Abstract
The quinones 1,4-naphthoquinone (NQ), tetramethyl-1,4-benzoquinone (DQ), 2-methyl-1,4-naphthoquinone (MNQ), 2,3-dimethoxy-5-methyl-1,4-benzoquinone (UBQ-0), 2,6-dimethylbenzoquinone (DMBQ), 2,6-dimethoxybenzoquinone (DMOBQ), and 9,10-phenanthraquinone (PHQ) enhance the rate of H2O2 reduction by ascorbate, under anaerobic conditions, as detected from the amount of methane produced after hydroxyl radical reaction with dimethyl sulfoxide. The amount of methane produced increases with an increase in the quinone one-electron reduction potential. The most active quinone in this series, PHQ, is only 14% less active than the classic Fenton reagent cation, Fe2+, at the same concentration. Since PHQ is a common toxin present in diesel combustion smoke, the possibility that PHQ-mediated catalysis of hydroxyl radical formation is similar to that of Fe2+ adds another important pathway to the modes in which PHQ can execute its toxicity. Because quinones are known to enhance the antitumor activity of ascorbate and because ascorbate enhances the formation of H2O2 in tissues, the quinone-mediated reduction of H2O2 should be relevant to this type of antitumor activity, especially under hypoxic conditions.
Introduction
Quinones form the second largest class of antitumor agents approved for clinical use in the U.S., and several antitumor quinones are in different stages of clinical and preclinical development.1 Many of these are metabolites of, or are, environmental toxins.2,3 A common feature of quinone-containing drugs is their ability to undergo reversible redox reactions to form semiquinone and oxygen radicals.4,5 One-electron reduction of a quinone (Q) gives the semiquinone radical (Q•– or QH•), whereas two-electron reduction gives the hydroquinone (QH2).5 The semiquinone can also be formed by a comproportionation reaction between a quinone and a hydroquinone (Reaction 1; the opposite of Reaction 1 is the semiquinone disproportionation reaction).
| 1 |
The catalytic enhancement of ascorbate (AscH–) oxidation by quinones has been previously observed.6 In addition, we have previously observed that quinones enhance the rates of ascorbate reduction of nitric oxide7 and S-nitrosothiols.8 In each of those works, the enhancement activity of quinones increases with its one-electron reduction potential within a certain range of one-electron reduction potential values. Thus, the semiquinone, the one-electron-reduced species, is postulated to be the actual intermediate responsible for reducing those targets. Furthermore, antitumor activity enhancement by quinones in the presence of ascorbate has been reported.9
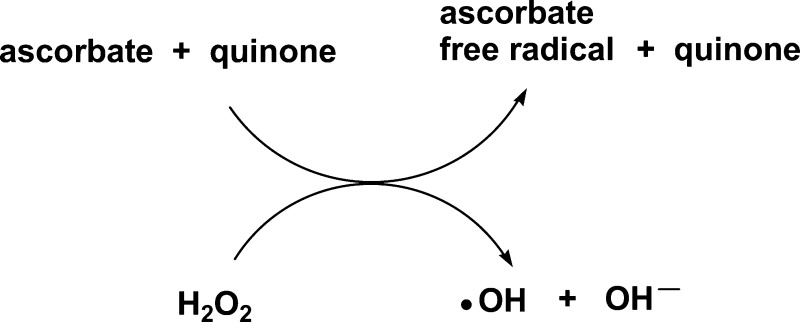
Hydrogen peroxide has important roles as a signaling molecule in the regulation of a variety of biological processes.10 Hydrogen peroxide also plays an important role in aging11 and cancer.12,13 Although quinone-enhanced ascorbate reduction reactions of the species mentioned above have been reported, very few works report the possibility of a semiquinone-mediated reduction of H2O2.14,15 In fact, Koppenol and Butler suggested that quinones with reduction potentials between −330 and −460 mV could be involved in metal-independent Fenton Reaction 2.16
| 2 |
However, there has not been any study comparing the reactivities among quinones with regard to reaction 2 with their dependence on the quinone one-electron reduction potential. Reaction 2 acquires more biomedical importance in view of the facts that parenteral administration of ascorbate generates H2O2 in tissues17 and that quinones enhance the antitumor activity of ascorbate.18 These observations suggest that quinones may be acting as H2O2 production promoters and/or as enhancers of hydroxyl radical production.
The metal-independent production of hydroxyl radicals by the reaction of H2O2 with halogenated quinones in the absence of reducing agents has been reported.19−21 This type of reaction was not detected for non-halogenated quinones, indicating the need for electron-withdrawing substituents at the quinone ring for this reaction to occur. In this work, we have determined the relative extent of hydroxyl radical production in the presence and absence of non-halogenated quinones, using ascorbate as the reducing agent. Hydroxyl radicals can be determined by spin trapping using nitrones, and the resulting nitroxide concentration can be measured by electron paramagnetic resonance (EPR) spectrometry.22 However, nitroxides are prone to reduction to the EPR-silent hydroxylamine in the presence of reducing agents. Therefore, in this work, we have determined the relative ability of quinones to enhance the rate of ascorbate reduction of H2O2 by measuring the amount of methane produced from the reaction of hydroxyl radicals with DMSO. A similar procedure for the selective detection of OH radical production, where DMSO is the hydroxyl radical scavenger and the produced methane gas is detected by gas chromatography (GC), has been previously used in several works.23−28 The mechanism of this reaction has been previously determined using EPR and radiolysis techniques.29,30
Materials and Methods
Chemicals
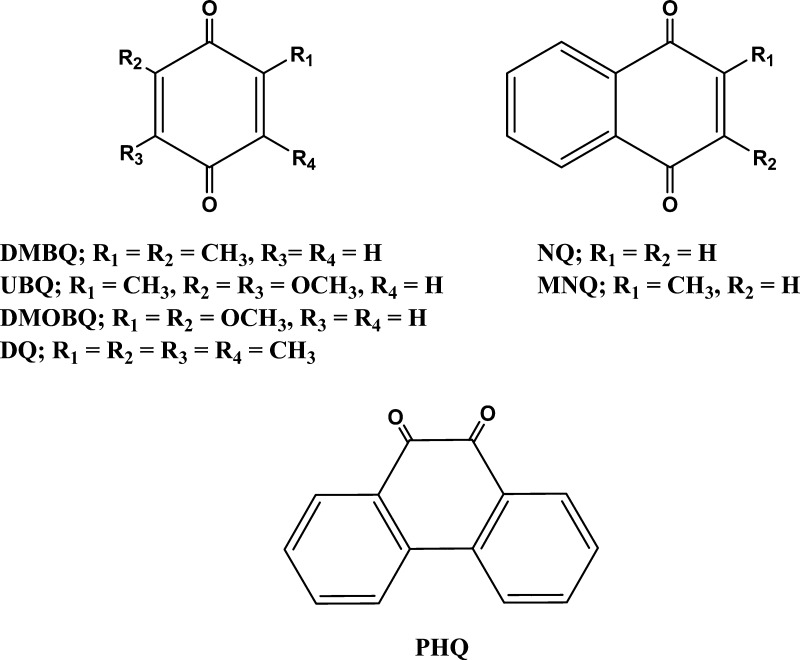
The quinones (Figure 1) were purchased from Sigma-Aldrich Corp. These quinones were selected because they have a wide range of one-electron reduction potentials, are commercially available, and were found to be active in the enhanced reduction of oxygen by ascorbate.6 Quinones were purified by sublimation. Methane (99.0% pure) was purchased in a gas lecture bottle from Sigma-Aldrich Corp. Stock solutions of quinones were prepared in water and used on the same day of their preparation. Deionized and Chelex-treated water was used in the preparation of all stock and sample solutions. Chelex treatment of water was monitored using the ascorbate test, as described by Buettner.31 Care was taken to minimize exposing quinone-containing solutions to light. Acid-washed Teflon needles and glass syringes were used to transfer solutions.
Figure 1.
Quinones used in this work.
Quinone-Enhanced H2O2 Reduction
Stock solutions of ascorbic acid, phosphate buffer (pH 7.4), DETAPAC, H2O2, DMSO, and quinone were deareated by purging with high-purity N2 and equilibrated to 37 °C in a temperature-controlled water bath. The reaction mixture most frequently used contained 10 μM quinone, 100 μM DETAPAC, 100 μM neocuproine, 10 mM ascorbate, and 2 mM H2O2 in a 1:3 DMSO/phosphate buffer (20 mM, pH 7.4) (v/v) mixture. The last reagent added was ascorbate. Variations in the concentrations of these reagents were also used. The reason for adding DETAPAC and neocuproine was to further inhibit transition metal catalysis in addition to that mediated by Chelex treatment of solutions.32,33 The total volume of the solutions was 1.50 mL. Nitrogen-saturated amber 3.00 mL septum-capped bottles were used with continuous spinning bar stirring. The reaction bottles were placed in a water-jacketed beaker with a constant temperature of 37 °C before addition of reagents. After the reaction, a single aliquot of 50 μL was withdrawn from the gas phase on top of the reaction mixture and injected, using a gastight syringe, into a gas chromatograph for analysis. The chromatographic peak retention time of CH4 was established by using a CH4 standard.
The chromatograph used was an Agilent 6890 with thermal conductivity detection and an Alltech 16226 Porapak Q column. Chromatographic conditions were as follows: flow rate of 5.0 mL of He/min, column temperature of 35 °C, and injector and detector temperatures of 120 and 150 °C, respectively.
Alternatively, the detection of hydroxyl radicals was also done by measuring the hydroxylation products of salicylic acid.34 A reaction mixture containing 10 μM PHQ or DQ or no quinone, 100 μM DETAPAC, 100 μM neocuproine, 10 mM ascorbate, 1 mM salicylic acid, and 2 mM H2O2 in 20 mM phosphate buffer, pH 7.4, was incubated for 40 min followed by HPLC determination of 2,3- and 2,5-dihydroxybenzoic acid (2,3-dHB and 2,5-dHB, respectively) in the sample.
Hydroquinone Formation
The hydroquinone of PHQ was prepared, as described elsewhere, by reducing the quinone with NaBH4.35,36 For this purpose, a N2-saturated hydroquinone aqueous stock solution was prepared by mixing a N2-saturated PHQ solution in methanol with solid NaBH4. The solution was then dried with a N2 stream followed by addition of a N2-saturated water/DMSO (1:1 v/v) mixture. The solution pH was decreased to 3 by HCl addition to destroy excess NaBH4 and to stabilize the hydroquinone solution.
Determination of Half-Wave Reduction Potentials (E1/2)
Half-wave reduction potentials were determined in nitrogen-purged acetonitrile solutions containing 1 mM quinone and 0.1 M tetra-n-butylammonium perchlorate (TBAP) using differential pulse voltammetry (DPV). A BAS CV 50W voltammetric analyzer using a glassy carbon working electrode was used for these determinations. An Ag/AgCl(sat) electrode was used as the reference electrode (E′ = +0.22 V vs NHE), and a platinum wire was used as the counter electrode. Differential pulse voltammograms were obtained in the potential range from −2.00 to 0.00 V, using a 50 mV pulse amplitude and 20 mV/s of scan rate. The reduction potential values were obtained from the DPV peak potential maxima. These are similar to the half-wave one-electron reduction potentials, E1/2, in normal polarographic measurements.37
HPLC Analyses
HPLC analyses were done using a HP Zorbax C-18 (4.6 × 250 mm) column and eluted using a gradient from 100% ammonium phosphate (pH 3.5) to 100% methanol. The flow rate of elution was 1.0 mL/min. An Agilent 1100 analytical HPLC system with absorption detection at 300 nm was used. The retention times of the corresponding PHQ, salicylic acid, and 2,3- and 2,5-dHB peaks were determined using commercial standards. All determinations were repeated at least three times, and the average of these determinations ± SD is reported.
Results and Discussion
Quinone-Enhanced Production of OH Radicals
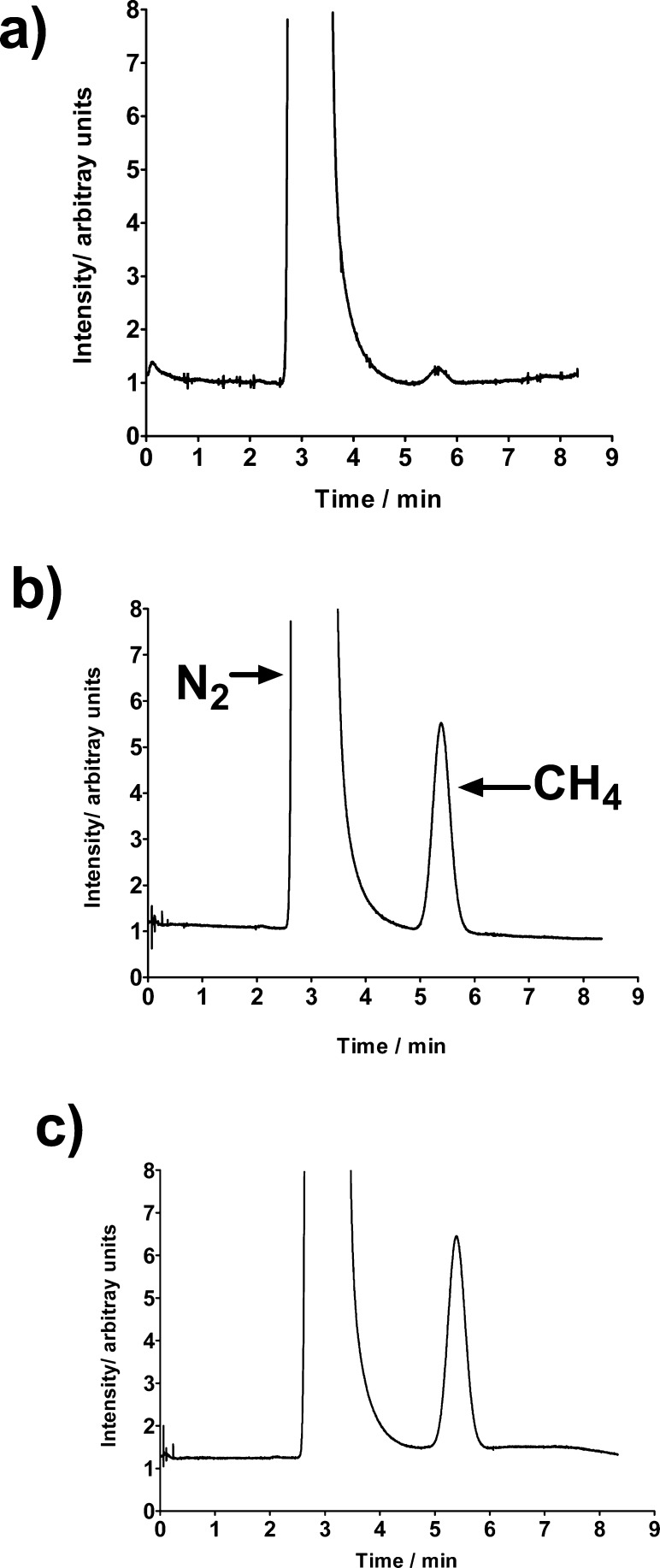
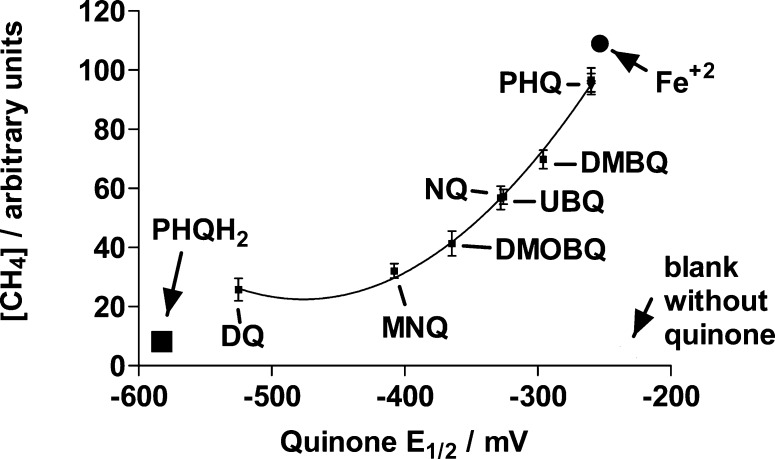
Upon adding a N2-saturated millimolar ascorbate solution to a N2-saturated solution containing DETAPAC, neocuproine, and H2O2 (millimolar) in a 1:3 DMSO/phosphate buffer (20 mM, pH 7.4) (v/v) mixture and incubating that solution for 40 min, a GC peak with a retention time of 5.4 min was detected. The identity of that peak was established by injecting pure methane to the GC. If an identical sample was prepared that instead contained 10 μM PHQ, then a methane GC peak was also detected that was 24 times more intense than that observed in the absence of quinone (Figure 2). Thus, PHQ strongly enhances the rate of hydroxyl radical formation, even at micromolar concentrations.
Figure 2.
Gas chromatograms of the gaseous phase of samples after 40 min of incubation. Samples contained (a) 100 μM DETAPAC, 100 μM neocuproine, 10 mM ascorbate, and 2 mM H2O2 in 1:3 DMSO/phosphate buffer (20 mM, pH 7.4) (v/v); (b) the same composition as in panel (a) but with 10 μM PHQ ; and (c) 10 μM FeCl2, 10 mM ascorbate, and 2 mM H2O2 in 1:3 DMSO/phosphate buffer (20 mM, pH 7.4) (v/v).
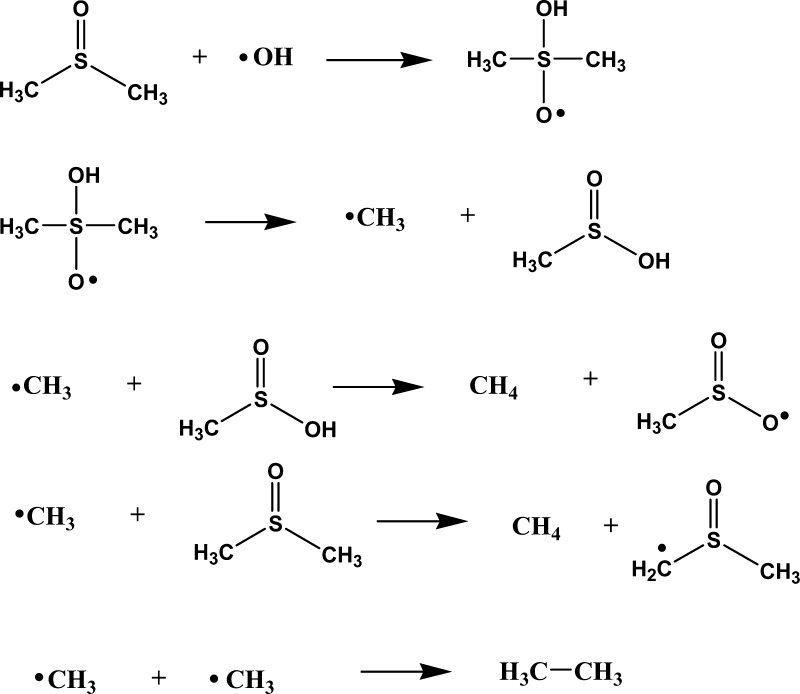
As indicated earlier, the indirect selective detection of OH radical production, where DMSO is the hydroxyl radical scavenger and the produced methane gas is detected by gas chromatography (GC), has been previously used in several works.23−28 The mechanism of this reaction has been previously determined using radiolysis and EPR techniques.29,30 The mechanism is illustrated in Scheme 1. Although ethane could be another observed product, its production is very limited at the high DMSO concentration used in this work (ca. 3.5 M) and thus the major gaseous product is methane.24
Scheme 1. Mechanism Proposed for the DMSO + OH Radical Reaction24.
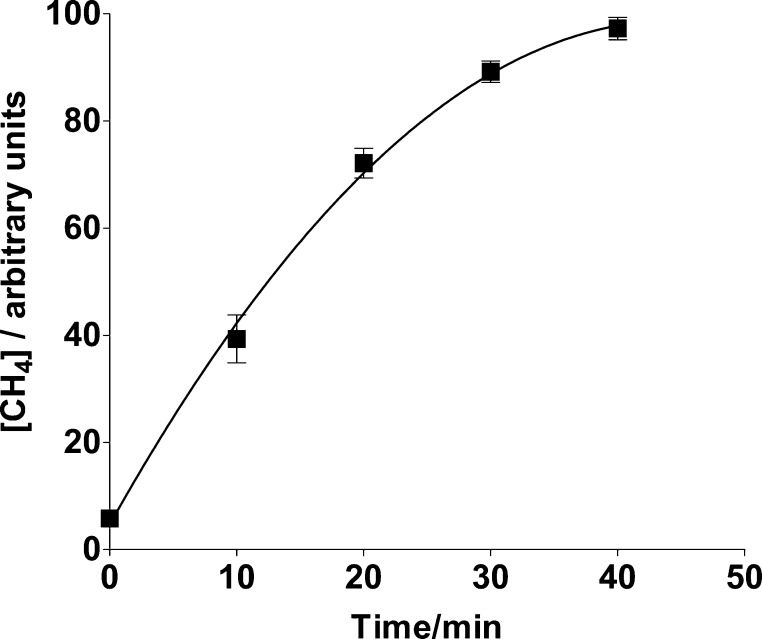
If PHQ was replaced by other quinones, then production of hydroxyl radicals was also detected in a manner that depends on the quinone one-electron reduction potential (Figure 3). Incubation times of 4 to 20 h were previously used in determining Henry’s constants for methane in water.38 The incubation time of 40 min used in the present work was selected in order to detect a relatively strong GC signal as well as to discriminate reactivity differences among the quinones used. A time course for CH4 generation by a sample, using PHQ as the quinone, is shown in Figure 4. Thus, although the GC CH4 peak areas do not correspond to the maximum areas that can be measured upon reaching methane solubility equilibrium, they reveal the catalytic effect of quinones on the production of hydroxyl radicals from H2O2 + ascorbate as well as their relative abilities to do so.
Figure 3.
Relative CH4 concentrations determined after a 40 min incubation of N2-saturated samples containing 10 μM quinone, 100 μM DETAPAC, 100 μM neocuproine, 10 mM ascorbate, and 2 mM H2O2 in 1:3 DMSO/phosphate buffer (20 mM, pH 7.4) (v/v). The symbols used to represent a blank sample without quinone, PHQH2, and Fe2+ are for solutions without quinone, with 10 μM PHQH2, or with 10 μM FeCl2, respectively, and correspond to the methane concentration, but not to the one-electron reduction potential, indicated by the position of the symbol. DETAPAC and neocuproine were not included in samples with FeCl2. The symbols ascribed to PHQ correspond to the same sample composition as that listed (■), with 10 μM DFO added (▲), or with 100 μM bathophenanthroline disulfonate and 100 μM ferrozine added (▼).
Figure 4.
Time dependence of CH4 concentrations determined from the headspace of a N2-saturated sample containing 10 μM PHQ, 10 mM ascorbate, 2 mM H2O2,100 μM DETAPAC, and 100 μM neocuproine in 1:3 DMSO/phosphate buffer (20 mM, pH 7.4) (v/v).
In order to further evidence hydroxyl radical formation, another probe for hydroxyl radicals was used, i.e., the hydroxylation of salicylic acid to form 2,3- and 2,5-dHB.34 Because the formation of 2,3-dHB is more prominent under our conditions, its chromatographic area was used as a measure of hydroxyl radical production. The ratio of the 2,3-dHB chromatographic peak area corresponding to a PHQ-containing sample to that of a DQ-containing sample (4.0 ± 0.2) was essentially the same as the ratio of the CH4 chromatographic peak area corresponding to a PHQ-containing sample to that of a DQ-containing sample (3.8 ± 0.3). Thus, the observed behavior between these 2 quinones is independent of the assay used to detect hydroxyl radical production.
In the absence of ascorbate, the CH4 GC peak areas obtained for samples containing PHQ, UBQ, MNQ, and DMOBQ are essentially the same as that of a blank sample in the absence of quinone. This indicates that the reaction reported for halogenated quinones involving the nucleophilic addition of HOO– to the quinone ring followed by hydroxyl radical production is not occurring. This is consistent with a previous report of the absence of hydroxyl radical production upon mixing H2O2 with DMBQ or DQ.19
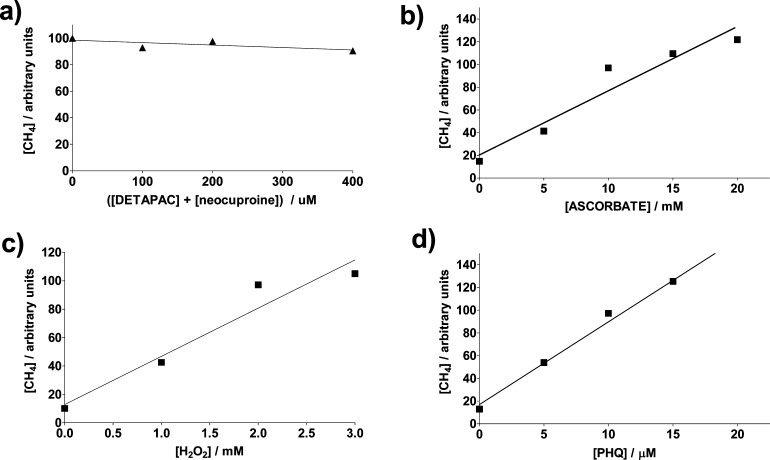
Varying chelating agent concentrations from 0 to 400 μM in samples containing the same amounts of ascorbate, H2O2, and PHQ, respectively, produced only a variation of up to ca. 10% in the GC peak areas (Figure 5a). Furthermore, addition of 10 μM of the iron chelator deferroxamine (DFO) or 100 μM of each of the nonhydroxamate iron chelators bathophenanthroline disulfonate and ferrozine to the sample in the presence of 100 μM DETAPAC and 100 μM neocuproine did not decrease the methane GC peak area compared to that in the absence of these chelators (Figure 3). The concentration of DFO used should be able to chelate any trace iron present after Chelex treatment of the water or solution.19 This is understandable due to the pretreatment of the water used. Thus, the dependence of the extent of methane production on the quinone one-electron reduction potential and the absence of variations in methane production as a function of transition metal chelator concentration indicate that the observed catalytic activity of the quinones is not metal-mediated. Substitution of PHQ with the same concentration of FeCl2 as that of the quinone, but excluding metal chelators in the incubation sample, exceeds the reactivity of 10 μM PHQ in producing hydroxyl radicals by 14% (Figure 3), as detected from the methane GC peak area. The Fenton reaction rate constant (Fe2+ + H2O2) is reported to be 2.0 × 104 M–1 s–1 in phosphate buffer.39 Assuming instantaneous semiquinone formation (see below) and similar rate constants for reactions after the hydroxyl radical production step, we can estimate the rate constant for the PHQ•–-assisted Fenton reaction (Reaction 2) from the GC peak area obtained from the PHQ-containing sample, compared to that of the Fe2+-containing sample, as k2 = (0.86) (2.0 × 104) = 1.7 × 104 M–1 s–1. Thus, PHQ reactivity is comparable to that of Fe2+. Because PHQ is a common toxin present in diesel combustion smoke, the possibility that PHQ-mediated catalysis of hydroxyl radical formation is similar to that of Fe2+ adds another important pathway to the modes in which PHQ can execute its toxicity. In an identical manner, values of k2 can be estimated for DMBQ, MNQ, and DQ as 1.3 × 104, 7.4 × 103, and 4.6 × 103 M–1 s–1, respectively. Rate constants, k2, for the electron transfer from the semiquinones of DMBQ, MNQ, and DQ to oxygen to form superoxide are 8.8 × 106, 2.4 × 108, and 2.2 × 108 M–1 s–1, respectively.40,41 Therefore, at equal concentrations of oxygen and H2O2, semiquinone reduction of oxygen is expected for these quinones instead of H2O2 reduction. Thus, H2O2 reduction by semiquinones should acquire more importance in hypoxic tissues whenever H2O2 concentrations exceed that of oxygen by several orders of magnitude.
Figure 5.
Relative CH4 concentrations determined after a 40 min incubation of N2-saturated samples containing (a) 10 μM PHQ, 10 mM ascorbate, 2 mM H2O2, and various concentrations of DETAPAC and neocuproine; (b) 10 μM PHQ, 100 μM DETAPAC, 100 μM neocuproine, 2 mM H2O2, and various concentrations of ascorbate; (c) 10 μM PHQ, 100 μM DETAPAC, 100 μM neocuproine, 10 mM ascorbate, and various concentrations of H2O2; and (d) 100 μM DETAPAC, 100 μM neocuproine, 10 mM ascorbate, 2 mM H2O2, and various concentrations of PHQ in 1:3 DMSO/phosphate buffer (20 mM, pH 7.4) (v/v).
Hydroquinone-Induced Production of OH Radicals
Replacing 10 μM PHQ with 10 μM of its hydroquinone in a sample containing 100 μM DETAPAC, 100 μM neocuproine, 10 mM ascorbate, and 2 mM H2O2 in a 1:3 DMSO/phosphate buffer (20 mM, pH 7.4) (v/v) mixture and incubating the mixture for 40 min produced a CH4 peak area that is only 14 ± 2% of that obtained in the PHQ-containing sample (Figure 3). Although this value is larger than that obtained in the absence of quinone, 2 ± 1%, it is consistent with the semiquinone, not the hydroquinone, being largely responsible for the enhanced production of hydroxyl radicals.
Further Evidence for the Semiquinone Role
In order to obtain further evidence that the semiquinone is the actual H2O2-reducing species, a PHQ solution containing DMSO, DETAPAC, H2O2, neocuproine, and phosphate buffer (pH 7.4) (v/v) was mixed with an equal volume of a PHQH2 solution in the absence of oxygen and ascorbate. The idea is to produce the PHQ semiquinone through the quinone–hydroquinone comproportionation reaction (Reaction 1). Final concentrations after mixing were 5 mM PHQ, 5 mM PHQH2, 100 μM DETAPAC, 100 μM neocuproine, and 2 mM H2O2 in 1:3 DMSO/phosphate buffer (20 mM, pH 7.4) (v/v). The last reagent added was H2O2. The PHQH2 concentration used contains the same amount of reducing equivalents as those involved in the one-electron oxidation of 10 mM ascorbate. The methane GC peak area obtained (93 ± 8 arbitrary units) after incubation for 40 min was essentially the same as that obtained when H2O2 reduction occurs by reacting ascorbate with PHQ (97 ± 4 arbitrary units, Figure 3). Because most para-semiquinones have pKa values for their protonated form, QH•, between 3 and 542−45 and those corresponding to ortho-semiquinones, between 4 and 5,46 the anion radical species is the most abundant species at physiological pH and thus is the carrier of electrons from ascorbate to H2O2.
The pKa value of H2O2 is 11.6.47 Thus, it is very unlikely that Michael addition of HOO– to the quinones occurs at the pH used for this work because very low concentrations of this anion will be present. Furthermore, the PHQ relative HPLC peak area of a H2O2-containing sample and that of a sample with no H2O2 and no ascorbate added, both containing phosphate buffer, DMSO, DETAPAC, and neocuproine, after 40 min of incubation, was 103.4 ± 0.5 and 100 ± 1 relative units, respectively. The latter shows that PHQ is not being transformed to another type of molecule during the incubation time in the presence of H2O2. Such a transformation would have made the dependence of the CH4 area on the E1/2 values of the quinones very erratic, as their identities in the reaction samples would have been different from those present during the E1/2 measurements.
Roles of Quinone, H2O2, and Ascorbate Concentrations
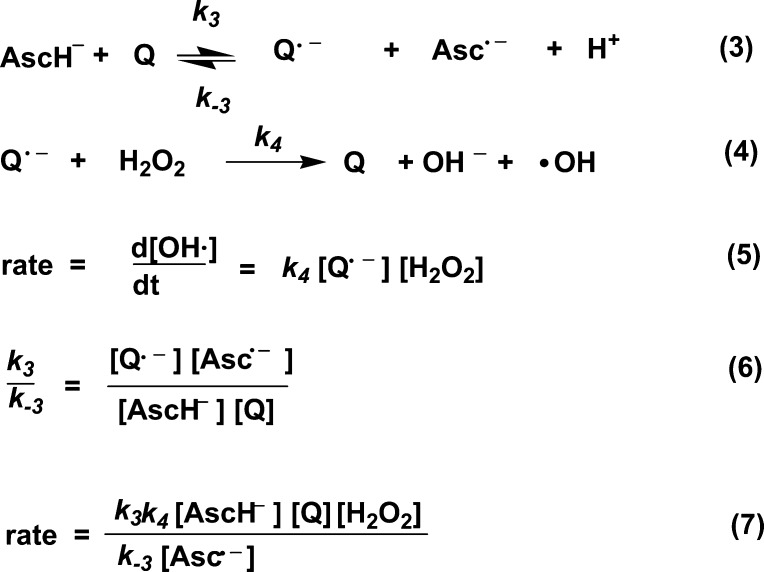
The rate of CH4 formation increases linearly with an increase in concentration of all reagents, i.e., PHQ, AscH–, and H2O2 (keeping the other reagents at constant concentrations) (Figure 5), indicating first-order kinetics with respect to each of these species. The fact that this rate depends on H2O2 concentration is in contrast to the zero-order behavior of the ascorbate autoxidation rate with respect to O2.6 If the semiquinone reduction of H2O2 is postulated to be the slow step in the mechanism, a rate equation can be obtained which is first-order with respect to each species, PHQ, AscH–, and H2O2 (Scheme 2). A constant steady-state concentration of the ascorbyl radical, Asc•–, has been observed in previous works during the quinone-enhanced48 as well as in the iron- and methylene blue-catalyzed49 ascorbate oxidation reactions. Thus, the k4k3/k–3[Asc•–] ratio is behaving as a constant.
Scheme 2. Postulated Mechanism for the Quinone-Enhanced Ascorbate Reduction of H2O2.
In summary, quinones reduce H2O2 in the presence of ascorbate in a quinone redox-potential-dependent manner and independent of transition metal traces. The most active quinone in the studied series, PHQ, is only 14% less active than the classic Fenton reagent cation, Fe2+, at the same concentration. These observations are relevant to the antitumor-enhancing activity of quinones in the presence of ascorbate and to the toxic activity of environmental quinone contaminants.
Glossary
Abbreviations
- 2,3-dHB
2,3-dihydroxybenzoic acid
- 2,5-dHB
2,5-dihydroxybenzoic acid
- AscH–
ascorbate
- Asc•–
ascorbyl radical
- DETAPAC
diethylenetriaminepentaacetic acid
- DPV
differential pulse voltammetry
- DMOBQ
2,6-dimethoxybenzoquinone
- UBQ-0
2,3-dimethoxy-5-methyl-1,4-benzoquinone
- DMBQ
2,6-dimethylbenzoquinone
- EPR
electron paramagnetic resonance
- QH2
hydroquinone
- PHQH2
hydroquinone of PHQ
- MNQ
methyl-1,4-naphthoquinone
- NQ
1,4-naphthoquinone
- PHQ
9,10-phenanthraquinone
- Q
quinone
- ROS
reactive oxygen species
- Q•–
semiquinone
- DQ
tetramethyl-1,4-benzoquinone
The authors express appreciation for grant nos. S06-GM008216 and P20 RR-016470 from the National Institutes of Health and University of Puerto Rico-FOPI program for financial support of this work.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Boyle R. G.; Travers S. (2006) Hypoxia: targeting the tumour. Anti-Cancer Agents Med. Chem. 6, 281–286. [DOI] [PubMed] [Google Scholar]
- Laskin J. D.; Rao N. R.; Punjabi C. J.; Laskin D. L.; Synder R. (1995) Distinct actions of benzene and its metabolites on nitric oxide production by bone marrow leukocytes. J. Leukocyte Biol. 57, 422–426. [DOI] [PubMed] [Google Scholar]
- Taguchi K.; Shimada M.; Fujii S.; Sumi D.; Pan X.; Yamano S.; Nishiyama T.; Hiratsuka A.; Yamamoto M.; Cho A. K.; Froines J. R.; Kumagai Y. (2008) Redox cycling of 9,10-phenanthraquinone to cause oxidative stress is terminated through its monoglucuronide conjugation in human pulmonary epithelial A549 cells. Free Radical Biol. Med. 44, 1645–1655. [DOI] [PubMed] [Google Scholar]
- O’Brien P. J. (1991) Molecular mechanisms of quinone cytotoxicity. Chem.–Biol. Interact. 80, 1–41. [DOI] [PubMed] [Google Scholar]
- Tudor G.; Gutierrez P.; Aguilera-Gutierrez A.; Sausville E. A. (2003) Cytotoxicity and apoptosis of benzoquinones: redox cycling, cytochrome c release, and BAD protein expression. Biochem. Pharmacol. 65, 1061–1075. [DOI] [PubMed] [Google Scholar]
- Roginsky V. A.; Barsukova T. K.; Stegmann H. B. (1999) Kinetics of redox interaction between substituted quinones and ascorbate under aerobic conditions. Chem.–Biol. Interact. 121, 177–197. [DOI] [PubMed] [Google Scholar]
- Alegría A. E.; Sanchez S.; Quintana I. (2004) Quinone-enhanced ascorbate reduction of nitric oxide: role of quinone redox potential. Free Radical Res. 38, 1107–1112. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cruz P.; Garcia C.; Alegría A. E. (2010) Role of quinones in the ascorbate reduction rates of S-nitrosoglutathione. Free Radical Biol. Med. 49, 1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrax J.; Stockis J.; Tison A.; Taper H. S.; Calderon P. B. (2006) Oxidative stress by ascorbate/menadione association kills K562 human chronic myelogenous leukaemia cells and inhibits its tumour growth in nude mice. Biochem. Pharmacol. 72, 671–680. [DOI] [PubMed] [Google Scholar]
- Cox A. G.; Winterbourn C. C.; Hampton M. B. (2009) Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem. J. 425, 313–325. [DOI] [PubMed] [Google Scholar]
- Afanas’ev I. (2010) Signaling and damaging functions of free radicals in aging-free radical theory, hormesis, and TOR. Aging Dis. 1, 75–88. [PMC free article] [PubMed] [Google Scholar]
- Du J.; Cullen J. J.; Buettner G. R. (2012) Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 1826, 443–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lazaro M. (2007) Dual role of hydrogen peroxide in cancer: Possible relevance to cancer chemoprevention and therapy. Cancer Lett. 252, 1–8. [DOI] [PubMed] [Google Scholar]
- Cadenas E. (1989) Biochemistry of oxygen toxicity. Annu. Rev. Biochem. 58, 79–110. [DOI] [PubMed] [Google Scholar]
- Nohl H.; Jordan W. (1985) The involvement of biological quinones in the formation of hydroxyl radicals via the Haber–Weiss reaction. Bioorg. Chem. 15, 374–382. [Google Scholar]
- Koppenol W. H.; Butler J. (1985) Energetics of interconversion reactions of oxyradicals. Adv. Free Radical Biol. Med. 1, 91–131. [Google Scholar]
- Ohno S.; Ohno Y.; Suzuki N.; Soma G.; Inoue M. (2009) High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer. Anticancer Res. 29, 809–815. [PubMed] [Google Scholar]
- Verrax J.; Beck R.; Dejeans N.; Glorieux C.; Sid B.; Pedrosa R. C.; Benites J.; Vásquez D.; Valderrama J. A.; Calderon P. B. (2011) Redox-active quinones and ascorbate: an innovative cancer therapy that exploits the vulnerability of cancer cells to oxidative stress. Anti-Cancer Agents Med. Chem. 11, 213–221. [DOI] [PubMed] [Google Scholar]
- Zhu B.-Z.; Zhao H.-T.; Kalyanaraman B.; Frei B. (2002) Metal-independent production of hydroxyl radicals by halogenated quinones and hydrogen peroxide: an ESR spin trapping study. Free Radical Biol. Med. 32, 465–473. [DOI] [PubMed] [Google Scholar]
- Zhu B.-Z.; Kalyanaraman B.; Jiang G.-B. (2007) Molecular mechanism for metal-independent production of hydroxyl radicals by hydrogen peroxide and halogenated quinones. Proc. Natl. Acad. Sci. U. S. A. 104, 17575–17578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B.-Z.; Mao L.; Huang C.-H.; Qin H.; Fan R.-M.; Kalyanaraman B.; Zhu J.-G. (2012) Unprecedented hydroxyl radical-dependent two-step chemiluminescence production by polyhalogenated quinoid carcinogens and H2O2. Proc. Natl. Acad. Sci. U.S.A. 109, 16046–16051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K.; Kanno T.; Ikai H.; Sato E.; Mokudai T.; Niwano Y.; Ozawa T.; Kohno M. (2012) Reevaluation of quantitative ESR spin trapping analysis of hydroxyl radical by applying sonolysis of water as a model system. Bull. Chem. Soc. Jpn. 83, 1037–1046. [Google Scholar]
- Repine J. E.; Eaton J. W.; Anders M. W.; Hoidal J. R.; Fox R. B. (1979) Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J. Clin. Invest. 64, 1642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt M. K.; Colina R. (1988) The reaction of OH radicals with dimethyl sulfoxide. A comparative study of Fenton’s reagent and the radiolysis of aqueous dimethyl sulfoxide solutions. J. Org. Chem. 53, 1071–1074. [Google Scholar]
- Roychoudhury S.; Ghosh S.; Chakraborti T.; Chakraborti S. (1996) Role of hydroxyl radical in the oxidant H2O2-mediated Ca2+ release from pulmonary smooth muscle mitochondria. Mol. Cell. Biochem. 159, 95–103. [DOI] [PubMed] [Google Scholar]
- Baser M. E.; Kennedy T. P.; Dodson R.; Rao N. V.; Rawlings W.; Hoidal J. R. (1990) Hydroxyl radical generating activity of hydrous but not calcined kaolin is prevented by surface modification with dipalmitoyl lecithin. J. Toxicol. Environ. Health 29, 99–108. [DOI] [PubMed] [Google Scholar]
- Fox R. B. (1984) Prevention of granulocyte-mediated oxidant lung injury in rats by a hydroxyl radical scavenger, dimethylthiourea. J. Clin. Invest. 74, 1456–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G.; Cederbaum A. I. (1979) Chemical evidence for production of hydroxyl radicals during microsomal electron transfer. Science 204, 66–68. [DOI] [PubMed] [Google Scholar]
- Gilbert B. C.; Norman R. O. C.; Sealy R. C. (1975) Electron spin resonance studies. Part XLIII. Reaction of dimethyl sulfoxide with the hydroxyl radical. J. Chem. Soc., Perkin Trans. 2, 303–308. [Google Scholar]
- Veltwisch D.; Janata E.; Asmus K.-D. (1980) Primary processes in the reaction of OH radicals with sulfoxides. J. Chem. Soc., Perkin Trans. 2, 146–153. [Google Scholar]
- Buettner G. R. (1988) In the absence of catalytic metals ascorbate does not autoxidize at pH 7: ascorbate as a test for catalytic metals. J. Biochem. Biophys. Methods 16, 27–40. [DOI] [PubMed] [Google Scholar]
- Buettner G. R.; Jurkiewicz B. A. (1996) Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat. Res. 145, 532–541. [PubMed] [Google Scholar]
- Goldstein S.; Czapski G. (1985) Kinetics of oxidation of cuprous complexes of substituted phenanthroline and 2,2′-bipyridyl by molecular oxygen and by hydrogen peroxide in aqueous solution. Inorg. Chem. 24, 1087–1092. [Google Scholar]
- Maskos Z.; Rush J. D.; Koppenol W. H. (1990) The hydroxylation of the salicylate anion by a Fenton reaction and Γ-radiolysis: a consideration of the respective mechanisms. Free Radical Biol. Med. 8, 153–162. [DOI] [PubMed] [Google Scholar]
- Alegría A. E.; Sanchez-Cruz P.; Lopez-Colon D. (2005) Sonochemically induced covalent binding of calf thymus DNA by aziridinylquinones. Radiat. Res. 164, 446–452. [DOI] [PubMed] [Google Scholar]
- Gutierrez P. L.; Balachandran Nayar M. S.; Nardino R.; Callery P. S. (1987) The chemical reduction of diaziquone: products and free radical intermediates. Chem.–Biol. Interact. 64, 23–37. [DOI] [PubMed] [Google Scholar]
- Sawyer D. T., and Roberts J. L. (1974) Experimental Electrochemistry for Chemists, Wiley and Sons, New York. [Google Scholar]
- Benson B. B.; Krause D. Jr.; Peterson M. A. (1979) The solubility and isotopic fractionation of gases in dilute aqueous solution. I. Oxygen. J. Solution Chem. 8, 655–690. [Google Scholar]
- Yamazaki I.; Piette L. H. (1990) ESR spin-trapping studies on the reaction of Fe2+ ions with H2O2-reactive species in oxygen toxicity in biology. J. Biol. Chem. 265, 13589–13594. [PubMed] [Google Scholar]
- Meisel D. (1975) Free energy correlation of rate constants for electron transfer between organic systems in aqueous solutions. Chem. Phys. Lett. 34, 263–266. [Google Scholar]
- Song Y.; Buettner G. R. (2010) Thermodynamic and kinetic considerations for the reaction of semiquinone radicals to form superoxide and hydrogen peroxide. Free Radical Biol. Med. 49, 919–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P. S.; Hayon E. (1973) Ionization constants and spectral characteristics of some semiquinone radicals in aqueous solution. J. Phys. Chem. 77, 2274–2276. [Google Scholar]
- Land E. J.; Mukherjee T.; Swallow J. (1983) Reduction of naphthazarin molecule as studied by pulse radiolysis. J. Chem. Soc., Faraday Trans. 79, 391–404. [Google Scholar]
- Mukherjee T.; Swallow A. J.; Guyan P. M.; Bruce J. M. (1990) One- and two-electron reduction of quinizarin and 5-methoxyquinizarin: a pulse radiolysis study. J. Chem. Soc., Faraday Trans. 86, 1483–1491. [Google Scholar]
- Ilan Y. A.; Czapski G.; Meisel D. (1976) The one-electron transfer redox potentials of free radicals. I. The oxygen/superoxide system. Biochim. Biophys. Acta, Bioenerg. 430, 209–224. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B. (1990) Characterization of o-semiquinone radicals in biological systems. Methods Enzymol. 186, 333–343. [DOI] [PubMed] [Google Scholar]
- Lamb F. S.; Moreland J. G.; Miller F. J. Jr. (2009) Electrophysiology of reactive oxygen production in signaling endosomes. Antioxid. Redox Signaling 11, 1335–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roginsky V. A.; Bruchelt G.; Stegmann H. B. (1998) Fully reversible redox cycling of 2,6-dimethoxy-1,4-benzoquinone induced by ascorbate. Biochemistry (Moscow) 63, 200–206. [PubMed] [Google Scholar]
- Roginsky V. A.; Stegmann H. B. (1994) Ascorbyl radical as natural indicator of oxidative stress: quantitative regularities. Free Radical Biol. Med. 17, 93–103. [DOI] [PubMed] [Google Scholar]