“History followed different courses for different peoples because of differences among peoples’ environments, not because of biological differences among peoples themselves.”
—Jared Diamond, Guns, Germs, and Steel: The Fates of Human Societies1
Twenty years ago, studies in a model of acute kidney injury (AKI) provided the first demonstration of the protective effects of heme oxygenase-1,2 an inducible enzyme now known to interrupt diverse pathways of injury (Figure 1).3–5 These studies emerged from an environment and tradition in nephrology that have long sought to understand renal responses in health and disease, and that hark back to the nineteenth-century physiologist Claude Bernard and his famous concept.6 Bernard wrote that “the constancy of the internal environment is the condition that life should be free and independent,” and “far from the higher animal being indifferent to the external world, it is on the contrary in a precise and informed relation with it, in such a way that its equilibrium results from the continuous and most delicate composition established as by the most sensitive of balances.”6 Bernard’s internal milieu was the forerunner to Cannon’s homeostasis,7 and, together, these elemental concepts have long imbued the study of the kidney, an organ that serves as one of “the most sensitive of balances.” Indeed, Homer Smith begins The Kidney: Structure and Function in Health and Disease by emphasizing the role of renal functional responses in maintaining homeostasis and the constancy of the internal environment.8
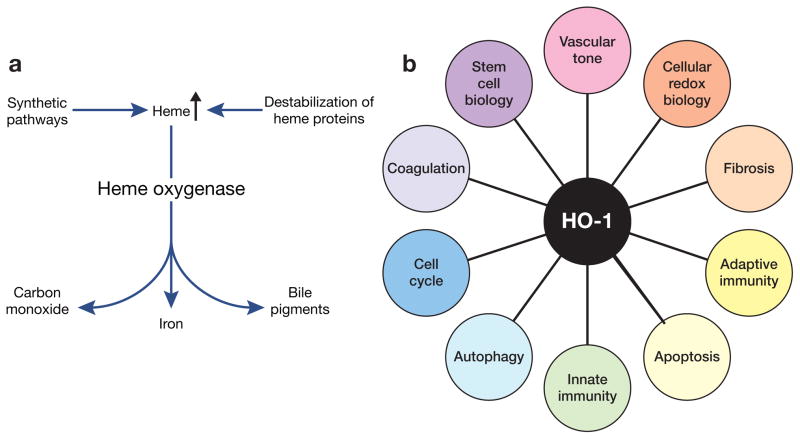
Figure 1. Overview of the biology of HO-1.
(a) Degradation of heme by heme oxygenase (HO). (b) Biologic processes relevant to tissue injury that are influenced by HO-1 and its products.
The nature of renal responses when the kidney itself is injured is also of long-standing and obvious clinical interest. A body of experimental work, extending more than a hundred years, has explored renal responses following exposure to a single insult, concomitant exposure to different insults, or sequential exposure to the same or different insults. The synergistic and adverse effects of such insults on renal function are well recognized and, indeed, underlie the current view that clinical AKI is commonly a multifactorial process. Less well appreciated is the fact that a given insult can condition the kidney to render it resistant to a subsequent insult; ironically, such acquired renal resistance was described a century ago by Suzuki in studies of the kidney exposed to uranyl salts.9 Subsequent studies confirmed this phenomenon and examined how the kidney can be conditioned against, or sensitized to, injury depending on the nature of the imposed insults (ischemic or nephrotoxic) and their relative severity, timing, and frequency.10,11 This led to the recognition that an acute ischemic insult may render the kidney resistant to subsequent ischemia,12 and such ischemic preconditioning is now broadly studied for insights it provides into the biology of cytoprotection.
By the late 1980s, studies of rodent models of AKI along with in vitro approaches substantially advanced the understanding of AKI and increasingly uncovered strategies that protected against renal injury.13,14 Such cytoprotective strategies encompassed, among others, the following: amino acids such as glycine; antioxidants such as glutathione; mitigation in elevations in intra-cellular calcium; augmentation of cellular purine nucleotide levels; the ameliorative effects of reducing temperature or pH; the administration of prostaglandin species, natriuretic peptides, or peptide growth factors; the eliciting of the heat-shock response; and the redressing of the vasoactive imbalance by inhibiting vasocon-stricting species, or by augmenting endothelium-dependent/endothelium-independent relaxation.13–15 The ambience of the AKI field at the time encouraged the search for renal cyto-protective strategies.
An insight relevant to renal cytoprotective strategies in AKI appeared in studies in chronic kidney disease (CKD) that proposed the hyper-filtration hypothesis.16 These studies not only substantiated a self-perpetuating mechanism for CKD but also categorized renal responses as either adaptive or maladaptive, on the basis of their sequential and integrated effects. For example, increased single nephron glomerular filtration rate (SNGFR) in nephrons surviving after subtotal renal ablation lessens the acute reduction in whole-kidney glomerular filtration rate (GFR). However, glomerular hemodynamic alterations that augment SNGFR can, in time, damage glomeruli, with consequent reduction in SNGFR and, ultimately, whole-kidney GFR.16 Thus, in the aftermath of renal injury, seemingly adaptive renal responses may ultimately prove maladaptive, a concept germane not only to putative pathways of protection in AKI, but also, given its current interest, to the transition from AKI to CKD.
In the 1980s, reactive oxygen species (ROS) were increasingly invoked as a mechanism of kidney injury.17,18 Responses to, and defenses against, ROS were also of interest, and such studies focused on antioxidants (such as glutathione) and enzymes that metabolize superoxide anion or hydrogen peroxide. ROS and other insults were known to induce a 32-kDa protein, which was eventually identified as heme oxygenase (HO);19 HO had become the focus of growing attention ever since its discovery in 1968 as the heme-degrading enzyme in microsomes.20–22 To account for the ubiquitous induction of HO in stressed tissue, Keyse and Tyrrell speculated that this response protected against ROS by producing bile pigments,19 metabolites shown previously to scavenge ROS in vitro.23 Stocker subsequently suggested that HO induction would also be salutary because of clearance of heme, a potentially toxic species released from destabilized heme proteins in injured cells.24 In 1991 Tyrrell’s group again demonstrated the inducibility of HO in ROS-exposed cells, but observed that “…the functional evidence for a protective role is still lacking.”25
This question regarding the functionality of HO was appealing because of ongoing interest in the role of endogenous antioxidant systems in protecting against AKI.26 However, it was quite unclear that induction of HO-1 would succor the injured kidney: degradation of heme by HO liberates iron, and the injurious effect of iron acting as a Fenton catalyst was, at the time, one of the major issues in the ROS field. Induction of HO-1 in the injured kidney may thus represent a maladaptive response rather than an adaptive response that mitigates the severity of such injury.
To examine the functional significance of HO, a strategy was based on a model of heme protein–induced AKI. Heme proteins appearing in plasma in large amounts are filtered and metabolized by the kidney, thus exposing the latter to large amounts of heme. By the dictates of the constancy of the internal environment and homeostasis, the presence of heme proteins in plasma and heme in copious quantities in the kidney would necessitate clearance of heme by increased renal HO activity.27 In this setting, the significance of HO activity in heme protein–induced renal injury could thereby be examined.
Interest in heme protein–induced human AKI dates back to the recognition of this syndrome in 1925 as a consequence of mismatched blood transfusions and attendant hemolysis, and in 1941 as a consequence of the crush syndrome as described during the London Blitz.28 An experimental model was subsequently introduced in which hypertonic glycerol was injected either subcutaneously to induce hemolysis, or intra-muscularly so that both hemolysis and rhabdomyolysis occurred.
Based on a multidisciplinary approach, studies of HO were undertaken in the glycerol model of AKI, the latter exhibiting robust induction of HO-1 mRNA and increased HO activity, measured by the generation of either bilirubin or carbon monoxide.2 Other antioxidant genes were not induced, underscoring the specificity of HO-1 induction. Inhibition of HO activity by a competitive inhibitor exacerbated AKI, thus indicating that loss of HO activity sensitized the kidney to heme proteins.2 A complementary approach used a nontoxic dose of hemoglobin to induce HO-1 in the kidney prior to AKI; such HO-1 induction conferred marked resistance to AKI.2 To address the fate of iron released by HO activity, the kidney content of ferritin, the principal iron-sequestering protein, was assessed. Ferritin expression was linked to HO activity, the former increasing with the prior induction of HO activity, and declining with inhibition of HO activity.2 This coupled response thus degraded heme, generated antioxidants, and chelated iron.2 Evidence for the cytoprotective effects of ferritin in heme-induced oxidative stress was subsequently demonstrated in endothelial cells in vitro by Balla et al.29
These studies on HO-1 and AKI, as emphasized here, drew on a rich tradition of research on mechanisms of renal injury. This long line of basic research in nephrology is clinically relevant, as cytoprotection and preconditioning adumbrate therapeutic strategies applicable to human disease. Receptivity to the question regarding the functional significance of HO-1 was developed by exposure to certain salient and emphasized themes in nephrology: Bernard’s constancy of the internal milieu, Cannon’s homeostasis, sensitivity and resistance to renal injury, the need for renal cytoprotective strategies, adaptive and maladaptive responses in the injured kidney, and ROS and catalytically active iron as pathways of renal injury. Thus, an appreciation of Bernard’s environment, and an environment created by the community of nephrology and its teachings, nurtured these studies that addressed the functional significance of HO-1 in tissue injury.
Acknowledgments
K.A.N. thanks the US National Institutes of Health (grant R37-DK47060) for support, Tammy Engel for secretarial expertise, and W.W. Norton for permission to reproduce the quotation from Guns, Germs, and Steel: The Fates of Human Societies by Jared Diamond.
Footnotes
DISCLOSURE
The author declared no competing interests.
References
- 1.Diamond J. Guns, Germs, and Steel: The Fates of Human Societies. W.W. Norton; New York/London: 1997. p. 25. [Google Scholar]
- 2.Nath KA, Balla G, Vercellotti GM, et al. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90:267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal A, Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 gene ablation and expression. J Am Soc Nephrol. 2000;11:965–973. doi: 10.1681/ASN.V115965. [DOI] [PubMed] [Google Scholar]
- 4.Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006;70:432–443. doi: 10.1038/sj.ki.5001565. [DOI] [PubMed] [Google Scholar]
- 5.Bolisetty S, Traylor AM, Kim J, et al. Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury. J Am Soc Nephrol. 2010;21:1702–1712. doi: 10.1681/ASN.2010030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernard C. Lessons on the phenomena of life common to animals and vegetables. In: Langley L, editor. Homeostasis: Origins of the Concept. Dowden, Hutchinson & Ross; Stroudsburg, PA: 1973. pp. 146–147. [Google Scholar]
- 7.Cannon W. Organization for physiological homeostasis. Physiol Rev. 1929;IX:399–431. [Google Scholar]
- 8.Smith H. The Kidney: Structure and Function in Health and Disease. Oxford University Press; New York: 1951. pp. v–vii. [Google Scholar]
- 9.Suzuki T. Zur Morphologie der Nierensekretion: Unter Physiologishchen und Pathologischen Bedingungen. Fischer Verlag; Jena: 1912. pp. 1–272. [Google Scholar]
- 10.Macnider WD. The functional and pathological response of the kidney in dogs subjected to a second subcutaneous injection of uranium nitrate. J Exp Med. 1929;49:411–433. doi: 10.1084/jem.49.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honda N, Hishida A, Ikuma K, et al. Acquired resistance to acute renal failure. Kidney Int. 1987;31:1233–1238. doi: 10.1038/ki.1987.136. [DOI] [PubMed] [Google Scholar]
- 12.Zager RA, Baltes LA, Sharma HM, et al. Responses of the ischemic acute renal failure kidney to additional ischemic events. Kidney Int. 1984;26:689–700. doi: 10.1038/ki.1984.204. [DOI] [PubMed] [Google Scholar]
- 13.Bonventre JV. Mediators of ischemic renal injury. Annu Rev Med. 1988;39:531–544. doi: 10.1146/annurev.me.39.020188.002531. [DOI] [PubMed] [Google Scholar]
- 14.Weinberg JM. The cell biology of ischemic renal injury. Kidney Int. 1991;39:476–500. doi: 10.1038/ki.1991.58. [DOI] [PubMed] [Google Scholar]
- 15.Fischereder M, Trick W, Nath KA. Therapeutic strategies in the prevention of acute renal failure. Semin Nephrol. 1994;14:41–52. [PubMed] [Google Scholar]
- 16.Hostetter TH, Olson JL, Rennke HG, et al. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981;241:F85–F93. doi: 10.1152/ajprenal.1981.241.1.F85. [DOI] [PubMed] [Google Scholar]
- 17.Shah SV. Effect of enzymatically generated reactive oxygen metabolites on the cyclic nucleotide content in isolated rat glomeruli. J Clin Invest. 1984;74:393–401. doi: 10.1172/JCI111434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci USA. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abraham NG, Lin JH, Schwartzman ML, et al. The physiological significance of heme oxygenase. Int J Biochem. 1988;20:543–558. doi: 10.1016/0020-711x(88)90093-6. [DOI] [PubMed] [Google Scholar]
- 22.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- 23.Stocker R, Yamamoto Y, McDonagh AF, et al. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 24.Stocker R. Induction of haem oxygenase as a defence against oxidative stress. Free Radic Res Commun. 1990;9:101–112. doi: 10.3109/10715769009148577. [DOI] [PubMed] [Google Scholar]
- 25.Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991;51:974–978. [PubMed] [Google Scholar]
- 26.Salahudeen AK, Clark EC, Nath KA. Hydrogen peroxide-induced renal injury. A protective role for pyruvate in vitro and in vivo. J Clin Invest. 1991;88:1886–1893. doi: 10.1172/JCI115511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pimstone NR, Engel P, Tenhunen R, et al. Inducible heme oxygenase in the kidney: a model for the homeostatic control of hemoglobin catabolism. J Clin Invest. 1971;50:2042–2050. doi: 10.1172/JCI106697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bywaters E, Beall D. Crush injuries with impairment of renal function. Br Med J. 1941;1:427–432. doi: 10.1136/bmj.1.4185.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balla G, Jacob HS, Balla J, et al. Ferritin: a cytoprotective antioxidant stratagem of endothelium. J Biol Chem. 1992;267:18148–18153. [PubMed] [Google Scholar]