Abstract
Objective
To describe the short-term changes in growth of uterine leiomyomata (fibroids).
Design
Prospective observational study
Setting
University Research Center
Patients
Premenopausal women with fibroids (n = 18 blacks and 18 whites) recruited through a physician network and community outreach.
Intervention(s)
Not applicable.
Main Outcome Measures
The volumes of 101 fibroids were measured at enrollment, 3, 6, and 12 months with magnetic resonance imaging (MRI), resulting in three interval-specific growth rates. Growth spurts were defined by interval growth rates ≥ 30% per 3 months and substantially greater than during other intervals of observation. An overall measure of short-term change in fibroid growth was calculated as the variance of the three interval-specific growth rates.
Results
Growth spurts were observed in 37 of the 101 fibroids, a prevalence nearly 10 times higher than that attributable to potential measurement error. Fibroids from the same women did not have similar short-term growth (p=0.27), nor were woman-specific factors (age, race/ethnicity, parity, body mass) or the fibroid position in the uterus important. However, large fibroids (>5 cm diameter) had less short-term change than smaller fibroids (p<0.001).
Conclusions
Short spurts of growth are common for fibroids, suggesting that tumor biology may change rapidly.
Keywords: uterine leiomyoma, tumor growth, fibroid size, ethnic disparity, magnetic resonance imaging, longitudinal study, short-term variability
Introduction
Uterine leiomyomata (fibroids) are hormonally dependent, benign tumors of the myometrial layer of the uterus (1). Symptoms include pelvic pain and heavy bleeding that often results in anemia. Fibroids are the leading indication for hysterectomy in the United States (2), with medical costs exceeding $2 billion per year (3). Fibroid tissue has been characterized by a low mitotic index (4), and the tumors are generally considered slow growing. Extracellular matrix comprises much of the volume of many fibroids (5). Despite the low proliferative index, fibroids can grow very large (>20 pounds) as documented by medical records from the early 1900s before surgery was a relatively safe treatment option (6).
The natural process of tumor growth can rarely be studied in humans. Fibroids are one of the few tumors that are allowed to grow naturally without treatment. Because the most effective treatments are invasive, watchful waiting is the standard of care until symptoms require intervention. We studied the growth of fibroids over a 12 month period in women who had been clinically diagnosed with fibroids. In our initial description of fibroid growth (7), we estimated each tumor's average growth rate during the study and found wide variation among tumors even for tumors from the same woman. Fibroid size and position within the uterus was not predictive of average growth rate.
The aim of the current analysis is to investigate short-term change in the growth rate of individual tumors. Magnetic resonance imaging (MRI) at enrollment, 3,6, and 12 months were used to determine growth rate data for the initial 3-month interval, the second 3-month interval, and the last 6-month interval for 101 tumors. We looked for spurts of growth and shrinkage; we compared short-term growth rate changes in tumors from the same woman; and we evaluated several woman-specific and tumor-specific factors to identify predictors of short-term change in tumor growth rates.
Materials and Methods
The data for analysis of short-term variability in tumor growth were collected for the National Institute of Environmental Health Sciences Fibroid Growth Study done in collaboration with the University of North Carolina Medical Center. Both institutional review boards gave approval, and all participants provided signed consent. The methods have been described previously (7, 8). Briefly, participants were premenopausal women with clinical ultrasound evidence of at least one fibroid ≥ 5 cm in diameter or the uterus enlarged to a size of a typical 12-week pregnancy (200-250 cm3) (9). Magnetic resonance imaging (MRI) scans of fibroids were taken up to four times (enrollment, 3, 6, and 12 months). The study did not involve treatment, and when a participant opted for treatment during the study, including GnRH agonist treatment, they were dropped from subsequent fibroid growth analyses. Of the 72 black and white women with two or more MRI visits, 19 could not be included in the current analysis because the field study ended before the fourth scheduled MRI visit, 10 opted for treatment during the year, 4 dropped out, and 3 had poor quality scans for one of the MRIs, leaving 36 women for the current analysis (18 blacks with 52 measured fibroids, 18 whites with 49 measured fibroids). The 36 women in this subsample were similar to the 72 women. A similar percentage reported symptoms (86% versus 88%). None became menopausal during the study.
Fibroid volume was determined at each MRI from T2-weighted images by use of a computer program developed for the study (Volume Estimation and Tracking Over Time) (10). Details of the MRI protocol have been published previously (7). In the parent study average growth rates for each tumor were determined by calculating the change in log volume during the interval between each MRI and averaging across the three intervals. The following characteristics of each fibroid were also determined for the parent study: tumor size at enrollment (volume, which was also converted to diameter measure based on the ellipsoid formula), type (submucosal, intramural, subserosal), and location (fundus, corpus, lower segment) (7).
For the current analysis the growth rate for each interval between MRIs (change in log volume per unit time in days) was converted to percent change in volume per 3 months by taking the exponent of the log volume difference metric. To describe the short-term changes in tumor growth rates, we defined growth spurts as growth that was substantially more rapid than during another period of observation for the same fibroid. Specifically, a ≥ 30% increase in volume per 3 months during one interval of observation, with the difference between that growth rate and at least one of the other two interval growth rates of ≥ 30%. For example, if the growth rates per 3 months for the three intervals were 32%, 12%, and 6% this tumor would not be designated as having a spurt because though the first interval's growth rate was >30%, the difference between the first interval's growth rate and the growth rates of the other intervals was less than 30%. However, if the growth rates were 32%, 1%, and negative 5%, then the 32% growth in the first interval would qualify as a spurt. Similarly, spurts of shrinkage were defined as an interval in which a shrinkage of ≥ 30% per 3 months occurred with the difference between that shrinkage rate and at least one of the other two interval growth rates of ≤ -30% per 3 months.
Though we chose a 30% change per three months as representing a change that would be much larger than variability from measurement error, we formally evaluated the possibility of chance findings from measurement error. The coefficient of variation for repeat measurements of a model system using the computer program developed for our volumetric measurements was slightly less than 3% (10). The coefficient of variation associated with additional possible tracing errors was <1% (7). Therefore, we estimated effects of a coefficient of variation of 5%. The “true” growth rate of each tumor was estimated by the rate of growth from enrollment until the fourth MRI (change in log volume per time unit converted to a percent change in volume). We then calculated the probability of finding a growth spurt (or spurt of shrinkage) just from measurement error for each tumor assuming that the measurement errors are independently distributed according to a Gaussian distribution. Applying the Benjamini-Hochberg procedure at a false discovery rate of 5%, we then estimated our maximum number of misidentified spurts.
To identify factors that might affect short-term variability in growth rate, we used an overall measure of this variability, the variance in the growth rates of the three time intervals (growth rate from enrollment to second MRI, and two subsequent time intervals). Mixed model regression, with woman entered as a random effect, was used to account for any correlation among tumors within a woman. Because the variance distribution was skewed to the right, we used the natural logarithm of the variance. This transformed distribution was approximately normal and homoscedastic. We first estimated the within and between woman variability in short-term variability in tumor growth. Then, we evaluated the woman-specific and tumor-specific factors. Factors were examined one at a time, but when size of fibroid was found to be significantly related to short-term variability in growth, the other factors were re-examined controlling for fibroid size. Because the actual value of the outcome measure does not convey an intuitive measure of short-term variability in tumor growth, we present the results as average ranks in the variance distribution (range = 1-101). Thus, a group of tumors whose average rank was 51 (the median) would represent average short-term variability in growth. In Table 2 we provide the average ranks for categories of women and also for categories of tumors. If a category has a large average rank relative to 51 then it would suggest that tumors for that particular category have a relatively high short-term variability in growth compared to other tumors. The P-values in Table 2 are based on the linear mixed effects analysis using the log of the variance as the outcome measure.
Table 2.
Association between characteristics of the participants and their tumors with degree of short-term variability in growth.
| Characteristic | Short-term Variability in Growth (average rank*) | P-value** | |
|---|---|---|---|
| Race/Ethnicity | 0.18 | ||
| Black | 55 | ||
| White | 47 | ||
| Age | 0.56 | ||
| < 35 | 56 | ||
| 35 to 44 | 48 | ||
| 45 and above | 51 | ||
| BMI | 0.64 | ||
| < 25 | 56 | ||
| 25 to 29 | 50 | ||
| 30 and above | 48 | ||
| Parity | 0.87 | ||
| Parity 0 | 52 | ||
| Parity 1 | 50 | ||
| Number of fibroids | 0.95 | ||
| < 5 | 53 | ||
| 5 to 10 | 52 | ||
| 11 and above | 49 | ||
| Fibroid size | <0.001 | ||
| <14.0 cm3 (<3.0 cm) | 55 | ||
| 14.0 - <65.0 cm3 (3.0 - 4.9 cm) | 54 | ||
| ≥ 65.0 cm3 (≥5.0 cm) | 34 | ||
| Fibroid type | 0.09 | ||
| Subserosal | 44 | ||
| Intramural | 54 | ||
| Fibriod location | 0.99 | ||
| Fundus | 52 | ||
| Corpus | 51 | ||
| Lower Segment | 51 | ||
Median rank is 51, so a lower average rank represent less short-term variability in tumor growth and a higher average rank represent more short-term variability in tumor growth.
P-value for importance of the factor in predicting short-term variability in tumor growth based on mixed model linear regression.
Results
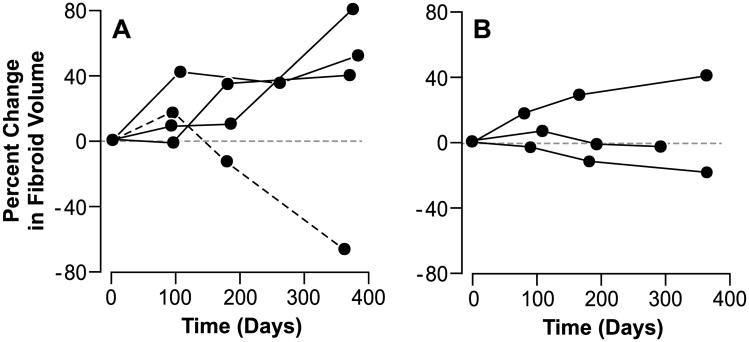
Table 1 shows the woman-specific and tumor-specific characteristics of the 101 tumors from 36 women in our sample. The median fibroid growth rate was 7% increase in volume per 3 months. Growth spurts were seen in 37 tumors (examples in Figure 1A). One tumor showed a spurt of shrinkage (Figure 1A). For this shrinking fibroid at the last MRI measurement, we found that the scan following contrast showed no blood flow to an estimated 90% of the fibroid. Sixty-three of the 101 tumors had no spurts of growth or shrinkage (examples shown in Figure 1B). While these tumors without spurts could be growing or shrinking during the year of observation, the growth rate or shrinkage rate remained relatively constant across the three observation intervals. Spurts could not be attributed to measurement error. The probability of measurement error producing a spurt was less than 1% for 65 of the 101 tumors, less than 2% for another 19 of the tumors, and less than 5% for all but 4 of the remaining tumors. The highest probability that measurement error would result in a spurt being observed was 13%. Applying the procedures for estimating false discovery rates, we estimate that our maximum number of misidentified spurts (false discovery rate) was 4 of the 38 we identified.
Table 1.
Characteristics of study participants and their fibroids.
| N | % | |
|---|---|---|
| Race/Ethnicity | ||
| Black | 18 | 50 |
| White | 18 | 50 |
| Age (years) | ||
| <35 | 9 | 25 |
| 35-44 | 13 | 31 |
| ≥ 45 | 14 | 39 |
| BMI (kg/m2) | ||
| <25 | 13 | 36 |
| 25.0-29.9 | 14 | 39 |
| ≥ 30 | 9 | 25 |
| Parity | ||
| 0 births | 22 | 61 |
| ≥ 1 birth | 14 | 39 |
| Number of fibroids | ||
| < 5 | 10 | 28 |
| 5 – 10 | 14 | 39 |
| ≥ 11 | 12 | 33 |
| Fibroid Characteristics (n = 101) Initial Size, volume (diameter) | n | % |
| <14.0 cm3 (<3.0 cm) | 48 | 48 |
| 14.0 - <65.0 cm3 (3.0 - 4.9 cm) | 30 | 30 |
| ≥ 65.0 cm3 (≥5.0 cm) | 23 | 23 |
| Type | ||
| Subserosal | 29 | 29 |
| Intramural* | 72 | 71 |
| Location | ||
| Fundus | 17 | 17 |
| Corpus | 66 | 65 |
| Lower segment | 18 | 18 |
Includes 3 submucosal fibroids.
Figure 1.
Panel A shows examples of short-term growth rate changes for three fibroids (solid lines) that exhibited growth spurts and the single fibroid that exhibited a spurt of shrinkage (dashed line). Panel B shows examples of short-term growth rate changes for three fibroids that showed no spurts.
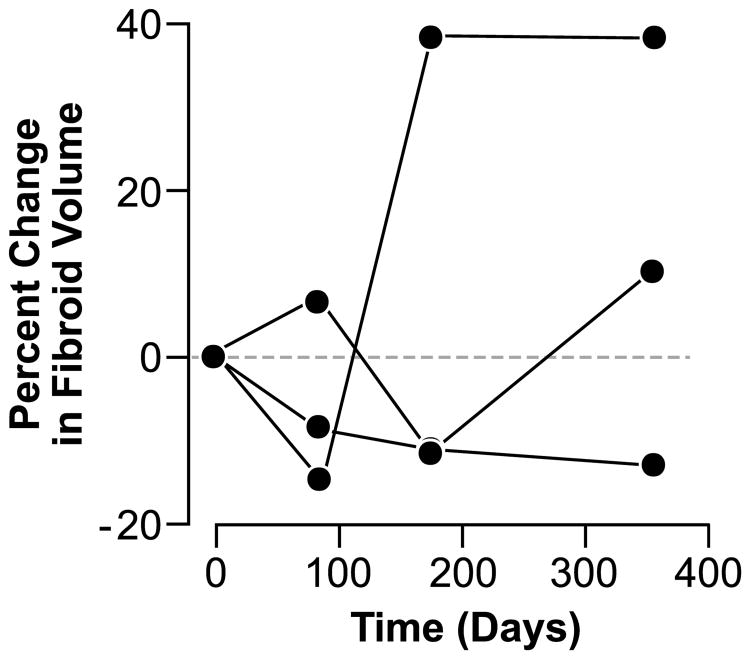
We estimated overall short-term growth variability by the variance in the 3 growth rates for each of the 101 tumors. As expected, the 38 tumors with spurts (37 growth spurts and 1 spurt of shrinkage) tended to be in the high end of the distribution (average rank = 80) compared to tumors without spurts (average rank = 33). Each of a woman's tumors showed its own short-term growth pattern, independent of the other tumors in the uterus (Figure 2). The component of the variance that could be attributed to woman-specific effects was only 7% and not significant (p=0.27), while the variance component due to differences among tumors within each woman was highly significant (p<0.0001), contributing 93% of the variance.
Figure 2.
Short-term growth rate changes for three fibroids from a single participant, illustrating the dissimilarity among fibroids from the same woman.
The association between woman-specific and tumor-specific factors and short-term variability in growth is shown in Table 2. Only tumor size was significantly associated with short-term variability in growth. Large tumors (≥ 5 cm diameter) were less likely to have short-term variability in growth (average rank = 34) compared to smaller tumors (average rank = 55) (p<0.001). We also examined the fibroids from exogenous hormone users most of whom were taking oral contraceptives (n = 10), phase of the menstrual cycle at MRI for women not on hormones, and the women who opted for treatment immediately after the fourth MRI (n = 5). None of these potentially confounding factors were important (all p-values ≥0.14).
Discussion
We examined short-term changes in fibroid growth for 101 tumors and found that nearly 40% exhibited growth spurts. When we compared the short-term growth of tumors from the same woman, each tumor had its own short-term growth patterns. Consistent with this, no woman-specific characteristics (e.g., age, ethnicity, or parity) were associated with degree of short-term growth variability. When we examined tumor-specific factors, we found that large tumors had significantly less short-term change in their growth rates.
Among the few studies that have looked at the natural history of fibroid growth (7, 11-13), none examined short-term growth rate changes. One study reported that a subset of tumors in their study exhibited short-term rapid growth, but the authors did not describe or compare growth rates across intervals (11). That study used ultrasound, and fibroid volume was estimated with the ellipsoid formula. All analyses were based on a dichotomous definition of growth (more than 30% increase in volume at the end of the one-year follow-up). Of the 101 fibroids monitored in that study, 27 increased in volume by more than 30% during the year, and 7 of these (26%) showed the >30% increase at the study's 3-month ultrasound. When we examined our data this way, our results were very similar (23% showed the >30% increase at the 3-month MRI).
Apparent spurts in growth or shrinkage can be artifacts of measurement error. As measurement error increases, the probability of artifacts increases. We chose a high growth rate to define our spurts in order to limit this problem. In addition, we evaluated factors related to short-term change in growth by calculating the variance in each fibroid's interval growth rates, which did not depend upon an arbitrary cutoff. To evaluate the problem further, we simulated growth rate data and found that spurts of the magnitude we observed were unlikely to be due to potential measurement error. We were unable to have women undergo repeated MRIs on the same day to estimate true measurement error, but repeat MRI measurements of a model system, showed measurement error substantially smaller than what we assumed in the simulation (coefficient of variation of 2.9% versus the 5% we assumed). Based on our simulation, only 4 of the 38 observed spurts could be attributed to potential measurement error.
The spurts we observed were short-lived. With longer time intervals between measurements, short-term spurts of rapid growth or shrinkage are diluted by periods when the tumors are not growing or growing very slowly. Our 3rd observation interval lasted 6 months (twice as long as each of the first two intervals), and during that interval we saw only 7 spurts compared with 31 in the first two intervals combined. Short-term growth spurts would not be seen often during routine clinical care, where follow-up intervals are generally six months or longer (14). Our data suggest that when a fibroid is clinically observed to be rapidly growing, further follow-up may reveal that this was only a short-term phenomenon.
If tumor biology is changing with growth spurts, heterogeneity among tumors in studies that examine molecular markers may be due at least in part to variation in growth phase. The biological basis of tumor growth spurts is of great interest. Fibroid tissue collected from surgical specimens is generally characterized by a low mitotic index (4). Fibroid growth spurts are common after GnRH agonist treatment as the tumors return to pretreatment size (1), but nontreatment-related spurts have not been described previously. The GnRH agonist treatment lowers estrogen and progesterone to postmenopausal levels, and given the hormonal dependency of fibroids, shrinkage generally occurs for all of a woman's tumors. The post-treatment growth also typically occurs in all the tumors. The molecular mechanism(s) involved in the naturally-occurring spurts we observed are unknown, but they are clearly tumor-specific rather than woman specific. Therefore, these growth patterns cannot be attributed to a global change in the hormonal milieu of the woman. Extracellular matrix makes up a large component of tumor volume for most fibroids (5), and the lability of this component compared to the cellular component would be important to characterize. Temporal variability in blood supply may also contribute. In addition, vascular instability associated with new vessel development within the tumor may allow for a rapid growth phase (15). If the mechanisms are local, the reason large fibroids may have reduced short-term change in growth may be that only portions of the fibroid are changing at any one time while the bulk of the fibroid remains relatively static.
Short-term variability in growth of other tumor types can rarely be studied because treatment usually starts as soon as possible after detection. Prostate cancer lesions may remain untreated and followed by changes in prostate specific antigen levels in serum, but growth (or shrinkage) of the lesion is not systematically monitored. Renal cancer can be managed by watchful waiting while growth is monitored (16, 17), but studies of short-term growth-rate variability have not been reported to our knowledge. Whether this is because growth spurts do not occur or have never been looked for is not known.
In summary, fibroids provide a rare opportunity to observe short-term changes in tumor growth. Much of the observed growth in fibroids less than 5 cm in diameter appears to be associated with growth spurts. Treatments that could prevent spurts could limit the number of tumors that become large enough to cause symptoms. The extent to which other types of tumors also grow in spurts is of great interest.
Acknowledgments
K. Haneke and H. Vahdat managed the field study. M. Turvey was the study nurse. J-P Guyon developed the computer program for measuring fibroid volume from MRI scans. P. Leppert, D. Dixon, and A. Wilcox reviewed an earlier version of the manuscript.
Research was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, NIH [Z01ES 101663-05], with partial funding from the National Center on Minority Health and Health Disparities grant #MO1RR00046 and NIEHS contracts #N01-ES-95446, #273-01-C-0157.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stewart EA. Uterine fibroids. Lancet. 2001;357:293–8. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 2.Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990-1997. Obstetrics and gynecology. 2002;99:229–34. doi: 10.1016/s0029-7844(01)01723-9. [DOI] [PubMed] [Google Scholar]
- 3.Flynn M, Jamison M, Datta S, Myers E. Health care resource use for uterine fibroid tumors in the United States. American journal of obstetrics and gynecology. 2006;195:955–64. doi: 10.1016/j.ajog.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Kawaguchi K, Fujii S, Konishi I, Nanbu Y, Nonogaki H, Mori T. Mitotic activity in uterine leiomyomas during the menstrual cycle. American journal of obstetrics and gynecology. 1989;160:637–41. doi: 10.1016/s0002-9378(89)80046-8. [DOI] [PubMed] [Google Scholar]
- 5.Fujita M. Histological and biochemical studies of collagen in human uterine leiomyomas. [Hokkaido igaku zasshi] The Hokkaido journal of medical science. 1985;60:602–15. [PubMed] [Google Scholar]
- 6.Kelly HA, Cullen TS. Myomata of the uterus. Philadelphia: Saunders; 1909. [Google Scholar]
- 7.Peddada SD, Laughlin SK, Miner K, Guyon JP, Haneke K, Vahdat HL, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proceedings of the National Academy of Sciences of the United States of America; 2008; pp. 19887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis BJ, Haneke KE, Miner K, Kowalik A, Barrett JC, Peddada S, et al. The fibroid growth study: determinants of therapeutic intervention. Journal of women's health. 2009;18:725–32. doi: 10.1089/jwh.2008.0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harb TS, Adam RA. Predicting uterine weight before hysterectomy: ultrasound measurements versus clinical assessment. American journal of obstetrics and gynecology. 2005;193:2122–5. doi: 10.1016/j.ajog.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Guyon JP, Foskey M, Kim J, Firat Z, Davis B, Aylward S. Volume estimation and tracking over time: Framework and validation. Lecture Notes Computer Science. 2003;2879(Pt 2):142–9. [Google Scholar]
- 11.Tsuda H, Kawabata M, Nakamoto O, Yamamoto K. Clinical predictors in the natural history of uterine leiomyoma: preliminary study. J Ultrasound Med. 1998;17:17–20. doi: 10.7863/jum.1998.17.1.17. [DOI] [PubMed] [Google Scholar]
- 12.Ichimura T, Kawamura N, Ito F, Shibata S, Minakuchi K, Tsujimura A, et al. Correlation between the growth of uterine leiomyomata and estrogen and progesterone receptor content in needle biopsy specimens. Fertility and sterility. 1998;70:967–71. doi: 10.1016/s0015-0282(98)00300-8. [DOI] [PubMed] [Google Scholar]
- 13.DeWaay DJ, Syrop CH, Nygaard IE, Davis WA, Van Voorhis BJ. Natural history of uterine polyps and leiomyomata. Obstetrics and gynecology. 2002;100:3–7. doi: 10.1016/s0029-7844(02)02007-0. [DOI] [PubMed] [Google Scholar]
- 14.Katz V, Lentz G. Congenital abnormalities of the female reproductive tract: Anomolies of the vagina, cervix, uterus, and adnex. In: Katz V, Lentz G, Gershenson D, editors. Comprehensive gynecology. St. Louis: Mosby; 2007. [Google Scholar]
- 15.Ergun S, Tilki D, Oliveira-Ferrer L, Schuch G, Kilic N. Significance of vascular stabilization for tumor growth and metastasis. Cancer Lett. 2006;238:180–7. doi: 10.1016/j.canlet.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 16.Chawla SN, Crispen PL, Hanlon AL, Greenberg RE, Chen DY, Uzzo RG. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol. 2006;175:425–31. doi: 10.1016/S0022-5347(05)00148-5. [DOI] [PubMed] [Google Scholar]
- 17.Crispen PL, Wong YN, Greenberg RE, Chen DY, Uzzo RG. Predicting growth of solid renal masses under active surveillance. Urol Oncol. 2008;26:555–9. doi: 10.1016/j.urolonc.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]