Abstract
Aim
A recent secular trend towards earlier thelarche has been suggested. The aim of this study is to examine normative ages of thelarche and menarche in contemporary US females.
Methods
Trained physicians documented Tanner breast stage by observation in a cross-sectional cohort. Age of menarche was self-reported. The subjects were healthy female children and adolescents. The mean age of thelarche was determined by probit analysis and the mean age of menarche was determined by using a normal time-to-event model.
Results
Mean age of thelarche was 9.7 years among 610 females aged 3.0–17.9 years (70% non-Hispanic Caucasian (NHC), 14% African-Americans, 7% Hispanic, 9% “other”). The mean age of menarche was 12.8 years for NHC, with African-Americans having menarche 0.6 years earlier.
Conclusions
Thelarche occurred earlier than recently reported, while age of menarche remained unchanged, this supported a persistent secular trend towards earlier thelarche but stable age of menarche. This suggests that the observed thelarche is gonadotropin-independent or the tempo of pubertal advancement has slowed.
Keywords: menarche, pubertal timing, puberty, thelarche
Introduction
The timing of female puberty has attracted considerable interest in both lay and medical communities. Better understanding of normative ranges informs clinical decisions as such data allows for the definition of both delayed and precocious puberty, this determines the need for an investigation, subspecialist referral, and/or therapeutic intervention. True central puberty results from activation of the hypothalamic-pituitary-gonadal (HPG) axis (1). In females, initial estrogenic activity results in thelarche, defined clinically as Tanner breast stage II (B2) (2). Classically, menarche occurs at a mean±standard deviation (SD) of 2.3±1.0 years after thelarche (3). Sexual maturity indicators are well established but are examiner dependent (2, 3).
A clear secular trend towards earlier age of thelarche over the past 40 years has been described by several large US studies. The Third National Health and Nutrition Examination Study (NHANES III), a population-based study conducted between 1988 and 1994, found the median age of thelarche for NHC to be 10.4 years (4), significantly earlier than previously reported (5). Similarly, the Pediatric Research in Office Settings (PROS) study reported a median age of thelarche for NHC of 10.0 years (6). Recent studies suggest a continuation of this trend as a higher prevalence of 7–8-year-old US girls have B2 (7), and in Denmark, the mean age of thelarche decreased by 1 year in a 15-year period (8).
Conversely, there has been little suggestion that the age of menarche has decreased. Both PROS and NHANES III found menarche to occur at mean ages of 12.9 and 12.7 years, respectively, in Caucasian females (6, 9, 10), essentially unchanged over the past 40 years (11, 12). Likewise, menarche has been stable at a mean age of 13.1 years over the past 15 years in Denmark (8). Taken together, these data support a persistent secular trend towards earlier thelarche with only a marginal change in menarchal age.
Here, we report a recent cross-sectional analysis of pubertal timing in US females, thus providing meaningful, normative ranges for the onset of thelarche and menarche in contemporary US females.
Methods
After obtaining approval by Hilltop Research from a Food and Drug Administration (FDA)-accredited institutional review board, healthy females were recruited as part of a larger study to determine pubertal stage and age specific IGF-1 reference ranges. While patient recruitment and physical exams were organized and conducted by a clinical research organization (HillTop Research, Inc., St. Petersburg, FL, USA), the study design, data analysis, and interpretation were performed entirely by the authors. Subjects were respondents to a newspaper advertisement for study participation from the suburban communities of St. Petersburg, FL, Scottsdale, AZ, and Miamiville, OH, USA. Inclusion criteria were age 3–18 years and the presence of a parent or legal guardian capable of providing informed consent. Exclusion criteria were history of growth promoting or sex hormone therapy, chronic illnesses, or current medication use excepting vitamins or antipyretics/analgesics. No information regarding socioeconomic status was collected. For this study, pediatricians at clinical centers in Arizona, Florida, and Ohio received training in Tanner staging by a single pediatric endocrinologist (GMB). Subjects received a one-time physical examination by the trained pediatrician, collecting height, weight, Tanner pubic hair stage, and Tanner breast stage. Tanner breast staging was performed by inspection except when the investigator felt palpation was necessary to properly distinguish a visible breast bud from simple adipose tissue. The age of menarche was obtained by self-report if the subject was post-menarchal at the time of encounter.
Statistical analysis
Probit analysis (SAS PROC LOGISTIC; SAS Institute Inc., Cary, NC, USA), the conventional way to determine the age of transition into any pubertal stage, was used to determine the cumulative percent-age of subjects by year of age entering into thelarche as has been previously described (4) and compared with a non-parametric curve as in (13). The statistical distribution of age of menarche was estimated using a normal time-to-event model (SAS PROC LIFEREG; SAS Institute Inc.) and confirmed as appropriate by comparison with a Kaplan-Meier curve. The standard deviation scores (SDS) (also called z-scores) for height (HT-SDS) and body mass index (BMI-SDS) were generated utilizing Centers for Disease Control (CDC) norms (14).
Descriptive statistics for anthropometric and other variables are presented as mean±SD, while percentile estimates for age of thelarche and menarche are presented as mean±standard error (SE).
Results
Over a 2-month period in the latter part of 2007, a total of 610 healthy females were examined and analyzed. The mean age was 11.4 years with a SD of 3.0 years (range 3.0–17.9 years). The study population self-identified as 70% non-Hispanic Caucasian (NHC), 14% black, 7% Hispanic, and 9% “other” that included subjects of mixed race/ethnicity. Mean±SD for HT-SDS was 0.2±1.1 and for BMI-SDS was 0.5±1.1. Table 1 summarizes HT-SDS, BMI-SDS, and parental HT-SDS for subjects at each Tanner breast stage. The prevalence in this population of obesity was 31%. Of the 610 girls, 204 (33%) reported age at menarche (range 9.0–15.4 years). Table 2 summarizes the mean age of thelarche and menarche obtained from probit and normal time-to-event analyses, respectively.
Table 1.
Height-SDS, BMI-SDS, and parental height-SDS by Tanner breast stage.
| Tanner breast stage |
na | Age, mean±SD |
HT-SDS, mean±SD |
BMI-SDS, mean±SD |
Maternal HT-SDS, mean±SD |
Paternal HT-SDS, mean±SD |
|---|---|---|---|---|---|---|
| Tanner 1 | 159 | 8.2±2.4 | −0.1±1.0 | 0.2±1.0 | 0.4±1.1 | 0.3±1.0 |
| Tanner 2 | 172 | 10.7±1.0 | 0.2±1.0 | 0.6±1.1 | 0.2±1.0 | 0.5±1.1 |
| Tanner 3 | 105 | 12.4±1.3 | 0.4±1.0 | 0.5±1.1 | 0.2±1.1 | 0.4±1.0 |
| Tanner 4 | 102 | 13.7±1.4 | 0.4±1.1 | 0.9±0.9 | 0.3±1.1 | 0.4±0.9 |
| Tanner 5 | 70 | 15.7±1.7 | 0.2±1.2 | 0.8±1.2 | 0.3±1.2 | 0.3±1.1 |
Two patients had unspecified Tanner breast stage and were excluded from thelarche analysis. SD, standard deviation; HT-SDS, height-standard deviation score; BMI-SDS, body mass index-standard deviation score.
Table 2.
Age at thelarche and menarche by race/ethnicity.
| Total n (n pre-, n post-thelarche or menarche) |
3rd percentile mean±SE |
50th percentile mean±SE |
97th percentile mean±SE |
|
|---|---|---|---|---|
| Age at thelarche, year | ||||
| NHC | 425(118, 307) | 7.34±0.25 | 9.75±0.13 | 12.16±0.36 |
| African-Americans | 83(14, 69) | 7.44±0.48 | 9.36±0.33 | 11.27±0.85 |
| Hispanics | 44(10, 34) | 8.13±0.52 | 9.60±0.27 | 11.06±0.69 |
| Alla | 608(159, 449) | 7.43±0.20 | 9.70±0.11 | 11.97±0.29 |
| Age at menarche, year | ||||
| NHC | 427(295, 132) | 10.66±0.12 | 12.77±0.09 | 14.88±0.17 |
| African-Americans | 83(43, 40) | 10.17±0.23 | 12.17±0.16 | 14.17±0.29 |
| Hispanics | 44(31, 13) | 10.57±0.34 | 12.47±0.25 | 14.38±0.51 |
| Allb |
Total of 610 females includes non-Hispanic Caucasians, African-Americans, Hispanics, as well as those self-identified as “other”.
Results not provided for all subjects combined because of statistically significant mean differences among groups. SE, standard error; NHC, non-Hispanic Caucasian.
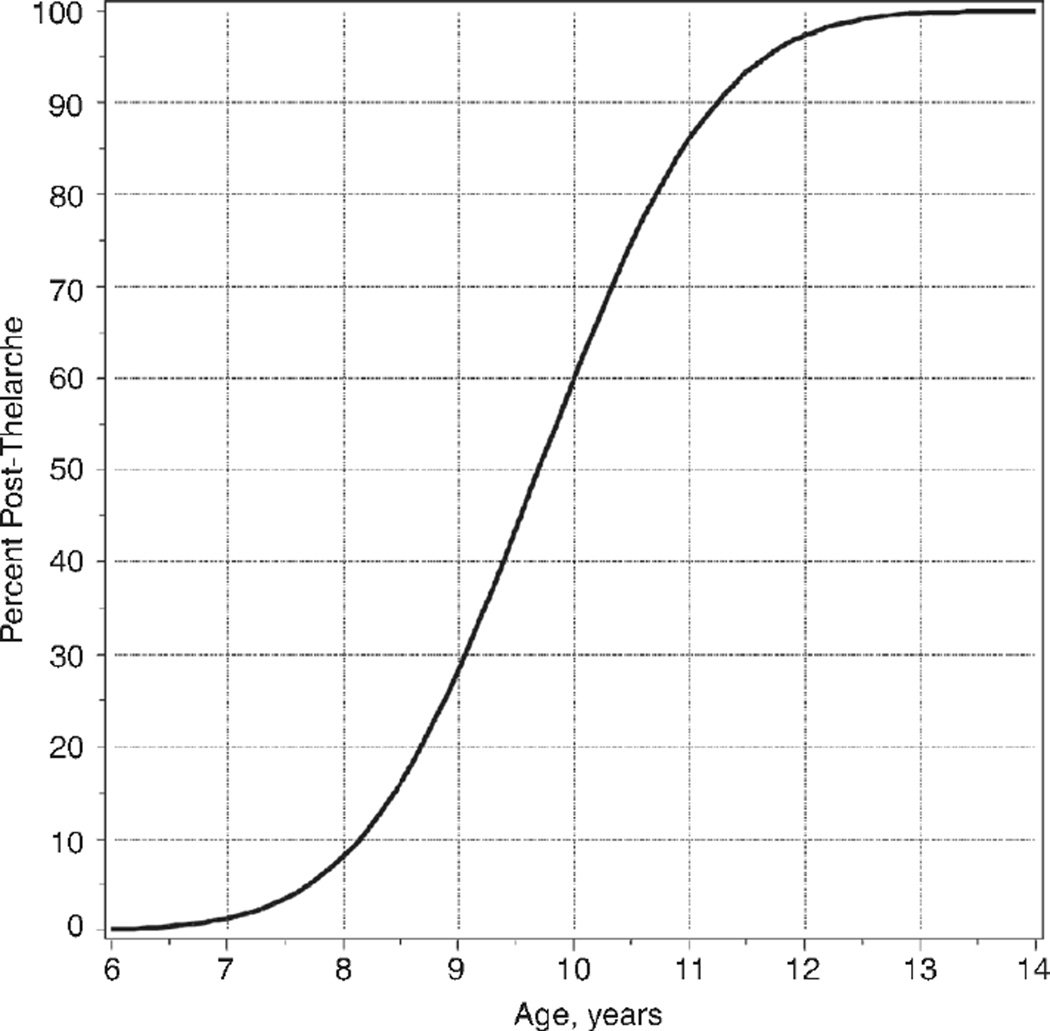
Thelarche occurred at a mean age±SE of 9.7±0.1 years. Across all race/ethnicities, the 3rd, 50th, and 97th percentiles for age of thelarche were 7.4, 9.7, and 12.0 years, respectively. There was no statistically significant difference in mean age of thelarche across racial/ethnic groups (p=0.21) or investigational sites (p=0.47) (Table 2 and Figure 1).
Figure 1.
Age at thelarche determined by probit analysis.
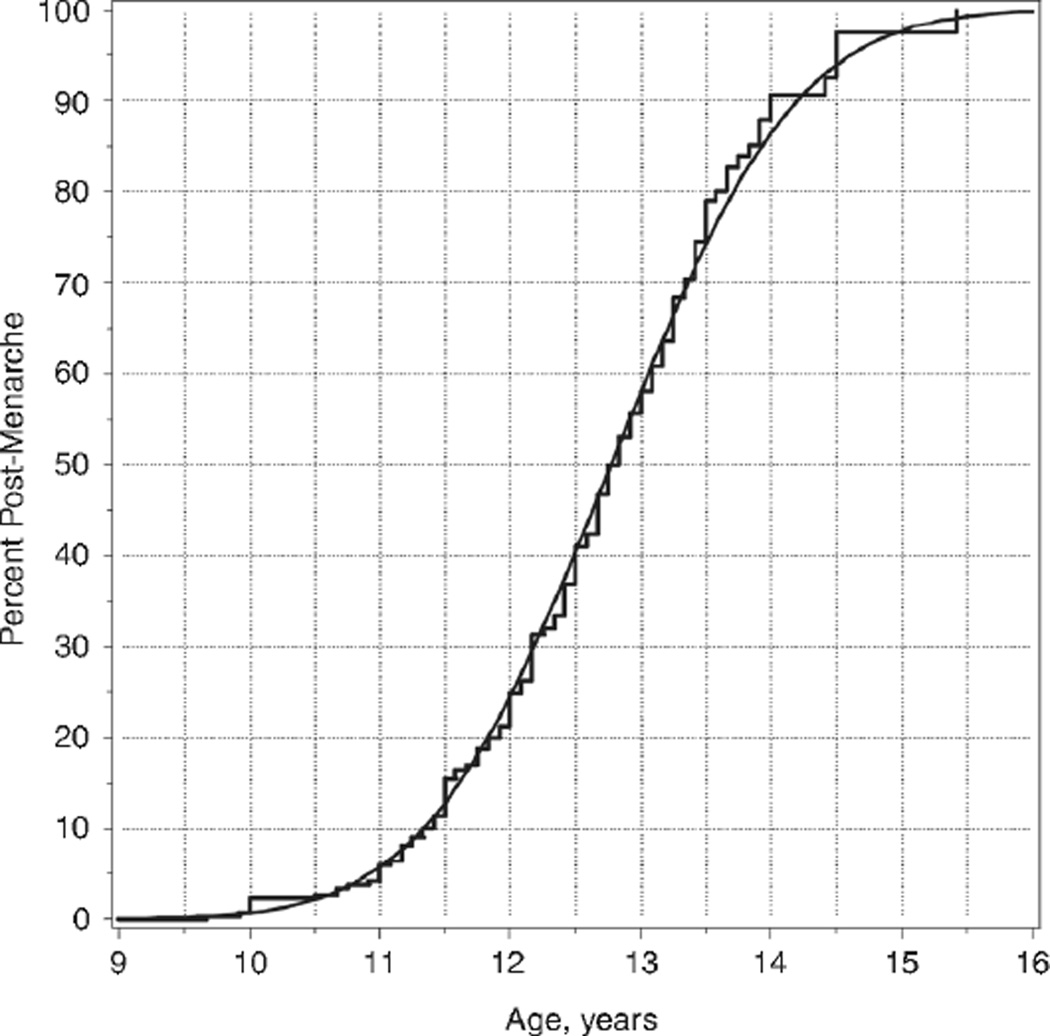
Figure 2 shows the Kaplan-Meier curve and smoothed time-to-event curve to menarche for NHC. As summarized in Table 2, African-Americans experienced menarche earlier than NHC (12.17 vs. 12.77 years, p=0.0010). Specific to NHC, the 3rd, 50th, and 97th percentiles for age at menarche were 10.7, 12.8 years, and 14.9 years, respectively. The difference in mean age of menarche between Hispanics and NHC was not significant (p=0.36). Given the significant mean differences among race/ethnic groups, a mean age of menarche was not calculated for all subjects combined. As with the thelarche data, the difference in mean age at menarche among investigational sites was not significant (p=0.39).
Figure 2.
Age at menarche for non-Hispanic Caucasians shown as a Kaplan-Meier step function and as a smooth curve from time-to-event analysis.
Discussion
This study relied on voluntary subjects at diverse sites in the USA. Inclusion and exclusion criteria were employed to minimize the number of subjects with medications or medical conditions that could interfere with the timing of pubertal events. The proportions of self-identified NHC and black subjects in this study population may not be representative, however, they are largely similar to those of the USA per 2010 US Census results (70% vs. 65% NHC, and 16% vs. 12% black, respectively), although Hispanics were under represented (7.2% vs. 16%) (15).
The CDC currently states that the obesity rate among American children is between 15%–17%, as defined by a BMI ≥95th percentile (equivalent to a BMI-SDS >1.645). The CDC norms for weight for age and BMI exclude data from NHANES III, and may under represent the degree of obesity among US children. Thus, the mean BMI-SDS among contemporary US children may actually now be greater than zero. Regardless, in our study population, 31% were obese, a rate twice that as quoted by the CDC. Therefore, our population was more obese than the general population and may not be completely generalizable.
The cross-sectional data from the number of subjects presented here cannot assess whether obesity leads to an earlier thelarche as the BMI data were determined after thelarche had occurred. It is not possible to distinguish an effect of body weight on thelarche from the effect of thelarche on body weight. The best approach to this question would be a longitudinal study, while a very large, cross-sectional study, such as NHANES, may be able to address this question. Such a study would be important, as it is unclear whether the onset of thelarche really indicates the onset of a pubertal HPG axis as menarche is not earlier. Hormone dynamics related to obesity is a potential factor to account for the earlier onset of thelarche, but without expected pubertal progression.
No socioeconomic data were gathered, therefore, no inference can be made on potential socioeconomic influences on the timing of pubertal events. The study sample size of 610 subjects was not determined in order to allow adequately powered, formal hypothesis testing for ethnic differences at each pubertal stage. Thus, ethnic variations included here are descriptive but may be used to generate hypotheses for studies with a larger sample size.
The method of examination may influence the determination of age at thelarche. In the Harpenden Longitudinal Study in which Tanner staging originated, Marshall and Tanner relied on photographs to determine the stage of breast development, that is, a method based on inspection (2, 3). A potential limitation of the inspection method is that, without palpation, the appearance of breast buds may be confounded by the presence of adipose tissue. In the present study, palpation was used for breast staging only when the examiner felt it necessary to distinguish a breast bud from adiposity. A more recent method to base age at thelarche on areolar changes was not used here (16).
The recall method for age at menarche has been proven to correlate closely to actual age at menarche, especially in females with close proximity to that event, as with this study population of children and adolescents (17, 18). The recall method is therefore an adequate and reliable means of assessing age at menarche, although admittedly a longitudinal study in which menarche is captured at time of occurrence would be superior.
There were no apparent differences in the age of pubertal event distributions among the three sites. While inter-rater concordance was not formally assessed, the lack of any significant difference in pubertal staging among investigational sites indicates good examiner reproducibility.
In this cohort of contemporary US females, thelarche was found to occur slightly earlier than reported 15–20 years ago (4, 6) and, specific to NHC, approximately 1.4 years earlier than found by Marshall and Tanner (3), thus providing further evidence of a down-ward secular trend in the age of thelarche among US females. However, the mean age of menarche was found to be essentially unchanged as has been documented in numerous studies over the past 20 years (4, 6, 8, 19). African-Americans were found to have menarche a mean of 0.6 years earlier than NHC, again comparable to other US studies (19).
The trend towards earlier thelarche without an accompanying earlier age of menarche begs the question of whether thelarche is evidence of true HPG activation or instead as a result of exogenous estrogen exposure, obesity, endocrine-disrupting chemicals, or gonadotropin-independent puberty. In fact, the Copenhagen Puberty Study found lower levels of circulating estradiol and non-stimulated gonadotropins despite earlier age of thelarche, suggesting thelarche in these individuals may indeed be gonadotropin independent (8). The finding of earlier age of thelarche and unchanged age of menarche in this cohort is in accordance with other recent cross-sectional studies (7, 8). The dissociation of B2 and menarche, and subsequent slowing of pubertal progression, suggests that the observed B2 is gonadotropin-independent. An earlier age at thelarche is now well established, but the proximate cause(s) remains to be determined. Questions regarding future health complications from earlier B2, including breast cancer and cardiovascular disease, remain unanswered.
In conclusion, the results of the current study confirm those from the NHANES III and PROS studies conducted during 1988–1994 (4, 6), but extend those findings to include contemporary US girls and define current statistical boundaries (3rd and 97th percentiles) for thelarche and menarche. These findings also confirm the dissociation (i.e., longer time interval) between thelarche and menarche and invite studies to determine why this is happening. Further studies should include concomitant evaluation of stimulated gonadotropins and estradiol levels to determine whether or not this earlier age of thelarche is truly gonadotropin-dependent. These data also provide estimates of prevalence and effects on ethnicity with which to complete sample size estimates for formal hypothesis testing. Importantly, these data should be useful to clinicians attempting to define the presence of precocious and delayed puberty. A further refinement in defining precocious and delayed puberty, based on Bayes' theorem (conditional probabilities) has recently been developed for use in longitudinal studies (13) and will help to better define the evolution of normative pubertal ranges.
Acknowledgments
The interpretation of the data, manuscript preparation, and decision to submit the paper were performed independently of the funding source for the study.
Conflict of interest statement: GMB has been an employee of Ipsen Biopharmaceuticals. GMB participated in data analyses. JF consults for Genentech, Inc. and Ipsen Biopharmaceuticals. SLB is a former employee of Ipsen Biopharmaceuticals. PAL has research support from Ipsen Biopharmaceuticals, NovoNordisk, Pfizer, and Abbott Laboratories and consults for NovoNordisk and Abbott Laboratories. Funding for the study, including design, collection, analysis, and interpretation was provided by Ipsen Biopharmaceuticals.
Contributor Information
Susanne M. Cabrera, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, USA
George M. Bright, San Mateo, CA, USA
James W. Frane, Vann-Frane Statistical Consulting, Santa Monica, CA, USA
Sandra L. Blethen, Belmont, CA, USA
Peter A. Lee, Department of Pediatrics, Pennsylvania State University College of Medicine, The Milton S. Hershey Medical Center, 500 University Drive, PO Box 850, Hershey, PA 17033, USA, Fax +1-717-531-6139, plee@psu.edu
References
- 1.Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2OO1;22:111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- 2.Tanner JM. Growth at adolescence. 2nd ed. Oxford: Blackwell; 1962. [Google Scholar]
- 3.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun SS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, et al. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics. 2002;110:911–919. doi: 10.1542/peds.110.5.911. [DOI] [PubMed] [Google Scholar]
- 5.Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008;121(Suppl 3):S172–S191. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- 6.Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99:505–512. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 7.Biro FM, Galvez MP, Greenspan LC, Succop PA, Vangeepuram N, et al. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics. 2010;126:e583–e590. doi: 10.1542/peds.2009-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aksglaede L, Sørensen K, Petersen JH, Skakkebaek NE, Juul A. Recent decline in age at breast development: the Copenhagen Puberty Study. Pediatrics. 2009;123:e932–e939. doi: 10.1542/peds.2008-2491. [DOI] [PubMed] [Google Scholar]
- 9.Chumlea WC, Schubert CM, Roche AF, Kulin HE, Lee PA, et al. Age at menarche and racial comparisons in US girls. Pediatrics. 2003;111:110–113. doi: 10.1542/peds.111.1.110. [DOI] [PubMed] [Google Scholar]
- 10.Wu T, Mendola P, Buck GM. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the Third National Health and Nutrition Examination Survey, 1988–1994. Pediatrics. 2002;110:752–757. doi: 10.1542/peds.110.4.752. [DOI] [PubMed] [Google Scholar]
- 11.MacMahon B. Age at menarche, United States, 1973. Rockville: National Center for Health Statistics; 1974. [PubMed] [Google Scholar]
- 12.Tanner JM, Pavies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107:317–329. doi: 10.1016/s0022-3476(85)80501-1. [DOI] [PubMed] [Google Scholar]
- 13.Frane J, Bright GM, Lee PA. A method to determine the likelihood of transition to puberty in a heterogeneous prepubertal age group. J Pediatr Endocrinol Metab. 2012;25:843–846. doi: 10.1515/jpem-2012-0116. [DOI] [PubMed] [Google Scholar]
- 14.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, et al. 2000 CDC growth charts for the United States: methods and development. National Center for Health Statistics. [Accessed 8 August 2013];Vital Health Stat. 2002 11 http://www.cdc.gov/growthcharts/2000growthchart-us.pdf. [PubMed] [Google Scholar]
- 15.US Census Bureau. 2010 Census Data. [Accessed 8 August 2013]; http://www.census.gov/2010census/data/.
- 16.Huang B, Biro FM, Dorn LD. Determination of relative timing of pubertal maturation through ordinaI logistic modeling: evaluation of growth and timing parameters. J Adolesc Health. 2009;45:383–388. doi: 10.1016/j.jadohealth.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damon A, Bayema CJ. Age at menarche of recall after 39 years. Human Biol. 1974;46:381–384. [PubMed] [Google Scholar]
- 18.Koprowski C, Coates RJ, Bernstein L. Ability of young women to recall past body size and age at menarche. Obesity Res. 2001;9:478–485. doi: 10.1038/oby.2001.62. [DOI] [PubMed] [Google Scholar]
- 19.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, et al. Relation of age at menarche to race, time period, and anthropometric dimensions: the Bogalusa Heart Study. Pediatrics. 2002;110:e43. doi: 10.1542/peds.110.4.e43. [DOI] [PubMed] [Google Scholar]