Abstract
Reptiles and amphibians constitute a significant portion of vertebrate biomass in terrestrial ecosystems and may be important arbovirus reservoirs. To investigate mosquito preference for ectothermic hosts, feeding indices were calculated from data collected in Tuskegee National Forest, Alabama, USA. Four mosquito species fed upon ectothermic hosts, with Cx. peccator and Cx. territans feeding primarily upon ectotherms. These two species appeared to target distinct species with little overlap in host choice. Culex peccator was a generalist in its feeding patterns within ectotherms, while Cx. territans appeared to be a more specialized feeder. Six of eleven ectotherm species fed upon by Cx. territans were fed upon more often than predicted based upon abundance. Spring Peepers were highly preferred over other host species by Cx. territans. Blood meals taken from each host species varied temporally, with some hosts being targeted fairly evenly throughout the season and others being fed upon in seasonal peaks.
Keywords: Host preference, Culex peccator, Culex territans, feeding index, blood meal analysis, reptile, amphibian
INTRODUCTION
Vector-host interactions are driving forces in the transmission of vector-borne pathogens.1 An important aspect of vector-host interactions is that of host preference. Each species of hematophagic arthropod feeds on a limited range of host species. Within a group of hosts, however, the degree to which a vector feeds on individual host species can vary tremendously. In addition, even closely related hosts can vary widely in pathogen reservoir competency. Therefore, in order to accurately describe an arthropod-borne-pathogen transmission cycle, it is critical to know which available host species are preferred by the corresponding vectors.
Many studies have reported on the host feeding patterns of mosquitoes.2, 3, 4, 5 Prior to 1999,6 host-preference studies were limited to class-level distinction among mosquito hosts. Although restricted by the available technology, these studies indicated that each species of mosquito fed predominantly on a limited range of hosts (e.g., mammals or birds). Species-level determination of blood meal source, however, suggests that mosquitoes discriminate among hosts beyond the level of class. Hassan and co-workers,5 reported that ornithophilic mosquitoes feed significantly more or less on available bird species than predicted based on biomass, surface area or relative abundance. This observation has been confirmed by a number of subsequent studies.7, 8, 9
For many of the vector-borne viral encephalitides, the classic transmission scenario involves ornithophilic mosquitoes vectoring virus among avian enzootic hosts in a cycle of amplification.10, 11 More generalist feeders carry the virus from birds to mammals, allowing the virus to escape the avian enzootic cycle of transmission. Ectotherms, however may also play a role in this cycle.12 Reptiles and amphibians make up a large component of vertebrate biomass in terrestrial biological systems,13, 14, 15, 16, 17 and, somewhat surprisingly, may represent important hosts for several arboviruses. For example, Western Equine Encephalitis (WEE) may over-winter in garter snakes, Thamnophis spp.18, 19 and can persist for prolonged periods in the Texas tortoise (Gopherus berlandieri).20 Eastern Equine Encephalitis virus (EEEV) has also been recovered from a number of wild ectotherms 21, 22, while alligators have been implicated as potential amplifying hosts for West Nile virus23. Finally, evidence for the presence of EEEV has been obtained from field-collected ectotherm-feeding mosquitoes, including Ochlerotatus Canadensis,24 Uranotaenia sapphirina,25 Culex peccator,12 and Culex territans (Burkett-Cadena et al., unpublished data).
In a previous study, we reported that polymerase chain reaction (PCR) assays targeting the cytochrome B gene of vertebrates could be used to identify blood meals derived from ectothermic hosts to the species level12 in much the same way that such assays have been widely applied to identify avian-derived blood meals. In this study we have employed these PCR-based assays together abundance data to determine whether ectotherm-feeding mosquitoes are generalists, feeding on available hosts in proportion to host abundance, or if they feed disproportionately on some hosts to a greater extent than would be predicted based upon abundance or biomass alone.
MATERIALS AND METHODS
Study site
Field work was conducted in Tuskegee National Forest, Macon Co., Alabama, USA, in an area of the forest with several ponds created by beaver (Castor canadensis) activity. All mosquito and ectotherm surveys were conducted within a circle of land (radius = 2 km) with center point on the banks of a beaver pond (N32°25.899′, W85°38.637′). A detailed description of the site may be found in a previous publication.25
Questing mosquitoes were collected using CDC light traps, as previously described,25 while blooded mosquitoes were collected from natural and artificial resting sites with a battery powered vacuum aspirator. Natural resting sites included cavities in living and dead hardwood trees, herbaceous vegetation, and holes in pond banks created by beavers. Artificial resting sites consisted of a variety of man-made resting shelters, including plastic garbage cans26 and wooden resting boxes.27 Collections were begun the first week of February, 2007 and continued weekly until October 31, 2007. Blood-engorged females were identified to species28 and stored individually at −70°C for subsequent blood meal identification.
Identification of blood meals
Blood meals were identified to the species level using a modification of a previously described PCR-based assay targeting the vertebrate cytochrome B gene.12 In brief, DNA was prepared from individual blooded mosquitoes using the Qiaquick kit (Qiagen) following the manufacturer’s protocol. A total of 2 μl of the resulting DNA was then used as a template in a 50 μl amplification reaction. The PCR amplifications were conducted in a solution containing 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 2 mM MgCl2, 200 μM of each dNTP, 0.5 μM of each primer and 1.25 units of Taq DNA polymerase (Invitrogen). Primers used in the PCR were taken from Kitano29 and were as follows: 5′ GCCTGTTTACCAAAAACATCAC 3′ and 5′ CTCCATAGGGTCTTCTCGTCTT 3′. Reactions began with incubation at 95 °C for 3.5 min, followed by 35 cycles consisting of 30 sec at 95 °C, 15 sec at 57 °C, and 30 sec at 72 °C. The reaction was completed by incubation at 72 °C for 5 min. The PCR products were visualized by 1.5% agarose gel electrophoresis and purified by using the QIAquick PCR purification kit (Qiagen). The purified PCR products were subject to direct DNA sequencing, as previously described.30
Estimation of amphibian and reptile abundance
Visual encounter surveys (VES) were used to estimate relative abundance of amphibians and reptiles at the study site. VES are similar to bird point count surveys, and have been widely used in herpetological research.31 Once each week, one diurnal and one nocturnal survey were conducted at five large ponds in the study site. Visual encounter surveys consisted of an observer(s) walking slowly along the water’s edge of a pond and noting all individuals of each species of amphibian or reptile seen. The amount of time spent searching was recorded for each sample period (this varied from 1--6 hours depending on conditions), and the relative abundance for each species was calculated as the number of individuals recorded per person-hour of searching.
Because male anurans vocalize, and because recent evidence has suggested that some mosquitoes may use these vocalizations as cues for host choice,32 we also estimated relative abundance of frogs from call surveys.31 These surveys were performed during each VES and consisted of recording the identity of each species heard calling. In addition to the identity of each species, the relative abundance of calling males was recorded in one of the following ways: 1) record of exact number of individuals heard calling (1--24); 2) estimate of 25 individuals calling when calls of individuals overlapped but calls of individuals were still distinguishable, 3) estimate of 50 individuals calling when calls had continuous overlap in which individuals were indistinguishable (see USGS Patuxent Wildlife Research Center, www.mbrpwrc.usgs.gov/wifrog/analysis, 2008).
Because amphibians and reptiles vary in size and abundance and because mosquitoes might in part select hosts on factors proportional to based on host biomass33, we also collected data to convert our VES estimates of abundance to values of biomass for each species. These values were based on size data from individuals captured during VES samples as well as opportunistic encounters with individuals at the study site. Each individual was captured by hand and secured in plastic containers for processing. Body mass, measured with a spring scale, was used to calculate biomass by multiplying mean mass of the species by its relative abundance as determined by the VES. Some specimens were transported to the laboratory to obtain blood samples and these animals were weighed using an electronic balance. Blood collections were carried out under procedures approved by the Institutional Animal Care and Usage Committee of Auburn University (protocol number 2008-1391). For species for which fewer than five mass measurements were obtained, body mass was estimated using the mass of species of similar size.
Feeding index calculation and statistical analysis
Feeding indices were calculated as previously described.5 Abundance data used to determine these indices were based on the maximum VES abundance or maximum call index noted for each species during the 2007 field season. Three types of feeding indices were produced. VES data of ectotherm hosts were used to calculate a visual count feeding index and a biomass-adjusted visual count feeding index. Auditory survey data of anuran hosts were used to calculate a calling-based feeding index. The calling-based index examined whether mosquitoes cue in on vocalizing male anurans regardless of their size and therefore was not adjusted for biomass. Calling-based feeding indexes were calculated for Cx. territans only, as this species was the only one found to feed upon significant numbers of anurans. All feeding index calculations included only those species actually detected by blood meal analysis. Likelihood ratio tests were used to compare feeding index values, as previously described.5 Confidence intervals surrounding the proportion of blood meals obtained from each host class were calculated as previously described.30
RESULTS
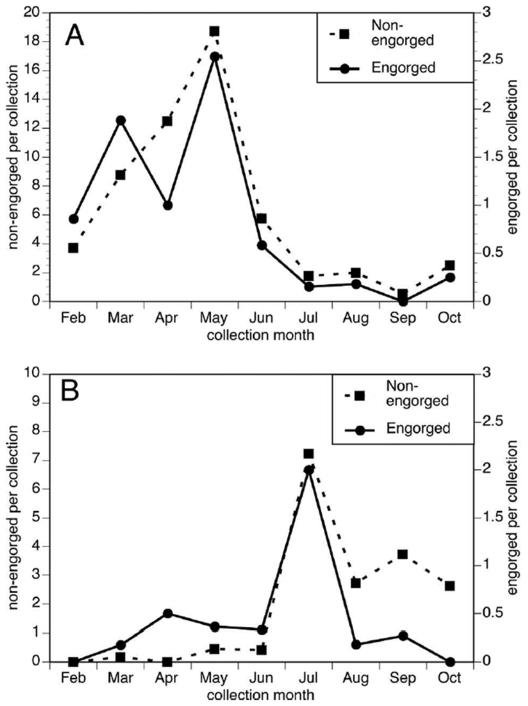
A total of 639 blood-engorged female mosquitoes of 13 species were collected during nine months of resting-site aspirations. DNA was extracted from 593 of these samples and subjected to PCR to identify to the species level the source of the blood meals. DNA was extracted from all individual mosquitoes, with the exception that only a random subset of Anopheles females were analyzed. Previous studies suggested that mosquitoes of this genus fed exclusively upon mammals.3, 30 Of the 593 individual mosquitoes from which DNA was extracted, the source of the blood meal was identified for 486 (78%). As expected, Anopheles crucians, Anopheles punctipennis and Anopheles quadrimaculatus fed exclusively on mammals (Table 1). Culex quinquefasciatus, Culex restuans and Culiseta melanura fed primarily on birds (Table 1). Four mosquito species (Cx. territans, Cx. peccator, Culex erraticus and Cs. melanura), fed at least some of the time upon ectothermic hosts (Table 1), with Cx. peccator and Cx. territans feeding primarily upon ectotherms (Table 1). The abundance of non-engorged females of Cx. territans peaked in May, while engorged Cx. territans exhibited two peaks, in March and in May (Figure 1, Panel A). In contrast, populations of both engorged and non-engorged Cx. peccator peaked in July (Figure 1, Panel B).
Table 1.
Host class of blood meals identified from field-collected mosquitoes, Tuskegee National Forest, AL, 2007.
| Species | Meals Identified |
% amphibian* | % avian* | % mammal* | % reptile* |
|---|---|---|---|---|---|
| Anopheles crucians | 10 | 0 | 0 | 100 | 0 |
| An. punctipennis | 7 | 0 | 0 | 100 | 0 |
| An. quadrimaculatus | 8 | 0 | 0 | 100 | 0 |
| Coquillettidia perturbans | 1 | 0 | 0 | 100 | 0 |
| Culiseta melanura | 6 | 0 | 83 (53-100) |
0 | 17 (0-47) |
| Culex erraticus | 336 | 1 (0-2) |
23 (19-27) |
71 (67-78) |
5 (3-7) |
| Cx. peccator | 33 | 36 (20-52) |
3 (0-9) |
9 (0-19) |
52 (35-69) |
| Cx. quinquefasciatus | 3 | 0 | 100 | 0 | 0 |
| Cx. restuans | 2 | 0 | 100 | 0 | 0 |
| Cx. territans | 78 | 86 (78-92) |
0 | 4 (0-8) |
10 (3-17) |
| Ochlerotatus sticticus | 1 | 0 | 0 | 100 | 0 |
Upper number represents the percentage of meals from a given class and lower numbers the 95% confidence interval surrounding that percentage.
Figure 1.
Temporal distribution of blood-engorged and non-engorged females of Cx. territans and Cx. peccator from Tuskegee National Forest, Macon Co., AL, 2007. Data are normalized for collection events. Panel A: Normalized collections of blood engorged and non-engorged Cx. territans. Panel B: Normalized collections of blood engorged and non-engorged Cx. peccator.
In addition to appearing at different times during the season, Culex peccator and Cx. territans appeared to partition the ectotherm host community. Of the 14 ectotherm species identified from blood-fed females, only three host species (the Bullfrog Rana catesbeiana, the Green Frog Rana clamitans, and the Southern Leopard Frog Rana sphenocephala) were utilized by both mosquito species. Culex peccator fed primarily upon reptiles, while Cx. territans fed primarily upon amphibians. Culex erraticus and Cx. peccator were the only species that fed on all four classes of vertebrates.
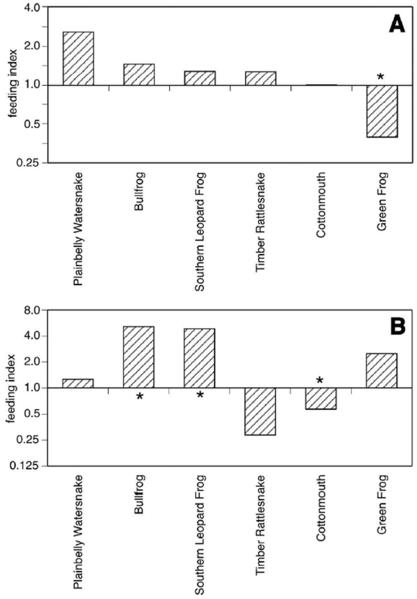
Feeding indices based on VES data suggested that Cx. peccator females generally fed on available ectotherm hosts in proportions that did not significantly differ from those predicted based upon relative abundance, with the exception of the Green Frog, which was fed upon significantly less (p=0.032) than was predicted based upon its abundance (Figure 2). When host relative abundances were adjusted for biomass, however, the adjusted feeding indices suggest that both the Bullfrog (p= 0.0048) and the Southern Leopard Frog (p=0.0064) were fed upon in greater proportion than predicted based upon their relative biomass, while Cottonmouths were fed upon less frequently (p=0.0048) than predicted by biomass (Figure 2).
Figure 2.
Feeding indices of ectothermic hosts of Cx. peccator from Tuskegee National Forest, AL, 2007. Feeding indices were calculated as described in Materials and Methods. In each panel, the feeding indices are shown on a log2 scale, so that species with feeding indices greater than 1 are indicated as positive bars and those with indices less than 1 as negative bars. Asterisks highlight species where the feeding index value was significantly different than 1.0 (p < 0.05). Panel A: Feeding indices calculated based upon raw counts from VES of host abundance. Panel B: Feeding indices calculated based upon biomass-adjusted visual encounter surveys of host abundance.
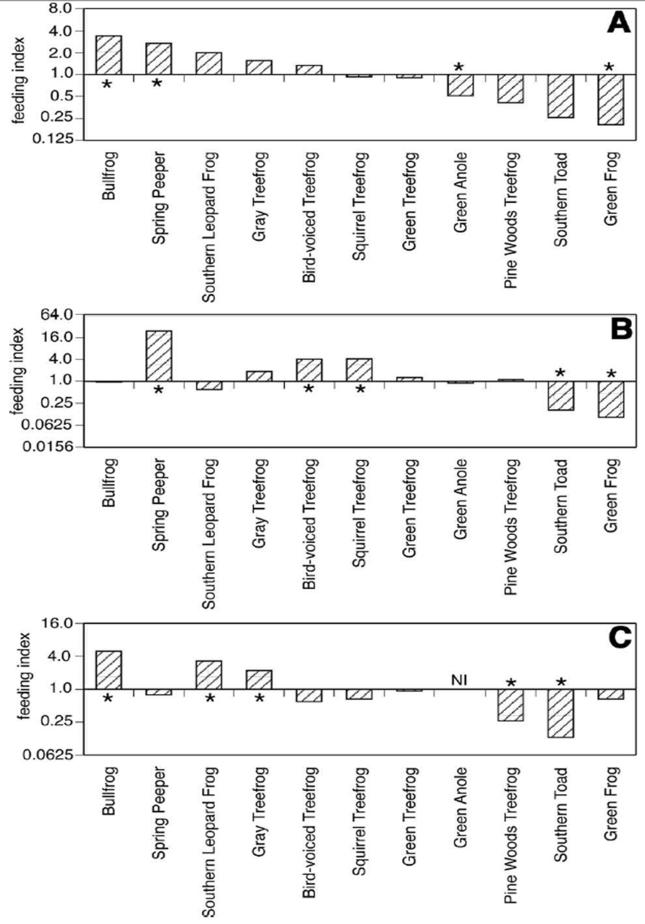
Culex territans was more specialized in its host feeding pattern than Cx. peccator. Six of eleven ectotherm host species were determined to be fed upon in proportions significantly greater than expected by Cx. territans by one or more of the three types of feeding indices (Figure 3). Feeding indices based on VES data suggested that Cx. territans females fed on the Spring Peeper Pseudacris crucifer (p= 0.014) and Bullfrogs (p= 0.016) in proportions that were significantly greater than that predicted based upon relative abundance alone. When abundance was adjusted for biomass, Spring Peepers (p< 0.001), Bird-voiced Treefrogs Hyla avivoca (p< 0.001), and Squirrel Treefrogs Hyla squirella (p= 0.047) were fed upon in proportions greater than predicted. In contrast, feeding indices calculated from calling data indicated that Gray Treefrogs Hyla chrysoscelis (p= 0.031), Bullfrogs (p= 0.001), and Southern Leopard Frogs (p= 0.041) were fed upon in proportions greater than expected. Two frog species (Bullfrogs and Spring Peepers) were found to be fed upon by Cx. territans in proportions significantly greater than predicted in more than one type of feeding index (Figure 3). Five host species (Green Anole Anolis carolinensis, Southern Toad Bufo terrestris, Green Treefrog Hyla cinerea, Pine Woods Treefrog Hyla femoralis, and Green Frog) were fed upon less than or equal to expected levels, as determined by all three types of feeding indices.
Figure 3.
Feeding indices of ectothermic hosts of Cx. territans from Tuskegee National Forest. Feeding indices were calculated as described in Materials and Methods. In each panel, the feeding indices are shown on a log2 scale, so that species with feeding indices greater than 1 are indicated as positive bars and those with indices less than 1 as negative bars. Asterisks highlight species where the feeding index value was significantly different than 1.0 (p < 0.05). Panel A: Feeding indices calculated based upon raw counts from VES of host abundance. Panel B: Feeding indices calculated based upon biomass-adjusted visual encounter surveys of host abundance. Panel C: Feeding indices calculated based upon calling survey data. NI = Not included in analysis (non-vocalizing species).
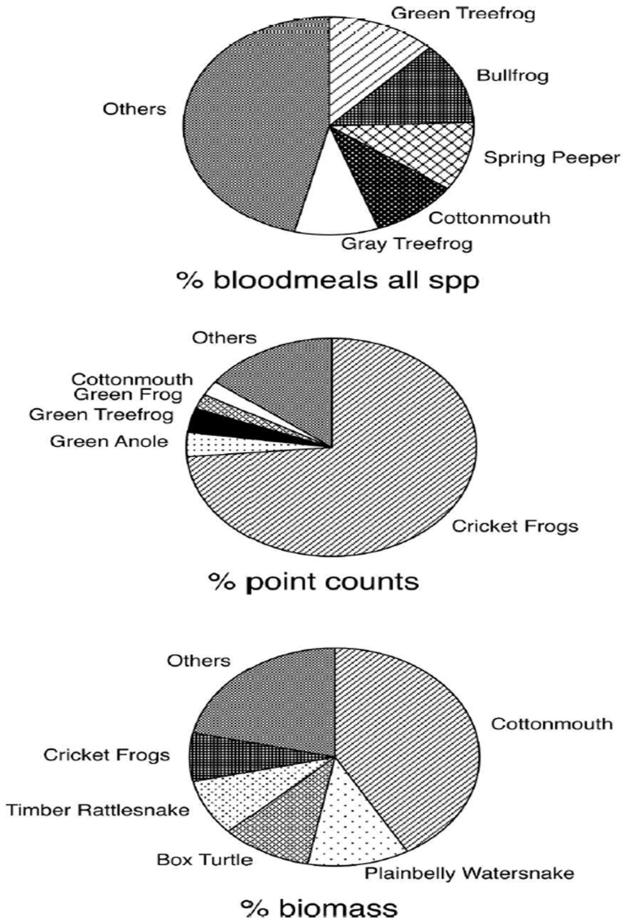
Several abundant species of reptiles and amphibians observed at the study site were not detected in blood meal analysis. For example, Cricket Frogs (Acris crepitans and Acris gryllus), were by far the most abundant species at the site, representing over 70% of the total animals observed, and roughly 7% of the total ectotherm biomass (Figure 4). No blood meals, however, were found to have come from these species. Turtles and salamanders, commonly observed throughout the site, were also not detected from blood meals. Spring Peepers, in contrast, were the third most commonly fed upon species, yet represented less than 1% of the animals observed and 0.1% of the ectotherm biomass at the site. Culex territans fed upon Spring Peepers 24 times more frequently than predicted, based upon biomass-adjusted abundance (Figure 3, Panel B).
Figure 4.
Blood meals, total abundance and total biomass from ectotherm hosts of Cx. peccator and Cx. territans from Tuskegee National Forest, AL, 2007.
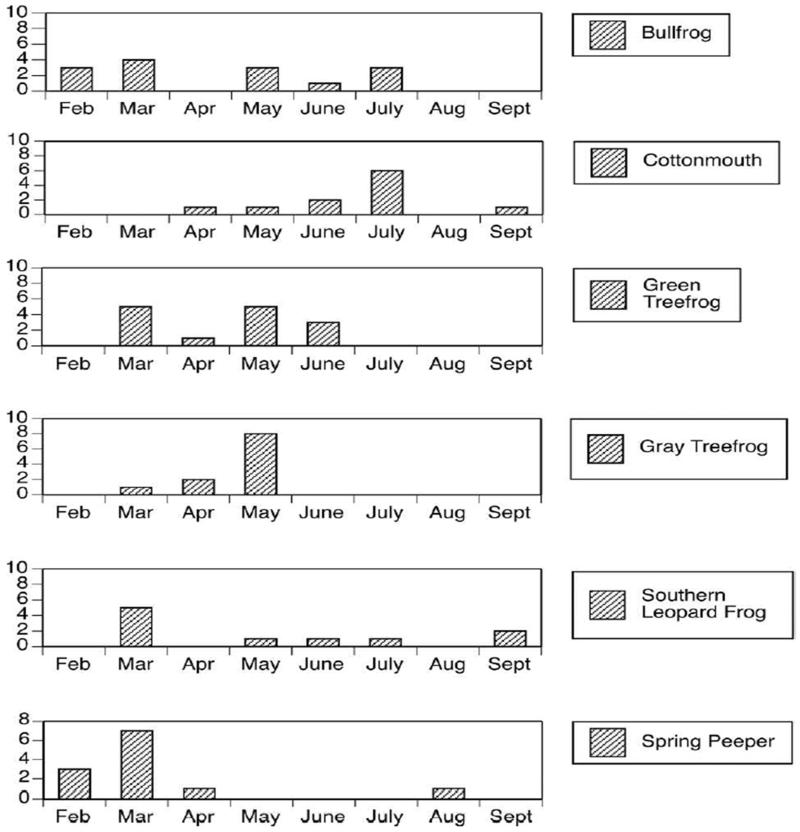
Blood meals from ectothermic hosts exhibited considerable temporal variation with respect to host species. Some hosts (such as Cottonmouths) were represented in mosquito blood meals fairly evenly throughout the collection season (Figure 5), while other species were unevenly distributed throughout the collection season. Blood meals from Spring Peepers, for example, were restricted primarily to the late winter and early spring, while blood meals from the Gray Treefrog were detected in March, April and May (Figure 5).
Figure 5.
Temporal distribution of blood meals taken from preferred ectotherm hosts of Cx. peccator and Cx. territans from Tuskegee National Forest, AL, 2007.
DISCUSSION
Previous studies on host preference have suggested that many mosquito species show distinct preferences for certain host species, such that the feeding pattern cannot be predicted based upon the abundance of the host species alone. This tendency has been demonstrated for mosquitoes that feed on birds5, 8, 9, 34 and for mosquitoes that feed on mammals.35 Our own findings indicate that the same is true for some species that feed on reptiles and amphibians. Culex territans, which feeds primarily on anurans, was found to be quite host specific, while Cx. peccator, which fed on all four classes of terrestrial vertebrates, was less host specific. Given the breadth of hosts from which Cx. peccator fed, it is perhaps not surprising that this species showed little apparent preference for one host versus another.
In a previous study conducted at the same site, Cottonmouths and Bullfrogs were found to be the host species most commonly fed upon by ectotherm-feeding mosquitoes (primarily Cx. peccator).12 At that time, however, data on the abundance of the various reptiles and amphibians at the site were not available. The current study supports the previous finding that both Bullfrogs and Cottonmouths are commonly targeted hosts. Cottonmouths, however, are very abundant at the TNF site, representing 41% of the ectotherm biomass. Thus, when considered in the context of their abundance, cottonmouths do not appear to be a preferred host. Bullfrogs, however, despite their relative rarity at the site (0.61% total abundance and 1.85% biomass), do appear to be a preferred host for both Cx. peccator and Cx. territans, with feeding indices of >1 in 3/5 of the analyses reported above.
Spring Peepers were another preferred host of Cx. territans, as determined by feeding indices calculated from VES data and VES data adjusted for overall biomass. When calling data were used to calculate feeding indices, however, Spring Peepers were not found to be a preferred host. In a recent laboratory study, in which Cx. territans females were allowed to choose between the calls of multiple frog species, females oriented towards the calls of Spring Peepers more than any other frog species.32 These findings and the results presented here, together suggest that Cx. territans might be specifically attracted to vocalizing Spring Peepers and other anurans, and may feed actively upon males that are attempting to attract a mate.
In other host groups, e.g., birds, it has been noted that some species are targeted to a lesser extent than would be predicted based upon their abundance. Perhaps the most striking example is that of the American Crow, which, although being a very common peri-urban bird and one that has been commonly employed as a sentinel for West Nile Virus in the USA, appears to be rarely fed upon by the mosquitoes that are the vectors for this virus.9, 36 Cricket Frogs appear to be in a similar position among reptiles and amphibians. Cricket Frogs, although abundant at the site, were not detected in mosquito blood meals. Although Cricket Frogs are (on average) the smallest frogs at the study site, it is unlikely that they are too small to be targeted by mosquitoes. Spring Peepers are only slightly larger, and were found to be preferred hosts. It is also unlikely that physical characteristics of the frog’s skin (thicker, warty skin in Cricket Frogs vs. thinner, nonwarty skin in Spring Peepers) are responsible for the observed patterns. While Cricket Frogs have warty skin (compared to Spring Peepers), Gray Treefrogs also have warty skin and were fed upon frequently relative to their abundance. Cricket Frogs are sympatric with other frog species at the study site, call during the daytime (as do Spring Peepers), and have call frequencies similar to that of Spring Peepers.32, 37 It is conceivable that Cricket Frogs are small enough that an approaching mosquito would be considered a prey item and eaten by the frogs. Unlike most hylid frogs (e.g., the Green Treefrog, or the Spring Peeper), Cricket Frogs apparently continue feeding throughout their extended breeding period.38 Alternatively, it is possible that Cricket Frogs have skin toxins or other chemical defenses that are repellent to foraging mosquitoes. Amphibians are characterized by a large repertoire of antimicrobial and anti-predatory skin toxins and peptides. The presence or absence of skin toxins has been associated with resistance to fish predation in tadpoles and salamander larvae.39 In addition, some frog skin toxins are associated with resistance to mosquito blood feeding.40 Skin semiochemicals associated with mosquito resistance/susceptibility could therefore explain the observed patterns of amphibian host avoidance, particularly in the case of Cricket Frogs.
Several frog species, including Spring Peepers, Gray Treefrogs and Green Treefrogs, were fed upon seasonally, and the peak of their appearances in the blood meals coincided with the peak of male calling. This indicates that the mosquitoes are finding the frogs at their breeding site, not at the places where they roost for the rest of the year. These temporal patterns provide further support to the hypothesis that Cx. territans females are foraging for reproductively active hosts and may locate those hosts by behaviors associated with mate finding. Previous studies have suggested that mosquito feeding upon preferred avian hosts is not evenly distributed temporally, but varies throughout the year. This phenomenon may be a function of the life history or behavior of the host animals8, 41 and may be an important component of arbovirus amplification. It may also play a role in the process that allows arboviruses to escape avian enzootic cycles and spill over into other vertebrate hosts.8 Because ectotherms are potentially important as reservoirs for certain mosquito-borne viruses, it is important to note that similar temporal variation was exhibited for mosquitoes feeding upon ectothermic hosts. That Cx. peccator and Cx. territans also fed on birds and mammals at our study site points to the possibility that these mosquitoes may be important in the transfer of arboviruses to and from ectotherms and other host groups.
In summary, the data presented above suggest that, like ornithophilic mosquitoes, species targeting ectothermic hosts appear to demonstrate significant biases in host choice. The proportion of blood meals taken from a given host is likely to be a complex function of a number of variables including the innate attractiveness of a species to the mosquito, the availability of that host to the mosquito, and behaviors that make the host either more or less vulnerable to being successfully fed upon by a mosquito. These factors, along with the innate susceptibility of a given vertebrate to a virus are likely to be important in determining the significance of a given species as a reservoir for an arthropod borne virus. In the case of EEEV, a significant amount of evidence has been mounting to support the theory that ectotherms may play a role (of an as yet undetermined magnitude) in the transmission cycle. Our findings that ectotherm feeding mosquitoes prefer some hosts over others could have important consequences for local transmission of this pathogen. Additional studies to test these hypotheses are currently underway.
ACKNOWLEDGEMENTS
The authors would like to thank Nathan Click and Katherine Gray for their help with mosquito collections, and Geoffrey Sorrell and Matthew McCurdy for assisting in ectotherm surveys. David Bayne graciously allowed access to his land, where a portion of the mosquitoes were collected.
FINANCIAL SUPPORT This research was supported by a grant from the National Institute of Allergy and Infectious Diseases, Project # R01AI049724 to TRU.
Contributor Information
Nathan D. Burkett-Cadena, Department of Entomology and Plant Pathology 301 Funchess Hall Auburn University Auburn, AL 36849 Ph: 334-844-5006 Fax: 334-844-5005
Sean P. Graham, Department of Biological Sciences 331 Funchess Hall Auburn University Auburn, AL 36849 Ph: 334-750-3774 FAX: 334-844-9234
Hassan K. Hassan, Global Infectious Disease Research Program Department of Global Health College of Public Health University of South Florida 3720 Spectrum Blvd., Suite 304 Tampa, FL 33612 Ph: 813-974-5233 Fax: 813-974-0992
Craig Guyer, Department of Biological Sciences 331 Funchess Hall Auburn University Auburn, AL 36849 Ph: 334-844-9232 FAX: 334-844-9234.
Micky D. Eubanks, Department of Entomology Texas A&M University TAMU 2475 College Station TX 77843-2475 Ph: 979-862-7847 FAX: 979-845-6305
Charles R. Katholi, Department of Biostatistics University of Alabama at Birmingham RPHB 327 Birmingham, AL 35294 Ph: 205-934-4500 FAX: 205-975-2540
Thomas R. Unnasch, Global Health Infectious Disease Research Program Department of Global Health College of Public Health University of South Florida 3720 Spectrum Blvd., Suite 304 Tampa, FL 33612
REFERENCES
- 1.LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci U S A. 2003;100:567–71. doi: 10.1073/pnas.0233733100. Epub 2003 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edman JD. Host-feeding patterns of Florida mosquitoes (Diptera: Culicidae) VI. Culex (Melanoconion) J Med Entomol. 1979;15:521–525. doi: 10.1093/jmedent/15.5-6.521. [DOI] [PubMed] [Google Scholar]
- 3.Irby WS, Apperson CS. Hosts of mosquitoes in the coastal plain of North Carolina. J Med Entomol. 1988;25:85–93. doi: 10.1093/jmedent/25.2.85. [DOI] [PubMed] [Google Scholar]
- 4.Christensen HA, de Vasquez AM, Boreham MM. Host-feeding patterns of mosquitoes (Diptera: Culicidae) from central Panama. Am J Trop Med Hyg. 1996;55:202–8. doi: 10.4269/ajtmh.1996.55.202. [DOI] [PubMed] [Google Scholar]
- 5.Hassan HK, Cupp EW, Hill GE, Katholi CR, Klingler K, Unnasch TR. Avian host preference by vectors of Eastern Equine Encephalomyelitis virus. Am J Trop Med Hyg. 2003;69:641–647. [PubMed] [Google Scholar]
- 6.Boakye D, Tang JM, Truc P, Merriweather A, Unnasch TR. Identification of blood meals in hematophagous diptera by polymerase chain reaction amplification and heteroduplex analysis. Med. Vet. Entomol. 1999;13:282–287. doi: 10.1046/j.1365-2915.1999.00193.x. [DOI] [PubMed] [Google Scholar]
- 7.Molaei G, Andreadis TA, Armstrong PM, Anderson JF, C.R V. Host Feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg Inf Dis. 2006;12:468–474. doi: 10.3201/eid1203.051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006;4:e82. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savage HM, Aggarwal D, Apperson CS, Katholi CR, Gordon E, Hassan HK, Anderson M, Charnetzky D, McMillen L, Unnasch EA, Unnasch TR. Host choice and West Nile virus Infection rates in blood fed mosquitoes, including members of the Culex pipiens complex, from Memphis and Shelby County, Tennessee 2002-2003. Vector Borne Zoonotic Dis. 2007;7:365–386. doi: 10.1089/vbz.2006.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shope RE, de Andrade AHP, Bensabath G, Causey OR, Humphrey PS. The epidemiology of EEE, WEE, SLE and Turlock viruses, with special reference to birds, in a tropical rain forest near Belem, Brazil. Am J Epidemiol. 1966;84:467–477. doi: 10.1093/oxfordjournals.aje.a120659. [DOI] [PubMed] [Google Scholar]
- 11.Dalrymple JM, Young OP, Eldridge BF, Russell PK. Ecology of arboviruses in a Maryland freshwater swamp. 3. Vertebrate hosts. Am J Epidemiol. 1972;96:129–40. doi: 10.1093/oxfordjournals.aje.a121439. [DOI] [PubMed] [Google Scholar]
- 12.Cupp EW, Zhang D, Yue X, Cupp MS, Guyer C, Korves T, Unnasch TR. Identification of reptilian and mphibian bloodmeals from mosquitoes in an Eastern Equine Encephalomyelitis virus focus in central Alabama. Am J Trop Med Hyg. 2004;71:272–276. [PMC free article] [PubMed] [Google Scholar]
- 13.Burton TM, Likens GE. Energy flow and nutrient cycling in salamander populations in the Hubbard Brook Experimental Forest, New Hampshire. Ecology. 1975;56:1068–1080. [Google Scholar]
- 14.Pough FH. Advantages to ectothermy for tetrapods. Am Nat. 1980;115:92–112. [Google Scholar]
- 15.Iverson J. Biomass and turtle populations: A neglected subject. Oecologia. 1982;55:69–76. doi: 10.1007/BF00386720. [DOI] [PubMed] [Google Scholar]
- 16.Petranka JW, Murray SS. Effectiveness of removal sampling for determining salamander density and biomass: A case study in an Appalachian streamside community. J Herpetol. 2001;35:36–44. [Google Scholar]
- 17.Gibbons JW, Winne CT, Scott DE, Willson JD, Glaudas X, Andrews KM, Todd BD, Fedewa LA, Wilkinson L, Tsaliagos RN, Harper SJ, Greene JL, Tuberville TD, Metts BS, Dorcas ME, Nestor JP, Young CA, Akre T, Reed RN, Buhlmann KA, Norman J, Croshaw DA, Hagen C, Rothermel BB. Remarkable amphibian biomass and abundance in an isolated wetland: Implications for wetland conservation. Conserv Biol. 2006;20:1457–1465. doi: 10.1111/j.1523-1739.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 18.Gebhardt LP, Hill DW. Overwintering of Western Equine Encephalitis virus. Proc Soc Exp Biol Med. 1960;104:695–8. doi: 10.3181/00379727-104-25955. [DOI] [PubMed] [Google Scholar]
- 19.Thomas LA, Eklund CM. Overwintering of Western Equine Encephalomyelitis virus in garter snakes experimentally infected by Culex tarsalis. Proc. Soc. Exp. Biol. Med. 1962;109:421–4. doi: 10.3181/00379727-109-27225. [DOI] [PubMed] [Google Scholar]
- 20.Bowen GS. Prolonged Western Equine Encephalitis viremia in the Texas tortoise (Gopherus berlandieri) Am J Trop Med Hyg. 1977;26:171–5. doi: 10.4269/ajtmh.1977.26.171. [DOI] [PubMed] [Google Scholar]
- 21.Karstad L. Reptiles as possible reservoir hosts for Eastern Encephalitis virus. Trans. 26th N. Am. Wildlife Conf..1961. pp. 186–202. [Google Scholar]
- 22.Hayes RO, Daniels JB, Maxfield HK, Wheeler RE. Field and laboratory studies on Eastern Encephalitis in warm- and cold-blooded vertebrates. Am J Trop Med Hyg. 1964;13:595–606. doi: 10.4269/ajtmh.1964.13.595. [DOI] [PubMed] [Google Scholar]
- 23.Klenk K, Snow J, Morgan K, Bowen R, Stephens M, Foster F, Gordy P, Beckett S, Komar N, Gubler D, Bunning M. Alligators as West Nile virus amplifiers. Emerg Inf Dis. 2004;10:2150–2155. doi: 10.3201/eid1012.040264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris CD, Caines AR, Woodall JP, Bast TF. Eastern Equine Encephalomyelitis in upstate New York 1972-1974. Am J Trop Med Hyg. 1975;24:986–91. doi: 10.4269/ajtmh.1975.24.986. [DOI] [PubMed] [Google Scholar]
- 25.Cupp EW, Klinger K, Hassan HK, Viguers LM, Unnasch TR. Eastern Equine Encephalomyelitis virus transmission in central Alabama. Am J Trop Med Hyg. 2003;68:495–500. [PMC free article] [PubMed] [Google Scholar]
- 26.Burkett-Cadena ND, Eubanks MD, Unnasch TR. Preference of female mosquitoes for natural and artificial resting sites. J Am Mosq Control Assoc. 2008;24:228–235. doi: 10.2987/5662.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edman JD, Evans FDS, J.A W. Development of a diurnal resting box to collect Culiseta melanura (Colquillett) Am J Trop Med Hyg. 1968;17:451–456. doi: 10.4269/ajtmh.1968.17.451. [DOI] [PubMed] [Google Scholar]
- 28.Darsie RF, Ward RA. Identification and geographical distribution of the mosquitoes of North America, north of Mexico. Mosq Systemat. 1981;1S:1–313. [Google Scholar]
- 29.Kitano T, Umetsu K, Tian W, Osawa M. Two universal primer sets for species identification among vertebrates. Int J Legal Med. 2007;121:423–7. doi: 10.1007/s00414-006-0113-y. [DOI] [PubMed] [Google Scholar]
- 30.Apperson CS, Harrison BA, Unnasch TR, Hassan HK, Irby WS, Savage HM, Aspen SE, Watson DW, Rueda LM, Engber BR, Nasci RS. Host-feeding habits of Culex and other mosquitoes (Diptera: Culicidae) in the borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. J Med Entomol. 2002;39:777–785. doi: 10.1603/0022-2585-39.5.777. [DOI] [PubMed] [Google Scholar]
- 31.Heyer W, Donnelly M, McDiarmid R, Hayek L, Foster M. Standard methods for amphibians. Smithsonian Institution Press; Washington DC: 1994. Measuring and monitoring biological diversity. [Google Scholar]
- 32.Bartlett-Healy K, Crans W, Gaugler R. Phonotaxis to amphibian vocalizations in Culex territans (Diptera: Culicidae) Ann Ent Soc Am. 2008;101:95–103. [Google Scholar]
- 33.Khan AA. Mosquito attractants and repellants. In: Shorey HH, J.J M, editors. Chemical Control of Insect Behavior: Theory and Application. John Wiley; New York: 1977. pp. 305–325. [Google Scholar]
- 34.Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. Host heterogeneity dominates West Nile virus transmission. Proc. R. Soc. Lond. B. Biol. Sci. 2006;273:2327–33. doi: 10.1098/rspb.2006.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hess AD, Hayes RO, Tempelis CH. The use of the foraging ratio technique in mosquito host preference studies. Mosq News. 1968;28:386–389. [Google Scholar]
- 36.Apperson CS, Hassan HK, Harrison BA, Aspen SE, Savage HM, Farajollahi A, Crans W, Daniels TJ, Falco RC, Benedict M, Anderson M, McMillen L, Unnasch TR. Host feeding patterns of probable vector mosquitoes of West Nile Virus in the eastern United States. Vector Borne Zoonotic Dis. 2004;4:71–82. doi: 10.1089/153036604773083013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McClelland BE, Wilczynski W, Ryan MJ. Correlations between call characteristics and morphology in male cricket frogs (Acris crepitans) J Exp Biol. 1996;199:1907–19. doi: 10.1242/jeb.199.9.1907. [DOI] [PubMed] [Google Scholar]
- 38.Wight AH. Life-histories of the frogs of Okefinokee Swamp, Georgia. Cornell University Press; Ithica, NY: 1932. [Google Scholar]
- 39.Kats C, Petranka J, Sih A. Antipredator defenses and the persistence of amphibian larvae with fishes. Ecology. 1988;69:1865–1870. [Google Scholar]
- 40.Williams CR, Smith BP, Best SM, Tyler MJ. Mosquito repellents in frog skin. Biol Lett. 2006;2:242–5. doi: 10.1098/rsbl.2006.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Unnasch RS, Cupp EW, Unnasch TR. Host selection and its role in transmission of arboviral encephalitides. In: Collinge SK, Ray C, editors. Disease Ecology: Community Structure and Pathogen Dynamics. Oxford University Press; Oxford, UK: 2005. pp. 73–89. [Google Scholar]