11.7, p < 0.01; Figure 1(B)), but there were no other main effects or interactions. All pups made more entries into the CS portion of the apparatus than entries into the no stimulus chamber. Control pups showed a main effect for compartment entries (F(1,30) = 13.1, p < 0.01), but no other main effects or interactions were found. PNS pup data analysis showed no main effects or interactions for compartment entries. Post-hoc paired samples t-tests indicate that control pups conditioned with the + CS showed significantly more entries into the CS portion compared to no stimulus compartment entries (t(19) = 4.43, p < 0.001). PNS pups, conditioned with the neutral CS, showed more CS compartment entries (t(19) = 2.6, p < 0.05, Figure 1(B)).
To determine differences in amount of activity during COP testing, the total number of compartment entries into either side of the testing chamber was assessed. A two-factor ANOVA revealed no significant differences in the number of total compartment entries for any of the dependent measures (PNS treatment or sex).
Familiar and novel odor approach
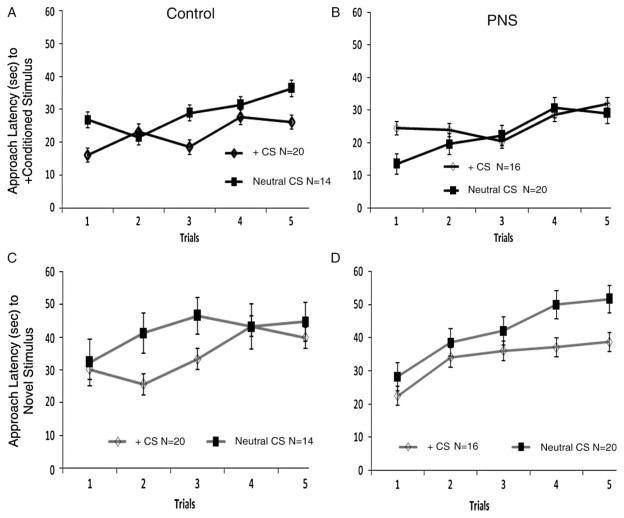
A three-way ANOVA indicated a significant main effect for latency to approach the CS (F(4, 248) = 5.03, p < 0.01; Figure 2(A),(B)). There were no main effects for gender, conditioning history, or treatment. With each trial, all pups displayed a longer latency to approach the goal (baited with the CS, lemon odor). There were no significant interactions.
Figure 2.
Approach latencies for CS and novel odors across five trials. (A) Approach latency for control pups (N = 34, + CS N = 20, Neutral CS N = 14) to the CS (lemon odor). (B) Approach latency for PNS pups (N = 36, + CS N = 16, Neutral CS N = 20) to the CS (lemon odor). (C) Approach latency for control pups to the novel odor (peppermint). (D) Approach latency for PNS pups to the novel odor (peppermint). Data are group means ± SEM. See Results text for ANOVA results, and Figure 3.
Similar to the CS, the pups showed a significantly longer latency to approach the goal end baited with the novel odor (peppermint) across trials (F(4, 248) = 9.01, p < 0.001, Figure 2(C),(D)). There was also a main effect for sex (F(1,62) = 4.8, p < 0.05). On average, males had longer approach latencies to the novel odor compared to females (Males: 42.4 ± 0.51 s; Females: 32.8 ± 0.54 s).
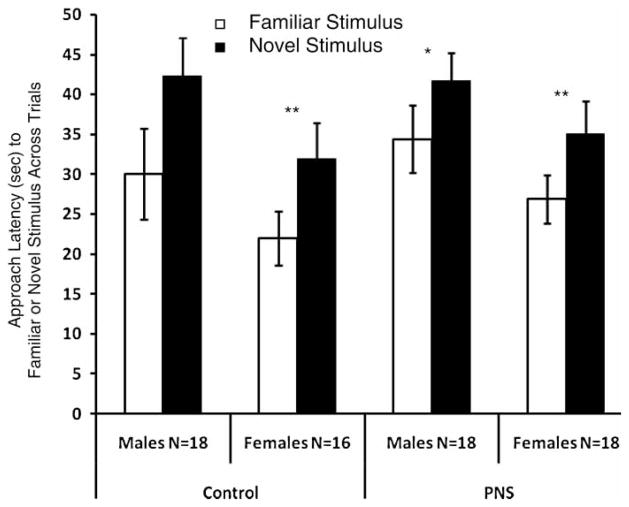
A final ANOVA was computed to compare the mean approach latency across repeated trials for both the CS and the novel stimulus for the prenatal treatment group (PNS compared to control) and the conditioning group ( + CS compared to Neutral CS) (Figure 3). The was a main effect for CS (F(1,62) = 18.86, p < 0.001, Figure 3). All pups approached the familiar odor more rapidly than they approached the novel odor. There was a main effect for sex (F(1,62) = 5.68, p < 0.05). Males had longer approach latencies to both the familiar and novel odors compared to females (Figure 3).
Figure 3.
Approach latency to a familiar and novel odor collapsed across the trials for control and PNS pups. Familiar odor is the CS (lemon) odor. The pups were not previously exposed to the novel odor (peppermint). Each group is subdivided by gender to reveal sex effects. Data are taken from Figure 2. Control: N = 34; PNS: N = 36. Data are group means ± SEM. Pairwise comparisons within subjects revealed significant differences: *p < 0.05, and **p < 0.01.
Discussion
The findings of the present study indicate that pups exposed to PNS, like controls. have a strong motivation to interact with the dam; however, PNS offspring may have deficits in associative-learning. This study has shown that pups exposed to a random variable stress paradigm during late gestation, compared to controls: (1) have a significant place preference for the CS regardless of the association cue, (2) show significantly more distress vocalizations at PND 10, and (3) show no significant difference on our measure of locomotor or ambulatory activity at PND 15.
Conditioned odor preference
All the PNS pups preferred the location of the CS of the place preference apparatus regardless of the associated pairing, positive (lemon-scented dam) or neutral (lemon-scented cotton ball). This could possibly be explained by neophobia and heightened emotionality in response to novelty that has been described by other researchers as a result of PNS (Fride et al. 1986; Deminiére et al. 1992). For example, Fride et al. (1986) reported increased emotionality in response to a novel situation by adult rats exposed to PNS. This could explain the behavior of our PNS pups in the COP paradigm.
For PNS rats, another possible interpretation for the absence of difference between the positive versus the neutral conditioning stimulus groups is that they failed to learn the associations between the dam or cotton ball and the lemon odor cue. Previous studies have linked PNS to impairments in learning (Lemaire et al. 2000; Aleksandrov et al. 2001; Nishio et al. 2001). Lemaire et al. (2000) found that prenatal restraint stress leads to delayed spatial learning in a place navigation task in adult male rats. Deficits in spatial learning seem unlikely as an explanation for the results of the present study: the testing in our study was on associative, not spatial learning. In addition, the PNS pups paired with the positive CS (lemon-scented dam) did show a slightly stronger preference for the CS portion compared to the PNS pups that were given the neutral stimulus, but further testing on an associated-odor learning task would elucidate this potential effect.
Distress vocalizations
Our results indicate that PNS pups show significantly more distress vocalizations compared to controls. Some studies have shown PNS pups show a decrease in distress calls (Takahashi et al. 1990; Morgan et al. 1998; Barron et al. 2000) while others have found no difference in the number of distress calls (Tonkiss et al. 2003). Our results are consistent with other studies demonstrating PNS pups show elevated levels of distress calls (Williams et al. 1998). We believe this is a result of the PNS pups experiencing significantly more distress in response to social isolation, as empirical research has demonstrated a positive relationship between distress vocalizations and anxiety levels (Insel et al. 1986; Insel and Harbaugh 1989) and HPA axis hyperactivity is consistently reported in PNS offspring (Henry et al. 1994). This conclusion is consistent with other researchers examining infantile vocalizations and PNS (Williams et al. 1998; Zimmerberg and Blaskey 1998). The increase in distress calls may be related to elevated HPA activity and therefore indicating significantly more anxiety or discomfort during this brief isolation period. Another interpretation for the elevated levels of distress calls is that the PNS pups are more strongly motivated to be retrieved to the nest and to their littermates. These distress calls have been shown to lead to increase pup retrieval to the nest (Litvin et al. 2007). It is possible that PNS pups are more strongly motivated to interact in a social environment and experience elevated distress when socially isolated.
As for the predictions for this study, we hypothesized elevated levels of distress calls in PNS pups, and this was empirically supported by our data. We also expected to see a strong place preference by the PNS pups which were exposed to the positive CS (scented dam). We observed a strong preference for the positive condition, but interestingly found that PNS pups exposed to the neutral stimulus showed this significant preference as well. Future tests of the effects of PNS on olfaction and associative learning are needed to clarify and more conclusively interpret these interesting findings. However, we conclude these findings further support the hypothesis that PNS leads to heightened emotionality in response to novelty that has been reported by others (Fride et al. 1986; Deminiére et al. 1992; Alonso et al. 1997).
Postnatal environment
Some interesting results that should be further explored are the effects of the maternal separation that occurred during the conditioning day of the COP test. The pups were isolated from their dam for approximately 9 h and the effect of this extent of separation has not been extensively studied. Though some studies have examined the effects of daily separation or the effects of complete maternal deprivation (Cameron et al. 2005; Estanislau and Morato 2005; Plotsky et al. 2005; Cannizzaro et al. 2006; Colorado et al. 2006; Burton et al. 2007), the amount of maternal deprivation in this study did not follow those protocols.
Additionally, the effects of PNS cannot be conclusively determined without a detailed analysis of the maternal care given to these pups. While PNS has been repeatedly demonstrated to alter behavioral and endocrinological development, quality of maternal care also importantly influences offspring development. Several researchers have attempted to address whether differences in offspring development are a consequence of antenatal conditions, or if the developmental differences are a result of variations in maternal care, or a consequence of a combination of both factors (Moore and Power 1986; Power and Moore 1986; Maccari et al. 1995; Meek et al. 2000; Pardon et al. 2000; Patin et al. 2002). This empirical research has produced mixed results; hence, further research on the effects of PNS on maternal care in the model used here is needed.
In conclusion, PNS can alter the formation of social motivation in the pups by lack of specificity in learning and responding in social relationships. Specifically, in this study, PNS led to an increase in isolation distress calls and a place preference for positive and neutral conditioned stimuli, in contrast with control pups that preferred cues associated with the dam. Continued research on PNS will elucidate the neural and hormonal mechanisms underlying these results.
Acknowledgments
K.M. Harmon received a J.P. Scott Center for Neuroscience, Mind and Behavior Graduate Student Fellowship that made this work possible. In addition, this work was partially supported by the Hope for Depression Research Foundation (New York, NY) and a Research Incentive Grant to H.C.C. from the Sponsored Programs and Research Office at Bowling Green State University. Also, a special thanks to Drs. Lee Meserve and Dara Musher-Eizenman for their contributions.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aleksandrov AA, Polyakova ON, Batuev AS. The effects of prenatal stress on learning in rats in a Morris maze. Neurosci Behav Physiol. 2001;31:71–74. doi: 10.1023/a:1026682415860. [DOI] [PubMed] [Google Scholar]
- Allin JT, Banks EM. Functional aspects of ultrasound production by infant albino rats (Rattus norvegicus) Anim Behav. 1972;20:175–185. doi: 10.1016/s0003-3472(72)80189-1. [DOI] [PubMed] [Google Scholar]
- Alonso SJ, Arevalo R, Alonso D, Rodriguez M. Effects of maternal stress during pregnancy on forces swimming test behavior of the offspring. Physiol Behav. 1997;50:511–517. doi: 10.1016/0031-9384(91)90538-y. [DOI] [PubMed] [Google Scholar]
- Barbazanges A, Piazza PV, Le Moal M, Maccari S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci. 1996;16:3943–3949. doi: 10.1523/JNEUROSCI.16-12-03943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. In utero programming of chronic disease. Clin Sci (Lond) 1998;95:115–128. [PubMed] [Google Scholar]
- Barron S, Segar TM, Yahr JS, Baseheart BJ, Willford JA. The effects of neonatal ethanol and/or cocaine exposure on isolation-induced ultrasonic vocalizations. Pharmacol Biochem Behav. 2000;67:1–9. doi: 10.1016/s0091-3057(00)00304-x. [DOI] [PubMed] [Google Scholar]
- Beversdorf DQ, Manning SE, Hillier A, Anderson SL, Nordgren RE, Walters SE, Nagaraja HN, Cooley WC, Gaelic SE, Bauman ML. Timing of prenatal stressors and autism. J Autism Dev Disord. 2005;35:471–478. doi: 10.1007/s10803-005-5037-8. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Alberts JR. On the significance of similarities between ultrasonic vocalizations of infant and adult rats. Neurosci Biobehav Rev. 1990;15:383–390. doi: 10.1016/s0149-7634(05)80031-4. [DOI] [PubMed] [Google Scholar]
- Brewster J, Leon M. Relocation of the site of mother-infant contact: Maternal transport behavior in Norway rats. J Comp Physiol Psychol. 1980;94:69–79. [Google Scholar]
- Brixey SN, Gallagher BJ, McFalls JA, Parmelee LF. Gestational and neonatal factors in the etiology of schizophrenia. J Clin Psychol. 1993;49:447–456. doi: 10.1002/1097-4679(199305)49:3<447::aid-jclp2270490321>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Brunelli SA, Shair HN, Hofer MA. Hypothermic vocalizations of rat pups (Rattus norvegicus) elicit and direct maternal search behavior. J Comp Psychol. 1994;108:298–303. doi: 10.1037/0735-7036.108.3.298. [DOI] [PubMed] [Google Scholar]
- Burton CL, Chatterjee D, Chatterjee-Chakraborty M, Lovic V, Grella SL, Steiner M, Flemming AS. Prenatal restrain stress and motherless rearing disrupts expression of plasticity markers and stress-induced corticosterone release in adult Sprague–Dawley rats. Brain Res. 2007;1158:28–38. doi: 10.1016/j.brainres.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Cameron NM, Champagne FA, Parent C, Fish EW, Ozaki-Kuroda K, Meaney MJ. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neurosci Biobehav Rev. 2005;29:843–865. doi: 10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Cannizzaro C, Plescia F, Martire M, Gagliano M, Cannizzaro G, Mantia G, Cannizzaro E. Single, intense prenatal stress decreases emotionality and enhances learning performance in the adolescent rat offspring: Interaction with a brief, daily maternal separation. Behav Brain Res. 2006;169:128–136. doi: 10.1016/j.bbr.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Clements AD. The incidence of attention deficit-hyperactivity disorder in children whose mothers experienced extreme psychological stress. Georgia Educational Researcher. 1992;91:1–14. [Google Scholar]
- Colorado RA, Shumake J, Conejo NM, Gonzalez-Pardo H, Gonzalez-Lima F. Effects of maternal separation, early handling, and standard facility rearing on orienting and impulsive behavior of adolescent rats. Behav Processes. 2006;71:51–58. doi: 10.1016/j.beproc.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Johnson A, McKnight L, Horinek M, Asbrock C, Burt S, Jolous-Jamshidi B, Meserve LA. Effects of polychlorinated biphenyls on maternal odor conditioning in rat pups. Physiol Behav. 2007;91:658–666. doi: 10.1016/j.physbeh.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Kang L, Wang L, Ma L. Different roles of dopamine receptor subtypes in footshock stress-induced enhancement of morphine conditioned place preference. Neurosci Lett. 2005;409:52–56. doi: 10.1016/j.neulet.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Deminiére JM, Piazza PV, Guégant G, Abrous N, Maccari S, Le Moal M, Simon H. Increased locomotor response to novelty and propensity to intravenous amphetamine self-administration in adult offspring of stressed mothers. Brain Res. 1992;586:135–139. doi: 10.1016/0006-8993(92)91383-p. [DOI] [PubMed] [Google Scholar]
- Estanislau C, Morato S. Prenatal stress produces more behavioral alterations than maternal separation in the elevated plus-maze and in the elevated T-maze. Behav Brain Res. 2005;163:70–77. doi: 10.1016/j.bbr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Fride E, Dan Y, Feldon J, Halevy G, Weinstock M. Effects of prenatal stress on vulnerability to stress in prepubertal and adult rats. Physiol Behav. 1986;37:681–687. doi: 10.1016/0031-9384(86)90172-1. [DOI] [PubMed] [Google Scholar]
- Grakalic I, Schindler CW, Baumann MH, Rice KC, Riley AL. Effects of stress modulation on morphine-induced conditioned place preferences and plasma corticosterone levels in Fischer, Lewis, and Sprague–Dawley rat strains. Psychopharmacology (Berl) 2006;189:277–286. doi: 10.1007/s00213-006-0562-5. [DOI] [PubMed] [Google Scholar]
- Harmon KM, Cromwell HC, Burgdorf J, Moskal JR, Brudzynski SM, Kroes RA, Panksepp JR. Rats selectively bred for low levels of 50 kHz ultrasonic vocalizations exhibit alterations in early social motivation. Dev Psychobiol. 2008;50(4):322–331. doi: 10.1002/dev.20294. [DOI] [PubMed] [Google Scholar]
- Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S. Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol. 1994;6:341–345. doi: 10.1111/j.1365-2826.1994.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Insel TR, Harbaugh CR. Central administration of corticotrophin releasing factor alters rat pup isolation calls. Pharmacol Biochem Behav. 1989;32:197–201. doi: 10.1016/0091-3057(89)90233-5. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hill JL, Mayer RB. Rat pup ultrasonic isolation calls: Possible mediation by the benzodiazepine receptor complex. Pharmacol Biochem Behav. 1986;24:1263–1267. doi: 10.1016/0091-3057(86)90182-6. [DOI] [PubMed] [Google Scholar]
- Koehl M, Darnaudery M, Dulluc J, Van Reeth O, Moal ML, Maccari S. Prenatal stress alters circadian activity of the hypothalamo-pituitary-adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. J Neurobiol. 1999;40:302–315. [PubMed] [Google Scholar]
- Kraszpulski M, Dickerson PA, Salm AK. Prenatal stress affects the developmental trajectory of the rat amygdala. Stress. 2006;9:85–95. doi: 10.1080/10253890600798109. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Dolnanasky ES, Hardin MT, Clubb M, Walkup JT, Stevenson J, Pauls DL. Perinatal factors in the expression of Tourette’s syndrome: An exploratory study. J Am Acad Child Adolesc Psychiatry. 1990;29:220–226. doi: 10.1097/00004583-199003000-00010. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvin Y, Blanchard DC, Blanchard RJ. Rat 22 kHz ultrasonic vocalizations as alarm cries. Behav Brain Res. 2007;182:166–172. doi: 10.1016/j.bbr.2006.11.038. [DOI] [PubMed] [Google Scholar]
- Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal M. Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. J Neurosci. 1995;15:110–116. doi: 10.1523/JNEUROSCI.15-01-00110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek LR, Dittel PL, Sheehan MC, Chan JY, Kjolhaug SR. Effects of stress during pregnancy on maternal behavior in mice. Physiol Behav. 2000;72:473–479. doi: 10.1016/s0031-9384(00)00431-5. [DOI] [PubMed] [Google Scholar]
- Meijer A. Child psychiatric sequelae of maternal war stress. Acta Psychiatr Scand. 1985;72:505–511. doi: 10.1111/j.1600-0447.1985.tb02647.x. [DOI] [PubMed] [Google Scholar]
- Moore CL, Power KL. Prenatal stress affects mother-infant interaction in Norway rats. Dev Psychobiol. 1986;19:235–245. doi: 10.1002/dev.420190309. [DOI] [PubMed] [Google Scholar]
- Morgan KN, Thayer JE, Frye CA. Prenatal stress suppresses rat pup ultrasonic vocalizations and myoclonic twitching in response to separation. Dev Psychobiol. 1998;34:205–215. doi: 10.1002/(sici)1098-2302(199904)34:3<205::aid-dev5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Nelson E, Panksepp J. Oxytocin mediate acquisition of maternally associated odor preferences in preweanling rat pups. Behav Neurosci. 1996;110:583–592. doi: 10.1037//0735-7044.110.3.583. [DOI] [PubMed] [Google Scholar]
- Nishio H, Kasuga S, Ushijima M, Harada Y. Prenatal stress and postnatal development of neonatal rats—sex-dependent effects on emotional behavior and learning ability of neonatal rats. Int J Dev Neurosci. 2001;19:37–45. doi: 10.1016/s0736-5748(00)00070-8. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Glover V. Maternal antenatal anxiety and behavioural/emotional problems in children: A test of a programming hypothesis. J Child Psychol Psychiatry. 2003;44:1025–1036. doi: 10.1111/1469-7610.00187. [DOI] [PubMed] [Google Scholar]
- Pardon MC, Gerardin P, Joubert C, Perez-Diaz F, Cohen-Salmon C. Influence of prepartum chronic ultramild stress on maternal pup care behavior in mice. Biol Psychiatr. 2000;47:858–863. doi: 10.1016/s0006-3223(99)00253-x. [DOI] [PubMed] [Google Scholar]
- Patin V, Lordi B, Vincent A, Thoumas H, Vaudry H, Caston J. Effects of prenatal stress on maternal behavior in the rat. Dev Brain Res. 2002;139:1–8. doi: 10.1016/s0165-3806(02)00491-1. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotrophin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Power KL, Moore CL. Prenatal stress eliminates differential maternal attention to male offspring in Norway rats. Physiol Beh. 1986;38:667–671. doi: 10.1016/0031-9384(86)90262-3. [DOI] [PubMed] [Google Scholar]
- Sales GD, Pye D. Ultrasonic communication by animals. London: Chapman and Hall; 1974. [Google Scholar]
- Salm AK, Pavelko M, Krouse EM, Webster W, Kraszpulski M, Birkle DL. Lateral amygdaloid nucleus expansion in adult rats is associated with exposure to prenatal stress. Dev Brain Res. 2004;148:159–167. doi: 10.1016/j.devbrainres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Bell RW, Starzec J, Elias J, Zachman TA. Maternal responses to infant vocalizations and olfactory cues in rats and mice. Behav Biol. 1974;12:55–66. doi: 10.1016/s0091-6773(74)91026-8. [DOI] [PubMed] [Google Scholar]
- Szuran TF, Pliska V, Pokorny J. Prenatal stress in rats: Effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav. 2000;71:353–362. doi: 10.1016/s0031-9384(00)00351-6. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Baker EW, Kalin NH. Ontogeny of behavioral and hormonal responses to stress in prenatally stressed rat pups. Physiol Behav. 1990;47:357–364. doi: 10.1016/0031-9384(90)90154-v. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Turner JG, Kalin NH. Prolonged stress-induced elevation in plasma corticosterone during pregnancy in the rat: implications for prenatal stress studies. Psychoneuroendocrinology. 1998;23:571–581. doi: 10.1016/s0306-4530(98)00024-9. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Bonnie KE, Hudson JL, Shultz PL, Duran P, Galler JR. Ultrasonic call characteristics of rat pups are altered following prenatal malnutrition. Dev Psychobiol. 2003;43:90–101. doi: 10.1002/dev.10124. [DOI] [PubMed] [Google Scholar]
- Watson JB, Mednick SA, Huttunen M, Wang X. Prenatal teratogens and the development of adult mental illness. Dev Psychopathol. 1999;11:457–466. doi: 10.1017/s0954579499002151. [DOI] [PubMed] [Google Scholar]
- Ward HE, Johnson EA, Salm AK, Birkle DL. Effects of prenatal stress on defensive withdrawal behavior and corticotrophin releasing factor systems in rat brain. Physiol Behav. 2000;70:359–366. doi: 10.1016/s0031-9384(00)00270-5. [DOI] [PubMed] [Google Scholar]
- Williams MT, Hennessy MB, Davis HN. Stress during pregnancy alters rat offspring morphology and ultrasonic vocalizations. Physiol Beh. 1998;63:337–343. doi: 10.1016/s0031-9384(97)00428-9. [DOI] [PubMed] [Google Scholar]
- Yang J, Li W, Liu X, Li H, Yang G, Xu L, Li L. Enriched environment treatment counteracts enhanced addictive and depressive-like behavior induced by prenatal chronic stress. Brain Res. 2006;1125:132–137. doi: 10.1016/j.brainres.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Blaskey LG. Prenatal effects are partially ameliorated by prenatal administration of the neurosteroid allopregnanolone. Pharmacol Biochem Behav. 1998;59:819–827. doi: 10.1016/s0091-3057(97)00540-6. [DOI] [PubMed] [Google Scholar]