Abstract
Background:
Fever and neutropenia (FN) often complicate anticancer treatment and can be caused by potentially fatal infections. Knowledge of pathogen distribution is paramount for optimal patient management.
Methods:
Microbiologically defined infections (MDI) in pediatric cancer patients presenting with FN by nonmyeloablative chemotherapy enrolled in a prospective multicenter study were analyzed. Effectiveness of empiric antibiotic therapy in FN episodes with bacteremia was assessed taking into consideration recently published treatment guidelines for pediatric patients with FN.
Results:
MDI were identified in a minority (22%) of pediatric cancer patients with FN. In patients with, compared with patients without MDI, fever [median, 5 (interquartile range: 3–8) vs. 2 (interquartile range: 1–3) days, P < 0.001] and hospitalization [10 (6–14) vs. 5 (3–8) days, P < 0.001] lasted longer, transfer to the intensive care unit was more likely [13 of 95 (14%) vs. 7 of 346 (2.0%), P < 0.001], and antibiotics were given longer [10 (7–14) vs. 5 (4–7) days, P < 0.001]. Empiric antibiotic therapy in FN episodes with bacteremia was highly effective if not only intrinsic and reported antimicrobial susceptibilities were considered but also the purposeful omission of coverage for coagulase-negative staphylococci and enterococci was taken into account [81% (95% confidence interval: 68–90) vs. 96.6% (95% confidence interval: 87–99.4), P = 0.004].
Conclusions:
MDI were identified in a minority of FN episodes but they significantly affected management and the clinical course of pediatric cancer patients. Compliance with published guidelines was associated with effectiveness of empiric antibiotic therapy in FN episodes with bacteremia.
Keywords: fever and neutropenia, pediatric oncology, bacteremia, infection
Fever and neutropenia (FN) frequently complicate anticancer treatment and can be caused by potentially fatal infections.1 Successful management of FN is based on empiric antimicrobial treatment.2 Knowledge of expected pathogens, local antimicrobial susceptibility patterns, and the clinical course of FN episodes is important to optimize empiric treatment. Shifts in the spectrum of bacterial pathogens causing infections in patients with cancer and the emergence of resistant pathogens fuel the need for a constant surveillance of local conditions.
The multicenter prospective Swiss Pediatric Oncology Group (SPOG) 2003 FN study was set up to develop prediction scores for adverse events3 and bacteremia4 and to do a randomized controlled trial of outpatient oral versus inpatient intravenous antibiotics in selected low-risk episodes of FN.5 We describe here FN episodes with microbiologically defined infections (MDI), their management and clinical course and the pathogens isolated in episodes with MDI in the SPOG 2003 FN study.
PATIENTS AND METHODS
Study Design
Details of the study design of the prospective multicenter SPOG 2003 FN study have been previously published.3,5 In brief, patients with cancer 1–18 years of age presenting with FN after nonmyeloablative chemotherapy were recruited by pediatric oncology centers in Switzerland and Germany between January 2004 and December 2007. Local and national ethics committees had approved the study.
Patients and Management of FN
In- and outpatients with cancer 1–18 years of age were included in the study if they had fever, defined either as axillary temperature ≥ 38.5°C once or ≥ 38.0°C for ≥ 2 hours,6 and neutropenia, defined as an absolute neutrophil count ≤ 0.5 G/L.6 At presentation with FN, all patients underwent a physical examination and blood was sampled for blood cultures and differential blood count. Empiric intravenous broad-spectrum antimicrobial therapy covering for Gram-positive [except methicillin-resistant Staphylococcus aureus, coagulase-negative staphylococci (CoNS) and Enterococcus species] and Gram-negative bacteria was started. The treating physician decided on further diagnostic procedures and supportive measures. Patients were observed as inpatients and reassessed within 8–24 hours after admission. If prespecified restrictive low-risk criteria were fulfilled, they were offered to participate in the randomized controlled trial mentioned above (NCT00107081).5 However, in the vast majority of episodes, patients stayed hospitalized and were offered all standard diagnostic and therapeutic measures. This included escalation of antimicrobial treatment in patients with persistent fever to cover for CoNS, methicillin-resistant Staphylococcus aureus, enterococci, resistant Gram-negative bacteria and eventually fungal infections.
Participating Centers
Eight pediatric oncology centers from Switzerland (centers A to E) and Germany (centers F to H) participated in the SPOG 2003 FN study. Centers A, B and C were located in the German-speaking part and centers D and E in the French-speaking part of Switzerland. All centers reported numbers and etiology of MDI, and results of antimicrobial susceptibility testing of bacterial pathogens isolated in blood cultures. Center A additionally reported time to positivity of pathogens isolated in blood cultures and was the only center to implement a low-threshold testing strategy for viral respiratory infections. There were no differences in antibiotic prophylaxis regimens between centers. Only standard pneumocystis jirovecii prophylaxis was used in this study.
Microbiologically Defined Infections
Details on MDI definitions have been published.3,4 Essentially, initial aerobic and anaerobic blood cultures were taken at presentation with FN before starting antimicrobial therapy in all patients. Further blood cultures were taken daily if fever persisted or the patient experienced shaking chills. Blood cultures were drawn from existing central venous catheters, if present, or a peripheral vein. Peripheral and central blood cultures were not performed in parallel. Bacteremia was defined as at least 1 positive blood culture, irrespective of the pathogen detected, using a qualitative automated culture system (BacT/ALERT, bioMérieux, Geneva, Switzerland; or BACTEC, Becton Dickinson, Basel, Switzerland).4 For the analysis of time to positivity in center A, the time from loading of the blood culture vial into the incubator until detection of growth of microorganisms was considered. All pathogens identified in blood cultures were analyzed separately, provided they differed in species, morphology, antimicrobial susceptibility testing or time point of sampling. Bacterial infections other than bacteremia were defined by a pathogen isolated from a normally sterile body fluid or compartment.3 Viral infections were defined by the detection of a viral antigen7 or product of polymerase chain reaction by a validated microbiologic method. For this analysis, invasive fungal infections were defined according to the revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group.8
Effectiveness of Empiric Antibiotic Treatment in FN Episodes With Bacteremia
Effectiveness of empiric antibiotic therapy was assessed in 3 different ways. In vitro effectiveness was assessed based on reported and intrinsic antimicrobial susceptibilities to the antibiotics given on the day of sampling of the first positive blood culture. Effectiveness because of compliance with pediatric FN guidelines was defined by in vitro effectiveness restricted to pathogens to be covered by empiric antibiotic therapy according to current treatment recommendations for pediatric FN.9,10 Therefore, effectiveness because of compliance with pediatric FN guidelines would be present even if a pathogen not covered by empiric antibiotic therapy (eg, CoNS or enterococci) was diagnosed from blood cultures sampled in the first 48 hours. Finally, microbiologic effectiveness was defined by the growth of bacterial pathogens in any blood culture after the initiation of empiric antibiotic therapy.
Comparison of Bacteremia Causing Pathogens to the Swiss Centre for Antibiotic Resistance Database
The Swiss Centre for Antibiotic Resistance (ANRESIS)11,12 is an ongoing laboratory-based representative surveillance system collecting antibiotic resistance data of actually 20 Swiss microbiology laboratories. For this analysis, the distribution and antibiotic resistance of blood-borne pathogens detected in centers A, B and C were compared with the corresponding population in the ANRESIS database. Data of centers D and E, located in the French-speaking part of Switzerland, were excluded from this analysis because of the very low numbers of FN episodes with bacteremia reported (1 and 4 episodes, respectively). The ANRESIS population was defined as all positive blood cultures in children 2–15 years of age isolated during the years 2004–2007 in 1 of the Swiss German university children’s hospitals participating also in the SPOG 2003 FN study. MDI with the same organism and the same resistance profile in the same patient were counted as different infections if the time interval between the 2 respective blood cultures exceeded 7 days. For this comparison, CoNS were judged as contaminants and excluded from analysis if isolated once within 7 days (ANRESIS population) or if clinically judged as nonsignificant, that is, not treated or treated <5 days with a glycopeptide antibiotic (SPOG 2003 FN study).
Statistics
Proportions and their exact 95% Blyth-Still-Casella confidence intervals (95% CI) were calculated. Nonparametric exact 2-sided tests were used throughout. Specifically, the Fisher test, Fisher-Freeman-Halton test, Mann-Whitney U test, Kruskall-Wallis test with Dunn’s multiple comparison tests and exact odds ratios (OR) of binomial proportions were calculated where applicable. For all statistical analyses, a 2-sided P value of 0.05 was considered significant. Statistical analyses were performed with GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA) and StatXact 10 (Cytel Inc., Cambridge, MA).
RESULTS
Patients and Episodes of FN
Eight German and Swiss pediatric oncology centers reported a total of 472 episodes of FN. Eligibility criteria were not fulfilled in 25 (5.3%) episodes, and follow up was missing in 4 (0.8%). Additionally, 2 (0.4%) duplicate episodes were excluded. The remaining 441 (93.4%) FN episodes were analyzed here. Centers A, B and C reported 171 (39%), 153 (35%) and 9 (2%) FN episodes, respectively, whereas centers D to H reported the remaining 108 (24%) FN episodes. They occurred in 209 patients [median, 2 episodes per patient, interquartile range (IQR), 1–3], with a median age of 7.0 years (IQR: 3.9–11.7) at the first FN episode. Further details have been published.3,4,13 An MDI was identified in 95 (22%; 95% CI: 18–26) of these 441 FN episodes, bacteremia in 67 (15%; 95% CI: 12–19), a focal bacterial infection in 8 (1.8%; 95% CI: 0.8–3.5), a viral infection in 29 (6.6%; 95% CI: 4.5–9.2) and a fungal infection in 5 (1.1%; 95% CI: 0.4–2.6). In 12 (2.7%; 95% CI: 1.5–4.6) of these 441 FN episodes, >1 MDI was identified.
Clinical Course of FN Episodes
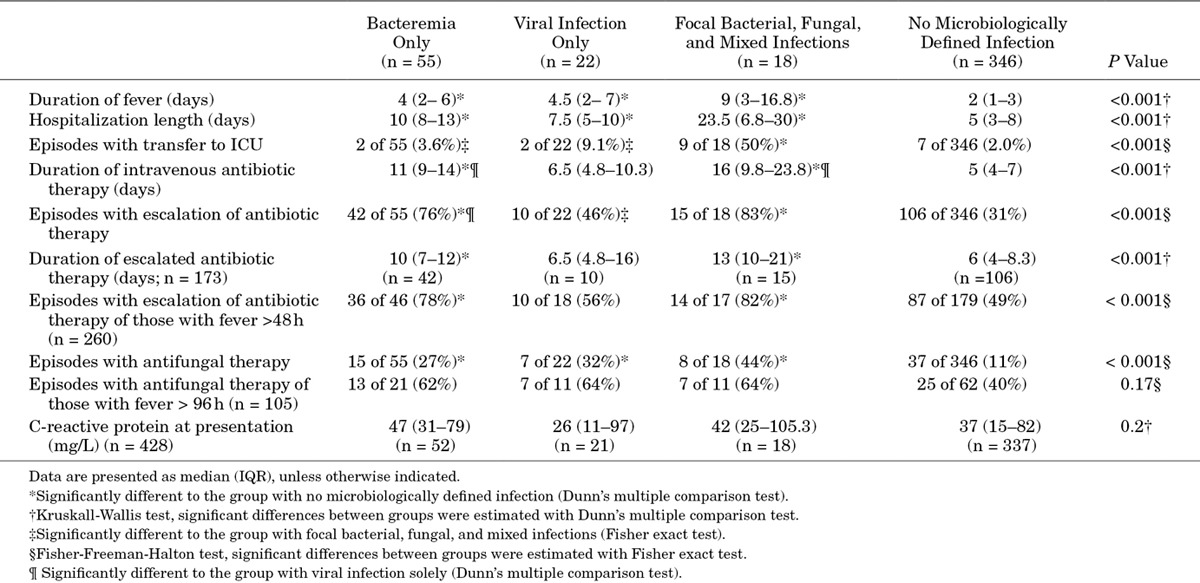
In FN episodes with versus without MDI fever persisted for a longer time [median, 5 (IQR: 3–8) vs. 2 (IQR: 1–3) days, P < 0.001], patients were hospitalized longer [median, 10 (IQR: 6–14) vs. 5 (IQR: 3–8) days, P < 0.001] and transferred more often to the intensive care unit [ICU; 13 of 95 (14%) vs. 7 of 346 (2.0%), OR: 7.7, 95% CI 2.7–23.3, P < 0.001]. Additionally, antimicrobial treatment in FN episodes with versus without MDI was more intensive as exemplified by a longer duration of intravenous antibiotic administration [median, 10 (IQR: 7–14) vs. 5 (IQR: 4–7) days, P < 0.001], higher proportion of empiric antibiotic therapy escalation [67 of 95 (71%) vs. 106 of 346 (31%), OR: 5.4, 95% CI: 3.2–9.2, P < 0.001] and a higher proportion of empiric antifungal treatment [30 of 95 (32%) vs. 37 of 346 (11%), OR: 3.9, 95% CI: 2.1–6.9, P < 0.001]. Table 1 shows clinical- and management-related characteristics for the different categories of MDI compared with FN episodes without MDI.
TABLE 1.
Differences of Clinical and Management Characteristics in 441 FN Episodes According to the Microbiologically Defined Infection
FN Episodes with Bacteremia
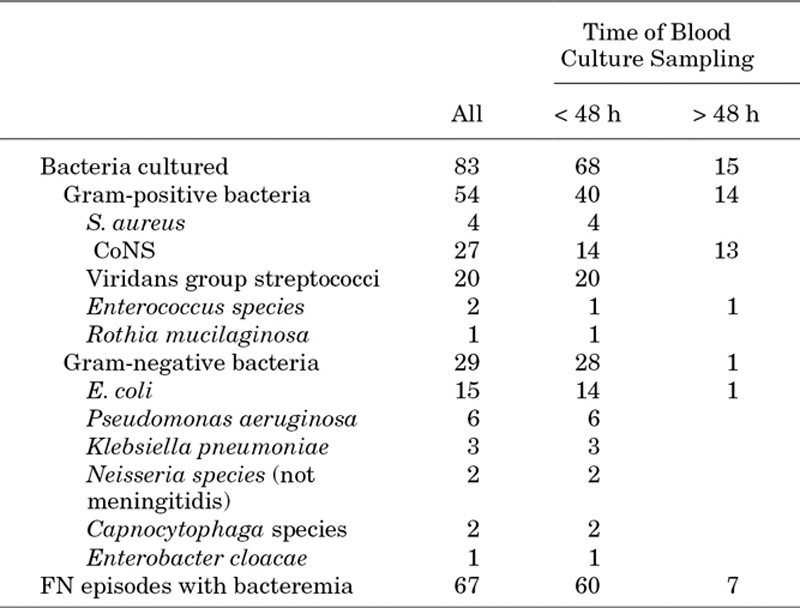
In 67 FN episodes with bacteremia, a total of 83 pathogens were identified; 54 (65%) Gram-positive bacteria and 29 (35%) Gram-negative bacteria (Table 2). There were no significant differences in fever duration, hospitalization length, proportion transferred to the ICU, duration of intravenous antibiotic treatment, escalation of antibiotic therapy and duration of escalated antibiotic therapy between Gram-positive and Gram-negative bacteria (details not shown). Antimicrobial susceptibility testing was available for 68 (82%) of 83 blood culture isolates, but the important heterogeneity of susceptibility results available precluded meaningful comparisons. Methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci or extended-spectrum, β-lactamase producing Gram-negative bacteria were not reported.
TABLE 2.
Positive Blood Culture Results Defining Bacteremia in 441 FN Episodes
Effectiveness of Empiric Antibiotic Therapy in FN Episodes With Bacteremia
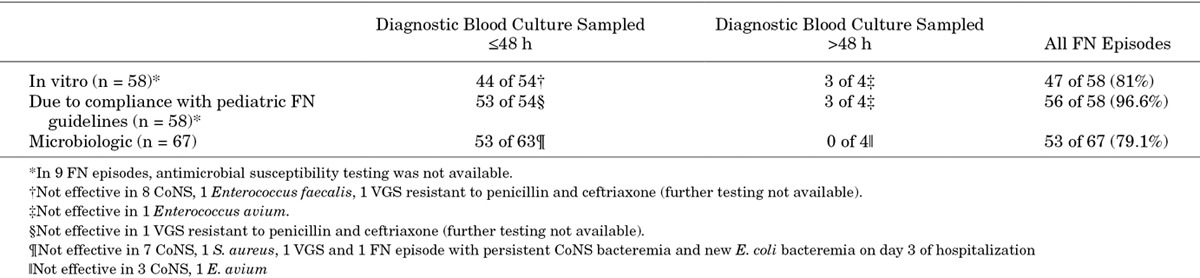
Table 3 specifies in vitro effectiveness versus effectiveness because of compliance with pediatric FN guidelines versus microbiologic effectiveness of empiric antibiotic therapy in the 67 FN episodes with bacteremia. In the 58 (87%) episodes with susceptibility results available, the effectiveness because of compliance with pediatric FN guidelines [56 of 58 episodes; 96.6% (95% CI: 87–99.4)] was significantly higher than the in vitro effectiveness [47 of 58 episodes; 81% (95% CI: 68–90), P = 0.004] and the microbiologic effectiveness [45 of 58 episodes; 78% (95% CI: 64–87), P = 0.003]. CoNS bacteremia was the major reason for both in vitro [8 of 11 (73%)] and microbiologic [11 of 14 (79%)] noneffectiveness. Applying only microbiologic effectiveness criteria, the initial empiric therapy failed in 10 (15%) of 67 FN episodes with bacteremia, while the escalated empiric therapy failed in 4 (7.7%) of 52 FN episodes. In 1 FN episode with clinical signs of a central venous catheter infection and empiric glycopeptide monotherapy, new Escherichia coli bacteremia was detected on day 3 of hospitalization. In both the in vitro and the microbiologic effectiveness scenarios, there were no significant differences in fever duration, hospitalization length and proportion transferred to the ICU between FN episodes with bacteremia treated with effective versus ineffective initial empiric antibiotic therapy (details not shown).
TABLE 3.
Effectiveness of Empiric Antibiotic Therapy in 67 FN Episodes With Bacteremia
Of note, antibiotic therapy was escalated in 106 (31%) of 346 FN episodes without MDI.
Time to Positivity in FN Episodes With Bacteremia
In center A, time to positivity was known in 33 (89%) of 37 blood cultures (31 of 34 FN episodes). In 27 (82%) of these 33 blood cultures, a pathogen grew within 24 hours. All blood cultures with a time to positivity >18 hours grew CoNS in monoculture (Fig. 1). Correspondingly, for blood cultures with monocultural growth of CoNS time to positivity [19.8 hours (IQR: 12.2–39)] was significantly longer than for blood cultures growing other Gram-positive bacteria [8.7 hours (IQR: 4.1–11.5), P = 0.021] and blood cultures growing Gram-negative bacteria [4.2 hours (IQR: 2.2–4.9), P = 0.001]. Compared with blood cultures with polymicrobial growth, only a trend was noted [7.9 hours (IQR: 3.5–8.7), P = 0.051].
FIGURE 1.
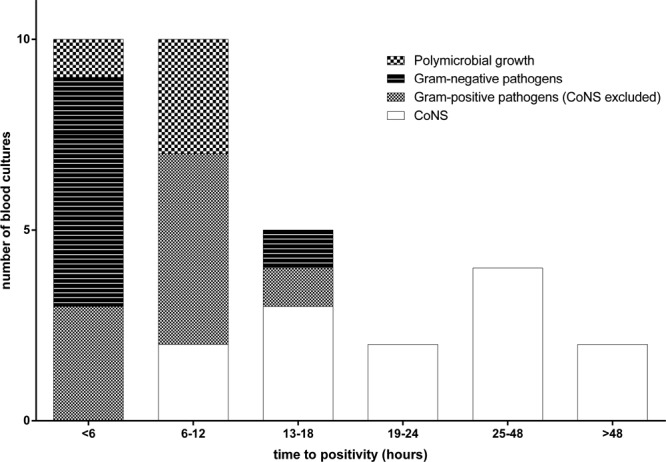
Distribution of time to positivity of 33 blood cultures processed in center A according to microbiologic etiology.
Comparison of Bacteremia Causing Pathogens to the Swiss Centre for Antibiotic Resistance Database
The distribution of pathogens in 58 (87%) of 67 FN episodes with bacteremia observed in 333 (76% of 441) FN episodes reported from centers A, B and C was significantly different from the distribution of pathogens causing bacteremia in the general pediatric population of the German-speaking part of Switzerland (P < 0.001; Table 4).
TABLE 4.
Comparison of the Distribution of Pathogens Causing Bacteremia in the German-speaking Part of Switzerland Between Centers A, B and C of the SPOG 2003 FN Study and the General Pediatric Population (ANRESIS 2004–2007)
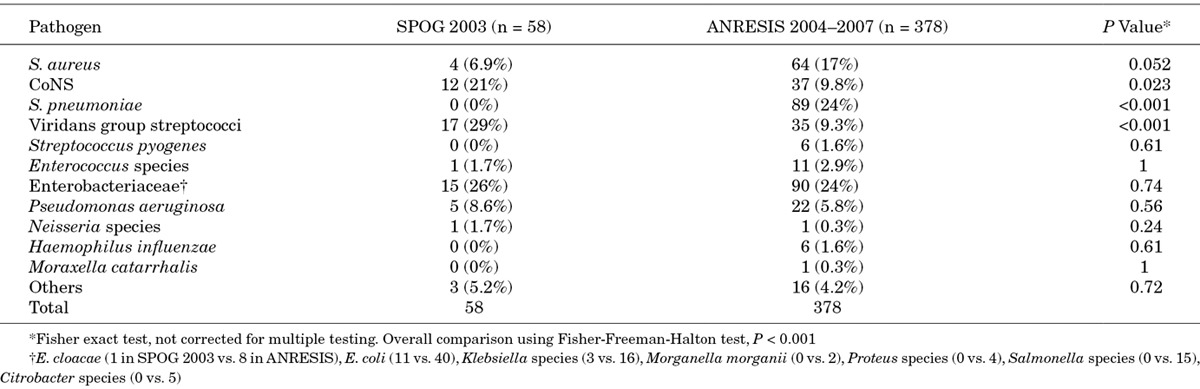
CoNS (21% vs. 9.8%, P = 0.023) and viridans group streptococci (VGS) (29% vs. 9.3%, P < 0.001) were detected more frequently in pediatric cancer patients with FN. In contrast, Streptococcus pneumoniae (0% vs. 24%, P < 0.001) was detected more frequently in the general pediatric population. Antimicrobial resistance did not differ significantly (details not shown).
Other MDI
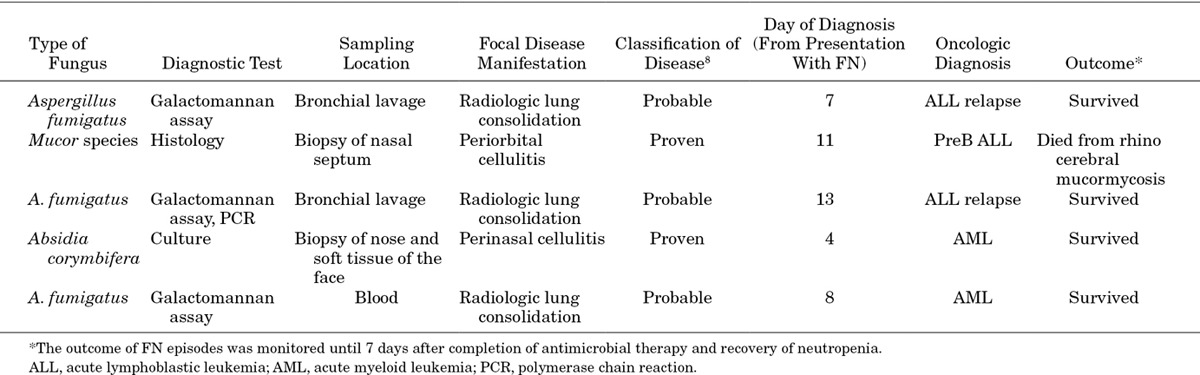
A focal bacterial infection was microbiologically proven in 8 (1.8%) of 441 FN episodes (2 urinary tract infections, 3 soft tissue infections and 3 pneumonias). A viral infection was microbiologically proven in 29 (6.6%) of 441 FN episodes (in 25 FN episodes by antigen detection and in 4 FN episodes by polymerase chain reaction). In center A, the single center with a low-threshold viral testing strategy, the proportion was 13% (23 of 171 FN episodes, 95% CI: 9–19%). In 16 FN episodes, an upper respiratory tract infection was diagnosed (1 adenovirus, later diagnosed with fatal systemic adenovirus infection,14 5 picornaviruses, 5 influenza A viruses, 1 influenza B virus, 1 parainfluenza virus, 2 respiratory syncytial viruses and 1 human metapneumovirus), in 6 FN episodes herpes simplex virus stomatitis was diagnosed, in 3 FN episodes a systemic viral infection was diagnosed (1 adenovirus, initially presented as respiratory tract infection,14 1 herpes simplex virus and 1 cytomegalovirus), in 4 episodes a viral gastroenteritis was diagnosed (3 adenoviruses, 1 rotavirus) and in 1 FN episode herpes simplex virus encephalitis was diagnosed. An invasive fungal infection was diagnosed in 5 FN episodes, in 2 FN episodes the fungal infection was histologically proven and in 3 probable (Table 5). All invasive fungal infections were reported by center A, with a proportion of 2.9% (5 of 171, 95% CI: 1.1–6.4%).
TABLE 5.
Invasive Fungal Infections Diagnosed in 5 of 441 FN Episodes
DISCUSSION
The aim of this study was to describe all MDI detected in the prospective multicenter SPOG FN 2003 study. In many FN episodes (78%), no MDI was detected. Also, lack of MDI detection was associated with shorter duration of fever and hospitalization, less need of intensive care and less intensive antimicrobial therapy compared with FN episodes with MDI.
We detected MDI in around every fourth FN episode. Other studies of FN in pediatric cancer patients have reported comparable frequencies of MDI.15,16 Nevertheless, the perception that infections can be documented only in a minority of pediatric cancer patients with FN has been challenged by studies relying on systematic identification of viral infections by molecular methods.17–19 In 2 recent studies, MDI were detected in 67% and 60% of FN episodes, with respiratory virus infections being detected by molecular methods in 57% and 46% of FN episodes, respectively.17,18 This difference in the frequency of MDI is best explained by different diagnostic policies and procedures used for the detection of respiratory virus infections. In our study, even in the center with the most comprehensive strategy for respiratory virus diagnostics, viral infections were only documented in 13% of all FN episodes.
The detection of any MDI versus no MDI was clearly associated with a more severe clinical course. The clinical course of FN episodes with bacteremia, however, did not relevantly differ from FN episodes with viral infection, except for a significantly longer administration and escalation of intravenous antibiotic therapy. The group with focal bacterial infections, fungal infections and mixed infections showed a more severe clinical course but the heterogeneity of this group prevented further conclusions. Nevertheless, a more severe clinical course of mixed viral and bacterial infections in FN has been reported before.17,18,20
Bacteremia was the most frequent and most reliably detected MDI in this study. Similar to other studies of FN in pediatric cancer patients, Gram-positive pathogens, mainly CoNS and VGS, constituted many isolated pathogens.15,16,21 E. coli was the most frequent Gram-negative pathogen causing bacteremia. As expected, the frequency of CoNS and VGS bacteremia differed significantly in patients with cancer and FN compared with the general pediatric population. Surprisingly, bacteremia caused by S. pneumoniae was not identified in our study population. Although, this contrasts with the general notion of cancer as a risk factor for invasive pneumococcal disease,22–24 it is strongly in line with other studies of pediatric cancer patients with FN.15,16,25 In other patient populations, repetitive antibiotic therapy has been shown to reduce the carriage of S. pneumoniae, whereas the effect of cotrimoxazole prophylaxis was less clear.26 It is unlikely that vaccination against S. pneumoniae played a role in this study as the Swiss Federal Office of Public Health first issued a general recommendation for pneumococcal vaccination of children <5 years of age only in 2006, and pediatric cancer patients were not specifically vaccinated against S. pneumoniae before chemotherapy start in this study.
In a subset of FN episodes with bacteremia, a short time to positivity for both Gram-positive, with the exception of CoNS, and Gram-negative pathogens was observed. Comparable results have been reported and may favor an initial inpatient treatment period of 24 hours for the evaluation of chemotherapy induced FN in pediatric cancer patients.15 It should be noted, however, that in the SPOG 2003 FN study, bacteremia was first detected in a subsequent blood culture in 11 (2.5%) of 441 FN episodes (16% of 67 FN episodes with bacteremia).4 A negative initial blood culture 24 hours after patient admission rendered a virulent Gram-negative or Gram-positive bacteremia unlikely but did not exclude bacteremia as such.
The effectiveness of empiric antibiotic therapy because of compliance with pediatric FN guidelines was high in this study. Nevertheless, in one FN episode, Gram-negative bacteremia was detected on the third day of hospitalization only. In this patient, glycopeptide monotherapy had been started because of clinical signs of catheter infection, clearly not fulfilling the predefined requirements on initial empiric antibiotic therapy in pediatric cancer patients with FN. Although CoNS bacteremia was proven in the blood culture sampled at presentation and on the catheter tip removed on the third day of hospitalization, E. coli bacteremia was identified in a blood culture sampled on the third day. This underlines once more the importance of empiric coverage of Gram-negative pathogens by the initial antibiotic therapy of FN.
CoNS bacteremia was the main reason for failure of empiric antibiotic therapy in this study. Empiric addition of glycopeptides to the first-line empiric therapy has been evaluated in adult patients with cancer, but was not found to be superior to standard regimens in a meta-analysis.27 Escalation of antibiotic therapy to include coverage for CoNS is recommended in pediatric cancer patients if fever persists for >24–72 hours, and they become clinically unstable.9,10
In this study, antibiotic therapy was escalated in almost one-third of FN episodes without MDI. Unfortunately, it was not possible to reliably determine in how many of these episodes no deterioration of clinical condition, and thereby overtreatment, occurred.
This study has several limitations. First, this was an ad hoc analysis for which the SPOG 2003 FN study was not designed for. Specifically, besides sampling of blood cultures all other microbiologic investigations, diagnostic modalities and clinical care practices were at the discretion of the treating physician. The low number of focal bacterial infections and viral infections identified in this study likely underestimates the actual burden of these MDI. Second, not all centers in this prospective multicenter study recruited patients consecutively and over the complete study duration. This may additionally favor underestimation of the burden of MDI, especially invasive fungal infections. Third, some analyses presented, for example, time to positivity, could only be performed in subgroups of the study population because of different laboratory data collection practices.
In this study, MDI were identified in only a minority of FN episodes but they significantly affected management and the clinical course of patients. Compliance with recently published guidelines9,10 was associated with effectiveness of empiric antibiotic therapy in FN episodes with bacteremia.
ACKNOWLEDGEMENTS
The authors thank all patients and parents for study participation; Annette Ridolfi Lüthy, MD, Hulya Oszahin, MD, and Pierre Wacker, MD, for help in protocol development and in the study committee; Eveline SJM de Bont, MD, for support in the study committee; and Andrea Wasem, RN, and Nadine Beusch, RN, for data administration.
Footnotes
This study was supported by unrestricted research grants from Oncosuisse/Swiss Cancer League (OCS-01466-02-2004), Bayer AG (Switzerland), and GSK AG (Switzerland). The Swiss Centre for Antibiotic Resistance (ANRESIS) is financially supported by the Swiss government and the University of Bern (Bern, Switzerland).
The authors have no conflicts of interest or funding to disclose.
REFERENCES
- 1.Bodey GP, Buckley M, Sathe YS, et al. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64:328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 2.Schimpff S, Satterlee W, Young VM, et al. Empiric therapy with carbenicillin and gentamicin for febrile patients with cancer and granulocytopenia. N Engl J Med. 1971;284:1061–1065. doi: 10.1056/NEJM197105132841904. [DOI] [PubMed] [Google Scholar]
- 3.Ammann RA, Bodmer N, Hirt A, et al. Predicting adverse events in children with fever and chemotherapy-induced neutropenia: the prospective multicenter SPOG 2003 FN study. J Clin Oncol. 2010;28:2008–2014. doi: 10.1200/JCO.2009.25.8988. [DOI] [PubMed] [Google Scholar]
- 4.Agyeman P, Aebi C, Hirt A, et al. Predicting bacteremia in children with cancer and fever in chemotherapy-induced neutropenia: results of the prospective multicenter SPOG 2003 FN study. Pediatr Infect Dis J. 2011;30:e114–e119. doi: 10.1097/INF.0b013e318215a290. [DOI] [PubMed] [Google Scholar]
- 5.Brack E, Bodmer N, Simon A, et al. First-day step-down to oral outpatient treatment versus continued standard treatment in children with cancer and low-risk fever in neutropenia. A randomized controlled trial within the multicenter SPOG 2003 FN study. Pediatr Blood Cancer. 2012;59:423–430. doi: 10.1002/pbc.24076. [DOI] [PubMed] [Google Scholar]
- 6.Koh AY, Pizzo PA. Infectious complications in pediatric cancer patients. In: Pizzo PA, Poplack DG, editors. Principles and Practices of Pediatric Oncology. Philadelphia, PA:: Lippincott Williams & Wilkins; 2010. pp. 1190–1242. [Google Scholar]
- 7.Sadeghi CD, Aebi C, Gorgievski-Hrisoho M, et al. Twelve years’ detection of respiratory viruses by immunofluorescence in hospitalised children: impact of the introduction of a new respiratory picornavirus assay. BMC Infect Dis. 2011;11:41. doi: 10.1186/1471-2334-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Pauw B, Walsh TJ, Donnelly JP, et al. European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehrnbecher T, Phillips R, Alexander S, et al. International Pediatric Fever and Neutropenia Guideline Panel. Guideline for the management of fever and neutropenia in children with cancer and/or undergoing hematopoietic stem-cell transplantation. J Clin Oncol. 2012;30:4427–4438. doi: 10.1200/JCO.2012.42.7161. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Care Excellence. Neutropenic Sepsis: Prevention and Management of Neutropenic Sepsis in Cancer Patients. London: National Institute for Health and Care Excellence; 2012. p. CG151. [PubMed] [Google Scholar]
- 11.Swiss Centre for Antibiotic Resistance. Bern: Institut für Infektionskrankheiten Universität Bern. Available at: http://www.anresis.ch/en/index.html. Accessed October 22, 2013.
- 12.Kronenberg A, Hilty M, Endimiani A, et al. Temporal trends of extended-spectrum cephalosporin-resistant Escherichia coli and Klebsiella pneumoniae isolates in in- and outpatients in Switzerland, 2004 to 2011. Euro Surveill. 2013:18. [PubMed] [Google Scholar]
- 13.Lüthi F, Leibundgut K, Niggli FK, et al. Serious medical complications in children with cancer and fever in chemotherapy-induced neutropenia: results of the prospective multicenter SPOG 2003 FN study. Pediatr Blood Cancer. 2012;59:90–95. doi: 10.1002/pbc.23277. [DOI] [PubMed] [Google Scholar]
- 14.Steiner I, Aebi C, Ridolfi Lüthy A, et al. Fatal adenovirus hepatitis during maintenance therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;50:647–649. doi: 10.1002/pbc.21120. [DOI] [PubMed] [Google Scholar]
- 15.Hakim H, Flynn PM, Knapp KM, et al. Etiology and clinical course of febrile neutropenia in children with cancer. J Pediatr Hematol Oncol. 2009;31:623–629. doi: 10.1097/MPH.0b013e3181b1edc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castagnola E, Fontana V, Caviglia I, et al. A prospective study on the epidemiology of febrile episodes during chemotherapy-induced neutropenia in children with cancer or after hemopoietic stem cell transplantation. Clin Infect Dis. 2007;45:1296–1304. doi: 10.1086/522533. [DOI] [PubMed] [Google Scholar]
- 17.Torres JP, Labraña Y, Ibañez C, et al. Frequency and clinical outcome of respiratory viral infections and mixed viral-bacterial infections in children with cancer, fever and neutropenia. Pediatr Infect Dis J. 2012;31:889–893. doi: 10.1097/INF.0b013e31825c4b7e. [DOI] [PubMed] [Google Scholar]
- 18.Lindblom A, Bhadri V, Söderhäll S, et al. Respiratory viruses, a common microbiological finding in neutropenic children with fever. J Clin Virol. 2010;47:234–237. doi: 10.1016/j.jcv.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koskenvuo M, Möttönen M, Rahiala J, et al. Respiratory viral infections in children with leukemia. Pediatr Infect Dis J. 2008;27:974–980. doi: 10.1097/INF.0b013e31817b0799. [DOI] [PubMed] [Google Scholar]
- 20.Koskenvuo M, Möttönen M, Rahiala J, et al. Mixed bacterial-viral infections in septic children with leukemia. Pediatr Infect Dis J. 2007;26:1133–1136. doi: 10.1097/INF.0b013e318146207c. [DOI] [PubMed] [Google Scholar]
- 21.Lehrnbecher T, Varwig D, Kaiser J, et al. Infectious complications in pediatric acute myeloid leukemia: analysis of the prospective multi-institutional clinical trial AML-BFM 93. Leukemia. 2004;18:72–77. doi: 10.1038/sj.leu.2403188. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan SL, Mason EO, Jr, Barson WJ, et al. Three-year multicenter surveillance of systemic pneumococcal infections in children. Pediatrics. 1998;102(3 pt 1):538–545. doi: 10.1542/peds.102.3.538. [DOI] [PubMed] [Google Scholar]
- 23.Meisel R, Toschke AM, Heiligensetzer C, et al. Increased risk for invasive pneumococcal diseases in children with acute lymphoblastic leukaemia. Br J Haematol. 2007;137:457–460. doi: 10.1111/j.1365-2141.2007.06601.x. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Vidal C, Ardanuy C, Gudiol C, et al. Clinical and microbiological epidemiology of Streptococcus pneumoniae bacteremia in cancer patients. J Infect. 2012;65:521–527. doi: 10.1016/j.jinf.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Simon A, Ammann RA, Bode U, et al. Healthcare-associated infections in pediatric cancer patients: results of a prospective surveillance study from university hospitals in Germany and Switzerland. BMC Infect Dis. 2008;8:70. doi: 10.1186/1471-2334-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mwenya DM, Charalambous BM, Phillips PP, et al. Impact of cotrimoxazole on carriage and antibiotic resistance of Streptococcus pneumoniae and Haemophilus influenzae in HIV-infected children in Zambia. Antimicrob Agents Chemother. 2010;54:3756–3762. doi: 10.1128/AAC.01409-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vardakas KZ, Samonis G, Chrysanthopoulou SA, et al. Role of glycopeptides as part of initial empirical treatment of febrile neutropenic patients: a meta-analysis of randomised controlled trials. Lancet Infect Dis. 2005;5:431–439. doi: 10.1016/S1473-3099(05)70164-X. [DOI] [PubMed] [Google Scholar]


