Abstract
Background
The literature on the relationship between diet and asthma has largely focused on individual nutrients, with conflicting results. People consume a combination of foods from various groups that form a dietary pattern. Studying the role of dietary patterns in asthma is an emerging area of research. The purpose of this study was to systematically review dietary patterns and asthma outcomes in adults and children, to review maternal diet and child asthma, and to conduct a meta-analysis on the association between asthma prevalence and dietary patterns in adults.
Methods
We searched Medline, Scopus, and ISI Web of Knowledge up to January 2014. Two researchers independently reviewed studies meeting the inclusion criteria using the American Dietetic Association quality criteria. A linear mixed model was used to derive the pooled effect size (95% confidence interval) for each of three dietary pattern categories (healthy, unhealthy, and neutral).
Results
Thirty-one studies were identified (16 cross-sectional, one case-control, 13 cohort, and one randomized controlled trial), including 12 in adults, 13 in children, five in pregnant woman–child pairs, and one in both children and pregnant woman–child pairs. Six of the 12 adult studies reported significant associations between dietary patterns and asthma outcomes (eg, ever asthma and forced expiratory volume in one second). Seven of ten studies examining the Mediterranean diet showed protective effects on child asthma and/or wheeze. Four of the six studies in mother-child pairs showed that maternal dietary patterns during pregnancy were not associated with child asthma or wheeze. The meta-analysis including six adult studies, the primary outcome of which was the prevalence of current or ever asthma, showed no association with healthy, unhealthy, or neutral dietary patterns.
Conclusion
The evidence suggests no association of dietary patterns with asthma prevalence in adults or of maternal diet with child asthma or wheeze. The Mediterranean diet in children may prevent asthma or wheeze, but randomized controlled trials are lacking.
Keywords: dietary pattern, asthma, systematic review, meta-analysis, adults, children
Video abstract
Introduction
Over the past few decades, the prevalence of asthma has markedly increased. In the USA, the number of people with asthma increased from 20.3 million (6.3 million children and 14 million adults) in 2001 to 25.6 million (6.8 million children and 18.7 million adults) in 2012.1,2 Worldwide, asthma affects approximately 300 million people, and this number is expected to reach 400 million by 2050.3
Although contributory, genetic factors alone cannot account for the rapid increase in the prevalence of asthma.4 It has been hypothesized this increase is largely caused by environmental changes (eg, urbanization) and modification of lifestyle behaviors (eg, dietary transition).5 Notably, the transition from a traditional to a modern diet is characterized by an increased intake of preserved foods, salt, refined sugar, and saturated fat, and a decreased intake of fruit, vegetables, milk, and dietary fiber.6
Previous studies of the relationship of diet and nutrition with asthma have focused on either individual nutrients (eg, long-chain polyunsaturated fatty acids, vitamin D, and antioxidants)6–8 or individual food groups (eg, fruit, vegetables, and fish).9–11 However, diet is a complex combination of foods from various groups and nutrients, and some nutrients are highly correlated. It would be challenging to separate the effect of a single nutrient or food group from that of others in free-living populations. Chance findings may arise from indiscriminate multiple statistical testing and from inadequate control for confounding in observational studies. The available evidence from intervention trials focusing on the efficacy of single nutrients as disease-modifying agents in asthma is largely inconsistent.12,13
More recently, a few studies have investigated the association between overall dietary patterns and asthma. Due to the rapid evolution of research on this topic, it is worth performing a comprehensive literature review. One recently published meta-analysis of eight cross-sectional studies in children concluded that the Mediterranean diet might protect against ever asthma and current wheeze.14 To date, no reviews of dietary pattern studies in adult asthma have been published. The objectives of this research were to systematically review the up-to-date findings on the effects of dietary patterns on asthma outcomes in adults and children as well as the effects of maternal dietary patterns on asthma outcomes in children, and to conduct a meta-analysis of published studies examining the effect of dietary patterns on asthma prevalence in adults.
Methods
We followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement to prepare the manuscript.15
Literature search strategy
An electronic literature search was conducted using Medline (PubMed), Scopus, and ISI Web of Knowledge in January 2014 and extended back to 1950. The terms used to search titles and abstracts were (asthma OR wheezing OR wheeze OR lung function) AND (diet OR dietary OR food pattern). Further details on the literature search are shown in the Appendix 1. Only original studies with human subjects and published in English were included. In addition, cross-referencing from the articles found was used to complete the search. To be included in the systematic review, a study needed to have at least one dietary pattern predefined and measured (eg, Mediterranean) or generated from usual dietary intake using a multivariate statistical method and examine the effect or association of the dietary pattern(s) with one or more asthma outcome.
Inclusion criteria
The meta-analysis included studies of dietary patterns and asthma prevalence in adults meeting the following criteria: primary outcomes included prevalence of current or ever asthma, which was most commonly reported in the reviewed studies for adults (the number of studies assessing other asthma outcomes was too small to perform a meta-analysis); a dietary pattern score was calculated or dietary patterns were identified using a statistical method such as principal component analysis (PCA; selective solo or oligo food groups, eg, fruit and vegetables or fruit and fish, were not eligible); and odds ratio (OR) was calculated to determine the association between the dietary pattern(s) and asthma prevalence. Two researchers (NL and LX) independently reviewed the identified relevant articles and judged whether they met the inclusion criteria for meta-analysis. Uncertainties and discrepancies were resolved by consensus after discussing with a senior researcher (JM).
Quality assessment
The same two researchers (NL and LX) independently rated all the research articles included in the systematic review using the American Dietetic Association Quality Criteria Checklist.16 The scientific soundness of the articles was rated using ten validity questions. Based on the answers, one of the three quality ratings was assigned: positive (answered “yes” to six or more validity questions, including four priority questions), negative (answered “no” to six or more validity questions), or neutral (the rest of the situations). Only articles with a positive or neutral quality rating were included in the meta-analysis.
Assessment of dietary patterns and data extraction
When statistically derived using PCA or factor analysis, usually multiple dietary patterns were reported. Two researchers (NL and LX) independently grouped all dietary patterns into three categories: healthy, unhealthy, or neutral, based on constituent foods of each pattern suggested by PCA or main factor loadings. Any disagreements were discussed with the senior researcher (JM). Table 1 shows dietary patterns, categories, and constituent foods. A healthy dietary pattern was characterized by high intakes of fruit, vegetables, whole grains, and/or fish. An unhealthy dietary pattern tended to have high loadings of refined grain, red meat, processed meat, fast foods, high sugar foods, and/or high fat foods. A neutral dietary pattern generally consisted of a mixture of healthy and unhealthy food items. The same two researchers (NL and LX) independently extracted the data to be used for meta-analysis.
Table 1.
Dietary patterns, categories, and constituent foods from six adult studies included in the meta-analysis
| Study | Category | Dietary pattern | Food |
|---|---|---|---|
| Varraso et al27 | Healthy | Prudent | Fruit and vegetables |
| Neutral | Nuts and wine | Nuts and seeds, salty biscuits, olives, wine, fortified wine | |
| Unhealthy | Western | Pizza/salty pies, desserts, cured meat, and pasta | |
| Bakolis et al20 | Healthy | Prudent | Wholemeal bread and rolls, yogurt, cheese, fish, salad vegetables, pasta, couscous, vegetable dishes, and French-type dressing |
| Healthy | Vegetables and fruit | Vegetables and fruit | |
| Unhealthy | Western | White bread and rolls, chips, roast potatoes, baked beans, processed meats, bacon, ham, crisps, meat dishes, fried snacks, chocolate bars, sponge puddings and cakes, ketchup, and Coke | |
| Neutral | Vegetarian | Cream crackers, crème fraiche, macaroni cheese, chick peas, hummus, lentils, nut roast, vegetables, nuts, and seeds | |
| Neutral | Traditional | High intake of vegetables, pork, beef, liver, lamb; low intake of naan, paratha and Bombay mix | |
| Hooper et al23 | Healthy | Fish, fruit, and vegetables | Fruits, vegetables, and fish |
| Unhealthy | Meat and potatoes | Sliced meat, beef, pork, bacon, sausage and fried egg/scrambled egg/omelet, potato or chips, bread, butter, biscuits, cakes | |
| McKeever et al26 | Healthy | Cosmopolitan | Higher intake of vegetables, fish, chicken, wine, rice; lower intake of high-fat dairy products, added fat, added sugar, and potato |
| Unhealthy | Traditional | Higher intake of red meat, processed meat, potato, boiled vegetables, added fat, coffee, beer; lower intake of soy products, low-fat dairy products, tea, breakfast cereal, brown rice, pizza, juice and fruit | |
| Unhealthy | Refined foods | Higher intake of mayonnaise, salty snacks, candy, high-sugar beverages, French fries, white bread, and pizza; lower intake of boiled vegetables, wholegrain bread, fruit, and cheese | |
| Rosenkranz et al (men)22 | Healthy | Fruit and vegetables | Cooked vegetables, raw vegetables, fruit |
| Unhealthy | Meat and cheese | Red meat, processed meat, cheese | |
| Neutral | Grains and alcohol | Brown/wholemeal bread, alcoholic drinks, breakfast cereal | |
| Healthy | Poultry and seafood | Poultry, fish, or seafood | |
| Rosenkranz et al (women)22 | Healthy | Fruit and vegetables | Cooked vegetables, raw vegetables, fruit |
| Unhealthy | Meats | Red meat, processed meat | |
| Unhealthy | Cereal and alcohol | Breakfast cereal, alcoholic drinks | |
| Healthy | Poultry and seafood | Poultry, fish, or seafood | |
| Healthy | Brown bread and cheese | Brown/wholemeal bread, cheese | |
| Shi et al31 | Healthy | Vegetable-rich | Whole grains, fruit, root vegetables, fresh and pickled vegetables, milk, eggs, and fish |
| Unhealthy | Macho | Animal foods and alcohol | |
| Unhealthy | Sweet tooth | Cake, milk, yoghurt, and drinks | |
| Neutral | Traditional | Loaded heavily on rice, fresh vegetables, and inversely on wheat flour |
Statistical analysis
We performed a meta-analysis to evaluate the association of dietary patterns with asthma prevalence in adults. Studies reported dietary pattern scores either as continuous variables or categorized them into tertiles or quintiles. Linear mixed models were used to derive the pooled effect sizes and 95% confidence intervals (CIs) for healthy, unhealthy, and neutral dietary patterns and to assess heterogeneity between studies.17 In addition, heterogeneity and publication bias were visually evaluated using Begg’s funnel plots, which displayed the scatter patterns of effect estimates against standard errors from the included studies, with a vertical line indicating the pooled estimate and diagonal lines showing the expected 95% CIs around the estimate.18 All statistical analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Search results
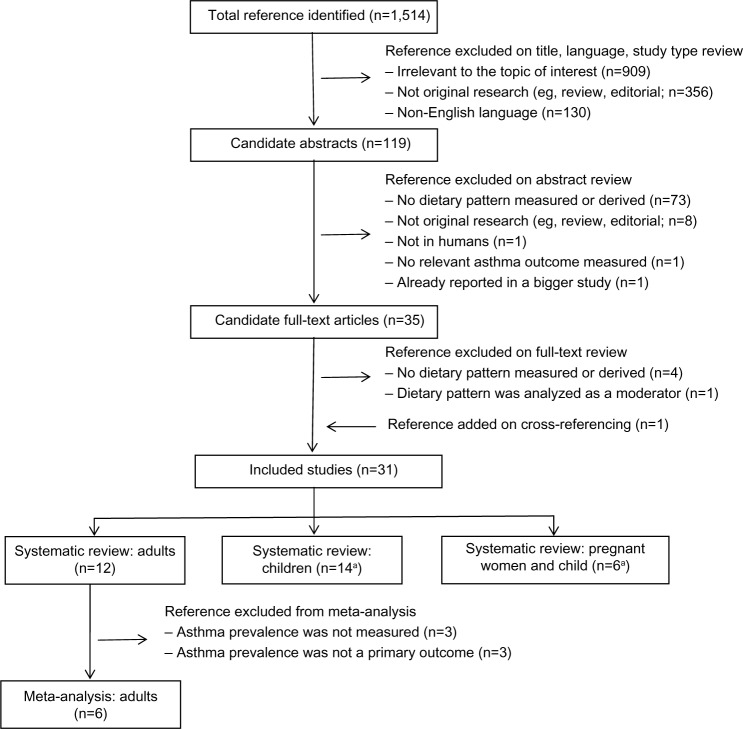
Of 1,514 references identified, 1,484 were excluded based on review of title (n=1,395), language (n=84), and study type, abstract, and full text (n=5), as shown in Figure 1. One reference was added on cross-referencing. Thirty-one studies were eligible for systematic review, including 12 in adults, 13 in children, five in pregnant woman–child pairs, and one in both children alone and pregnant woman–child pairs. Six studies in adults were excluded from meta-analysis because asthma prevalence was not measured (three studies assessed asthma control, quality of life, lung function, and/or inflammatory markers) or was not a primary outcome (three studies assessed chronic obstructive pulmonary disease or persistent cough with phlegm as a primary outcome).
Figure 1.
Results of search for relevant studies.
Note: aOne study investigated the effects of both children’s and maternal dietary patterns on asthma prevalence in children.
Scientific quality
One19 of the 31 studies was published as a conference abstract only and was not included in our quality review. Twenty-eight studies were rated positive (scoring 8 or 9 out of 10; Tables 2–4). One20 of the two studies rated as neutral was a case-control study that received a quality score of 8, but did not clearly describe whether potential confounding factors were comparable for the cases and controls, although the potential confounding variables were adjusted for in the analyses. The other study21 was a randomized controlled trial (RCT) that did not clearly describe the method of randomization or the amount of exposure to intervention. No study was excluded from meta-analysis based on quality ratings.
Table 2.
Summary of 12 studies reporting an association between dietary patterns and asthma outcomes in adults
| Studya and location | Sample and study design | Asthma outcome | Dietary pattern | Primary results | Adjusted confounders | Quality score (rating)b |
|---|---|---|---|---|---|---|
| Rosenkranz et al22 Australia |
n=156,035; male 62.2±10.6 years; female 60.2±10.2 years; 55% female; cross-sectional | Ever asthma | PCA: 4 dietary patterns for men (“fruit and vegetable”, “meats and cheese”, “grains and alcohol”, “poultry and seafood”) and 5 for women (“fruit and vegetables”, “meats”, “poultry and seafood”, “cereal and alcohol”, “brown bread and cheese”); 12-item FFQ | For men, a risk factor for asthma was meat/cheese (OR 1.18, 95% CI 1.08–1.28). For women, protective factor for asthma was cheese/brown bread (OR 0.88, 95% CI 0.82–0.94). | Age group, education, weight status, physical activity weekly minutes quartile, smoking status | 8 (positive) |
| Shi et al31 People’s Republic of China |
n=1,486, cohort | Ever asthma | PCA: 4 dietary patterns (“macho”, “traditional”, “sweet tooth”, “vegetable rich”); 33-item FFQ | “Traditional” pattern (rice, fresh vegetables) was positively associated with ever asthma (OR 2.25, 95% CI 1.45–3.51). | Age, sex, smoking, income, manual job, BMI, energy, MSG intake, all other dietary pattern scores | 8 (positive) |
| Bakolis et al20 UK |
599 cases and 854 controls; 16–50 years; 60% female; case-control study | (Current) asthma, quality of life | PCA: 5 dietary patterns (“prudent”, “vegetable and fruit”, “Western”, “vegetarian”, “traditional”); over 200-item FFQ | No clear relation between the dietary patterns and asthma outcomes was observed. | Age, sex, BMI, social class, housing tenure, employment status, whether a single parent, smoking, passive smoke exposure at home, total energy intake, ethnicity, number of siblings, paracetamol and supplement use, all other dietary patterns | 8 (neutral) |
| Hooper et al23 Germany, UK, Norway |
n=1,174; 29–55 years; cross-sectional | Current asthma, asthma symptom score, FEV1 | PCA: 2 dietary patterns (“meats and potatoes” and “fish, fruit and vegetables”); 158-, 198-, or 204-item FFQ | No association was observed between the dietary patterns and current asthma, asthma symptoms, and FEV1 | Age, sex, social class, smoking status, exercise, BMI, quintiles of total energy intake, other dietary pattern | 8 (positive) |
| McKeever et al26 The Netherlands |
Cross-sectional: n=12,648; 41.5±11.2 years; 52% female; cohort: n=2,911; 45.0±9.5 years; 50% female | Cross-sectional: FEV1, prevalence of asthma and wheeze; cohort: FEV1 decline | PCA: 3 dietary patterns: (“cosmopolitan”; “traditional”; “refined foods”); 178-item FFQ | “Traditional diet” (high intake of meat and potatoes and lower intake of soy and cereal) was negatively associated with FEV1 (5th versus 1st quintile −94.4 mL; 95% CI −123.4, −65.5 mL). “Cosmopolitan” was positively associated with wheeze (OR 1.3, 95% CI 1–1.5) and asthma (OR 1.4, 95% CI 1–1.9). No dietary patterns were associated with lung function decline. | Age, sex, smoking status, pack-years of smoking, education level, location | 8 (positive) |
| Varraso et al27 France |
n=54, 672; asthmatics 52.5±6.5 years; nonasthmatics 52.7±6.5 years; 100% female; cohort | Current asthma, adult-onset asthma, and frequency of asthma attacks | Factor analysis: 3 dietary patterns: (“prudent”; “Western”; “nuts and wine”); 66-item FFQ | No dietary pattern was associated with asthma incidence or current asthma. Western pattern (pizza, salty pies, desserts, and cured meat) was a risk factor for asthma attacks (highest versus lowest tertile OR 1.79, 95% CI 1.11–3.73) while nuts and wine pattern was protective (highest versus lowest tertile OR 0.65, 95% CI 0.31–0.96). | Age, energy intake, BMI, smoking, physical activity, menopausal status, education, multivitamin supplement use | 8 (positive) |
| Sexton et al21 New Zealand |
38 adults with symptomatic asthma; high intervention: 38.0±4.2 years, 88% female; low intervention: 37.0±4.0 years, 67% female; control: 40.2±4.0 years, 67% female; 12-week RCT | Asthma control (ACQ), asthma-related quality of life, FEV1, FVC, inflammatory markers. | Mediterranean diet; 142-item FFQ | With significantly increased Mediterranean score, the high intervention group achieved small but insignificant improvement in asthma-related quality of life and prebronchodilator FEV1 and FVC. No changes were observed in asthma control or inflammatory markers. | Age, sex | 6 (neutral) |
| Shaheen et al24 UK |
n=2,942; male 65.7±2.9 years; female 66.6±2.7 years; 47% female; cross-sectional | FEV1, FVC, FEV1/FVC | PCA: 2 dietary patterns (“prudent”; “traditional”); 129-item FFQ | A “prudent” pattern was positively associated with FEV1 (men, adjusted coefficient 0.18 L, 95% CI 0.08–0.28 L; women, adjusted coefficient 0.08 L, 95% CI 0.00–0.16 L) and FVC (men, adjusted coefficient 0.03; 95% CI 0.00–0.05; women, adjusted coefficient 0.03; 95% CI 0.01–0.04) in both men and women. | Age, height, smoking status, pack-years, smoke in home, age left education, home ownership status, number of rooms, number of cars, social class, fat mass, activity score, energy intake, alcohol, dietary supplement use, birth weight, father’s social class at birth, inhaled or oral steroid use, paracetamol use | 8 (positive) |
| Barros et al25 Finland |
n=174; 40±15 years; 82% female; cross-sectional | Asthma control (controlled defined as FEV1 ≥80%, FeNO ≤35 ppb, and ACQ score <1) | Mediterranean diet; 86-item FFQ | Mediterranean diet reduced 78% (OR 0.22, 95% CI 0.05–0.85) the risk of uncontrolled asthma. | Sex, age, education, inhaled corticosteroids, energy intake | 8 (positive) |
| Varraso et al28 USA |
n=72,043; 30–55 years; 100% female; cohort | Prevalence of adult-onset asthma between 1984 and 2000 | PCA: 2 dietary patterns (“prudent”; “Western”); 116-item FFQ | Dietary patterns were not associated with adult-onset asthma. | Age, smoking status, pack-years, pack-years2, exposure to secondhand tobacco smoke, menopausal status, race-ethnicity, spouse’s educational attainment, physician visits, US region, BMI, physical activity, multivitamin use, energy intake | 8 (positive) |
| Varraso et al29 USA |
n=42,917; 40–75 years; 100% men; cohort | Prevalence of adult-onset asthma between1986 and 1998 | PCA: 2 dietary patterns (“prudent”; “Western”); 131-item FFQ | Dietary patterns were not associated with adult-onset asthma. | Age, smoking, pack-years, pack-years2, race/ethnicity, physician visits, US region, BMI, physical activity, multivitamin use, energy intake | 8 (positive) |
| Butler et al30 Singapore |
n=52,325; 45–74 years; cohort | Prevalence of new-onset asthma between baseline and follow-up | PCA: 2 dietary patterns(“meat-dim sum”; “vegetable-fruit-soy”); 165-item FFQ | Dietary patterns were not associated with new-onset asthma. | Age, total energy intake, dialect group, sex, smoking status, age at starting to smoke, cigarettes per day, adult environmental tobacco smoke exposure, education, (nonstarch polysaccharide intake) | 8 (positive) |
Notes:
The first six studies were included in meta-analysis because all examined ever or current asthma as the primary outcome
quality was scored and rated independently using the American Dietetic Association Quality Criteria Checklist. Pack-years was defined as the number of packs smoked per day multiplied by the number of years smoked. The authors adjusted for both pack-years and the square of pack-years (pack-years2).
Abbreviations: ACQ, asthma control questionnaire; BMI, body mass index; CI, confidence interval; FFQ, food frequency questionnaire; OR, odds ratio; PCA, principal component analysis; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; MSG, monosodium glutamate; RCT, randomized controlled trial.
Table 4.
Summary of six studies reporting the association between maternal dietary patterns and wheeze in children
| Study and location | Sample and study design | Outcome | Dietary pattern | Primary results | Adjusted confounders | Quality score (rating)a |
|---|---|---|---|---|---|---|
| Chatzi et al45 Spain, Greece |
n=2,516 pregnant woman-infant pairs; cohort | Wheeze in the first year of life | Mediterranean diet; 100-item FFQ | Adherence to Mediterranean diet during pregnancy was not associated with wheeze in the first year of life | Maternal age; education; maternal history of asthma; smoking during pregnancy; parity; duration of breastfeeding; child’s age at assessment; child’s sex | 8 (positive) |
| Miyake et al49 Japan |
n=763 pregnant woman-infant pairs; cohort | Wheeze in toddlers aged 16–24 months | Factor analysis: 3 dietary patterns (“healthy”, “Western” and “Japanese”); 150-item FFQ | Only maternal “Western” dietary pattern was a protective factor of child wheezing (OR 0.59, 95% CI 0.35–0.98) | Maternal age, gestation at baseline, residential municipality at baseline, family income, maternal and parental education, maternal and parental history of asthma, atopic eczema and allergic rhinitis, changes in maternal diet in the previous one month, season at baseline, maternal smoking during pregnancy, baby’s older siblings, baby’s sex, baby’s birth weight, household smoking in the same room as infant, breastfeeding duration, and age of infant at third survey | 8 (positive) |
| Castro-Rodriguez et al46 Spain |
n=1,409 pregnant woman-infant pairs (mean age, 16.6±2.5 months); cohort | Ever wheezing during the first year | Mediterranean diet; FFQ (number of item unreported) | Mediterranean diet score (excluding olive oil) was not associated with infants’ ever wheezing during the first year. However, olive oil was protective of ever wheezing (OR 0.57, 95% CI 0.4–0.9) | Sex, exclusive breastfeeding, day care attendance, eczema, maternal asthma, smoking during pregnancy, siblings, mold on household wall, preterm birth, olive oil | 8 (positive) |
| Lange et al48 USA |
n=1,376 pregnant woman-child pairs; cohort | Recurrent wheeze at 3 years | Mediterranean diet; Alternate Healthy Eating Index modified for pregnancy; PCA: 2 dietary patterns (“prudent” and “Western”); 166-item FFQ | No maternal dietary pattern was associated with recurrent wheeze in children | Child sex, maternal race, maternal education level, household income, maternal and paternal history of asthma, presence of children <12 years of age at home, maternal prepregnancy BMI, breast-feeding duration, and passive smoke exposure | 8 (positive) |
| Shaheen et al47 UK |
n=14,541 pregnant women and 14,062 children; cohort | Early wheezing phenotypes at 2.5 years; wheezing at 3.5 years; asthma, wheezing at 7 years; lung function and bronchial responsiveness at 8–9 years | PCA: 5 dietary patterns (“health conscious”, “traditional”, “processed”, “vegetarian” and “confectionery”); 51-item FFQ | Maternal dietary patterns were not associated with asthma and related outcomes after adjusting for confounding variables | Energy intake, maximum smoked, infections, antibiotics and paracetamol use during pregnancy; maternal education level, housing tenure, financial difficulties, prepregnancy BMI, ethnicity, age, parity, history of asthma, eczema, rhinoconjunctivitis, migraine; child’s sex, gestational age, breast fed in first 6 months, day care at 8 months, multiple pregnancy, pets in infancy, damp/condensation/mold, child exposed to environmental tobacco smoke, season of birth, season of FFQ completion, birth weight, head circumference, birth length | 8 (positive) |
| Chatzi et al38 Spain |
n=507 pregnant women and 460 children; cohort | Persistent wheeze, atopic wheeze in children at 6.5 years | Mediterranean diet; 42-item FFQ | Higher adherence of Mediterranean diet was a protective factor of persistent wheeze (OR 0.22, 95% CI0.08–0.58) and atopic wheeze (OR 0.30, 95% CI0.10–0.90) at age 6.5 years | Sex, maternal and paternal asthma, maternal social class and education, BMI, total energy intake, children adherence to Mediterranean diet at age 6.5 | 8 (positive) |
Notes:
Quality was scored and rated independently using the American Dietetic Association Quality Criteria Checklist.
Abbreviations: BMI, body mass index; CI, confidence interval; FFQ, food frequency questionnaire; OR, odds ratio; PCA, principal component analysis.
Adult dietary patterns and asthma
Systematic review
Table 2 shows the main characteristics and results of the 12 studies in adults, published between 2006 and 2013. These papers included four cross-sectional studies,22–25 six cohort studies,26–31 one case-control study,20 and one RCT.21 Six of them were conducted in Europe,20,23–27 two in Australia,21,22 two in the USA,28,29 and two in Asia.30,31 Sample sizes ranged from 38 in the RCT to 156,035 in the cross-sectional studies. Two studies27,28 included female subjects only and one29 male subjects only, while the remaining examined both sexes, with one22 examining men and women separately.
All studies used food frequency questionnaires (FFQs) to measure dietary intakes, with the number of food items or groups ranging from 12 to over 200. Ten studies20,22–24,26–31 derived at least two dietary patterns a posteriori using PCA (n=9) or factor analysis (n=1). Two studies21,25 calculated a Mediterranean diet score defined a priori.
Asthma outcomes evaluated in these studies included prevalence of ever or current asthma, asthma-related quality of life, asthma symptoms, lung function (forced expiratory volume in one second, forced vital capacity), frequency of asthma attacks, asthma control (Asthma Control Questionnaire alone or plus fractional exhaled nitric oxide), and asthma-related inflammatory markers. The findings were mixed. Among the 12 studies, six reported significant association between dietary patterns and ever asthma,22,31 forced expiratory volume in one second,24,26 frequency of asthma attacks,27 and risk of uncontrolled asthma.25 Although the asthma outcomes varied across these studies, potentially protective dietary patterns tended to include cheese/brown bread, nuts and wine, a prudent pattern diet (fruit, vegetables, oily fish, and wholemeal cereals), and the Mediterranean diet. At the same time, potentially risky dietary patterns tended to include meats/cheese, Chinese traditional pattern (rice and fresh vegetables), the Netherlands traditional diet (meat and potatoes), and Western pattern (pizza, salty pies, desserts, and cured meat). In contrast, one cross-sectional,23 one case-control,20 and three cohort studies28–30 reported no association. An RCT with 38 adults who had symptomatic asthma showed no effect of two Mediterranean diet interventions on asthma control, lung function, asthma-related quality of life, or inflammatory markers compared with no-intervention control.21
Results of meta-analysis
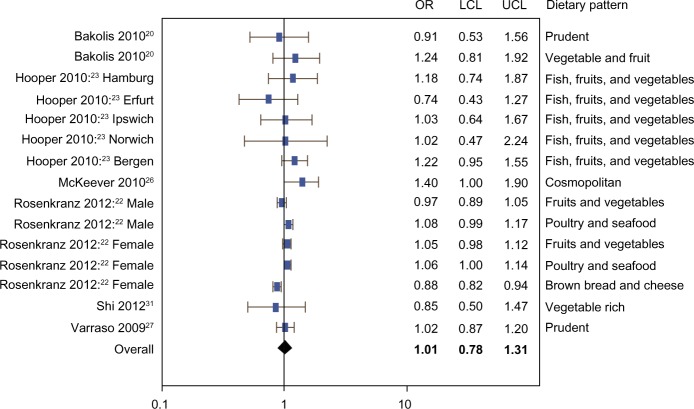
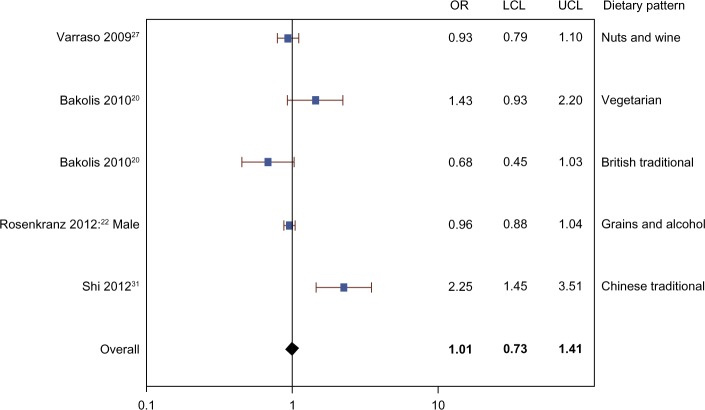
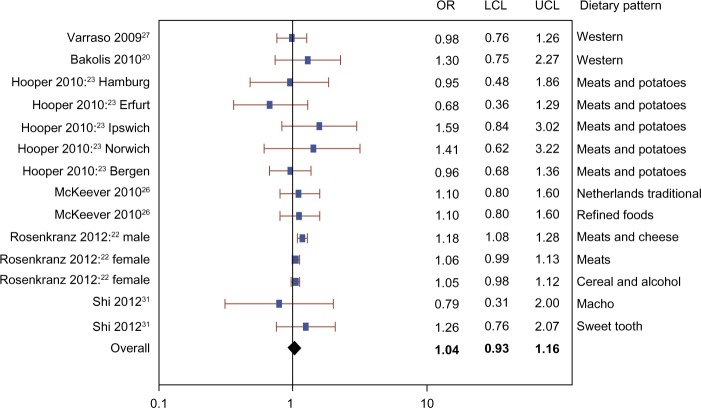
The meta-analysis included three cohort studies,26,27,31 two cross-sectional studies,22,23 and one case-control study.20 Figures 2–4 show that there was no evidence of association between the prevalence of current or ever asthma and healthy (OR 1.01, 95% CI 0.78–1.31), unhealthy (OR 1.04, 95% CI 0.93–1.16), or neutral (OR 1.01, 95% CI 0.73–1.41) dietary patterns. The mixed model results show that the random effect estimates were zero, suggesting very small or negligible variance between versus within studies.
Figure 2.
Meta-analysis of observational studies examining the association between healthy dietary patterns and prevalence of current or ever asthma.
Abbreviations: OR, odds ratio; LCL, lower confidence limit; UCL, upper confidence limit.
Figure 4.
Meta-analysis of observational studies examining the association between neutral dietary patterns and prevalence of current or ever asthma.
Abbreviations: OR, odds ratio; LCL, lower confidence limit; UCL, upper confidence limit.
Publication bias and heterogeneity
Figure 5 shows funnel plots of studies examining the association between healthy, unhealthy, and neutral dietary patterns separately and the prevalence of current or ever asthma. The plots were roughly symmetrical, suggesting little evidence of publication bias, and almost all the studies lay within the diagonal lines indicating 95% CIs, suggesting negligible between-study heterogeneity, which is consistent with the model-based results described above.
Figure 5.
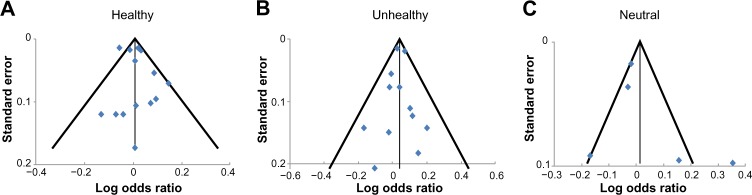
Funnel plots of studies in meta-analysis.
Child dietary patterns and asthma
The main characteristics and results of the 14 studies in children, published between 2006 and 2013, are shown in Table 3. Twelve studies were cross-sectional and two32,33 were cohort studies. Nine of them were conducted in Europe,19,32,34–40 three in America (one in Brazil41 and two in Mexico33,42), and one in Asia.43 One other study44 was an international study conducted in 20 countries. All studies examined both boys and girls with sample sizes ranging from 158 (cohort) to 50,004 (cross-sectional).
Table 3.
Summary of 14 studies reporting the association between dietary patterns and asthma outcomes in children
| Studya and location | Sample and study design | Asthma outcome | Dietary pattern | Primary results | Adjusted confounders | Quality score (rating)b |
|---|---|---|---|---|---|---|
| de Cássia Ribeiro Silva et al41 Brazil |
n=1,187; 6–12 years; 47% girls; cross-sectional | Current wheezing | PCA: 2 dietary patterns (“prudent” and “Western”); 97-item FFQ | Western pattern was positively associated with wheeze (OR 1.77, 95% CI 1.10–2.84). | Age, sex, education of caregivers, per capita income, number of people living in the household, presence of smokers in the house, BMI, stages of sexual maturity, physical activity, energy intake, the other dietary pattern | 8 (positive) |
| Lee et al43 Taiwan, People’s Republic of China |
n=2,082; 8.5±1.7 years; 47% girls; cross-sectional | Current asthma, current severe asthma, nocturnal cough, exercise-induced wheeze, ever asthma | RRR: 1 unhealthy dietary pattern; 21-item FFQ | The unhealthy dietary pattern (high consumption of fast foods, high fat snacks, candy, and cheese, low consumption of fruits, vegetables, and rice) was positively associated with current asthma (OR 2.42, 95% CI 1.19–4.93), current severe asthma (OR 4.45, 95% CI 1.59–12.5), and nocturnal cough (OR 1.82, 95% CI 1.07–3.11). | Age, sex, BMI z-score, older sibling number, mother’s education level, parental asthma history, ambient nitrogen oxides concentration, seasonal effect | 8 (positive) |
| Tromp et al32 The Netherlands |
n=2,173; cohort | Wheezing, shortness of breath at the age of 2, 3, and 4 years | PCA: 2 dietary patterns (“health conscious” and “Western”); 211-item FFQ | Higher adherence to “Western” pattern was positively associated with frequent wheeze (RR 1.39; 95% CI 1.02–1.89) at 3 years of age and frequent shortness of breath (RR 1.44; 95% CI 1.03–2.01) at 4 years of age. These associations were partially explained by energy intake. | Maternal age, maternal socioeconomic status, smoking during pregnancy, parental history of atopy, multiple parities, standard deviation score birth weight, sex, breastfeeding, vitamin D supplementation at 6–12 months, day care attendance in the first 2 years of life, and history of cow’s milk allergy in the first year | 9 (positive) |
| Grigoropoulou et al34 Greece |
n=1,125; 10–12 years; 53% girls; cross-sectional | Ever asthma (symptoms) | Mediterranean diet; 63-item FFQ | Higher Mediterranean score was associated with a lower prevalence of ever asthma (OR 0.84, 95% CI 0.77–0.91). | Environmental factors (details unknown) | 9 (positive) |
| Gonzalez Barcala et al35 Spain |
n=14,700: 6–7-year age group, 6.5±0.5 years, 49% girls; 13–14-year group, 13.5±0.5 years; 51% girls; cross-sectional | Prevalence and severity of ever asthma | Mediterranean diet (quartiles); FFQ (number of items unknown) | No protective effect of Mediterranean diet was found. Higher adherence of the diet was associated with a higher risk of severe asthma (OR 2.26, 95% CI 1.21–4.22) in girls aged 6–7 years. |
BMI, parental smoking, maternal education | 8 (positive) |
| Romieu et al33 Mexico |
n=158 asthmatic (mean 9.6 years; 38% girls) and 50 nonasthmatic (mean 9.3 years; 60% girls); cohort | Inflammatory response (IL-8) and lung function (FEV1, FVC) | Fruit and vegetable index and a Mediterranean diet index; 108-item FFQ | In asthmatic children, fruit and vegetable index was negatively associated with IL-8 levels in nasal lavage (P=0.013); Mediterranean diet index was positively associated with FEV1 (P=0.045) and FVC (P=0.018). Fruit and vegetable index was a significant modifier for the effect of ozone on FEV1 (P=0.023) and FVC (P=0.008) and Mediterranean diet was a modifier for the effect of ozone on FVC (P=0.02). | Sex, BMI, previous day minimum temperature, corticoid use, chronological time | 8 (positive) |
| Arvaniti et al36 Greece |
n=700; 10–12 years; 54% girls; cross-sectional | Ever asthma, asthma symptom, ever wheeze, exercise wheeze | Mediterranean diet; 63-item FFQ | Higher adherence to Mediterranean diet was associated with a lower prevalence of ever asthma, any asthma symptom, ever wheeze, and exercise wheeze (all P<0.005). | Age, sex, BMI, physical activity status, energy intake | 8 (positive) |
| Nagel et al44 20 countries |
n=50,004; 8–12 years; cross-sectional | Ever asthma, current wheeze, and atopic wheeze | Mediterranean diet; FFQ (number of items unreported) | Higher adherence to Mediterranean diet was associated with a lower prevalence of ever asthma (OR 0.95, 95% CI 0.92–0.99) and current wheezing (OR 0.97, 95% CI 0.94–0.99). | Age, sex, environmental tobacco smoke, parental atopy, exercise, number of siblings | 8 (positive) |
| Castro-Rodriguez et al37 Spain |
n=1,784; 4.08±0.8 years; cross-sectional | Current wheeze | Mediterranean diet; FFQ (number of items unreported) | Mediterranean diet was a protective factor for current wheezing (OR 0.54, 95% CI 0.33–0.88). | Age, birth weight, livestock during pregnancy, delivery by cesarean, antibiotic consumption during the first year, acetaminophen consumption during the previous 12 months, rhinoconjunctivitis, dermatitis, paternal asthma, maternal asthma, maternal age, maternal education level, current paternal smoking, current maternal smoking, vigorous physical activity frequency, cats at home in the last 12 months | 8 (positive) |
| De Batlle et al42 Mexico |
n=1,476; 6–7 years; cross-sectional | Ever asthma, ever wheezing, current wheezing | Mediterranean diet; 70-item FFQ | Adherence to Mediterranean diet was negatively associated with ever asthma (OR 0.60, 95% CI 0.40–0.91) and ever wheezing (OR 0.64, 95% CI 0.47–0.87). | Sex, maternal education, exercise, current tobacco smoking at home, maternal asthma, maternal rhinitis | 8 (positive) |
| Chatzi et al38 Spain |
n=460 children aged 6.5 years; cross-sectional | Persistent wheeze, atopic wheeze | Mediterranean diet; 96-item FFQ | Adherence to Mediterranean diet was not associated with either asthma outcome. | Sex, maternal and paternal asthma, maternal social class and education, BMI, total energy intake | 8 (positive) |
| Chatzi et al39 Greece |
n=690; 7–18 years; 52% girls; cross-sectional | Current and ever wheezing, wheezing ever with atopy | Mediterranean diet; 58-item FFQ | High adherence to Mediterranean diet was not associated with current and ever wheezing. | Age categories, sex, BMI, parental asthma, number of older siblings | 8 (positive) |
| Garcia-Marcos et al40 Spain |
n=20,106; 6–7 years; cross-sectional | Current occasional asthma, current severe asthma | Mediterranean diet; 15-item FFQ | Mediterranean diet was a protective factor for current severe asthma in girls (OR 0.90, 95% CI 0.82–0.98). | Older and younger siblings, maternal smoking | 8 (positive) |
| Sanchez-Solis et al19 Spain |
n=683; 6–8 years; cross-sectional | Clinical significant asthma | Mediterranean diet; 26-item FFQ | Mediterranean diet was a protective factor for clinical significant asthma, independent of percent body fat (OR 0.78, 95% CI 0.61–0.97). | Adjusted, but not specified | NAc |
Notes:
Last 8 studies were included in a previously published meta-analysis study;14
quality was scored and rated independently using the American Dietetic Association Quality Criteria Checklist
this conference abstract was not rated due to insufficient information.
Abbreviations: BMI, body mass index; CI, confidence interval; FFQ, food frequency questionnaire; OR, odds ratio; PCA, principal component analysis; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; IL, interleukin; RR, relative risk; RRR, reduced rank regression.
Eight of these studies (shown in the footnote to Table 2) were included in a recent meta-analysis14 to investigate whether the Mediterranean diet has a protective effect on ever asthma and current wheeze and whether these relationships were specific to the Mediterranean regions. The meta-analytic results showed adherence to the Mediterranean diet was negatively associated with current wheeze (OR 0.79, 95% CI 0.66–0.94; P=0.009) and current severe wheeze (OR 0.66, 95% CI 0.48–0.90; P=0.008) in Mediterranean regions, and with ever asthma (OR 0.86, 95% CI 0.75–0.98; P=0.027) in non-Mediterranean regions. When considering all regions together, the authors concluded adherence to the Mediterranean diet tended to have a protective effect on current wheeze and ever asthma but not on current severe wheeze.
The meta-analysis14 excluded six studies for the following reasons: three studies derived dietary patterns a posteriori,32,41,43 one measured lung function as the lone outcome,33 and two34,35 reported data published in other studies that were included in the meta-analysis. The limited additional studies on dietary patterns and child asthma or wheeze precluded another meta-analysis. Among these six studies, five found either a beneficial effect of the Mediterranean diet or a detrimental effect of an unhealthy (eg, Western) pattern. Interestingly, one study reported that the Mediterranean diet was a risk factor for severe asthma in girls aged 6–7 years.35 The authors speculated this could be due to a reverse causal effect (families of children with severe asthma may improve their diet) and residual confounding.
Overall, the meta-analysis and six additional studies suggest that the Mediterranean diet is potentially protective against child asthma.
Maternal dietary patterns and child asthma
Table 4 shows the main characteristics and results of the six studies reporting an association between maternal dietary patterns and asthma prevalence in children. All were cohort studies published between 2008 and 2013, with four conducted in Europe,38,45–47 one in the USA,48 and one in Asia.49 Sample sizes ranged from 460 to 14,062.
All studies used FFQs to measure dietary intakes, with number of food items or groups ranging from 42 to 166. Three studies38,45,46 calculated a Mediterranean diet score defined a priori. Two studies47,49 derived dietary patterns using PCA or factor analysis. One study48 used a combination of both a posteriori and a priori approaches to measure dietary patterns.
These studies examined the association between maternal dietary patterns and wheezing prevalence in children between the ages of one and 7 years. The heterogeneity of the study populations and outcomes precluded a meta-analysis. However, four of the six studies did not find any association between maternal dietary patterns and prevalence of wheezing. One study38 reported a protective effect of maternal Mediterranean diet pattern on persistent wheeze (OR 0.22, 95% CI 0.08–0.58) and atopic wheeze (OR 0.30, 95% CI 0.10–0.90) in offspring at age 6.5 years. Another study49 conducted in Japan found a beneficial effect of maternal “Western” dietary pattern on wheeze (OR 0.59, 95% CI 0.35–0.98; P=0.02) in toddlers aged 16–24 months. The authors noted that this “Western” dietary pattern in Japan might be comparatively healthier than the typical Western dietary pattern in the USA, because it was characterized by low intake of soft drinks, confectionery, and fruit, in addition to high intake of vegetable oil, salt-containing seasonings, beef, pork, processed meat, eggs, chicken, and white vegetables. Adherence to this “Western” dietary pattern was actually positively associated with a high intake of α-linolenic acid, vitamin E, and β-carotene, which were shown in some studies to have a beneficial effect on asthma and wheezing.7,9,50,51 The Japanese study suggests that dietary patterns are region-specific and population-specific, and that caution is necessary when interpreting the results of studies in diverse populations. Overall, these studies show weak evidence of any association between maternal dietary pattern and child wheezing.
Discussion and conclusion
Taken together, studies in adults and pregnant woman–child pairs failed to show that dietary patterns were associated with asthma outcomes. Only studies in children suggested a protective effect of the Mediterranean diet on current wheeze and ever asthma.
Compared with studying individual foods or nutrients, an evaluation of dietary patterns may shed light on the combinatorial effects of foods and/or nutrients on the health outcome of interest. Studying the overall effect of dietary patterns on asthma is an emerging literature; however, the findings so far have been inconsistent. We offer several possible explanations for the inconsistency and suggestions for future research.
First, the heterogeneous results may be partially explained by the notable variation in FFQs used for measuring dietary intakes and statistical approaches for deriving dietary patterns. For example, in adult studies, the FFQs included between 12 and over 200 food items or groups, which could influence the selection of foods loaded on the dietary patterns. Although most of the FFQs were validated, the dietary patterns derived from them explained only a small to medium percentage of total variance, ranging from 11% to 58% in the five adult studies that reported this information.
Two approaches, a priori and a posteriori, have been used to generate dietary patterns. Both approaches have strengths and weaknesses. The a priori approach focuses on a predefined dietary pattern based on prior knowledge of a specific diet (eg, Mediterranean) and its relationship to disease. Therefore, this approach is limited by current knowledge and could involve uncertainties in selecting individual components of the diet index and subjective decisions of defining cutoffs.52 In contrast, the a posteriori approach provides opportunities to open up new areas of diet-disease research and detect dietary patterns specific to the region and/or population of interest. However, it involves important but arbitrary decisions, including the number of components to extract, the method of rotation, consolidation of food items into groups, and labeling of the components.53 Researchers should choose the appropriate method according to the study objectives and dietary characteristics of the study population.
Most studies in children (eleven of 14) and pregnant woman–child pairs (four of six) used an a priori approach and defined the dietary pattern using a Mediterranean diet index, whereas ten of 12 studies in adults utilized an a posteriori approach to derive dietary patterns statistically (eg, with PCA). In addition to the Mediterranean diet, future studies may also examine the association between healthy dietary patterns in other regions (eg, the widely promoted Dietary Approaches to Stop Hypertension in the USA54) or alternative diet quality indices (eg, the Healthy Eating Index55) and asthma outcomes. If using the a posteriori approach, the reliability and validity of the dietary patterns generated from FFQs can be examined using a different source of dietary data (eg, dietary records). To examine the reproducibility of the dietary patterns, sensitivity analyses can be performed to test whether the arbitrary choices made during PCA or factor analysis influence the results and whether similar dietary patterns can be obtained using randomly split samples.52
Statistical approaches used to derive dietary patterns a posteriori have included PCA, factor analysis, cluster analysis, and to a lesser extent, reduced rank regression. Some review papers detailed each of these approaches.56,57 Different from the exploratory approaches (eg, PCA, factory analysis, and cluster analysis), reduced rank regression defines linear combinations of food intakes that maximally explain the outcome variable (eg, an asthma outcome). In other words, in contrast with PCA and factor analysis, which derive dietary patterns to maximally explain the variance in food intake among participants, reduced rank regression identifies dietary patterns to maximally explain the outcome variable. Among the studies reviewed in this paper, most used PCA, two used factor analysis, and only one in children used reduced rank regression. This may be another reason why many studies of dietary patterns using PCA or factor analysis found no associations with asthma outcomes. The appropriate statistical method should be chosen based on study objectives. In doing so, one must keep in mind that PCA, factor analysis, and cluster analysis identify existing dietary patterns while reduced rank regression is likely to yield useful information for hypothesis generation but may not describe actual intake patterns in the population.58
Second, the heterogeneity of reported results is also possibly attributable to the varied number of confounders controlled for in the studies. For example, the number of confounders controlled for ranged from five to 19 in the observational studies among adults. Confounding could pose challenges for interpretation of the diet–asthma relationship. Nurmatov et al59 have proposed a comprehensive list of primary and secondary confounders that should be considered in future epidemiologic studies examining the early-life diet and asthma relationship in children. The authors suggested that the primary confounders should account for maternal and child characteristics, socioeconomic status, environmental exposures, and dietary factors, while the secondary confounders could be confirmed using appropriate statistical tests. Confounders in the diet–asthma relationship are different between children and adults; therefore, further research is needed to investigate confounders in the adult population. Future observational studies should select confounders based on existing knowledge of the causal mechanism in the diet– asthma relationship and suggestive evidence from statistical analysis. The criteria for selection of confounders should also be reported in observational studies, so readers can be well informed to reach a valid and reliable interpretation of findings.60 In addition, very few population-based studies have been conducted to investigate the association between dietary patterns and asthma outcomes. National survey data (eg, the National Health and Nutrition Examination Survey) may be leveraged to examine the diet–asthma association and identify potential covariates.
Lastly, the inconsistent findings underline the importance of prospective studies and RCTs in helping to better understand the role of dietary patterns in the etiology and disease course of asthma. People’s dietary patterns often change over time through the lifespan and because of changes in socioeconomic and/or health status. Most of the studies reviewed were cross-sectional, precluding investigation of a temporal or causal relationship between dietary patterns and asthma. The cumulative effects of diet on asthma warrant prospective studies. Also, to date, only two RCTs have been designed to evaluate the impact of a healthy dietary pattern on asthma. One is a Mediterranean diet intervention study21 recently completed in New Zealand and the other is an ongoing Dietary Approaches to Stop Hypertension intervention study61 in the USA. More experimental studies like these are needed to elucidate the causal relationship.
This systematic review and meta-analysis has a number of strengths and limitations. This is a comprehensive review of the literature on dietary patterns and asthma from 1950 to 2014; however, it was limited to studies published in English. Because of the institutional subscription limitation, we did not include Embase as one of the databases searched for this review. Although Scopus overlaps substantially with Embase,62,63 any studies only indexed in Embase would have been missed. The funnel plots suggested no evidence of publication bias. Nonetheless, the meta-analysis was limited by the abovementioned inherent limitations of individual studies, including a low percentage of total variance explained by the dietary patterns, and inconsistent and possibly incomplete adjustment for potential confounders. Regardless of the limitations, this paper shows that the results of existing studies do not reveal a clear and consistent relationship between dietary patterns and asthma outcomes. Although higher adherence to the Mediterranean diet may be associated with reduced asthma risk in children, more well designed and controlled studies are needed to provide solid evidence and explore whether other healthy dietary patterns are associated with asthma outcomes in children and adults.
Figure 3.
Meta-analysis of observational studies examining the association between unhealthy dietary patterns and prevalence of current or ever asthma.
Abbreviations: OR, odds ratio; LCL, lower confidence limit; UCL, upper confidence limit.
Appendix
Appendix 1.
Search strategy
| Search strategy | Number of references |
|---|---|
|
PubMed: (asthma[TIAB] OR wheezing[TIAB] OR wheeze[TIAB] OR lung function[TIAB]) AND (diet[TIAB] OR dietary[TIAB] OR food pattern[TIAB]) Limit: Species (Human) |
1,307 |
|
Scopus: ((TITLE({asthma}) OR ABS({asthma})) OR (TITLE({wheezing}) OR ABS({wheezing})) OR (TITLE({wheeze}) OR ABS({wheeze})) OR (TITLE({lung function}) OR ABS({lung function}))) AND ((TITLE({diet}) OR ABS({diet})) OR (TITLE({dietary}) OR ABS({dietary})) OR (TITLE({food pattern}) OR ABS({food pattern}))) Limit: Subject Area (Medicine; Immunology and Microbiology; Agricultural and Biological Sciences; Nursing; Pharmacology, Toxicology and Pharmaceutics; Health Professions; Environmental Science; Social Science; Multidisciplinary) and Document Type (article; conference paper) |
1,272 |
|
ISI Web of Knowledge: TS=(“asthma” OR “wheezing” OR “wheeze” OR “lung function”) AND TS=(“diet” OR “dietary” OR “food pattern”) Limit: Web of Science Categories (Allergy; Immunology; Respiratory System; Nutrition Dietetics; Pediatrics; Medicine General Internal; Public Environmental Occupational Health; Critical Care Medicine) and Document Type (article; proceedings paper; meeting abstract) |
1,195 |
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.National Center for Health Statistics National Health Interview Survey. 2001. [Accessed March 7, 2014]. Available from: http://www.cdc.gov/asthma/nhis/01/table3-1.htm.
- 2.National Center for Health Statistics National Health Interview Survey. 2012. [Accessed March 7, 2014]. Available from: http://www.cdc.gov/asthma/nhis/2012/table3-1.htm.
- 3.Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma (GINA) Program The global burden of asthma: Executive Summary of the GINA Dissemination Committee Report. Allergy. 2004;59(5):469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 4.Skadhauge LR, Christensen K, Kyvik KO, Sigsgaard T. Genetic and environmental influence on asthma: a population-based study of 11,688 Danish twin pairs. Eur Respir J. 1999;13(1):8–14. doi: 10.1183/09031936.99.13100899. [DOI] [PubMed] [Google Scholar]
- 5.Kim JH, Ellwood PE, Asher MI. Diet and asthma: looking back, moving forward. Respir Res. 2009;10:49. doi: 10.1186/1465-9921-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torres-Borrego J, Moreno-Solis G, Molina-Teran AB. Diet for the prevention of asthma and allergies in early childhood: much ado about something? Allergol Immunopathol (Madr) 2012;40(4):244–252. doi: 10.1016/j.aller.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Allan K, Devereux G. Diet and asthma: nutrition implications from prevention to treatment. J Am Diet Assoc. 2011;111(2):258–268. doi: 10.1016/j.jada.2010.10.048. [DOI] [PubMed] [Google Scholar]
- 8.Allen S, Britton JR, Leonardi-Bee JA. Association between antioxidant vitamins and asthma outcome measures: systematic review and meta-analysis. Thorax. 2009;64(7):610–619. doi: 10.1136/thx.2008.101469. [DOI] [PubMed] [Google Scholar]
- 9.Nurmatov U, Devereux G, Sheikh A. Nutrients and foods for the primary prevention of asthma and allergy: systematic review and meta-analysis. J Allergy Clin Immunol. 2011;127(3):724–733. e721–e730. doi: 10.1016/j.jaci.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Iikura M, Yi S, Ichimura Y, et al. Effect of lifestyle on asthma control in Japanese patients: importance of periodical exercise and raw vegetable diet. PLoS One. 2013;8(7):e68290. doi: 10.1371/journal.pone.0068290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uddenfeldt M, Janson C, Lampa E, et al. High BMI is related to higher incidence of asthma, while a fish and fruit diet is related to a lower– results from a long-term follow-up study of three age groups in Sweden. Respir Med. 2010;104(7):972–980. doi: 10.1016/j.rmed.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Woods RK, Thien FC, Abramson MJ. Dietary marine fatty acids (fish oil) for asthma in adults and children. Cochrane Database Syst Rev. 2002;3:CD001283. doi: 10.1002/14651858.CD001283. [DOI] [PubMed] [Google Scholar]
- 13.Milan SJ, Hart A, Wilkinson M. Vitamin C for asthma and exercise-induced bronchoconstriction. Cochrane Database Syst Rev. 2013;10:CD010391. doi: 10.1002/14651858.CD010391.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Marcos L, Castro-Rodriguez JA, Weinmayr G, Panagiotakos DB, Priftis KN, Nagel G. Influence of Mediterranean diet on asthma in children: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2013;24(4):330–338. doi: 10.1111/pai.12071. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Academy of Nutrition and Dietetics . Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process. Chicago, IL, USA: ADA Research and Strategic Business Development; 2012. [Google Scholar]
- 17.Sheu CF, Suzuki S. Meta-analysis using linear mixed models. Behav Res Methods. 2001;33(2):102–107. doi: 10.3758/bf03195354. [DOI] [PubMed] [Google Scholar]
- 18.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 19.Sanchez-Solis M, Valverde-Molina J, Pastor MD, et al. Mediterranean diet is a protective factor for asthma in children 6–8 years old. Eur Respir J. 2006;50(Suppl):850s. [Google Scholar]
- 20.Bakolis I, Hooper R, Thompson RL, Shaheen SO. Dietary patterns and adult asthma: population-based case-control study. Allergy. 2010;65(5):606–615. doi: 10.1111/j.1398-9995.2009.02215.x. [DOI] [PubMed] [Google Scholar]
- 21.Sexton P, Black P, Metcalf P, et al. Influence of Mediterranean diet on asthma symptoms, lung function, and systemic inflammation: a randomized controlled trial. J Asthma. 2013;50(1):75–81. doi: 10.3109/02770903.2012.740120. [DOI] [PubMed] [Google Scholar]
- 22.Rosenkranz RR, Rosenkranz SK, Neessen KJ. Dietary factors associated with lifetime asthma or hayfever diagnosis in Australian middle-aged and older adults: a cross-sectional study. Nutr J. 2012;11:84. doi: 10.1186/1475-2891-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooper R, Heinrich J, Omenaas E, et al. Dietary patterns and risk of asthma: results from three countries in European Community Respiratory Health Survey-II. Br J Nutr. 2010;103(9):1354–1365. doi: 10.1017/S0007114509993266. [DOI] [PubMed] [Google Scholar]
- 24.Shaheen SO, Jameson KA, Syddall HE, et al. The relationship of dietary patterns with adult lung function and COPD. Eur Respir J. 2010;36(2):277–284. doi: 10.1183/09031936.00114709. [DOI] [PubMed] [Google Scholar]
- 25.Barros R, Moreira A, Fonseca J, et al. Adherence to the Mediterranean diet and fresh fruit intake are associated with improved asthma control. Allergy. 2008;63(7):917–923. doi: 10.1111/j.1398-9995.2008.01665.x. [DOI] [PubMed] [Google Scholar]
- 26.McKeever TM, Lewis SA, Cassano PA, et al. Patterns of dietary intake and relation to respiratory disease, forced expiratory volume in 1 s, and decline in 5-y forced expiratory volume. Am J Clin Nutr. 2010;92(2):408–415. doi: 10.3945/ajcn.2009.29021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varraso R, Kauffmann F, Leynaert B, et al. Dietary patterns and asthma in the E3N study. Eur Respir J. 2009;33(1):33–41. doi: 10.1183/09031936.00130807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varraso R, Fung TT, Barr RG, Hu FB, Willett W, Camargo CA., Jr Prospective study of dietary patterns and chronic obstructive pulmonary disease among US women. Am J Clin Nutr. 2007;86(2):488–495. doi: 10.1093/ajcn/86.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varraso R, Fung TT, Hu FB, Willett W, Camargo CA. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US men. Thorax. 2007;62(9):786–791. doi: 10.1136/thx.2006.074534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butler LM, Koh WP, Lee HP, et al. Prospective study of dietary patterns and persistent cough with phlegm among Chinese Singaporeans. Am J Respir Crit Care Med. 2006;173(3):264–270. doi: 10.1164/rccm.200506-901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Z, Yuan B, Wittert GA, et al. Monosodium glutamate intake, dietary patterns and asthma in Chinese adults. PLoS One. 2012;7(12):e51567. doi: 10.1371/journal.pone.0051567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tromp II, Kiefte-de Jong JC, de Vries JH, et al. Dietary patterns and respiratory symptoms in pre-school children: the Generation R Study. Eur Respir J. 2012;40(3):681–689. doi: 10.1183/09031936.00119111. [DOI] [PubMed] [Google Scholar]
- 33.Romieu I, Barraza-Villarreal A, Escamilla-Nunez C, et al. Dietary intake, lung function and airway inflammation in Mexico City school children exposed to air pollutants. Respir Res. 2009;10(1):122. doi: 10.1186/1465-9921-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grigoropoulou D, Priftis KN, Yannakoulia M, et al. Urban environment adherence to the Mediterranean diet and prevalence of asthma symptoms among 10- to 12-year-old children: the Physical Activity, Nutrition, and Allergies in Children Examined in Athens study. Allergy Asthma Proc. 2011;32(5):351–358. doi: 10.2500/aap.2011.32.3463. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez Barcala FJ, Pertega S, Bamonde L, et al. Mediterranean diet and asthma in Spanish schoolchildren. Pediatr Allergy Immunol. 2010;21(7):1021–1027. doi: 10.1111/j.1399-3038.2010.01080.x. [DOI] [PubMed] [Google Scholar]
- 36.Arvaniti F, Priftis KN, Papadimitriou A, et al. Adherence to the Mediterranean type of diet is associated with lower prevalence of asthma symptoms, among 10–12 years old children: the PANACEA study. Pediatr Allergy Immunol. 2011;22(3):283–289. doi: 10.1111/j.1399-3038.2010.01113.x. [DOI] [PubMed] [Google Scholar]
- 37.Castro-Rodriguez JA, Garcia-Marcos L, Alfonseda Rojas JD, Valverde-Molina J, Sanchez-Solis M. Mediterranean diet as a protective factor for wheezing in preschool children. J Pediatr. 2008;152(6):823–828. e821–e822. doi: 10.1016/j.jpeds.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Chatzi L, Torrent M, Romieu I, et al. Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax. 2008;63(6):507–513. doi: 10.1136/thx.2007.081745. [DOI] [PubMed] [Google Scholar]
- 39.Chatzi L, Apostolaki G, Bibakis I, et al. Protective effect of fruits, vegetables and the Mediterranean diet on asthma and allergies among children in Crete. Thorax. 2007;62(8):677–683. doi: 10.1136/thx.2006.069419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Marcos L, Canflanca IM, Garrido JB, et al. Relationship of asthma and rhinoconjunctivitis with obesity, exercise and Mediterranean diet in Spanish schoolchildren. Thorax. 2007;62(6):503–508. doi: 10.1136/thx.2006.060020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Cassia Ribeiro Silva R, Assis AM, Cruz AA, et al. Dietary patterns and wheezing in the midst of nutritional transition: a study in Brazil. Pediatr Allergy Immunol Pulmonol. 2013;26(1):18–24. doi: 10.1089/ped.2012.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Batlle J, Garcia-Aymerich J, Barraza-Villarreal A, Anto JM, Romieu I. Mediterranean diet is associated with reduced asthma and rhinitis in Mexican children. Allergy. 2008;63(10):1310–1316. doi: 10.1111/j.1398-9995.2008.01722.x. [DOI] [PubMed] [Google Scholar]
- 43.Lee SC, Yang YH, Chuang SY, Liu SC, Yang HC, Pan WH. Risk of asthma associated with energy-dense but nutrient-poor dietary pattern in Taiwanese children. Asia Pac J Clin Nutr. 2012;21(1):73–81. [PubMed] [Google Scholar]
- 44.Nagel G, Weinmayr G, Kleiner A, Garcia-Marcos L, Strachan DP; Group IPTS. Effect of diet on asthma and allergic sensitisation in the International Study on Allergies and Asthma in Childhood (ISAAC) Phase Two. Thorax. 2010;65(6):516–522. doi: 10.1136/thx.2009.128256. [DOI] [PubMed] [Google Scholar]
- 45.Chatzi L, Garcia R, Roumeliotaki T, et al. Mediterranean diet adherence during pregnancy and risk of wheeze and eczema in the first year of life: INMA (Spain) and RHEA (Greece) mother-child cohort studies. Br J Nutr. 2013;110(11):2058–2068. doi: 10.1017/S0007114513001426. [DOI] [PubMed] [Google Scholar]
- 46.Castro-Rodriguez JA, Garcia-Marcos L, Sanchez-Solis M, Perez-Fernandez V, Martinez-Torres A, Mallol J. Olive oil during pregnancy is associated with reduced wheezing during the first year of life of the offspring. Pediatr Pulmonol. 2010;45(4):395–402. doi: 10.1002/ppul.21205. [DOI] [PubMed] [Google Scholar]
- 47.Shaheen SO, Northstone K, Newson RB, Emmett PM, Sherriff A, Henderson AJ. Dietary patterns in pregnancy and respiratory and atopic outcomes in childhood. Thorax. 2009;64(5):411–417. doi: 10.1136/thx.2008.104703. [DOI] [PubMed] [Google Scholar]
- 48.Lange NE, Rifas-Shiman SL, Camargo CA, Jr, Gold DR, Gillman MW, Litonjua AA. Maternal dietary pattern during pregnancy is not associated with recurrent wheeze in children. J Allergy Clin Immunol. 2010;126(2):250–255. e251–e254. doi: 10.1016/j.jaci.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyake Y, Okubo H, Sasaki S, Tanaka K, Hirota Y. Maternal dietary patterns during pregnancy and risk of wheeze and eczema in Japanese infants aged 16–24 months: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol. 2011;22(7):734–741. doi: 10.1111/j.1399-3038.2011.01176.x. [DOI] [PubMed] [Google Scholar]
- 50.Allan K, Kelly FJ, Devereux G. Antioxidants and allergic disease: a case of too little or too much? Clin Exp Allergy. 2010;40(3):370–380. doi: 10.1111/j.1365-2222.2009.03413.x. [DOI] [PubMed] [Google Scholar]
- 51.Varraso R. Nutrition and asthma. Curr Allergy Asthma Rep. 2012;12(3):201–210. doi: 10.1007/s11882-012-0253-8. [DOI] [PubMed] [Google Scholar]
- 52.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Martinez ME, Marshall JR, Sechrest L. Invited commentary: factor analysis and the search for objectivity. Am J Epidemiol. 1998;148(1):17–19. doi: 10.1093/oxfordjournals.aje.a009552. [DOI] [PubMed] [Google Scholar]
- 54.US Department of Health and Human Services, US Department of Agriculture Dietary Guidelines for Americans. 2010. 2011. [Accessed December 17, 2013]. Available from: http://www.cnpp.usda.gov/dietaryguidelines.html.
- 55.Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc. 1995;95(10):1103–1108. doi: 10.1016/S0002-8223(95)00300-2. [DOI] [PubMed] [Google Scholar]
- 56.Barbaresko J, Koch M, Schulze MB, Nothlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. 2013;71(8):511–527. doi: 10.1111/nure.12035. [DOI] [PubMed] [Google Scholar]
- 57.Fung TT, Brown LS. Dietary patterns and the risk of colorectal cancer. Curr Nutr Rep. 2013;2(1):48–55. doi: 10.1007/s13668-012-0031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tucker KL. Dietary patterns, approaches, and multicultural perspective. Appl Physiol Nutr Metab. 2010;35(2):211–218. doi: 10.1139/H10-010. [DOI] [PubMed] [Google Scholar]
- 59.Nurmatov U, Nwaru BI, Devereux G, Sheikh A. Confounding and effect modification in studies of diet and childhood asthma and allergies. Allergy. 2012;67(8):1041–1059. doi: 10.1111/j.1398-9995.2012.02858.x. [DOI] [PubMed] [Google Scholar]
- 60.von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma J, Strub P, Lavori PW, et al. DASH for asthma: a pilot study of the DASH diet in not-well-controlled adult asthma. Contemp Clin Trials. 2013;35(2):55–67. doi: 10.1016/j.cct.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elsevier Training and Support. Frequently asked questions. [Accessed April 29, 2014]. Available from: http://www.elsevier.com/online-tools/embase/training-and-support.
- 63.Burnham JF. Scopus database: a review. Biomed Digit Libr. 2006;3:1. doi: 10.1186/1742-5581-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]