Abstract
Introduction
Somatostatin receptor subtype 5 (SSTR5) mediates the inhibitory effect of somatostatin on insulin expression/secretion and cell proliferation. A number of single nucleotide polymorphisms (SNPs) of SSTR5 have been identified, including P335L, a non-synonymous SNP located in the protein C-terminal region and encrypted by the codons CCG (proline) or CTG (leucine). In the present study, we sought to determine if the P335L SNP affected the cellular function of SSTR5 in human pancreatic cancer as has been reported previously for neuropsychiatric diseases and pituitary adenomas.
Methods
The P335L germline genotype of 246 patients with pancreatic cancer (213 Caucasians, 16 Hispanics and 17 African Americans), and 17 human pancreatic cell lines was determined with the TaqMan SNP Genotyping assay. Human SSTR5 leucine variant (L335) was generated by performing site-directed mutagenesis using SSTR5 proline variant (P335) as a template. Transient transfections were performed in HEK293, Mia PaCa-2 and β-TC-6 cells using Lipofectamine 2000. The expression of SSTR5 L335 was determined with a mouse monoclonal anti-SSTR5 L335 antibody generated in our laboratory. The cell proliferation rate was measured by performing MTS assays. Insulin concentration was measured by performing ELISA assays.
Results
1. Genotyping of the patients' blood indicated that the frequency of the T allele (CT and TT genotypes) in codon 335 of SSTR5 in Caucasians, Hispanics and African Americans was 52%, 69% and 35%, respectively. Statistical analysis indicated no significant association existed between the frequency of the T allele and the existence of pancreatic cancer in each race. 2. Of the 17 tested human pancreatic cancer cell lines, 5 cell lines (CAPAN-2, HPAF-II, Panc03.27, Panc-1, and -3) had the homozygote TT genotype and 9 cell lines including Mia PaCa-2 were heterozygote (CT genotype). 3. Over-expression of SSTR5 L335 in Mia PaCa-2 cells enhanced cell proliferation compared to over-expression of SSTR5 P335; 4. Over-expression of SSTR5 P335 enhanced the inhibitory effect of SSTR5 agonist RPL-1980 on cell proliferation of Mia PaCa-2 cells and glucose-stimulated insulin secretion from mouse insulinoma cells, while over-expression of SSTR5 L335 blocked the inhibitory effect of RPL-1980. 5. Over-expression of SSTR5 L335 enhanced PDX-1 expression in Mia PaCa-2 cells.
Conclusion
SSTR5 P335L SNP widely exists in the human population; in patients with pancreatic cancer, which are race-dependent; and in human pancreatic cancer cell lines. In contrast to SSTR5 P335, over-expression of SSTR5 L335 variant resulted in cellular proliferation and PDX-1 over-expression in human pancreas cancer cells and blocked the inhibitory effect of an SSTR5-specific analogue on human pancreas cancer cell proliferation and glucose-stimulated insulin secretion from mouse insulinoma cells. These data suggest that SSTR5 P335L is a hypofunctional protein with a potential harmful effect on function, as well as potential latent effect, and therefore could affect the clinical response to somatostatin analogue therapy for patients with pancreas cancer.
Introduction
Somatostatin receptor 5 (SSTR5) is one member of a group of five G protein-coupled receptors (SSTR1-5) (1-5) that mediate the cellular functions of somatostatin (6). SSTR5 is one of the major SSTRs in the islets of Langerhans as it is present in 87% of insulin-producing β-cells, 35% of glucagon-producing alpha cells, and 75% of somatostatin-producing delta cells. The major role of SSTRs in the islets of Langerhans is the negative regulation of insulin expression/secretion and islet cell proliferation (7). SSTR5 also contributes to decreased pancreatic carcinogenesis (8-10), decreased islet angiogenesis (11) and increased apoptosis (12). SSTR5 exerts its cellular effect through a wide variety of mechanisms including increased production of retinoblastoma tumor suppressor protein and p21 (cyclin dependent kinase inhibitor), which induces cell cycle arrest at the G1 phase (13), inhibition of the kinase activation of mitogen-activated protein kinase (MAPK) extracellular regulated kinase (ERK) (14), activation of the inositol phospholipids/calcium pathway (15, 16), interfering the coupling of the receptor to guanylate cyclase (17, 18), the activation of the SAPK/JNK signaling pathway via G protein α-subunits, and the activation of the nitric oxide (NO) signaling (19).
Single nucleotide polymorphisms (SNPs) are the most common type of genetic variations in the human genome, which can occur in all coding, non-coding and regulatory regions of a gene. A single base polymorphism is referred to as a SNP when the frequency of the minor allele exceeds 1% in at least one population; otherwise it is considered a mutation (20). When a SNP occurs within a coding region, it can have a silent effect (no change in protein sequence), a harmless effect (subtle changes in protein, but no impact on function), a harmful effect (functional impact), or a latent effect (the change in coding or regulatory regions is not harmful on its own, but is harmful under certain conditions). The clinical consequences of SNPs depend on two factors: 1) where in the genome the SNP occurs and 2) the exact nature of the SNP. A number of SNPs of SSTR5 have been identified, including 20 missense variations (A19T, P34S, G37R, A40T, L48M, A52V, W105R, P109S, V180M, R229K, R234C, R248C, L251S, V267I, R312C, A327V, T333M, P335L, R339K and G357R) (21). Among them, SSTR5 L48M is associated with circulating levels of insulin-like growth factor-I (IGF-1) and IGFBP3 and potential prostate cancer risk (22). The SSTR5 P335L SNP resulting from a C to T change at the 1004th nucleotide of the human SSTR5 gene was associated with neuropsychiatric diseases (23, 24). Loss of heterozygosity at the SSTR5 P335L gene locus was found in pituitary adenomas and was associated with a more aggressive phenotype (25). A recent study demonstrates an association of SSTR5 P109S, L48m and P335L with incidence of pancreatic cancer (26). Moreover, SSTR5 P335L and P109S also were associated with the overall survival of patients with resectable pancreatic cancer (26). In the present study, we sought to investigate the differential presence of SSTR5 P335L SNP in patients of different races diagnosed with pancreatic cancer and determine the effect of this missense polymorphism on the cellular function of SSTR5.
Materials and methods
Reagents
QuikChange XL site-directed mutagenesis kit was purchased from Stratagene (La Jolla, CA). An enhanced chemiluminescence (ECL) detection kit was purchased from Amersham Biosciences Corp (Piscataway, NJ). Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA). Anti-HA antibody (12CA5) was purchased from Boehringer-Mannheim (Indianapolis, IN). Anti-PDX-1 antibody and anti-SSTR5 antibody were described previously (27, 28). Mercodia Ultrasensitive Mouse Insulin kit was purchased from Mercodia AB (Sylveniusgatan, Sweden). SSTR5-specific analogue PRL-1980 was a gift from Dr. David Coy (Tulane University, New Orleans, LA). All other chemical reagents were purchased from Sigma (St. Louis, MO) unless otherwise noted.
Sample collection and processing
Informed consent from 246 patients with resectable pancreatic exocrine adenocarcinoma was obtained under an IRB-approved protocol. One of the two following methods were used to collect and isolate the DNA from the patient blood: 1) the blood was collected in PAXgene Blood DNA tubes and the DNA was isolated with the PAXgene Blood DNA kit (PreAnalytiX, Qiagen, Valencia, CA); or 2) the blood was collected in heparinized vacutainers (BD Biosciences, Franklin Lakes, NJ). The peripheral mononuclear cells were immediately separated by Ficoll-Histopaque (Amersham Pharmacia Biotech, Piscataway, NJ) density gradient centrifugation and the DNA was extracted with the FlexiGene DNA kit (Qiagen).
Genomic DNA samples for the normal control were obtained from established lymphoblast cell lines from the BPR collection which contains de-identified healthy adults from three self-declared ethnic groups (Caucasian, Hispanic, and African American) recruited in Houston, Texas. The Caucasian group also contained unrelated individuals from the Coriell Institute (CEPH Utah HapMap samples).
Genotype was determined with the TaqMan SNP Genotyping assay (Applied Biosystems, Foster City, CA). The reactions were prepared using 10 ng of gDNA, TaqMan universal master mix (Applied Biosystems), and SNP genotyping assay mix (Primers and TaqMan MGB probes) (Applied Biosystems) in a final volume of 5 μl. Allele discrimination was accomplished by running end point detection using ABI Prism 7900HT Sequence Detection System, and SDS 2.3 software (Applied Biosystems).
Cells, transfection, Western blotting, immunohistochemistry, site-directed mutagenesis, cell proliferation assay and insulin measurement
Capan-1, BxPC-3, CFPAC-1, Mia PaCa-2, AsPC-1, Colo357, HPAC, Hs776T, Panc-28, Panc-48, PL45, Su86.86, Panc-1, Panc03.27, Panc-3, Capan-2, HPAF-II, β-TC-6 and HEK293 cells were obtained from the American Type Culture Collection (Manassas, VA). HEK293, Panc-1, Mia PaCa-2 and mouse insulinoma β-TC-6 cells were grown and maintained in DMEM supplemented with 10% FBS and penicillin/streptomycin. Transient transfection was performed in HEK293, Mia PaCa-2 and β-TC-6 cells using Lipofectamine 2000 according to the manufacturer's instruction. Western blotting was performed using an enhanced chemiluminescence (ECL) detection kit according to the manufacturer's protocols. Immunohistochemistry staining was performed as described previously (29) using an anti-SSTR5 L335 antibody (see below). Simultaneous fluorescence microscopy observation and photography were carried out using an Olympus IX70 microscope. Human SSTR5 L335 was generated from SSTR5 P335 by performing PCR-mediated, site-directed mutagenesis as instructed by the manufacturer's manual. The cell proliferation rate was measured by performing MTS assays (Promega, Madison, WI) as described previously (30) using a Multiskan EX System from Thermo Electron Co. (Franklin, Massachusetts). Insulin concentration was measured by performing ELISA assays using a Mercodia Ultrasensitive Mouse Insulin ELISA kit (Uppsala, Sweden).
Generation of monoclonal anti-SSTR5 L335 antibody
BALB/cJ mice were injected with 50 μg of SSTR5 L335-derived peptide-KLH conjugate in Freund's complete adjuvant. The peptide NH2-CDADATELRPDRIRQ-COOH was synthesized by Pi Proteomics, LLC (Huntsville, AL). The first injection was subcutaneous (SC), followed by sequential intraperitoneal injection (IP)/SC boosters at two-week intervals. Mouse sera were screened after the last boost using both a peptide ELISA and Western blotting against SSTR5 P335 and SSTR5 L335. A single mouse with the best titer was given a final boost (IP) four days before sacrifice for fusions. The mouse spleen cells were fused with mouse THT myeloma cells as described previously (31). The resultant hybridomas were screened using an ELISA for antibodies that preferentially interact with BSA-SSTR5 L335 peptide conjugate, but not with BSA-SSTR5 P335 peptide conjugate. A secondary confirmation ELISA was done with free SSTR5 P335 and SSTR5 L335 peptide-coated plates. Western blotting of extracts from HEK293 cells expressing SSTR5 P335 and SSTR5 L335 were used as a tertiary screening for monoclonal antibody reaction with the appropriate intact SSTR5 native proteins. The hybridomas that were found to produce antibodies exhibiting the desired specificity for the SSTR5 L335 variant, but not for SSTR5 P335 variant, were cloned using limiting dilution, re-characterized and used for large-scale production of antibodies.
Statistical Analysis
Chi2 test/Fisher's exact test was used for statistical analysis of the association between the SSTR5 P335L missense polymorphism and the pancreatic cancer risk and progression with P < 0.05 indicating significance. Proportion test (R version 2.10.1) was used for the statistical analysis of the percentage of allele T in each race and stage of PC with P < 0.05 indicating significance. The unpaired Student t test was used for statistical analyses of cell proliferation rates and insulin levels, with P < 0.05 indicating significance.
Results
Wide occurrence of SSTR5 P335L SNP
Since SSTR5 is widely expressed in human cancers and plays an essential role in mediating somatostatin's anti-proliferative effect, we were prompted to explore if SSTR5 P335L was one of the genetic variations of SSTR5 that contributes to tumorigenesis. We applied a TaqMan Allelic Discrimination assay to determine the distribution of SSTR5 P335L CC, CT, and TT in patients with pancreatic cancer. A total of 246 patients with pancreatic cancer (213 Caucasians, 16 Hispanics and 17 African Americans), were included in this study. As shown in Table 1, in the Caucasian cohort of 213 pancreatic cancer patients, the proportion of patients with a CC genotype (expressing the SSTR5 P335 variant only) was slightly lower than the TT genotype (expressing the SSTR5 L335 variant only). A similar, but more pronounced pattern, was observed in the Hispanic cohort (6% CC versus 44% TT). The African American cohort was totally shifted with a high proportion of patients with a CC genotype (41% CC versus 12% TT). An equivalent proportion of patients were heterozygote in the three populations. This translated in a frequency of T allele in Caucasians, Hispanics and African Americans as 52%, 69% and 35%, respectively. The distribution of SSTR5 P335L among ethnic groups also was significantly different in the control group. The frequency of the T allele in the African American population was 24% compared to 58% in the Caucasian population. Statistical analysis indicated that, compared to control normal genotypes, the association between genotype and incidence of pancreatic cancer in each race was not significant.
Table 1. Genotype and allele distribution of the SSTR5 P335L SNP in human pancreatic adenocarcinoma.
| Caucasian | Hispanic | African American | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| PC patient (n=213) | Normal control (n=237) | PC patient (n=16) | Normal control (n=95) | PC patient (n=17) | Normal control (n=87) | |
|
| ||||||
| CC (freq) | 47 (0.22) | 37 (0.16) | 1 (0.06) | 24 (0.25) | 7 (0.41) | 52 (0.6) |
| CT (freq) | 110 (0.52) | 125 (0.53) | 8 (0.5) | 44 (0.46) | 8 (0.47) | 29 (0.33) |
| TT (freq) | 56 (0.26) | 75 (0.31) | 7 (0.44) | 27 (0.28) | 2 (0.12) | 6 (0.07) |
| T% | 52 | 58 | 69 | 51 | 35 | 24 |
|
| ||||||
| P-value | 0.163 | 0.167 | 0.357 | |||
PC: pancreatic cancer
We also analyzed the genotypes of 17 human pancreatic cancer cell lines. As shown in Table 2, three cell lines (CAPAN-1, BxPc-3 and CFPAC-1) were homozygote for CC, while five cell lines (Panc03.27, Panc-1, Panc-3, Capan-2 and HPAF-II) were homozygote for TT. The remaining nine cell lines (Mia PaCa-2, AsPC-1, Colo357, HPAC, Hs776T, Panc-28, Panc-48, PL45 and Su86.86) were heterozygous (CT). This confirms the existence of the three genotypes in pancreatic cancer cell lines, which would be important for use in functional studies using somatostatin analogues.
Table 2. Genotype of the SSTR5 P335L SNP in human pancreatic cancer cell lines.
| Cells | Genotype | Amino acids |
|---|---|---|
| Capan-1 | CC | Proline |
| BxPC-3 | CC | |
| CFPAC-1 | CC | |
|
| ||
| MiaPaCa-2 | CT | Proline/Leucin |
| AsPC-1 | CT | |
| Colo357 | CT | |
| HPAC | CT | |
| Hs776T | CT | |
| Panc-28 | CT | |
| Panc-48 | CT | |
| PL45 | CT | |
| Su86.86 | CT | |
|
| ||
| Panc03.27 | TT | Leucine |
| Panc-1 | TT | |
| Panc-3 | TT | |
| Capan-2 | TT | |
| HPAF-II | TT | |
Generation of a SSTR5 L335-specific monoclonal antibody
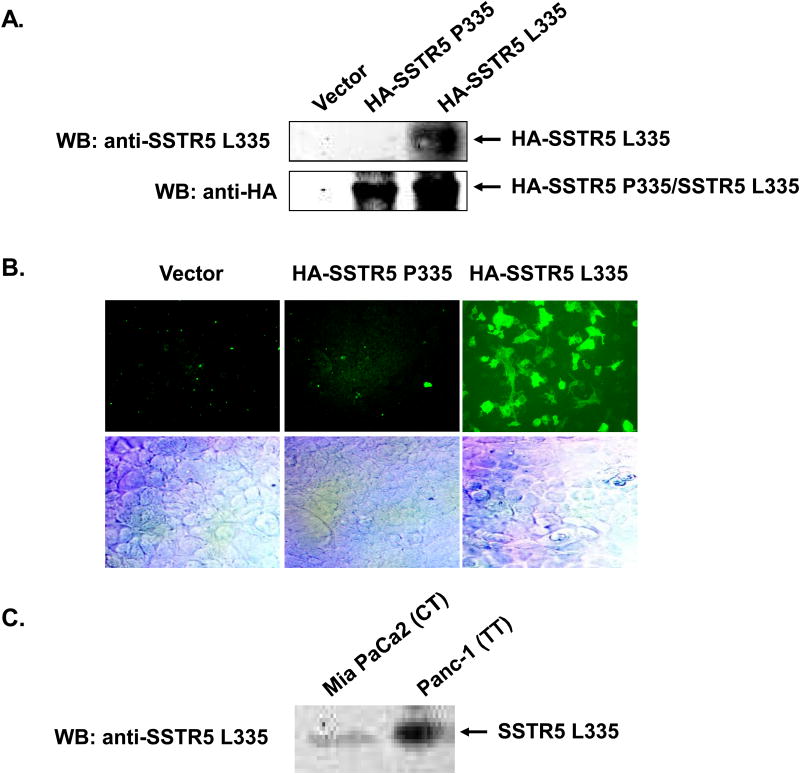
The association of SSTR5 P335L SNP with neuropsychiatric diseases (23, 24), the aggressiveness of pituitary adenomas (25) and the wide presence of SSTR5 P335L in pancreatic cancer strongly suggest SSTR5 P335L as a potential and promising biomarker for these human diseases. We therefore were prompted to generate an anti-SSTR5 L335 monoclonal antibody to determine the presence of SSTR5 P335L SNP expression in human pancreatic cancer cell lines. As shown in Fig. 1, we successfully generated a mouse monoclonal anti-SSTR5 L335 antibody that recognized only SSTR5 L335, but not SSTR5 P335. HEK293 cells were transfected with empty vector, HA-SSTR5 P335 or HA-SSTR5 L335. Western blot analysis with an anti-HA antibody showed that comparable levels of HA-SSTR5 P335 and HA-SSTR5 L335 were expressed in HEK293 cells (Fig. 1A, lower panel). However, only HEK293 cells over-expressing SSTR5 L335, but not HEK293 cells over-expressing empty vector or SSTR5 P335, were given a positive signal for Western blotting using the anti-SSTR5 L335 antibody (Fig. 1A, upper panel), indicating that the antibody recognized only SSTR5 L335, but not SSTR5 P335. The specificity of this antibody toward SSTR5 L335 was further confirmed by immunohistochemical analysis. As shown in Fig. 1B, only HEK293 cells over-expressing SSTR5 L335, but not the HEK293 cells over-expressing empty vector or SSTR5 P335, were immunostained by the anti-SSTR5 L335 antibody. Next, we used this SSTR5 L335-specific antibody to examine the expression of SSTR5 L335 in pancreatic cancer Mia PaCa-2 and Panc-1 cells. Western blot analysis showed that both Mia PaCa-2 and Panc-1 cells expressed SSTR5 L335, with higher levels in Panc-1 cells (Fig. 1C). These results are consistent with the genotypes of these cells (Table 2): CT genotype in Mia PaCa-2 cells expressed both SSTR5 P335 and SSTR5 L335 variants, while TT genotype in Panc-1 cells expressed SSTR5 L335 variant only.
Fig. 1. Generation of a monoclonal anti-SSTR5 L335 antibody that specifically recognizes SSTR5 L335, but not SSTR5 P335.
A, 5 μg of empty vector, HA-SSTR5 P335 or HA-SSTR5 L335 was transfected into HEK293 cells (1.5 × 106 in 100 mm dishes) using Lipofectamine 2000. 50 μg of the whole cell lysates were subjected to SDS-PAGE, followed by Western blotting with a monoclonal anti-SSTR5 L335 antibody. The expression levels of HA-SSTR5 P335 and HA-SSTR5 L335 proteins were examined by Western blotting using an anti-HA antibody. B, HEK293 cells were grown on 8 Chamber Polystyrene Vessel Tissue Culture treated Glass Slide, followed by transfection with empty vector, HA-SSTR5 P335 or HA-SSTR5 L335. Immunohistochemical staining was performed 24 h after transfection using a monoclonal anti-SSTR5 L335 antibody (10 μg/ml). Fluorescence was developed using cy3-conjugated secondary antibody. Simultaneous fluorescence microscopy observation and photography were carried out using an Olympus IX70 microscope. C, 100 μg of the whole cell lysates from Mia PaCa-2 cells expressing both SSTR5 P335 and SSTR5 L335, and Panc-1 cells expressing SSTR5 L335 only were subjected to SDS-PAGE, followed by Western blotting with a monoclonal anti-SSTR5 L335 antibody.
SSTR5 L335 enhances cellular proliferation of Mia PaCa-2 cells
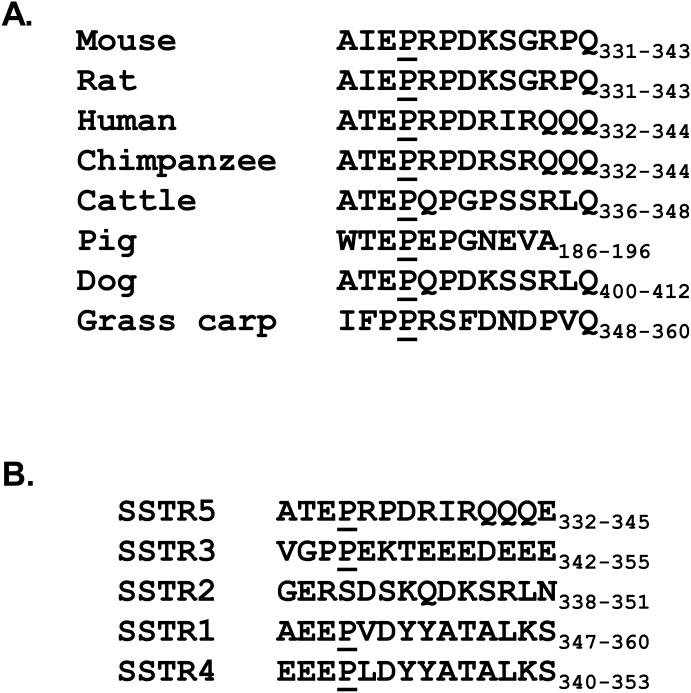
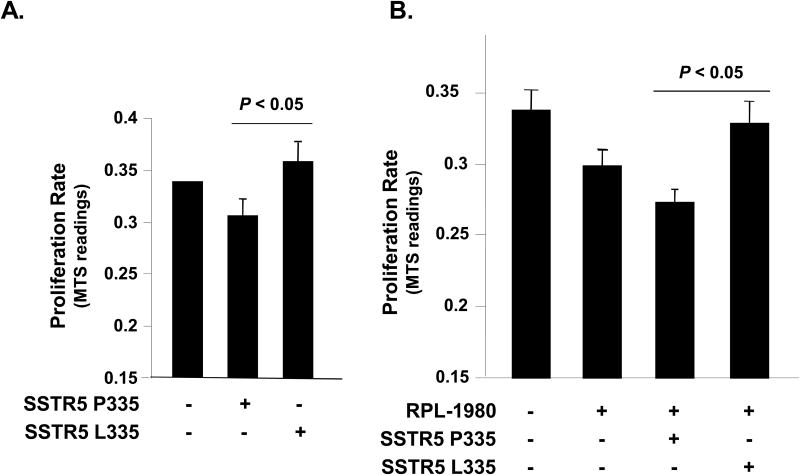
Sequence alignment analysis indicated that the proline 335 residue is highly conserved in SSTR5 among human, mouse, rat, chimpanzee, cattle, pig, dog and grass carp (Fig. 2A). Proline 335 also is highly conserved among different SSTRs, except SSTR2 (Fig. 2B). Therefore, we were prompted to study the effect of the leucine 335 polymorphism on the cellular function of SSTR5. Given that SSTR5 is one of the major SSTRs that mediates the anti-proliferative effect of somatostatin (32), we first examined the effect of the SSTR5 L335 variant on cellular proliferation of Mia PaCa-2 cells in comparison to the SSTR5 P335 variant. We transfected Mia PaCa-2 cells with empty vector, SSTR5 P335 or SSTR5 L335 and examined the cell proliferation rates of these cells by performing MTS assays. As shown in Fig. 3A, Mia PaCa-2 cells over-expressing the SSTR5 P335 variant had a decreased proliferation rate, while the cells over-expressing the SSTR5 L335 variant had an enhanced proliferation rate compared to the cells over-expressing empty vector. These data indicate that SSTR5 L335 adversely affects the cellular function of SSTR5 and suggests that SSTR5 L335 acted as a hypofunctional protein compared to SSTR5 P335.
Fig. 2. Proline 335 is a highly conserved residue in SSTRs.
A, Amino acid sequences of SSTR5 from different species were aligned. Proline 335 in human SSTR5 and its corresponding residue in mouse, rat, chimpanzee, cattle, pig, dog and grass carp were underlined. B, Amino acid sequences of human SSTR1, 2, 3, 4 and 5 were aligned. Proline 335 in human SSTR5 and its corresponding residue in SSTR1, 2, 3 and 4 were underlined.
Fig. 3. SSTR5 L335 enhances cellular proliferation of Mia PaCa-2 cells.
A, Mia PaCa-2 cells were transfected with empty vector, HA-SSTR5 P335 or HA-SSTR5 L335. Twenty-four hours after transfection, cells were re-plated into 96-well cell culture plates at 5 × 103 cells/well. Cell proliferation rate was determined by MTS assay. B, SSTR5 L335 abolishes the inhibitory effect of RPL-1980 on proliferation of Mia PaCa-2 cells. Mia PaCa-2 cells were transfected with empty vector, HA-SSTR5 P335 or HA-SSTR5 L335 using Lipofectamine 2000. Twenty-four hours after transfection, the cells were treated with 10-6 M of SSTR5 agonist RPL-1980 for 24 h. The cells were then re-plated into 96-well cell culture plates at 5 × 103 cells/well. Cell proliferation rate was determined by performing MTS assay.
RPL-1980 is a SSTR5-specific analogue. Treatment of Mia PaCa-2 cells with RPL-1980 inhibited cellular proliferation (Fig. 3B, column 2). Over-expression of the SSTR5 P335 enhanced the inhibitory effect of RPL-1980 on cellular proliferation of Mia PaCa-2 cells (Fig. 3, column 3), while over-expression of SSTR5 L335 abolished the anti-proliferative effect of RPL-1980 (Fig. 3, column 4). These data indicate that SSTR5 L335 adversely affects the inhibitory function of the SSTR5-specific analogue, RPL-1980, in a human pancreatic cancer cell lines, and therefore could potentially affect the clinical response to somatostatin analogue therapy.
SSTR5 leucine 335 variant has no enhancing effect on RPL-1980-induced inhibition of glucose-stimulated insulin secretion in β-TC-6 cells
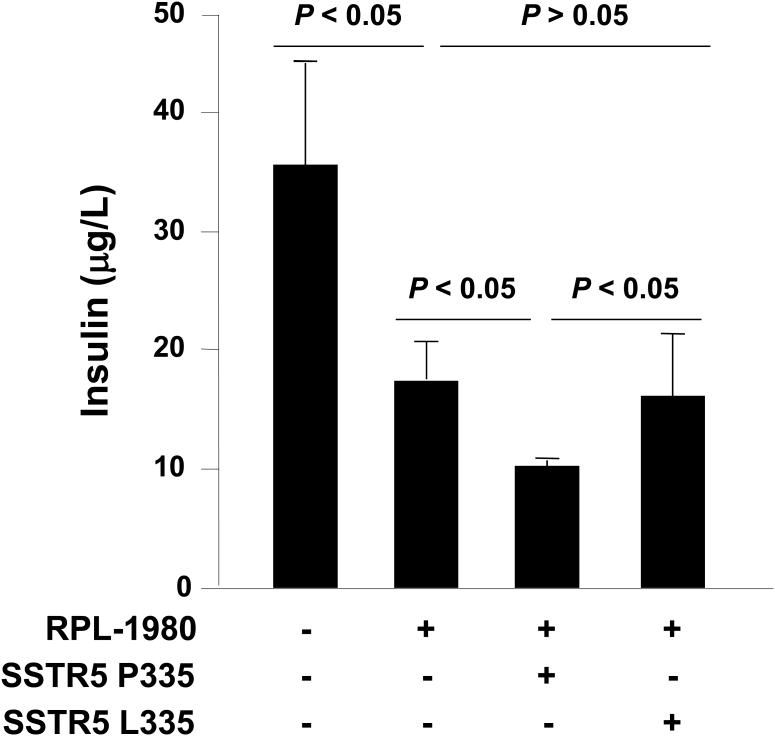
Our previous studies have shown that SSTR5 mediates the inhibitory effect of somatostatin on insulin secretion within the mouse islet (7, 33). Therefore, we examined the effect of the leucine 335 variant on RPL-1980-induced inhibition of glucose-stimulated insulin secretion in mouse insulinoma β-TC-6 cells. β-TC-6 cells have a P335L CC genotype, which expressed only SSTR5 P335 variant. Treatment of β-TC-6 cells with RPL-1980 inhibited insulin secretion and over-expression of SSTR5 P335 enhanced the inhibitory effect of RPL-1980 on insulin secretion (Fig. 4, column 2 and 3, respectively). However, over-expression of SSTR5 L335 had no such enhancing effect (Fig. 4, column 4). These data suggest that SSTR5 L335 is hypofunctional compared to SSTR5 P335 in negatively regulating insulin secretion in a mouse insulinoma cell line.
Fig. 4. SSTR5 L335 has no effect on the inhibition of glucose-stimulated insulin secretion by RPL-1980 in insulinoma β-TC6 cells.
β-TC6 cells were transfected with empty vector, HA-SSTR5 P335 or HA-SSTR5 L335 using Lipofectamine 2000. Twenty-four (24) h after transfection, the cells were washed twice with Krebs-Ringer bicarbonate (KRB) buffer and incubated in KRB-BSA for 1 h. The cells were then added to 11.1 mM of glucose and 10-6 M of SSTR5 analogue PRL-1980 and incubated for 2 h. After incubation, the media were collected and centrifuged at 600 g for 5 min. The insulin concentrations in the media were measured by ELISA.
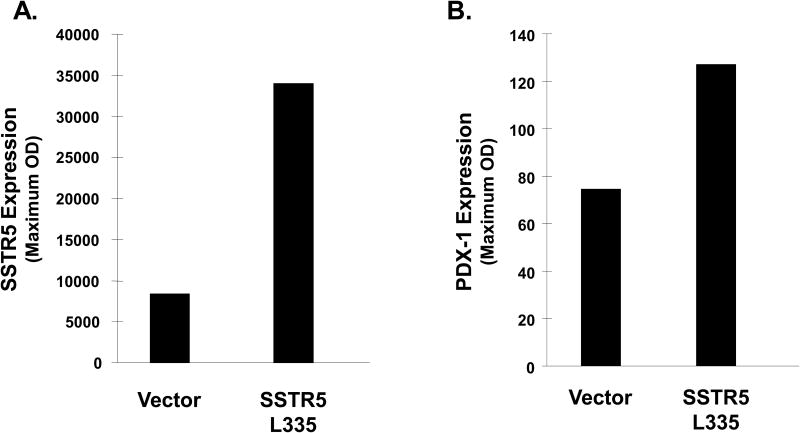
SSTR5 L335 variant enhances PDX-1 expression in Mia PaCa-2 cells
Pancreatic and duodenal homeobox-1 (PDX-1) is a homeodomain-containing transcription factor that plays an essential role in insulin expression and secretion (34). Our previous studies show that PDX-1 has oncogenic potential and is associated with enhancing pancreatic cancer cell proliferation, invasion and tumor growth in vivo (35). In an effort to explore the underlying mechanism by which SSTR5 L335 enhanced cell proliferation, we examined the effect of SSTR5 L335 on PDX-1 expression. Western blotting showed that over-expression of SSTR5 L335 increased PDX-1 expression in Mia PaCa-2 cells (Fig. 5), suggesting that SSTR5 is a potential negative regulator for PDX-1 expression and that the SSTR5 L335 variant acts as a hypofunctional protein, resulting in increased PDX-1 expression.
Fig. 5. PDX-1 expression is increased in SSTR5 L335-overexpressing Mia PaCa-2 cells.
Fifty (50) μg of the whole cell lysates from Mia PaCa-2 cells overexpressing empty vector or HA-SSTR5 L335 were subjected to SDS-PAGE, followed by Western blotting with an anti-SSTR5 antibody (A) or an anti-PDX-1 antibody (B).
Discussion
In this study, we examined the presence of SSTR5 P335L, a non-synonymous SNP, in patients with and without pancreatic cancer, as well as in human pancreatic cancer cell lines. Consistent with the SNP NCBI database and the HapMap data on European, African, and Asiatic population (36), our results showed that the distribution of SSTR5 P335L among ethnic groups is significantly different. The frequency of the T allele in the African American control population was 24% compared to 58% in the Caucasian control population. More than half of the patients with pancreatic cancer have a T allele having either a heterozygous (CT) or homozygous (TT) genotype. However, statistical analysis did not indicate significance in the association between the SSTR5 P335L SNP and the incidence of pancreatic cancer in the cohort of the Caucasian group. It could not be determined whether the SSTR5 P335L SNP is functionally associated with the incidence of pancreatic cancer in Hispanics and African Americans due to the small sample sizes in this study. It is, therefore, necessary to increase the sample sizes to determine if the presence of SSTR5 P335L SNP is associated with the incidence of pancreatic cancer. In a recent study, three non-synonymous SNPs (P109S, L48M and P335L) of the SSTR5 gene were found in pancreatic cancer (25). The SSTR5 P109S variant allele was associated with an increased risk of pancreatic cancer, whereas a combination of SSTR5 L48M AC variant and smoking on risk of pancreatic cancer was observed. In addition, there was a combined genotype effect of P109S CC and SSTR5 P335L CC on reduced survival of patients with resectable pancreatic cancer. These data suggest that genomic variations of SSTR5 could be important in the development and progression of pancreatic cancer.
For this study, we focused on the functional role of the SSTR5 P335L SNP, since SSTR5 P335L SNP is associated with neuropsychiatric disease and aggressiveness of pituitary tumors, yet little is known about the functional consequence of this SNP. We found that, in contrast to SSTR5 P335 variant, over-expression of the SSTR5 L335 variant enhanced cell proliferation in Mia PaCa-2 cells. Given the role of SSTR5 in mediating the anti-proliferative effect of somatostatin, the results of this study suggest that the SSTR5 L335 variant is a hypofunctional protein. In contrast to the proline 335 variant that enhanced the inhibitory effect of the SSTR5 analogue RPL-1980 on cell proliferation, the leucine variant abolished the inhibitory effect of RPL-1980 on cell proliferation in Mia PaCa-2 cells. These results are consistent with previous studies of the C-terminal region of SSTR5, which include proline 335 residue, which is required for octreotide-induced induction of retinoblastoma protein and G1 cell cycle arrest (37). Moreover, we found that the proline 335 variant enhanced the inhibitory effect of RPL-1980 on insulin secretion in β-TC-6 cells, while the leucine 335 variant had no such effect. This is important since we have previously shown that SSTR5 is one of the major SSTRs in islets that mediate the inhibitory effect of somatostatin on insulin secretion (7). Together, these results further support that SSTR5 L335 acts as a hypofunctional protein, and therefore could potentially have a harmful effect.
The underlying mechanism by which the SSTR5 leucine 335 variant acts as a hypofunctional protein remains unknown. SSTR5 is a G protein-coupled receptor for somatotatin (38). The initial events by which SSTR5 mediates somatostatin signaling include ligand (somatostatin) binding, homo/heterodimerization, internalization and protein-protein interaction (39, 40). It is reasonable to speculate that the presence of the leucine may interfere with one or more of these initial SST/SSTR5 signaling events. It has been shown that residues 328-347 within the C-terminus of SSTR5 play an inhibitory role in SSTR5 internalization (41). Therefore, it would be particularly interesting to investigate the effect of the P335L variation on SSTR5 internalization.
PDX-1 is a homeodomain-containing transcription factor (34, 42) that plays an essential role in pancreatic development, islet β-cell differentiation and maintaining mature β-cell functions by regulating a group of β-cell-specific genes (e.g., insulin, glucose transporter 2, islet amyloid polypeptide and glucokinase) (43-47). Our previous studies also show that PDX-1 is oncogenic and regulates cell proliferation and invasion of human pancreatic cancer cells as well as xenograft tumor growth in mice (30). Furthermore, aberrant over-expression of PDX-1 is found in a number of human cancers such as pancreatic, breast, colon, prostate, kidney, liver, lung, and ovarian (27, 48-51). In this study, over-expression of SSTR5 L335 variant increased PDX-1 expression in a human pancreatic cancer cell lines. Given the essential role of PDX-1 in cell proliferation and insulin secretion, a potential mechanism is that the SSTR5 leucine 335 variant enhanced cell proliferation through up-regulating PDX-1 expression, and therefore has a potential latent effect.
It has been well established in many different experimental tumor models that somatostatin is an endogenous antiproliferative agent, and thus a promising antitumor agent (39, 52-54). Somatostatin exerts its antitumor function through both a direct and an indirect way. The direct antitumor activities of somatostatin include blockade of autocrine/paracrine growth-promoting hormone and growth factor production, inhibition of growth factor-mediated mitogenic signals and induction of apoptosis. The indirect antitumor effects of somatostatin include inhibition of growth-promoting hormone and growth factor secretion, and antiangiogenic actions. However, the highly significant antiproliferative effect of somatostatin in preclinical studies becomes much less convincing when the laboratory data are translated to clinical trials. For example, approximately 50% of patients with insulinoma are not responsive to the treatment of somatostatin analogue and lack long-term responsiveness. The underlying molecular basis for the lack of therapeutic effect of somatostatin remains a mystery. SSTR5 and other SSTRs are widely and variably expressed in a variety of tumors such as gastroenteropancreatic tumors, pituitary tumors, and carcinoid tumors. These receptors thus represent the molecular basis for the clinical use of somatostatin analogs in the treatment of endocrine tumors and their in vivo localization. It could be due to the lack of expression of the target SSTR on the tumor surface, since 10-50% of these tumors do not express SSTRs.
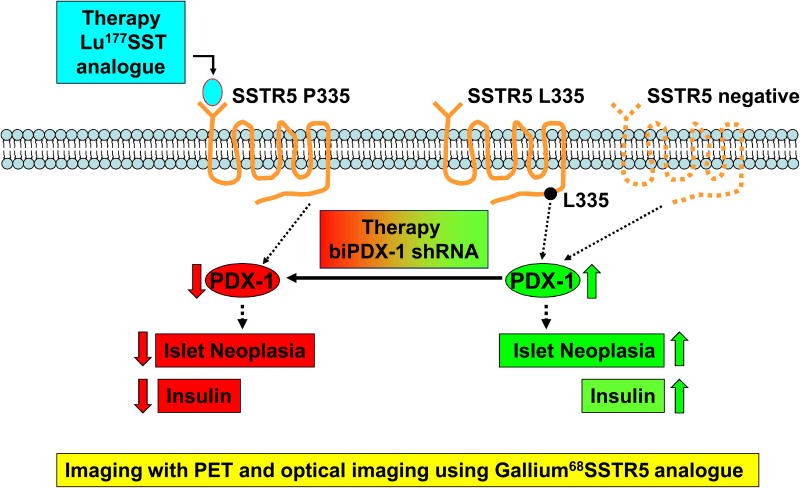
Our studies showed that SSTR5 P335L widely existed in human pancreatic cancer and that the leucine 335 variant abolished the inhibitory effect of SSTR5 agonist on cell proliferation. It is thus reasonable to speculate that such a hypofunctional protein may contribute to the lack of therapeutic effect of somatostatin analogues that mainly target SSTR5. Thus, at least two host factors may affect the therapeutic effect of a somatostatin analogue. One is the expression level of the analogue-targeted receptor. The other is the genetic variation(s) within the receptor that may affect its cellular functions such as the SSTR5 L335 variant. As a result, we developed a SSTR5-based tumor therapy approach (e.g., insulinoma) (Fig. 6). First, a SSTR5-specific Lu177-SST analogue will be used as a therapy under the condition that SSTR5 is expressed in the tumor cells (hSSTR5 P335 variant vs. SSTR5 negative). PDX-1 will be down-regulated, leading to the inhibition of islet neoplasia and insulin secretion. Second, once the SSTR5 L335 variant exists, an alternative approach will be applied. A bi-functional PDX-1 (biPDX-1) shRNA approach will be used to knockdown PDX-1 to inhibit islet neoplasia and insulin secretion. Thus, personalized genomic information may help serve to guide the choice of therapeutic treatments.
Fig. 6. A model of SSTR5-based tumor therapy approach.
Cancer patients exhibit a wide heterogeneity in the anti-cancer responses to chemotherapy. Today clinicians primarily employ the empirical “trial-and-error” approach to chemotherapy for their cancer patients due to a lack of reliable molecular determinants to predict the responsiveness to different drugs. There is a mandate from the U. S. Department of Health and Human Services (HHS) to develop Personalized Health Care, which is largely based on the foundation of our rapidly growing understanding of the human genome and the potential for health information technology (55). It is expected that personalized genomic medicine will profoundly impact as well as provide new opportunities for the future of cancer research (56, 57).
In summary, our study identified SSTR5 P335L as a genetic variation widely existing in the human population and in patients with pancreatic cancer. The leucine 335 variant lost the ability seen with the proline variant to enhance the inhibitory effect of SSTR5 agonist on cell proliferation and insulin secretion, thus acting as a hypofunctional protein. The functional information of the SSTR5 L335 variant will help guide the choice of therapy for tumor treatments.
Acknowledgments
We thank Dr. Dean Edwards and Dr. Kurt R. Christensen for their help in generation of monoclonal anti-SSTR5 L335 variant antibody, Dr. David Coy for providing RPL-1980, Dr. John Belmont for providing the BPR control DNA samples, and Dr. Hao Liu and Eunji Jo for statistical analysis. Gratitude is extended to Katie Elsbury for her editorial assistance and Priscilla Massey for her administrative assistance.
This work was supported by the National Institutes of Health (NIH) grant NIDDK R01-DK46441, the Vivian Smith Foundation, the Elkins Pancreas Center at Baylor College of Medicine, the generosity of Mr. and Mrs. Walter Hecht (to F.C.B.) and the BCM Seed Fund – the Caroline Wiess Law Fund for Molecular Medicine (to G. Z.).
The abbreviations used are
- SSTR
somatostatin receptor
- SSTR1-5
somatostatin receptor subtype 1-5
- SST
somatostatin
- L
leucine
- P
proline
Footnotes
This work was presented at the Molecular Surgeon Symposium on Personalized Genomic Medicine and Surgery, Baylor College of Medicine, Houston, TX, USA, May 7, 2010.
References
- 1.Moldovan S, DeMayo F, Brunicardi FC. Cloning of the mouse SSTR5 gene. J Surg Res. 1998;76:57–60. doi: 10.1006/jsre.1998.5286. [DOI] [PubMed] [Google Scholar]
- 2.Raynor K, O'Carroll AM, Kong H, Yasuda K, Mahan LC, Bell GI, Reisine T. Characterization of cloned somatostatin receptors SSTR4 and SSTR5. Mol Pharmacol. 1993;44:385–392. [PubMed] [Google Scholar]
- 3.Yamada Y, Post SR, Wang K, Tager HS, Bell GI, Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci U S A. 1992;89:251–255. doi: 10.1073/pnas.89.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasuda K, Rens-Domiano S, Breder CD, Law SF, Saper CB, Reisine T, Bell GI. Cloning of a novel somatostatin receptor, SSTR3, coupled to adenylylcyclase. J Biol Chem. 1992;267:20422–20428. [PubMed] [Google Scholar]
- 5.Reisine T, Bell GI. Molecular biology of somatostatin receptors. Endocr Rev. 1995;16:427–442. doi: 10.1210/edrv-16-4-427. [DOI] [PubMed] [Google Scholar]
- 6.Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- 7.Fagan S, A A, Moldovan S, Ray M, Adrian T, Ding X, et al. Insulin secretion is inhibited by subtype five somatostatin receptor in the mouse. Surgery. 1998;124:254–259. [PubMed] [Google Scholar]
- 8.Reubi JC, Horisberger U, Essed CE, Jeekel J, Klijn JG, Lamberts SW. Absence of somatostatin receptors in human exocrine pancreatic adenocarcinomas. Gastroenterology. 1988;95:760–763. doi: 10.1016/s0016-5085(88)80025-8. [DOI] [PubMed] [Google Scholar]
- 9.Player A, Gillespie J, Fujii T, Fukuoka J, Dracheva T, Meerzaman D, Hong KM, Curran J, Attoh G, Travis W, Jen J. Identification of TDE2 gene and its expression in non-small cell lung cancer. Int J Cancer. 2003;107:238–243. doi: 10.1002/ijc.11391. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg L, Lipsett M, Yoon JW, Prentki M, Wang R, Jun HS, Pittenger GL, Taylor-Fishwick D, Vinik AI. A pentadecapeptide fragment of islet neogenesis-associated protein increases beta-cell mass and reverses diabetes in C57BL/6J mice. Ann Surg. 2004;240:875–884. doi: 10.1097/01.sla.0000143270.99191.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zatelli MC, Tagliati F, Taylor JE, Rossi R, Culler MD, degli Uberti EC. Somatostatin receptor subtypes 2 and 5 differentially affect proliferation in vitro of the human medullary thyroid carcinoma cell line tt. J Clin Endocrinol Metab. 2001;86:2161–2169. doi: 10.1210/jcem.86.5.7489. [DOI] [PubMed] [Google Scholar]
- 12.Qiu CZ, Wang C, Huang ZX, Zhu SZ, Wu YY, Qiu JL. Relationship between somatostatin receptor subtype expression and clinicopathology, Ki-67, Bcl-2 and p53 in colorectal cancer. World J Gastroenterol. 2006;12:2011–2015. doi: 10.3748/wjg.v12.i13.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li K, L S, Brunicardi FC, Seu P. Use of RNA Interference to Target Cyclin E-Overexpressing. Cancer Res. 2003;13:3593–3597. [PubMed] [Google Scholar]
- 14.Takeda S, N A, Miyoshi K, Takagi H. Gene therapy for pancreatic cancer. Semin Surg Oncol. 1998;15:57–61. doi: 10.1002/(sici)1098-2388(199807/08)15:1<57::aid-ssu10>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 15.Hannon GJ, Rossi JJ. Unlocking the potential of the human genome with RNA interference. Nature. 2004;431:371–378. doi: 10.1038/nature02870. [DOI] [PubMed] [Google Scholar]
- 16.Deng X, Ewton DZ, Li S, Naqvi A, Mercer SE, Landas S, Friedman E. The kinase Mirk/Dyrk1B mediates cell survival in pancreatic ductal adenocarcinoma. Cancer Res. 2006;66:4149–4158. doi: 10.1158/0008-5472.CAN-05-3089. [DOI] [PubMed] [Google Scholar]
- 17.Westphal EM, G J, Catchpole JR, Ford M, Kenney SC. The nitroreductase/CB1954 combination in Epstein-Barr virus-positive B-cell lines: induction of bystander killing in vitro and in vivo. Cancer Gene Ther. 2000;7:97–106. doi: 10.1038/sj.cgt.7700102. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka S, P S, Taguchi K, Shimada M, Mori M, Krag DN, Arii S. Specific peptide ligand for Grb7 signal transduction protein and pancreatic cancer metastasis. J Natl Cancer Inst. 2006;98:491–498. doi: 10.1093/jnci/djj105. [DOI] [PubMed] [Google Scholar]
- 19.Cordelier P, Esteve JP, Najib S, Moroder L, Vaysse N, Pradayrol L, Susini C, Buscail L. Regulation of neuronal nitric-oxide synthase activity by somatostatin analogs following SST5 somatostatin receptor activation. J Biol Chem. 2006;281:19156–19171. doi: 10.1074/jbc.M602024200. [DOI] [PubMed] [Google Scholar]
- 20.Botstein D, Risch N. Discovering genotypes underlying human phenotypes: past successes for mendelian disease, future approaches for complex disease. Nat Genet. 2003;33(Suppl):228–237. doi: 10.1038/ng1090. [DOI] [PubMed] [Google Scholar]
- 21.http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?chooseRs=all&go=Go&locusId=6755
- 22.Johansson M, McKay JD, Wiklund F, Rinaldi S, Hallmans G, Balter K, Adami HO, Gronberg H, Stattin P, Kaaks R. Genetic variation in the SST gene and its receptors in relation to circulating levels of insulin-like growth factor-I, IGFBP3, and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18:1644–1650. doi: 10.1158/1055-9965.EPI-08-0893. [DOI] [PubMed] [Google Scholar]
- 23.Barnby G, Abbott A, Sykes N, Morris A, Weeks DE, Mott R, Lamb J, Bailey AJ, Monaco AP. Candidate-gene screening and association analysis at the autism-susceptibility locus on chromosome 16p: evidence of association at GRIN2A and ABAT. Am J Hum Genet. 2005;76:950–966. doi: 10.1086/430454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nyegaard M, Borglum AD, Bruun TG, Collier DA, Russ C, Mors O, Ewald H, Kruse TA. Novel polymorphisms in the somatostatin receptor 5 (SSTR5) gene associated with bipolar affective disorder. Mol Psychiatry. 2002;7:745–754. doi: 10.1038/sj.mp.4001049. [DOI] [PubMed] [Google Scholar]
- 25.Filopanti M, Ballare E, Lania AG, Bondioni S, Verga U, Locatelli M, Zavanone LM, Losa M, Gelmini S, Peri A, Orlando C, Beck-Peccoz P, Spada A. Loss of heterozygosity at the SS receptor type 5 locus in human GH- and TSH-secreting pituitary adenomas. J Endocrinol Invest. 2004;27:937–942. doi: 10.1007/BF03347536. [DOI] [PubMed] [Google Scholar]
- 26.Li D, T M, Brunicardi FC, Fisher WE, Gibbs RA, Gingras MC. Association of somatostatin receptor 5 gene polymorphisms with pancreatic cancer risk and survival. Cancer in press. 2010 doi: 10.1002/cncr.25858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang XP, Li ZJ, Magnusson J, Brunicardi FC. Tissue MicroArray analyses of pancreatic duodenal homeobox-1 in human cancers. World J Surg. 2005;29:334–338. doi: 10.1007/s00268-004-7823-4. [DOI] [PubMed] [Google Scholar]
- 28.Wang XP, Y J, Norman MA, Magnusson J, DeMayo FJ, Brunicardi FC. SSTR5 ablation in islet results in alterations in glucose homeostasis in mice. FEBS Lett. 2005;579:3107–3114. doi: 10.1016/j.febslet.2005.04.069. [DOI] [PubMed] [Google Scholar]
- 29.Wang XP, Norman M, Yang J, Liu SH, Magnusson J, DeMayo FJ, Brunicardi FC. The effect of global SSTR5 gene ablation on the endocrine pancreas and glucose regulation in aging mice. J Surg Res. 2005;129:64–72. doi: 10.1016/j.jss.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, Ballian N, Belaguli NS, Patel S, Li M, Templeton NS, Gingras MC, Gibbs R, Fisher W, Brunicardi FC. PDX-1 acts as a potential molecular target for treatment of human pancreatic cancer. Pancreas. 2008;37:210–220. doi: 10.1097/MPA.0b013e31816a4a33. [DOI] [PubMed] [Google Scholar]
- 31.Weigel NL, B C, Estes PA, Prendergast P, Altmann M, Christensen K, Edwards DP. Ligands induce conformational changes in the carboxyl-terminus of progesterone receptors which are detected by a site-directed monoclonal antibody. Mol Endocrinol. 1992;6:1585–1597. doi: 10.1210/mend.6.10.1448113. [DOI] [PubMed] [Google Scholar]
- 32.Barbieri F, Pattarozzi A, Gatti M, Porcile C, Bajetto A, Ferrari A, Culler MD, Florio T. Somatostatin receptors 1, 2, and 5 cooperate in the somatostatin inhibition of C6 glioma cell proliferation in vitro via a phosphotyrosine phosphatase-eta-dependent inhibition of extracellularly regulated kinase-1/2. Endocrinology. 2008;149:4736–4746. doi: 10.1210/en.2007-1762. [DOI] [PubMed] [Google Scholar]
- 33.Tirone TA, Norman MA, Moldovan S, DeMayo FJ, Wang XP, Brunicardi FC. Pancreatic somatostatin inhibits insulin secretion via SSTR-5 in the isolated perfused mouse pancreas model. Pancreas. 2003;26:e67–73. doi: 10.1097/00006676-200304000-00025. [DOI] [PubMed] [Google Scholar]
- 34.Ashizawa S, Brunicardi FC, Wang XP. PDX-1 and the pancreas. Pancreas. 2004;28:109–120. doi: 10.1097/00006676-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Feanny MA, Fagan SP, Ballian N, Liu SH, Li Z, Wang X, Fisher W, Brunicardi FC, Belaguli NS. PDX-1 expression is associated with islet proliferation in vitro and in vivo. J Surg Res. 2008;144:8–16. doi: 10.1016/j.jss.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 36.http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=169068
- 37.Sharma K, Patel YC, Srikant CB. C-terminal region of human somatostatin receptor 5 is required for induction of Rb and G1 cell cycle arrest. Mol Endocrinol. 1999;13:82–90. doi: 10.1210/mend.13.1.0220. [DOI] [PubMed] [Google Scholar]
- 38.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 39.Florio T. Molecular mechanisms of the antiproliferative activity of somatostatin receptors (SSTRs) in neuroendocrine tumors. Front Biosci. 2008;13:822–840. doi: 10.2741/2722. [DOI] [PubMed] [Google Scholar]
- 40.Strowski MZ, Blake AD. Function and expression of somatostatin receptors of the endocrine pancreas. Mol Cell Endocrinol. 2008;286:169–179. doi: 10.1016/j.mce.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Peverelli E, Mantovani G, Calebiro D, Doni A, Bondioni S, Lania A, Beck-Peccoz P, Spada A. The third intracellular loop of the human somatostatin receptor 5 is crucial for arrestin binding and receptor internalization after somatostatin stimulation. Mol Endocrinol. 2008;22:676–688. doi: 10.1210/me.2007-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaneto H, Miyatsuka T, Shiraiwa T, Yamamoto K, Kato K, Fujitani Y, Matsuoka TA. Crucial role of PDX-1 in pancreas development, beta-cell differentiation, and induction of surrogate beta-cells. Curr Med Chem. 2007;14:1745–1752. doi: 10.2174/092986707781058887. [DOI] [PubMed] [Google Scholar]
- 43.Watada H, Kajimoto Y, Miyagawa J, Hanafusa T, Hamaguchi K, Matsuoka T, Yamamoto K, Matsuzawa Y, Kawamori R, Yamasaki Y. PDX-1 induces insulin and glucokinase gene expressions in alphaTC1 clone 6 cells in the presence of betacellulin. Diabetes. 1996;45:1826–1831. doi: 10.2337/diab.45.12.1826. [DOI] [PubMed] [Google Scholar]
- 44.Bretherton-Watt D, Gore N, Boam DS. Insulin upstream factor 1 and a novel ubiquitous factor bind to the human islet amyloid polypeptide/amylin gene promoter. Biochem J. 1996;313(Pt 2):495–502. doi: 10.1042/bj3130495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carty MD, Lillquist JS, Peshavaria M, Stein R, Soeller WC. Identification of cis- and trans-active factors regulating human islet amyloid polypeptide gene expression in pancreatic beta-cells. J Biol Chem. 1997;272:11986–11993. doi: 10.1074/jbc.272.18.11986. [DOI] [PubMed] [Google Scholar]
- 46.Serup P, Jensen J, Andersen FG, Jorgensen MC, Blume N, Holst JJ, Madsen OD. Induction of insulin and islet amyloid polypeptide production in pancreatic islet glucagonoma cells by insulin promoter factor 1. Proc Natl Acad Sci U S A. 1996;93:9015–9020. doi: 10.1073/pnas.93.17.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watada H, Kajimoto Y, Kaneto H, Matsuoka T, Fujitani Y, Miyazaki J, Yamasaki Y. Involvement of the homeodomain-containing transcription factor PDX-1 in islet amyloid polypeptide gene transcription. Biochem Biophys Res Commun. 1996;229:746–751. doi: 10.1006/bbrc.1996.1875. [DOI] [PubMed] [Google Scholar]
- 48.Leys CM, N S, Rudzinski E, Kaminishi M, Montgomery E, Washington MK, Goldenring JR. Expression of PDX-1 in human gastric metaplasia and gastric adenocarcinoma. Hum Pathol. 2006;37:1162–1168. doi: 10.1016/j.humpath.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 49.Sakai H, E Y, Li XL, Akiyama Y, Miyake S, Takizawa T, Konishi N, Tatematsu M, Koike M, Yuasa Y. PDX-1 homeobox protein expression in pseudopyloric glands and gastric carcinomas. Gut. 2004;53:323–330. doi: 10.1136/gut.2003.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyatsuka T, K H, Shiraiwa T, Matsuoka TA, Yamamoto K, et al. Persistent expression of PDX-1 in the pancreas causes acinar-to-ductal metaplasia through Stat3 activation. Genes Dev. 2006;20:1435–1440. doi: 10.1101/gad.1412806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jonmarker S, Glaessgen A, Culp WD, Pisa P, Lewensohn R, Ekman P, Valdman A, Egevad L. Expression of PDX-1 in prostate cancer, prostatic intraepithelial neoplasia and benign prostatic tissue. APMIS. 2008;116:491–498. doi: 10.1111/j.1600-0463.2008.01020.x. [DOI] [PubMed] [Google Scholar]
- 52.Bodei L, Ferone D, Grana CM, Cremonesi M, Signore A, Dierckx RA, Paganelli G. Peptide receptor therapies in neuroendocrine tumors. J Endocrinol Invest. 2009;32:360–369. doi: 10.1007/BF03345728. [DOI] [PubMed] [Google Scholar]
- 53.Modlin IM, Pavel M, Kidd M, Gustafsson BI. Review article: somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Aliment Pharmacol Ther. 31:169–188. doi: 10.1111/j.1365-2036.2009.04174.x. [DOI] [PubMed] [Google Scholar]
- 54.Pyronnet S, Bousquet C, Najib S, Azar R, Laklai H, Susini C. Antitumor effects of somatostatin. Mol Cell Endocrinol. 2008;286:230–237. doi: 10.1016/j.mce.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 55.http://www.hhs.gov/myhealthcare
- 56.Brunicardi FC, Gibbs RA, Fisher W, Chen C. Overview of the Molecular Surgeon Symposium on Personalized Genomic Medicine and Surgery. World J Surg. 2009;33:612–614. doi: 10.1007/s00268-008-9861-9. [DOI] [PubMed] [Google Scholar]
- 57.Collins F. Has the revolution arrived? Nature. 464:674–675. doi: 10.1038/464674a. [DOI] [PMC free article] [PubMed] [Google Scholar]