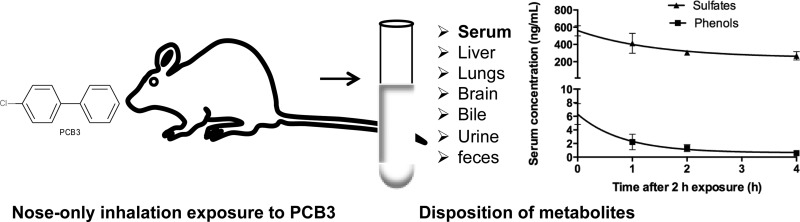
Abstract
PCBs, such as PCB3, are air contaminants in buildings and outdoors. Metabolites of PCB3 are potential endocrine disrupting chemicals and genotoxic agents. We studied the disposition of phenolic and sulfated metabolites after acute nose-only inhalation exposure to airborne PCB3 for 2 h in female rats. Inhalation exposure was carried out in three groups. In the first group, rats exposed to an estimated dose of 26 μg/rat were euthanized at 0, 1, 2, and 4 h after exposure. Highest concentrations of phenols and sulfates were observed at 0 h, and the values were 7 ± 1 and 560 ± 60 ng/mL in serum, 213 ± 120 and 842 ± 80 ng/g in liver, 31 ± 27 and 22 ± 7 ng/g in lung, and 27 ± 6 and 3 ± 0 ng/g in brain, respectively. First-order serum clearance half-lives of 0.5 h for phenols and 1 h for sulfates were estimated. In the second group, rats exposed to an estimated dose of 35 μg/rat were transferred to metabolism cages immediately after exposure for the collection of urine and feces over 24 h. Approximately 45 ± 5% of the dose was recovered from urine and consisted mostly of sulfates; the 18 ± 5% of the dose recovered from feces was exclusively phenols. Unchanged PCB3 was detected in both urine and feces but accounted for only 5 ± 3% of the dose. Peak excretion of metabolites in both urine and feces occurred within 18 h postexposure. In the third group, three bile-cannulated rats exposed to an estimated dose of 277 μg/rat were used for bile collection. Bile was collected for 4 h immediately after 2 h exposure. Biliary metabolites consisted mostly of sulfates, some glucuronides, and lower amounts of the free phenols. Control rats in each group were exposed to clean air. Clinical serum chemistry values, serum T4 level, and urinary 8-hydroxy-2′-deoxyguanosine were similar in treated and control rats. These data show that PCB3 is rapidly metabolized to phenols and conjugated to sulfates after inhalation and that both of these metabolites are distributed to liver, lungs, and brain. The sulfates elaborated into bile are either reabsorbed or hydrolyzed in the intestine and excreted in the feces as phenols.
Introduction
Polychlorinated biphenyls (PCBs) are a group of 209 synthetic chemicals, named PCB1 to PCB209, according to the number and position of chlorine atoms on the biphenyl ring.1 The intentional commercial production of PCBs was banned in 1977 in the United States. They were formerly used in a variety of items as lubricants, flame retardants, coolants, insulating agents, or plasticizer chemicals.2 PCBs in general are persistent chemicals, as they are often resistant to degradation by environmental processes.3 Legacy PCBs contaminate food and water, which are already well-known sources of oral exposures. Detection of PCBs in the air samples from both indoor and outdoor environments has raised a concern for inhalation exposures in the recent years.4 Airborne PCBs are mostly lower chlorinated congeners.5 These PCBs have high vapor pressures.6 PCB3, with its one chlorine atom, is more volatile than most other PCB congeners. PCB3 has been detected in homes,7,8 as well as in outdoor air samples.9 This congener was a major constituent of Aroclor 1221 and 1232 and is present in many commercial PCB mixtures.1
Phenolic metabolites of PCBs are endocrine disrupting chemicals (EDCs). Exposure to both PCB3 and its metabolites caused a significant increase in estradiol level in the medium of granulosa and theca cells derived from porcine ovary.10 Estrogenic effects of many OH-PCBs also occur through the inhibition of enzymes involved in steroid hormone metabolism.11 Phenolic metabolites of PCBs also disrupt thyroid hormone homeostasis by inhibition of thyroid hormone sulfation12 and by interfering with transthyretin in the transport of thyroid hormones in circulation.13 A recent study shows that PCB3 metabolites are high-affinity ligands of transthyretin in vitro.14 Exposure to the environmental EDCs may not be manifested in a clinical disease but may be an underlying cause of reproductive and developmental abnormalities later in life.15
According to the International Agency for Research in Cancer, PCBs are human carcinogens.16 Metabolically active, lower chlorinated PCBs are bioactivated to genotoxic agents. Bioactivation of PCB3 occurs by the formation of electrophiles, such as arene oxides and PCB quinones. An arene oxide of PCB3 is formed during the initial oxidation of PCB3 by cytochrome P450 enzymes.17,18 PCB quinones are formed by the oxidation of dihydroxylated PCBs by peroxidases such as prostaglandin synthase19 and myeloperoxidase.20 PCB quinones produce reactive oxygen (ROS) species through a futile redox cycling with the semiquinones.21 Taken together, formation of ROS and electrophiles are the basis of PCB3-induced genotoxicity in various cell lines.22,23
Although bioactivation and toxicity of OH-PCBs have been the focus of many studies, toxicologic properties of sulfated PCBs are largely unreported. OH-PCBs are good substrates for purified rat and human SULT1A and SULT2A isoforms in vitro.24,25 Sacco et al. found that liver cytosol prepared from polar bear can efficiently form sulfate conjugates of many OH-PCBs.26 Previously, we showed that a sulfate conjugate is a major urinary metabolite of PCB3 in rats and that the serum concentrations of sulfates were 64 times higher than the free phenolic forms.27 Interestingly, plants, including poplar trees, can form sulfated PCBs after exposure through their roots.28 We tested stability of mono- to pentachlorinated PCB sulfates in human urine and found that they are stable for a prolonged time at room temperature without any preservatives.29 Incubation of sulfated PCBs with transthyretin in vitro has resulted in high-affinity binding at thyroid hormone (T4) binding sites, indicating the possibility of transport of these metabolites into the brain and disruption of thyroid hormone homeostasis.14 These studies indicate that PCB sulfates are biologically formed, stable metabolites and are potential EDCs.
Few studies exist in the literature describing the disposition of PCBs after inhalation exposure.4 On the basis of the concentration of PCBs in indoor and outdoor air samples and dietary sources, Currado et al. estimated that inhalation exposure may contribute significantly (6 to 64%) to the body burden of PCBs in adults in the UK.30 Previously, Hu et al. carried out inhalation exposure studies in rats using Aroclor 1242,31 a mixture of PCBs resembling Chicago air profile,32 and PCB11.33 These inhalation exposures in rats have demonstrated that lower chlorinated PCBs are quickly absorbed and distributed to tissues from the lungs. In a subsequent study, Hu et al. reported that 99.8% of PCB11 was absorbed from the lungs and was excreted mostly as conjugated metabolites in urine and feces.34 We recently reported that sulfate conjugates are the metabolite markers of inhalation exposure to PCB3 in rats.29 In this study, we further characterize the inhalation exposure of lower chlorinated PCBs by measuring the disposition of PCB3 metabolites in tissues, serum, bile, urine, and feces.
We carried out nose-only inhalation exposure to PCB3 in bile-cannulated and intact rats. In addition to disposition and excretion of metabolites, we also measured urinary 8-oxo-dG as a marker of PCB3 bioactivation to electrophilic species and serum T4 level as an indicator of interaction of PCB sulfates with transthyretin.
Materials and Methods
Standards and Chemicals
PCB3, 4′OH-PCB3, 3′4′-diOH-PCB3, 2′5′-diOH-PCB3, 2′3′-diOH-PCB3, 3PCB3 sulfate, 3′PCB3 sulfate, 4′PCB3 sulfate, 3-F,4′OH-PCB3 (Internal standard, IS), and 3-F,4′PCB3 sulfate (IS) were synthesized as described previously.27,35,36 PCB3 purity was 99.7%, as determined by gas chromatography (GC). Stock solution of standards was prepared in methanol. All solvents used were HPLC grade and purchased from Fisher Scientific Co. (St. Louis, MO). Weak anion exchange (WAX) cartridges, a solid-phase extraction column of 1 mL (30 mg, 30 μM) or 6 mL (150 mg, 30 μM) capacity, were purchased from Water Oasis (Milford, MA,). Sulfatase (sulfatase type H-2 crude solution from Helix pomatia), glucuronidase (β-glucuronidase type XI from Escherichia coli), and d-saccharic-1,4-lactone were obtained from Sigma-Aldrich (St. Louis, MO). All other chemicals were of the highest purity commercially available.
Generation of PCB3 Vapor
The studies presented here were carried out in three separate experiments (groups 1, 2, and 3) in a 6 m3 secondary containment area with appropriate personal protection for laboratory staff. PCB3 (2 g) was dissolved in diethyl ether (5 mL) and added to a round-bottomed flask (500 mL) containing glass beads (300 g, 4.75 mm diameter). PCB3 was coated on the glass beads by completely removing the solvent in a rotary evaporator. The flask was fitted with a glass stopper having inlet and outlet inline tubes as illustrated in Figure 1. The outlet of the flask was connected to the nose-only inhalation system (InTox, Inc., Albuquerque, NM, for groups 1 and 2; SCIREQ Inc., Montreal, Canada, for group 3). The flow of air through the inhalation system was maintained at 11 L/min in the InTox system and 14 L/min in the SCIREQ system by a vacuum pump. The air containing PCB3 was sampled by adsorption onto polymeric resin (10 g, Amberlite XAD-2, Supelco Analytical, Bellefonte, PA) packed in a stainless steel cartridge. The flask was kept in a precision water bath maintained at 25 °C (Thermo Scientific, Portsmouth, NH). To ensure that the animals were exposed only to PCB3 vapor and not to the particulate matter, a layer of glass wool was placed over the glass beads. Concentration of PCB3 in air (Cair) was determined from PCB3 captured in XAD divided by the air volume sampled during exposure.
Figure 1.
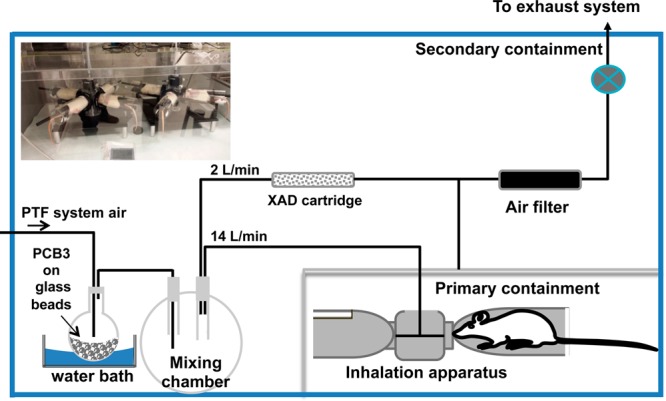
Illustration of nose-only inhalation system.
Animal Exposure
All animal experiments were conducted under approved protocols by the Institutional Animal Care and Use Committee of the University of Iowa. Female Sprague–Dawley (SD) rats were purchased from Harlan Sprague–Dawley Inc. (Indianapolis, IN). Upon arrival to our vivarium, animals were individually housed in cages with food and water ad libitum and allowed to acclimatize to the new environment for 3 days. The animals were kept in a room that was maintained at 22 °C with a 12 h light–dark cycle. The day before the experiment, rats were transferred to metabolism cages and also given training to adjust to the nose-only inhalation system by keeping them there for 2 h. All exposure experiments were 2 h in duration.
In the first group (n = 15, 190–225 g), three animals were euthanized at each of four time points: 0, 1, 2, and 4 h after exposure concluded. Three control rats were similarly held in the nose-only inhalation tube and exposed to laboratory air in an adjacent room and euthanized at 0 h. Lungs, liver, brain, and serum were collected from these animals.
In the second group (n = 6, 190–225 g), rats were exposed to PCB3 or laboratory air and transferred to metabolism cages immediately after exposure. Urine and feces were collected every 6 h over a 24 h period.
In the third group (n = 9, 260–290 g), three bile-cannulated and three intact rats were exposed to PCB3 for 2 h. Three intact rats were used as controls. Immediately after exposure, intact rats were transferred to metabolism cages for the collection of urine. Bile-cannulated rats were held on the bench, and bile was collected for 4 h from the surgically attached cannula. Bile was also collected before the exposure and used as baseline data.
The dose of PCB3 exposure was estimated using the following equation29,31
For these experiments, we assumed a frequency of breathing (f) of 94 breaths/min, tidal volume (Vt) of 1.5 mL/breath, and uptake from the lungs (α) of 99.89%.34Cair is the exposure concentration of PCB3, and time (T) is the duration of animal exposure.
Extraction of PCB3 and Its Metabolites from Tissue
Simultaneous extraction of PCB3, hydroxylated PCB3, and sulfated PCB3 from tissues and fluids was carried out according to a previously described procedure with some changes.29 A flow diagram of all steps of the extraction is presented in Figure 2. Urine, serum, or bile (1 mL) was acidified by adding glacial acetic acid (20 μL) and extracted in acetonitrile (2 mL). Sodium acetate buffer (25 mM, pH 4.0) was added to make the final volume 8 mL. Tissues (1–2 g) and feces (1–3 g) were homogenized in Milli-Q water (5 mL), acidified by adding glacial acetic acid (100 μL), extracted in acetonitrile (40 mL), and concentrated to 10 mL. Sodium acetate buffer (25 mM, pH 4.0) was added to make the final volume 40 mL.
Figure 2.

Flow diagram for simultaneous extraction of PCB3 and its metabolites from serum, urine, and bile. Tissues and fecal samples were extracted in 40 mL of acetonitrile, and 6 mL capacity WAX columns were used for cleanup. The volume of solvents required for conditioning, washing, and eluting were increased to 6 mL.
The buffered urine, bile, or serum sample was applied to a preconditioned 1 mL WAX column. The column was washed with 1 mL of methanol in water (50:50, v/v) containing 2% NH4OH and dried for 1 h. The metabolites were eluted from the column with 1 mL of methanol in acetonitrile (20:80, v/v) containing 2% NH4OH. A final elution with hexane (1 mL) was carried out to completely elute PCB3 from the column. Buffered tissue or fecal sample was applied to a preconditioned 6 mL capacity column. Washing and eluting volumes were increased to 6 mL. Final WAX extract was evaporated nearly to dryness, reconstituted in 35% acetonitrile in water (200 μL), and transferred into an autosampler vial for analysis of sulfates and phenols. For the analysis of sulfates, an internal standard (50 μL, 3-F, 4′PCB3 sulfate at 0.5 μg/mL in 35% acetonitrile in water) was added, and 5 μL was injected into the liquid chromatography/mass spectrometry (LC/MS) system. To the same vial, after the analysis of sulfates, an internal standard (50 μL, 3-F-4′OH-PCB3 at 0.5 μg/mL in methanol) was added, and phenols were analyzed. For the analysis of PCB3 in feces and urine, final acetonitrile extract was concentrated to exactly 500 μL, and 2 μL was injected into gas chromatography/mass spectrometry (GC/MS) system. The recovery for PCB sulfates and phenols from spiked matrices is presented in the Supporting Information.
Enzyme Incubation
The WAX column-cleaned acetonitrile extracts of bile or urine were aliquoted equally to three clean vials, uncapped, and kept in a fume hood until the solvent evaporated completely. Reference samples contained 100 ng of authentic 4′PCB3 sulfate standard in 300 μL of acetonitrile. The vials were reconstituted in 100 μL of sodium acetate buffer (25 mM, pH 7.0) containing no enzymes, 100 μL of sodium acetate buffer (25 mM, pH 5.0) containing 100 units β-glucuronidase, or 100 μL of sodium acetate buffer (25 mM, pH 5.0) containing 25 units sulfatase and d-saccharic acid-1,4-lactone (20 mM). The mixture was incubated at 37 °C overnight to allow complete hydrolysis of conjugated metabolites.27 The reactions were terminated by adding an equal volume of methanol. The final volume (200 μL) was centrifuged at 12 000g for 10 min, the supernatant fraction (50 μL) was transferred to an autosampler vial, an internal standard (50 μL, 3F′-4′OH-PCB3 at 0.5 μg/mL in methanol) was added, and the sample analyzed by LC/MS.
LC/MS
Analysis of PCB3 phenols and sulfates was carried out with a Waters Acquity Tandem Quadrupole Detector (TQD) instrument (Waters, USA). Chromatographic separation was achieved on an Agilent RP C18 column (4.6 × 250 mm, 5 μm) at ambient temperature. Mobile phases were 100% acetonitrile (A) and 5 mM ammonium acetate in water (B). Sulfated metabolites were eluted from the column using an isocratic mobile phase containing 35% of A and a flow rate of 1 mL/min. Phenols were eluted using an isocratic mobile phase containing 80% of A and a flow rate of 1 mL/min. The mass spectrometer was used in selected-ion monitoring (SIM) mode to analyze the (M – H)− of interest by negative electrospray ionization. Other TQD parameters were as follows: 120 °C, source temperature; 600 °C, dessolvation gas temperature; 2.6 kV, cone voltage; 100 L/h, cone gas flow; and 600 L/h, dessolvation gas flow.
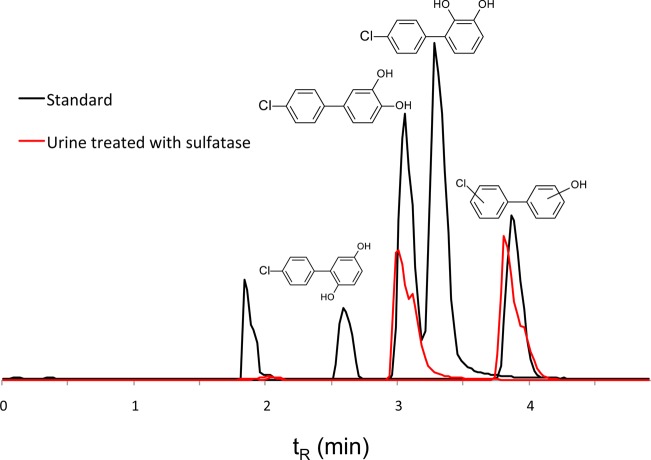
Representative SIM chromatograms for authentic standards of mono- and dihyroxylated PCB3s are shown in Figure 3. All three dihydroxylated PCB3 compounds (2′5′-diOH-PCB3, 3′4′-diOH-PCB3, and 2′3′-diOH-PCB3) were completely separated. We could not separate the isomers of monohydroxylated PCB3, and they eluted as a single peak at 3.8 min. We quantified this peak from a calibration curve prepared from 4′OH-PCB3.
Figure 3.
Chromatograms of phenolic metabolites. SIM chromatograms showing the separation of dihydroxylated (m/z 219) and monohydroxylated (m/z 203) metabolites of PCB3. A representative chromatogram of urine sample treated with sulfatase has been overlaid on the chromatogram of authentic standards to show different dihydroxylated metabolites formed in vivo. Chromatography was performed on a RP C18 column with an isocratic mobile phase of 80% acetonitrile in water (5 mM ammonium acetate) and a flow rate of 1 mL/min. This method could not separate isomers of monohydroxylated PCB3, and all isomers eluted as a single peak at 3.8 min. A peak at 1.9 min resulted from sulfate in the mixture of standards. Concentration of free phenolic forms was significantly lower than that of the conjugated forms in serum, urine, and bile.
Representative SIM chromatograms for authentic standards of PCB3 sulfates are shown in Figure 4. The elution order of sulfates detected in bile and urine was 3PCB3 sulfate, 3′PCB3 sulfate, catechol sulfate (m/z 299), and 4′PCB3 sulfate. When operated in full-scan mode, a putative glucuronide eluted before the 3PCB3 sulfates (Supporting Information).
Figure 4.
Chromatograms of sulfated metabolites. SIM chromatograms showing the separation of PCB3 sulfates (m/z 283). A representative chromatogram of sulfates in lungs and brain are overlaid on authentic standards to show the distribution of sulfates in tissues other than liver. Chromatography was performed on a RP C18 column with an isocratic mobile phase of 35% acetonitrile in water (5 mM ammonium acetate) and a flow rate of 1 mL/min.
XAD Extraction and Analysis of PCB3
PCB3 was extracted from XAD resin by pressurized liquid extraction (ASE 200, Dionex, Sunnyvale, CA) using acetone/hexane (1:1, v/v), as described previously.5 Briefly, the extraction cell was preheated at 80 °C for 6 min, followed by a static extraction for 5 min at a pressure of 1500 psi. Quality control samples, prepared by spiking 4 mg of PCB3 in 10 g of XAD resin, were also extracted together. The volume of ASE extract was adjusted to 40 mL, and 1 mL was transferred to an autosampler vial for analysis. Internal standard (50 μL, PCB2 at 10 μg/mL in hexane) was added, and 1 μL was injected into GC/MS (Agilent 7890A GC system). The MS detector was operated in selected-ion monitoring (SIM) mode to detect ions of m/z 188 by positive electrospray ionization of the parent analyte. A capillary GC column (DB5-MS, Agilent, Santa Clara, CA) (30 m × 0.25 mm i.d. × 0.25 μm film thickness) was used. The temperature of the column was initially held at 50 °C for 1 min, increased at a rate of 10 °C/min to 280 °C, and then finally kept at 280 °C for 5 min. The flow rate of the helium carrier gas was 1 mL/min.
Urinary 8-oxo-dG Analysis
A composite 24 h urine sample was prepared by proportionately mixing the urine collected at each time point. Urinary creatinine level was determined as per the instructions in the kit (no. 500701, Cayman, USA). Urine (0.1 mL) was treated with ice-cold acetone (0.9 mL) and centrifuged at 12 000g for 10 min. Clear supernatant was transferred to a clean 2 mL glass vial, uncapped, and kept under a chemical fume hood until the solvent was completely evaporated. It was reconstituted in EIA buffer and analyzed per the instructions mentioned in DNA/RNA oxidative damage EIA kit (no. 589320, Cayman, USA).
Serum Chemistry and Serum Thyroxine (T4) Level
For determination of serum chemistry and serum T4 levels, serum samples were sent to the Department of Veterinary Pathology at the University of Illinois, Urbana–Champaign. Details of the laboratory tests procedures can be found at http://vetmed.illinois.edu/vdl/index.html.
Quality Assurance
Analyses of tissue samples derived from PCB3-exposed animals were accompanied by solvent blanks, spiked solvent or calibration standards, control tissue/fluid samples, and spiked tissue/fluid samples. A spiked tissue/fluid sample was prepared by spiking a known amount of analyte (0.5 μg) in the tissue or fluid derived from control rats. Extraction efficiency, which was defined as the percentage recovery of the analytes from different matrices, is presented in the Supporting Information. All measurements in the samples were corrected for extraction efficiency. Using Microsoft Excel, a linear calibration line ranging from 1 to 500 ng/mL was generated for each analyte by plotting a known concentration on the x axis and the area under the chromatogram or response (Y) on the y axis. Expected response (Y′) was calculated from the regression line. Error of response for each Y measurement (y) was determined by subtracting Y′ from Y. LOQ was defined as 10((Sy)/K), where Sy is the standard deviation in y and K is the slope of the regression line.29 LOQs were 5, 2, 8, 16, and 12 ng/mL for 3PCB3 sulfate, 3′PCB3 sulfate, 4′PCB3sulfate, 4′OH-PCB3, and 3′4′diOH-PCB3, respectively. Up to 2 g of tissue or 2 mL of urine was taken for extraction, and the final extract was concentrated to as low as 200 μL to get responses above the LOQ range.
Results
Estimate of Inhalation Dose
A schematic of the inhalation exposure apparatus is shown in Figure 1. The measured air concentration of PCB3 was 1.52, 2.06, and 16.33 mg/m3 in the first, second, and third groups of animal experiments, respectively. This gave an estimated cumulative inhalation dose of approximately 26, 35, and 277 μg/rat for the first, second, and third groups, respectively.
Tissue Disposition
Disposition of phenol and sulfate metabolites in tissues was quantified in the first group of rats that received an estimated dose of 26 μg/rat. A selected-ion monitoring (SIM) chromatogram showing the presence of sulfated metabolites in lungs and brain is shown in Figure 4. Peak disposition of both phenol and sulfate metabolites in tissue was observed immediately after 2 h exposure (Table 1).
Table 1. Disposition of PCB3 Metabolites in Tissuea.
| 0 h |
1 h |
2 h |
4 h |
|||||
|---|---|---|---|---|---|---|---|---|
| phenols | sulfates | phenols | sulfates | phenols | sulfates | phenols | sulfates | |
| liver | 213 ± 123 | 842 ± 80 | 48 ± 25 | 584 ± 123 | 39 ± 51 | 572 ± 89 | 34 ± 17 | 292 ± 23 |
| lungs | 31 ± 27 | 22 ± 7 | 17 ± 11 | ND | 7 ± 7 | ND | 4 ± 3 | ND |
| brain | 27 ± 6 | 3 ± 0.3 | 11 ± 6 | ND | ND | ND | ND | ND |
Concentration is expressed as ng/g of wet tissue. The estimated inhalation dose of PCB3 was 26 μg/rat. Values are mean ± SD, n = 3, and are corrected for extraction efficiency. ND, not detected.
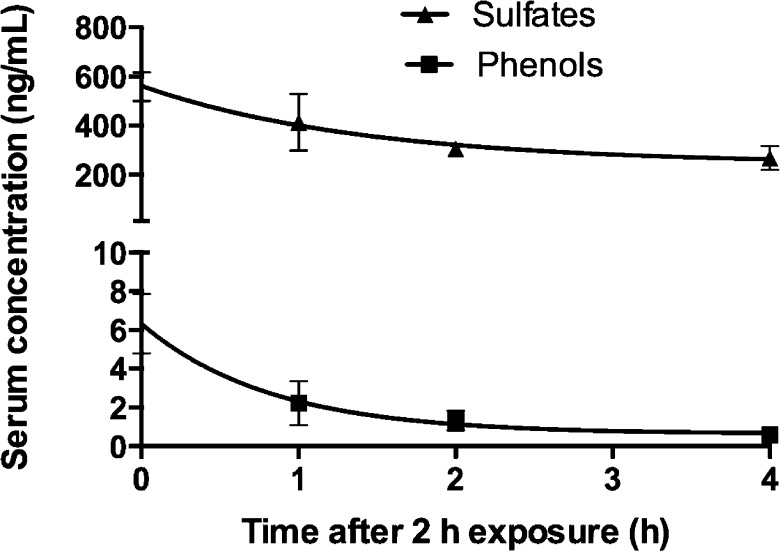
Serum Clearance
Serum analysis of phenol and sulfate metabolites was carried out after 0, 1, 2, and 4 h postexposure in the groups of rats that received an estimated inhalation dose of 26 μg/rat and is presented in Figure 5. At 0 h postexposure, serum concentrations of 3PCB3 sulfate, 3′PCB3 sulfate, 4′PCB3 sulfate, and 4′OH-PCB3 were 28 ± 6, 61 ± 3, 470 ± 56, and 7 ± 1 ng/mL, respectively. The total concentration of sulfates was calculated by adding the concentration of 3PCB3 sulfate, 3′PCB3 sulfate, and 4′PCB3 sulfate for each time point. When data were fit to first-order elimination kinetics (goodness of fit, R2, was 0.80 for sulfates and 0.88 for phenols), estimated half-lives were 1 h for sulfates and 0.5 h for phenols.
Figure 5.
Plasma clearance. First-order elimination kinetics of metabolites showed elimination half-lives of 1 h for sulfates and 0.5 h for phenols. The serum concentration of sulfates (sum of 3PCB sulfate, 3′PCB3 sulfate, and 4′PCB3 sulfate) was 560 ± 60 ng/mL, whereas the concentration of 4′OHPCB3 was only 7 ± 1 ng/mL at 0 h postexposure. Serum was collected at 0, 1, 2, and 4 h from the first group of rats that received an estimated dose of 26 μg/rat. The kinetic analysis was done in GraphPad using a nonlinear fit of one phase exponential decay (goodness of fit, R2, is 0.80 for sulfates and 0.88 for phenols). Values are mean ± SD, n = 3, and are corrected for extraction efficiency.
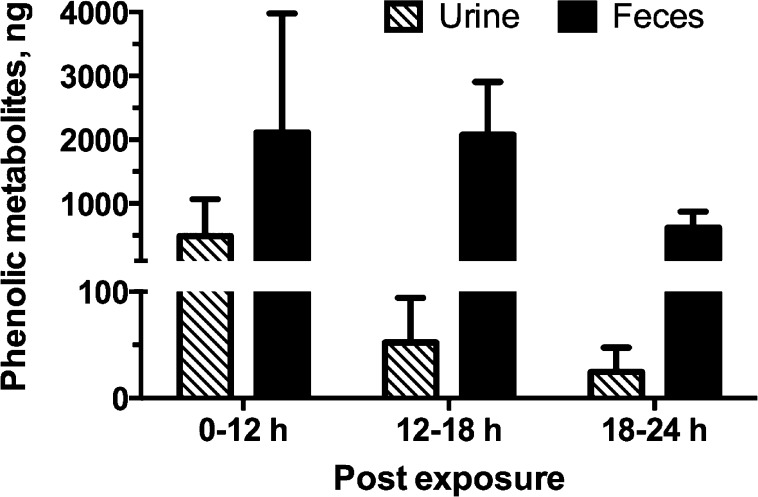
Peak Excretion in Urine and Feces
The amounts of free phenolic metabolites of PCB3 excreted in urine and feces at different time points over 24 h postexposure are shown in Figure 6. The maximum excretion of 4′OH-PCB3 occurred within 12 h in urine, and maximum excretion in feces was observed within 18 h postexposure. Peak excretion of sulfates in urine was also observed within 6–12 h postexposure, published previously.29
Figure 6.
Peak excretion of metabolites in feces and urine occurred within 24 h. Amount of 4′OH-PCB3 recovered from urine and feces from samples collected at different time after exposure showed that a peak excretion occurred from 0 to 12 h in urine and 0–18 h in feces. Peak excretion of sulfates also occurred from within 0–12 h postexposure, which has been published previously.29 No conjugated metabolites were detected in feces at any time point. Values are mean ± SD, n = 3, and are corrected for extraction efficiency.
Biliary Excretion
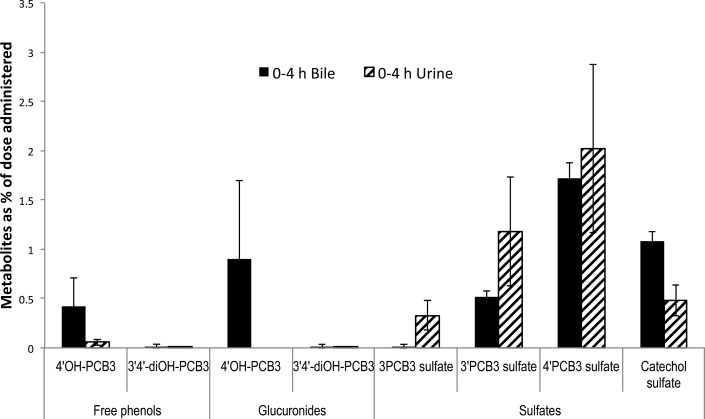
Because conjugated metabolites were not seen in the feces, we were interested in the biliary excretion of metabolites. Rats in this group inhaled an estimated dose of 277 μg/rat. Interestingly, we found 3′PCB3 sulfate (m/z 283), 4′PCB3 sulfate (m/z 283), putative PCB3-catechol sulfate (m/z 299), and glucuronide (m/z 379) as conjugated metabolites in bile (Supporting Information). For quantification of putative glucuronides and catechol sulfate, for which no standards were available, samples were incubated with hydrolytic enzymes, and the conjugates were quantified by comparison with the corresponding phenolic forms before and after hydrolysis. Figure 7 shows the relative disposition of free phenolic forms, glucuronides, and sulfates in bile and urine collected from 0 to 4 h postexposure. The ratio of free 4′OH-PCB3/glucuronide conjugate of 4′OH-PCB3/sulfate conjugate of 4′OH-PCB3 was approximately 1:2:4. Disposition of the catechol sulfate in bile was twice that in urine during this time. Altogether, 5.0 ± 0.6% of the administered dose was seen in bile, and 4.0 ± 0.2% of the dose was recovered in urine during the 4 h period. Over this period, 6.0 ± 0.6 mL of bile and 2.0 ± 0.05 mL of urine were collected. Glucuronide conjugates related to dihydroxylated metabolites were not found in either urine or bile.
Figure 7.
Metabolites excreted in 0–4 h bile and urine. Sulfated forms were the major metabolites in both urine and bile followed by some glucuronide conjugates and free phenols. Approximately 4 ± 2 and 5 ± 0.6% of the dose in urine and bile were recovered in 0–4 h, respectively. Approximately 6 ± 0.6 mL of bile and 2 ± 0.05 mL of urine was collected in 4 h. Estimated inhaled dose was 277 μg/rat. Values are mean ± SD, n = 3, and are corrected for extraction efficiency.
Excretion Urine and Feces over 24 h
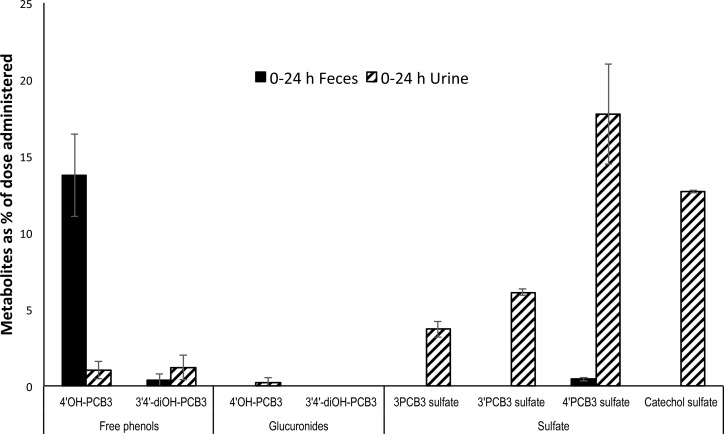
An estimated excretion of PCB3 in urine and feces from the second group of animals that received a calculated inhalation dose of 35 μg/rat is presented in Figure 8. Approximately 45 ± 5% of the dose was seen in urine, and 18 ± 5% of the dose was recovered in feces. Together, this accounted for 62 ± 9% of the inhaled dose. Unchanged PCB3 was found in both urine (2.0 ± 1%) and feces (4.0 ± 2%) at very low concentrations compared to that of metabolites. Major metabolites excreted in urine were 4′PCB3 sulfate (18 ± 3%), PCB3 catechol sulfate (13 ± 0.07%), 3′PCB3 sulfate (6.0 ± 0.2%), 3PCB3 sulfate (4.0 ± 0.5%), free 4′OH-PCB3 (1.0 ± 0.5%), and free 3′4′-diOH-PCB3 (1.0 ± 0.8%). Over this period, 13 ± 2 mL of urine and 6.0 ± 1 g of feces were collected.
Figure 8.
Percentage of dose excreted in 0–24 h feces and urine. Approximately 45 ± 5% of the dose in urine and 18 ± 5% in feces were recovered in 24 h. Sulfated forms were the major metabolites in urine. Unmetabolized PCB3 contributed 2 ± 1% in urine and 4 ± 2% in feces over 24 h. Estimated inhaled dose was 35 μg/rat. Approximately 13 ± 2 mL of urine and 6 ± 1 g of feces were collected in 24 h. Values are mean ± SD, n = 3, and are corrected for extraction efficiency.
Bioactivation and Toxicity Assessment
Serum chemistry parameters measured (renal function, serum protein, T4, electrolyte balance, pancreas function, liver function, and lipid metabolism) were within normal ranges in both control rats and in rats exposed to PCB3. Free serum T4 levels in serum were 39 ± 13 pmol/L in control rats and 31 ± 14 pmol/L in rats euthanized immediately after 2 h exposure to PCB3. This difference was not statistically significant (Student’s t test, p = 0.409). The 8-oxo-dG levels in 24 h urine were 125 ± 24 pg/mg creatinine in control and 160 ± 35 pg/mg creatinine in exposed rats, and this difference was also not statistically significant (Student’s t test, p = 0.23). Serum chemistry and T4 values are presented in the Supporting Information.
Discussion
Previously, we reported that a sulfate metabolite was a major biotransformation product of PCB3 applied in vivo via i.p. injection to male Sprague–Dawley rats.27 Sulfated metabolites of PCBs are high-affinity ligands for the thyroid hormone transport protein transthyretin.14 Therefore, sulfated PCBs represent a potential biomarker of exposure for metabolically active PCBs,29 and their potent interactions with transthyretin may suggest toxic consequences of exposure. This study extends our knowledge of the metabolism of PCB3 after inhalation by reporting the disposition of sulfated and phenolic metabolites in liver, lungs, and brain and provides serum clearance half-lives for these metabolites as well as their biliary, urinary, and fecal excretion rates.
We exposed female SD rats to air containing 1.52–16.33 mg/m3 of PCB3. The highest concentration of ∑PCBs in indoor air reported so far is 20 800 ng/m3 in an old school building in Germany.37 The concentration of ∑PCBs measured across five public schools in New York City had a median value of 257 ng/m3 before remediation.38 Hu et al. reported the ∑PCBs in the atmosphere of Chicago ranging 75–5500 pg/m3.9 These values are either the sum of all congeners or estimates from the measurement of few indicator congeners, and the fraction of PCB3 in these measurements would be very small. Davis et al. reported 215 ng/m3 of monochlorinated PCBs (PCB1 was 158 ng/m3 and PCB3 was 57 ng/m3) in an old residential building in California.8 Given that an average adult of 70 kg body weight breathes 16 m3 of air per day, a person living in the home that has a concentration of 215 ng/m3 of monochlorinated PCBs would be exposed to 3.4 μg/day (0.05 μg/kg/day). A dose of 26 μg to a rat of 200 g is equal to 130 μg/kg body weight. Human exposure to the dose given to the rat would be achieved by living in the place that has a PCB3 concentration of 215 ng/m3 for at least 7 years. Human dosimetry of PCBs should include factors such as exposure through the diet, time spent indoors and outdoors, concentration of PCBs outdoors, and age.30,39 Therefore, the comparison of the animal dose to the human exposure scenario should be interpreted carefully, as accurate exposure–dose modeling is beyond the scope of this article.
In 1959, probably the first study in the metabolism of PCBs, Block and Cornish administered 1 g of PCB3 in rabbits orally and recovered up to 64% percent of the dose in the form of conjugated metabolites from urine over 4 days.40 Pereg et al. dosed radiolabeled PCB3 in female mice i.p. at 600 μmol/kg and observed a peak distribution and disappearance in lungs, liver, and kidney within 24 h.41 In this study, we also observed a rapid metabolism of PCB3 after inhalation.
PCB3 has been used as a model compound to understand the mechanism of cytochrome P450 (CYP)-catalyzed formation of phenolic metabolites of PCBs in a variety of species.40,42,43 These studies have reported that 4′OH-PCB3 > 3′4′-diOH-PCB3 > 3′OH-PCB3 are three major phenolic metabolites in naïve animals. Rats induced with phenobarbital (PB) and 3-methylcholanthrene (3-MC) exhibited enhanced metabolism of PCBs, suggesting a role for hepatic CYP1A and 2B isoforms in the formation of phenolic metabolites.44 CYPs in the liver microsomes of induced rats could further add a second hydroxyl group on 3′OH-PCB3, forming 2′3′-diOH-PCB3, or on 2′OH-PCB3, forming both 2′3′-diOH-PCB3 and 2′5′-diOH-PCB3.17,45 This study confirms that 4′OH-PCB3 and 3′4′-diOH-PCB3 are major phenolic metabolites and 3′OH-PCB3 and 3OH-PCB3 are minor phenolic metabolites of PCB3. We did not find strong evidence for the formation of 2′3′-diOH-PCB3 and 2′5′-diOH-PCB3 in vivo. Because we observed both hydroxylated and sulfated metabolites in lungs and brain, we recognize the possibility of in situ metabolism of PCB3 in tissues other than liver.
Hydroxylated metabolites are converted to more water-soluble forms by cytosolic sulfotransferases (SULTs) and UDP glucuronosyl transferases (UGTs) to respective O-sulfate and O-glucuronide conjugates. Although both SULTs and UGTs act upon the same substrate, there is a complex mechanism that governs the substrate preference of these enzymes. Many OH-PCBs of lower chlorinated congeners are good substrates for both SULTs and UGTs in vitro, although some of them have been described as inhibitors for both enzymes.24,46−49
We found that the major conjugated metabolites of PCB3 in female rats were 4′PCB3 sulfate and PCB3 catechol sulfate, although small amounts of the glucuronide of monohydroxylated PCB3 were also measured in bile. The dominance of the sulfation pathway over glucuronidation is similar to our previous study in male rats after a high dose i.p. exposure.27 These bile-cannulated rats were 12–14 week old females. Studies have shown that expression of SULT1A1, a major enzyme for sulfation of phenols, is significantly lower in adult female rats compared to its level in adult male and young female rats.50,51 Therefore, one possible explanation for formation of glucuronides in the adult female rats could be that they compensate for a deficiency in sulfation by utilizing UGTs.
Besides sulfates, PCB3 mercapturate (m/z 348) is a major urinary product of PCB3 formed following glutathione conjugation to PCB3-arene oxide in rats.27 PCB mercapturate is a precursor for the formation of methyl sulfone metabolites.52 Methyl sulfone is a major metabolic product of PCBs in marine animals,53 and it is bioaccumulative in the food chain.54 Formation of methyl sulfones of PCB11 has also been reported.34 Our calculation of the fraction of the dose excreted in urine or feces over 24 h does not account for these metabolites because of the lack of authentic standards, but these metabolites may not account for more than 40% of the administered dose.
Glucuronide conjugates of many drugs and xenobiotics are preferentially excreted into bile cannaliculi.55,56 The absorption of a xenobiotic into the hepatocyte occurs by passive diffusion due to higher lipophilicity. Once it is metabolized to the less lipophilic form, it requires energy-dependent transporters to be eliminated from hepatocytes. The interaction of the metabolites with transporters present at the basolateral membrane will eliminate them into the systemic circulation, and they will subsequently be excreted via urine, whereas their interaction with the transporters at the apical membrane is expected to deliver them into the bile. Our data indicate that glucuronide and catechol sulfates are preferentially excreted into bile. A mechanistic study on the interaction of these metabolites with hepatic transporter protein is indicated.
Despite the very low concentration of free phenols in bile, metabolites detected in feces were all phenols. Hydrolysis of conjugated metabolites by gut microflora is very common.57 Therefore, it is likely that OH-PCB3s are regenerated in the intestine from the sulfates or glucuronides elaborated in the bile. Biliary secretion and enterohepatic reabsorption of PCB and its metabolites have been described.58 Previously, Hass et al. also observed that PCB3 excreted in bile over 4 h was more than the cumulative amount excreted in feces over 48 h.43 Therefore, it is also likely that PCB3 metabolites undergo enterohepatic cycling.
Urinary 8-hydroxy-2′-deoxyguanosine (8-oxo-dG) is a common marker of exposure to environmental chemicals that produce oxidative stress.59 Metabolic bioactivation of PCB3 occurs by the formation of arene oxides and PCB quinones. These electrophiles have been shown to generate various degrees of oxidative stress in many cell lines.22,23 However, in this study, we measured the level of 8-oxo-dG in a composite urine collected over 24 h and did not find a significant difference between PCB3-exposed and control rats.
The serum chemistry of PCB3-exposed rats was similar to that of control rats. Although a depletion of T4 from circulation might be expected due to the high affinity of sulfated PCB metabolites to transthyretin,14 no significant difference in free T4 levels was observed in serum from control and PCB3-exposed rats. Although this study did not observe toxic effects of PCB metabolites, the lack of toxicity after acute inhalation exposure could result from the rapid clearance of the metabolites from serum. We suggest that toxic effects of PCB metabolites might be revealed by a chronic exposure experiment, which would provide a closer representation of an exposure scenario that allows the PCB metabolites to remain in the circulation for a longer period.
In conclusion, we exposed female SD rats to airborne PCB3 via nose-only inhalation for 2 h and investigated the disposition of phenolic and sulfated metabolites of PCB3. We measured the concentration in tissues, half-lives of clearance from serum, biliary excretion, and amounts excreted in 24 h feces and urine. After inhalation, PCB3 was rapidly metabolized to phenol and conjugated mostly to sulfates. Over 60% of the inhaled dose of PCB3 was recovered over 24 h postexposure from urine and feces. This study has shown, for the first time, the disposition of PCB sulfates in the lungs and brain.
Acknowledgments
We thank Drs. Xueshu Li and Xianran He for the synthesis of authentic standards used in these studies. We thank Dr. Xueshu Li further for the determination of the purity of PCB3. We also thank members of our laboratory for help with the animal studies. We are very grateful to the reviewers for their thorough and critical review of the manuscript. These studies form a portion of the dissertation research of K.D. The opinions expressed are solely those of the authors and do not reflect an official policy of the granting agencies.
Glossary
Abbreviations
- 3-MC
3-methylcholanthrene
- 8-oxo-dG
8-hydroxy-2′-deoxyguanosine
- ASE
accelerated solvent extraction
- CYPs
cytochrome P450
- EDC
endocrine disrupting chemical
- EIA
enzyme immunoassay
- i.p.
intraperitoneal
- IS
internal standard
- LC-PCBs
lower chlorinated PCBs
- LOQ
limit of quantification
- PB
phenobarbital
- PCB
polychlorinated biphenyl
- PCB3
4-chlorobiphenyl
- SIM
selected-ion monitoring
- SULTs
sulfotransferase
- T4
thyroxine
- TQD
tandem quadrupole detector
- UGTs
UDP-glucuronosyltransferases
- WAX
weak anion exchange
Supporting Information Available
Recovery of the authentic standards of PCB3 and metabolites from the spiked matrices, serum chemistry and serum free thyroxine levels, and SIM chromatograms and mass spectra showing putative PCB3 glucuronide and catechol sulfate in bile and urine. This material is available free of charge via the Internet at http://pubs.acs.org.
This study was supported by funding from NIH (P42 ES013661 and P30 ES005605). K.D. gratefully acknowledges support from the Iowa Superfund Research Program (P42 ES013661) Training Core.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Ballschmiter K.; Zell M. (1980) Analysis of polychlorinated-biphenyls (PCB) by glass-capillary gas-chromatography: composition of technical aroclor-PCB and clophen-PCB mixtures. Fresenius' Z. Anal. Chem. 302, 20–31. [Google Scholar]
- Erickson M. D.; Kaley R. G. (2011) Applications of polychlorinated biphenyls. Environ. Sci. Pollut. Res. 18, 135–151. [DOI] [PubMed] [Google Scholar]
- Jones K. C.; de Voogt P. (1999) Persistent organic pollutants (POPs): state of the science. Environ. Pollut. 100, 209–221. [DOI] [PubMed] [Google Scholar]
- Robertson L. W.; Ludewig G. (2011) Polychlorinated biphenyl (PCB) carcinogenicity with special emphasis on airborne PCBs. Gefahrstoffe - Reinhalt. Luft 71, 25–32. [PMC free article] [PubMed] [Google Scholar]
- Hu D.; Martinez A.; Hornbuckle K. C. (2008) Discovery of non-aroclor PCB (3,3′-dichlorobiphenyl) in Chicago air. Environ. Sci. Technol. 42, 7873–7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman W. T.; Bidleman T. F. (1985) Vapor pressure estimates of individual polychlorinated biphenyls and commercial fluids using gas chromatographic retention data. J. Chromatogr. A 330, 203–216. [Google Scholar]
- Wilson L. R.; Palmer P. M.; Belanger E. E.; Cayo M. R.; Durocher L. A.; Hwang S. A. A.; Fitzgerald E. F. (2011) Indoor air polychlorinated biphenyl concentrations in three communities along the upper Hudson River, New York. Arch. Environ. Contam. Toxicol. 61, 530–538. [DOI] [PubMed] [Google Scholar]
- Davis B.; Beach J.; Wade M.; Klein A.; Hoch K. (2002) Risk assessment of polychlorinated biphenyls (PCBs) in indoor air. Toxicol. Sci. 66, 516. [Google Scholar]
- Hu D.; Lehmler H.-J.; Martinez A.; Wang K.; Hornbuckle K. C. (2010) Atmospheric PCB congeners across Chicago. Atmos. Environ. 44, 1550–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak A.; Ludewig G.; Lehmler H. J.; Wojtowicz A. K.; Robertson L. W.; Gregoraszczuk E. L. (2005) Comparison of the actions of 4-chlorobiphenyl and its hydroxylated metabolites on estradiol secretion by ovarian follicles in primary cells in culture. Reprod. Toxicol. 20, 57–64. [DOI] [PubMed] [Google Scholar]
- Kester M. H.; Bulduk S.; Tibboel D.; Meinl W.; Glatt H.; Falany C. N.; Coughtrie M. W.; Bergman A.; Safe S. H.; Kuiper G. G.; Schuur A. G.; Brouwer A.; Visser T. J. (2000) Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs. Endocrinology 141, 1897–1900. [DOI] [PubMed] [Google Scholar]
- Schuur A. G.; Brouwer A.; Bergman A.; Coughtrie M. W.; Visser T. J. (1998) Inhibition of thyroid hormone sulfation by hydroxylated metabolites of polychlorinated biphenyls. Chem.–Biol. Interact. 109, 293–297. [DOI] [PubMed] [Google Scholar]
- Gutleb A. C.; Cenijn P.; Velzen M.; Lie E.; Ropstad E.; Skaare J. U.; Malmberg T.; Bergman A.; Gabrielsen G. W.; Legler J. (2010) In vitro assay shows that PCB metabolites completely saturate thyroid hormone transport capacity in blood of wild polar bears (Ursus maritimus). Environ. Sci. Technol. 44, 3149–3154. [DOI] [PubMed] [Google Scholar]
- Grimm F. A.; Lehmler H. J.; He X.; Robertson L. W.; Duffel M. W. (2013) Sulfated metabolites of polychlorinated biphenyls are high-affinity ligands for the thyroid hormone transport protein transthyretin. Environ. Health Perspect. 121, 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin H. J.; Guillette L. J. (2011) Embryos as targets of endocrine disrupting contaminants in wildlife. Birth Defects Res., Part C 93, 19–33. [DOI] [PubMed] [Google Scholar]
- Lauby-Secretan B.; Loomis D.; Grosse Y.; Ghissassi F. E.; Bouvard V.; Benbrahim-Tallaa L.; Guha N.; Baan R.; Mattock H.; Straif K. (2013) Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol. 14, 287–288. [DOI] [PubMed] [Google Scholar]
- Wyndham C.; Safe S. (1978) In vitro metabolism of 4-chlorobiphenyl by control and induced rat liver microsomes. Biochemistry 17, 208–215. [DOI] [PubMed] [Google Scholar]
- Ludewig G.; Robertson L. W. (2013) Polychlorinated biphenyls (PCBs) as initiating agents in hepatocellular carcinoma. Cancer Lett. 334, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangpradit O.; Mariappan S. V. S.; Teesch L. M.; Duffel M. W.; Norstrom K.; Robertson L. W.; Luthe G. (2009) Oxidation of 4-chlorobiphenyl metabolites to electrophilic species by prostaglandin H synthase. Chem. Res. Toxicol. 22, 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaro A. R.; Oakley G. G.; Bauer U.; Spielmann H. P.; Robertson L. W. (1996) Metabolic activation of PCBs to quinones: reactivity toward nitrogen and sulfur nucleophiles and influence of superoxide dismutase. Chem. Res. Toxicol. 9, 623–629. [DOI] [PubMed] [Google Scholar]
- Song Y.; Wagner B. A.; Lehmler H. J.; Buettner G. R. (2008) Semiquinone radicals from oxygenated polychlorinated biphenyls: electron paramagnetic resonance studies. Chem. Res. Toxicol. 21, 1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. M.; Mapuskar K. A.; Marek R. F.; Xu W. J.; Lehmler H. J.; Robertson L. W.; Hornbuckle K. C.; Spitz D. R.; Aykin-Burns N. (2013) A new player in environmentally induced oxidative stress: polychlorinated biphenyl congener, 3,3-dichlorobiphenyl (PCB11). Toxicol. Sci. 136, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri L.; Sarsour E. H.; Goswami P. C. (2010) 2-(4-Chlorophenyl)benzo-1,4-quinone induced ROS-signaling inhibits proliferation in human non-malignant prostate epithelial cells. Environ. Int. 36, 924–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Smart J. T.; Song Y.; Lehmler H. J.; Robertson L. W.; Duffel M. W. (2009) Structure–activity relationships for hydroxylated polychlorinated biphenyls as substrates and inhibitors of rat sulfotransferases and modification of these relationships by changes in thiol status. Drug Metab. Dispos. 37, 1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Lehmler H. J.; Robertson L. W.; Duffel M. W. (2011) Physicochemical properties of hydroxylated polychlorinated biphenyls aid in predicting their interactions with rat sulfotransferase 1A1 (rSULT1A1). Chem.–Biol. Interact. 189, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco J. C.; James M. O. (2005) Sulfonation of environmental chemicals and their metabolites in the polar bear (Ursus maritimus). Drug Metab. Dispos. 33, 1341–1348. [DOI] [PubMed] [Google Scholar]
- Dhakal K.; He X.; Lehmler H. J.; Teesch L. M.; Duffel M. W.; Robertson L. W. (2012) Identification of sulfated metabolites of 4-chlorobiphenyl (PCB3) in the serum and urine of male rats. Chem. Res. Toxicol. 25, 2796–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai G.; Lehmler H. J.; Schnoor J. L. (2013) Sulfate metabolites of 4-monochlorobiphenyl in whole poplar plants. Environ. Sci. Technol. 47, 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakal K.; Adamcakova-Dodd A.; Lehmler H. J.; Thorne P. S.; Robertson L. W. (2013) Sulfate conjugates are urinary markers of inhalation exposure to 4-chlorobiphenyl (PCB3). Chem. Res. Toxicol. 26, 853–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currado G. M.; Harrad S. (1998) Comparison of polychlorinated biphenyl concentrations in indoor and outdoor air and the potential significance of inhalation as a human exposure pathway. Environ. Sci. Technol. 32, 3043–3047. [Google Scholar]
- Hu X.; Adamcakova-Dodd A.; Lehmler H. J.; Hu D.; Kania-Korwel I.; Hornbuckle K. C.; Thorne P. S. (2010) Time course of congener uptake and elimination in rats after short-term inhalation exposure to an airborne polychlorinated biphenyl (PCB) mixture. Environ. Sci. Technol. 44, 6893–6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X.; Adamcakova-Dodd A.; Lehmler H. J.; Hu D.; Hornbuckle K.; Thorne P. S. (2012) Subchronic inhalation exposure study of an airborne polychlorinated biphenyl mixture resembling the Chicago ambient air congener profile. Environ. Sci. Technol. 46, 9653–9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X.; Lehmler H. J.; Adamcakova-Dodd A.; Thorne P. S. (2013) Elimination of inhaled 3,3′-dichlorobiphenyl (CB11) and the formation of the 4-hydroxylated metabolite. Environ. Sci. Technol. 47, 4743–4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X.; Adamcakova-Dodd A.; Thorne P. S. (2014) The fate of inhaled (14)C-labeled PCB11 and its metabolites in vivo. Environ. Int. 63, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espandiari P.; Glauert H. P.; Lehmler H. J.; Lee E. Y.; Srinivasan C.; Robertson L. W. (2004) Initiating activity of 4-chlorobiphenyl metabolites in the resistant hepatocyte model. Toxicol. Sci. 79, 41–46. [DOI] [PubMed] [Google Scholar]
- Li X.; Parkin S.; Duffel M. W.; Robertson L. W.; Lehmler H. J. (2010) An efficient approach to sulfate metabolites of polychlorinated biphenyls. Environ. Int. 36, 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl B.; Schettgen T.; Kerscher G.; Broding H. C.; Otto A.; Angerer J.; Drexler H. (2004) Evidence for increased internal exposure to lower chlorinated polychlorinated biphenyls (PCB) in pupils attending a contaminated school. Int. J. Hyg. Environ. Health 207, 315–324. [DOI] [PubMed] [Google Scholar]
- Thomas K., Xue J., Williams R., Jones P., and Whitaker D. (2012) Polychlorinated biphenyls (PCBs) in school buildings: sources, environmental levels, and exposures. U.S. EPA Report, Washington, DC, http://www.epa.gov/pcbsincaulk/pdf/pcb_EPA600R12051_final.pdf.
- Norström K.; Czub G.; McLachlan M. S.; Hu D.; Thorne P. S.; Hornbuckle K. C. (2010) External exposure and bioaccumulation of PCBs in humans living in a contaminated urban environment. Environ. Int. 36, 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block W. D.; Cornish H. H. (1959) Metabolism of biphenyl and 4-chlorobiphenyl in the rabbit. J. Biol. Chem. 234, 3301–3302. [Google Scholar]
- Pereg D.; Tampal N.; Espandiari P.; Robertson L. W. (2001) Distribution and macromolecular binding of benzo[a]pyrene and two polychlorinated biphenyl congeners in female mice. Chem.–Biol. Interact. 137, 243–258. [DOI] [PubMed] [Google Scholar]
- Safe S.; Ruzo L. O. (1975) The metabolism of 4-chlorobiphenyl in the pig. Can. J. Physiol. Pharmacol. 53, 392–396. [DOI] [PubMed] [Google Scholar]
- Hass J. R.; Jao L. T.; Wilson N. K.; Matthews H. B. (1977) Metabolism of 4-chlorobiphenyl and 4,4′-dichlorobiphenyl in the rat: qualitative and quantitative aspects. J. Agric. Food Chem. 25, 1330–1333. [DOI] [PubMed] [Google Scholar]
- Kennedy M. W.; Carpentier N. K.; Dymerski P. P.; Adams S. M.; Kaminsky L. S. (1980) Metabolism of monochlorobiphenyls by hepatic microsomal cytochrome P-450. Biochem. Pharmacol. 29, 727–736. [DOI] [PubMed] [Google Scholar]
- McLean M. R.; Bauer U.; Amaro A. R.; Robertson L. W. (1996) Identification of catechol and hydroquinone metabolites of 4-monochlorobiphenyl. Chem. Res. Toxicol. 9, 158–164. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Apak T. I.; Lehmler H. J.; Robertson L. W.; Duffel M. W. (2006) Hydroxylated polychlorinated biphenyls are substrates and inhibitors of human hydroxysteroid sulfotransferase SULT2A1. Chem. Res. Toxicol. 19, 1420–1425. [DOI] [PubMed] [Google Scholar]
- Sacco J. C.; James M. O. (2004) Glucuronidation in the polar bear (Ursus maritimus). Mar. Environ. Res. 58, 475–479. [DOI] [PubMed] [Google Scholar]
- van den Hurk P.; Kubiczak G. A.; Lehmler H. J.; James M. O. (2002) Hydroxylated polychlorinated biphenyls as inhibitors of the sulfation and glucuronidation of 3-hydroxy-benzo[a]pyrene. Environ. Health Perspect. 110, 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampal N.; Lehmler H. J.; Espandiari P.; Mahnberg T.; Robertson L. W. (2002) Glucuronidation of hydroxylated polychlorinated biphenyls (PCBs). Chem. Res. Toxicol. 15, 1259–1266. [DOI] [PubMed] [Google Scholar]
- Chen G.; Baron J.; Duffel M. W. (1995) Enzyme- and sex-specific differences in the intralobular localizations and distributions of aryl sulfotransferase IV (tyrosine-ester sulfotransferase) and alcohol (hydroxysteroid) sulfotransferase a in rat liver. Drug Metab. Dispos. 23, 1346–1353. [PubMed] [Google Scholar]
- Klaassen C. D.; Liu L.; Dunn R. T. 2nd (1998) Regulation of sulfotransferase mRNA expression in male and female rats of various ages. Chem.–Biol. Interact. 109, 299–313. [DOI] [PubMed] [Google Scholar]
- Letcher R., Klasson-Wehler E., and Bergman A. (2000) Methyl sulfone and hydroxylated metabolites of polychlorinated biphenyls. The Handbook of Environmental Chemistry (Hutzinger O., and Paasivirta J., Eds.) pp 315–359, Springer: Berlin/Heidelberg. [Google Scholar]
- Stapleton H. M.; Letcher R. J.; Baker J. E. (2001) Metabolism of PCBs by the deepwater sculpin (Myoxocephalus thompsoni). Environ. Sci. Technol. 35, 4747–4752. [DOI] [PubMed] [Google Scholar]
- Letcher R. J.; Norstrom R. J.; Muir D. C. G. (1998) Biotransformation versus bioaccumulation: sources of methyl sulfone PCB and 4,4′-DDE metabolites in the polar bear food chain. Environ. Sci. Technol. 32, 1656–1661. [Google Scholar]
- Hjelle J. J.; Klaassen C. D. (1984) Glucuronidation and biliary excretion of acetaminophen in rats. J. Pharmacol. Exp. Ther. 228, 407–413. [PubMed] [Google Scholar]
- Zamek-Gliszczynski M. J.; Hoffmaster K. A.; Nezasa K.; Tallman M. N.; Brouwer K. L. (2006) Integration of hepatic drug transporters and phase II metabolizing enzymes: mechanisms of hepatic excretion of sulfate, glucuronide, and glutathione metabolites. Eur. J. Pharm. Sci. 27, 447–486. [DOI] [PubMed] [Google Scholar]
- Mani S.; Boelsterli U. A.; Redinbo M. R. (2014) Understanding and modulating mammalian–microbial communication for improved human health. Annu. Rev. Pharmacol. Toxicol. 54, 559–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandacek R. J.; Heubi J. E.; Buckley D. D.; Khoury J. C.; Turner W. E.; Sjödin A.; Olson J. R.; Shelton C.; Helms K.; Bailey T. D.; Carter S.; Tso P.; Pavuk M. (2014) Reduction of the body burden of PCBs and DDE by dietary intervention in a randomized trial. J. Nutr. Biochem. 25, 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziech D.; Franco R.; Georgakilas A. G.; Georgakila S.; Malamou-Mitsi V.; Schoneveld O.; Pappa A.; Panayiotidis M. I. (2010) The role of reactive oxygen species and oxidative stress in environmental carcinogenesis and biomarker development. Chem.–Biol. Interact. 188, 334–339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.