Abstract
Preclinical data suggest that combined epidermal growth factor receptor (EGFR) targeting with an EGFR tyrosine kinase inhibitor and an anti-EGFR monoclonal antibody may be superior over single-agent targeting. Therefore, as part of a phase I study, we analyzed the outcome of 20 patients with non-small cell lung cancer treated with the combination of erlotinib and cetuximab. EGFR mutation status was ascertained in a CLIA-approved lab. There were 10 men; median number of prior therapies, 5. Overall, 2 of 20 patients (10%) achieved partial response (PR), one of whom had a TKI-resistant EGFR insertion in exon 20, time to treatment failure (TTF) = 24+ months; and, the other patient had squamous cell histology (EGFR wild-type), TTF=7.4 months. In addition, 3 of 20 patients (15%) achieved stable disease (SD) ≥6 months (one of whom had wild-type EGFR and squamous cell histology, and two patients had an EGFR TKI-sensitive mutation, one of whom had failed prior erlotinib therapy). Combination therapy with ertotinib plus cetuximab was well tolerated. The most common toxicities were rash, diarrhea, and hypomagnesemia. The recommended phase II dose was erlotinib 150 mg oral daily and cetuximab 250 mg/m2 IV weekly. In summary, erlotinib and cetuximab treatment was associated with SD ≥6 months/PR in 5 of 20 patients with non-small cell lung cancer (25%), including individuals with squamous histology, TKI-resistant EGFR mutations, and wild-type EGFR, and those who had progressed on prior erlotinib after an initial response. This combination warrants further study in select populations of non-small cell lung cancer.
Keywords: cetuximab, EGFR, erlotinib, NSCLC, resistance
Introduction
Lung cancer is the leading cause of cancer-related death in the United States(1). Recent progress in understanding the biology of this tumor has led to the development of targeted agents that demonstrate improved response rates in patients with non-small cell lung cancer (NSCLC)(2, 3).
There is a broad literature on the efficacy of EGFR inhibitors in NSCLC(4-7). Currently, two distinct classes of drugs are used to target EGFR(8). EGFR tyrosine kinase inhibitors (TKI's)- erlotinib and gefitinib- bind to the intracellular tyrosine kinase domain and block the enzymatic function of the receptor. Cetuximab, a monoclonal antibody, binds to the extracellular ligand-binding domain of EGFR, suppressing EGFR-dependent signaling through inhibition of ligand-dependent activation and receptor dimerization, and induction of antibody-dependent cell-mediated cytotoxicity(9).
Resistance to EGFR therapy represents a major clinical problem. Primary resistance to EGFR inhibitors can be mediated by certain insertion mutations in exon 20 and other concomitant mutations such as those in the KRAS gene(10). While many EGFR mutation-positive patients demonstrate tumor regression initially with EGFR TKI treatment, most will relapse within one year due to acquired resistance(10-13). About 50% of erlotinib-resistant cases of NSCLC demonstrate the emergence of a second TKI-resistant mutation (T790M) in exon 20(11, 13, 14).
While preclinical studies have demonstrated that combination therapy with two different classes of EGFR antagonists can be synergistic(15, 16), clinical trials have to date demonstrated minimal activity(17, 18). We conducted a phase I study to evaluate the combination of EGFR TKI erlotinib with anti-EGFR monoclonal antibody cetuximab in patients with advanced cancer(19). Herein, we report the results of the subset of 20 patients with NSCLC who were treated on this study.
Patients and Methods
Eligibility Criteria
To be eligible for this study, patients must have had pathologically confirmed advanced or metastatic cancer, refractory to standard therapy; Eastern Cooperative Oncology Group (ECOG) performance status(20) ≤2. Other key inclusion criteria were absolute neutrophil count ≥ 1000/mL; platelets ≥50,000/mL; serum creatinine ≤2times upper limit of normal; total bilirubin ≤2 mg/dL, alanine amino transferase (ALT) ≤3 times the upper limit of normal. In the presence of liver metastases, total bilirubin can be ≤3 and ALT ≤5 times the upper limit of normal. In the dose escalation cohorts, neither presence of EGFR mutation nor prior EGFR inhibitor therapy was required. Patients who were pregnant or unwilling to use contraception, a history of cerebrovascular accidents or myocardial infarction within 6 months, or known hypersensitivity to any component of the drugs tested were excluded from the study. The study and all treatments were conducted in accordance with the guidelines of the MD Anderson Institutional Review Board and written informed consent was obtained from all the patients before study related procedures were started.
Study design
Patients were enrolled in a phase I, open-label, dose-escalation study with a standard 3 + 3 design conducted by the Department of Investigational Cancer Therapeutics at MD Anderson Cancer Center (MDACC) beginning May, 2009. Erlotinib was given orally daily with cetuximab given intravenously on days 1, 8, 15, and 22 of a 28 day cycle. Patients were treated on one of the 2 dose levels in 28 day cycles (Table 1). Patients remained on the study until disease progression, unacceptable toxicity, death, or withdrawal of consent. Primary endpoints were to establish the maximum tolerated dose (MTD) and to characterize toxicity profiles. Secondary endpoints included a preliminary assessment of biologic activity.
Table 1. Dose-escalation schedule for erlotinib and cetuximab.
| Dose level | Erlotinib PO daily (mg) | Cetuximab IV on days 1, 8, 15, and 22 (mg/m2) | |
|---|---|---|---|
|
| |||
| Loading dose | Maintenance dose | ||
| Level -2 | 50 | 200 | 125 |
| Level -1 | 75 | 200 | 125 |
| Level 1 | 100 | 200 | 125 |
| Level 2 | 150 | 400 | 250 |
Abbreviations: PO, per os (by mouth); IV, intravenous
Dose-limiting toxicity and maximum tolerated dose
Dose limiting toxicity (DLT) was defined as any grade 3 or 4 non-hematologic toxicity as defined in the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) Version 3.0(21), any grade 4 hematologic toxicity lasting two weeks or longer (as defined by the NCI-CTCAE) despite supportive care, grade 4 nausea or vomiting >5 days despite maximum anti-nausea regimens, or any severe/life-threatening complication not defined in the NCI-CTCAE that was attributable to the therapy during the first treatment cycle. Correctable electrolyte imbalances and alopecia were not considered DLTs.
Dose levels were escalated in cohorts of three patients as long as no DLT was observed. If a DLT was observed in one patient at a particular dose level, three more patients were treated at this dose level. If no additional patients in the expanded cohort of six patients experienced a DLT, dose escalation resumed. If a second patient enrolled at the same dose level experienced a DLT, the MTD was considered to have been exceeded. The next lower dose level was considered the MTD, and an additional three patients were treated at the MTD level unless six patients were already treated at that dose level. The maximum tolerated dose was the highest dose at which no more than one of every six patients had a DLT. Dose escalation was not permitted for individual patients.
Toxicity evaluation
Adverse events were recorded from day 1 of each cycle, and up to 30 days after last dose on study. Severity of the events was assessed using the NCI-CTCAE v3.0(21). MTD was defined by DLTs that occurred during only the first cycle of therapy.
Assessment of anti-tumor efficacy
Treatment efficacy was evaluated by computed tomography (CT) scans and/or magnetic resonance imaging (MRI) studies according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.0(22) criteria at baseline before treatment initiation and then every three cycles (8–12 weeks) and were reported as best response. All radiographs were read in the Department of Radiology at MDACC and reviewed in the Department of Investigational Cancer Therapeutics tumor measurement clinic. Responses were categorized per RECIST 1.0 criteria. In brief, complete response was defined as the disappearance of all measurable and non-measurable disease; partial response (PR) was defined as at least a 30% decrease in the sum of the longest diameter of measurable target lesions; progressive disease (PD) was defined as at least a 20% increase in the sum of the longest diameter of measurable target lesions, or unequivocal progression of a non-target lesion, or the appearance of a new lesion; and stable disease (SD) was defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD. A waterfall plot was used to illustrate antitumor efficacy, as previously described(23).
Molecular assays
All histologies were centrally reviewed at MD Anderson Cancer Center. Mutation testing was performed in the Clinical laboratory Improvement Amendment (CLIA) -certified Molecular Diagnostic Laboratory at MDACC. Polymerase Chain Reaction (PCR)-based DNA sequencing analysis was done on DNA extracted from paraffin-embedded or tissue from fine-needle aspiration or surgical biopsies. Analysis was performed on exons 18 to 21 of the kinase domain of the EGFR gene, the sites of the most common mutations observed in lung adenocarcinomas. The lower limit of sensitivity of detection was approximately one mutated cell per five total cells in sample (20%). Whenever possible, in addition to EGFR, we tested for other mutations such as PIK3CA (codons 532 to 554 in exon 9 and codons 1011 to 1062 in exon 20), KRAS/NRAS (codons 12, 13, and 61), TP53 (exons 4 to 9), and AKT1 (exon 4 and 7 of AKT gene). PTEN expression was assessed, if tissue was available, using immunohistochemistry and the DAKO antibody (Carpentaria, Ca.)(24).
Statistical analysis
Descriptive statistics were used to summarize patient characteristics and adverse events. Fisher's exact test was used to assess the association between categorical variables. Time to treatment failure (TTF) was defined as the time interval between the start of therapy and the date of disease progression or death or removal from study for any reason, whichever occurred first. Patients who were alive and on study were censored at the time of their last follow-up.
Results
Patient Characteristics
As part of a dose escalation study(19), 20 patients with NSCLC were enrolled on the study. Two patients were enrolled on dose level 1 (erlotinib 100 mg oral daily and cetuximab 125 mg/m2 IV on days 1, 8, 15, and 22 after a loading dose of 200 mg/m2 IV) and 18 patients on dose level 2 (erlotinib 150 mg oral daily and cetuximab 250 mg/m2 IV on days 1, 8, 15, and 22 after a loading dose of 400 mg/m2 IV). Demographics and baseline characteristics of the 20 NSCLC patients are summarized in Table 2.
Table 2. Demographics and pre-treatment characteristics of 20 patients with NSCLC.
| Characteristics | No. of patients (%) |
|---|---|
| Age, Years | |
| Median | 66 |
| Range | 32-82 |
| ≤60 years | 8 (40) |
| >60 years | 12 (60) |
|
| |
| Sex | |
| Female | 10 (50) |
| Male | 10 (50) |
|
| |
| Race | |
| White | 13 (65) |
| Asian | 4 (20) |
| Black | 3 (15) |
|
| |
| Histology | |
| Adenocarcinoma | 15 (75) |
| Squamous cell | 4 (20) |
| Adenosquamous | 1 (5) |
|
| |
| EGFR mutation | |
| Mutation in exon 19 only | 5 (25) |
| Mutation in exon 20 only | 1 (5) |
| Mutation in exon 21 only | 2 (10) |
| Mutations in exon 19 and 20 | 1 (5) |
| Wild-type | 8 (40) |
| Unknown | 3 (15) |
|
| |
| KRAS mutation | |
| Present | 2 (10) |
| Wild-type | 11 (55) |
| Unknown | 7 (35) |
|
| |
| PIK3CA mutation | |
| Present | 0 (0) |
| Wild-type | 10 (50) |
| Unknown | 10 (50) |
|
| |
| Smoking history | |
| No | 10 (50) |
| Yes | 10 (50) |
|
| |
| Prior Therapies | |
| Median | 4.5 |
| Range | 2-9 |
| <3 | 5 (25) |
| ≥3 | 15 (75) |
|
| |
| Prior EGFR therapy | |
| No | 5 (25) |
| Yes | 15 (75) |
|
| |
| ECOG PS | |
| 0 | 3 (15) |
| 1 | 12 (60) |
| 2 | 5 (25) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR, Epidermal Growth Factor Receptor; KRAS, V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog; PS, Performance status; PIK3CA, Phosphatidylinositol 3-kinase, catalytic, alpha polypeptide
EGFR mutations
Of 20 patients with NSCLC, EGFR mutations were assessed in 17 patients. Ten EGFR mutations were seen in nine patients (Table 3). More specifically, known EGFR TKI-sensitive mutations were observed in eight patients, including six patients with deletions in exon 19 (cases #3, 5, 6, 8, 16 and 19, Table 3) and two patients (cases #17 and 18, Table 3) with point mutations in exon 21 (L858R). One of these eight patients had a co-existing TKI-resistant mutation, T790M in exon 20 (case #5, Table 3). One other patient (case #2, Table 3) had an EGFR TKI-resistant insertion, D770>GY in exon 20. The only significant association that was noted between patient characteristics and EGFR mutation status, was that of non-smokers and EGFR mutation-positive status (p-value =0.015).
Table 3. Genomic, proteomic and histopathologic characterization and corresponding responses in 20 patients with NSCLC treated with erlotinib and cetuximab.
| Case No. | Tumor-histology | EGFR mutation | Sensitive/Resistant to EGFR TKI | KRAS mutation | TTF-Prior EGFR therapy (months) | erlotinib+cetuximab | |||
|---|---|---|---|---|---|---|---|---|---|
| Dose level | TTF (months)a | Best Responseb | Recist % | ||||||
| 1 | Squamous cell carcinoma | unknown | NA | unknown | 1.9 | 1 | 2.0 | PD | +40 |
| 2 | Adenocarcinoma | D770>GY insertion (exon 20) | resistant | wild-type | NA | 2 | 24.2+ | PR | -33 |
| 3 | Adenocarcinoma | deletion (exon 19) | sensitive | wild-type | 24.7 | 2 | 0.9 | PD* | +20 |
| 4 | Adenocarcinoma | unknown | NA | unknown | 22.8 | 2 | 1.2 | PD* | +20 |
| 5 | Adenocarcinoma | deletion (exon 19) T790M (exon 20) | sensitive; resistant | wild-type | 39.0 | 2 | 1.8 | PD** | +20 |
| 6 | Adenocarcinoma | deletion (exon 19) | sensitive | unknown | 16.3 | 2 | 2.1 | PD* | +20 |
| 7 | Adenocarcinoma | wild-type | NA | wild-type | NA | 2 | 1.9 | PD** | +20 |
| 8 | Adenosquamous | deletion (exon 19) | sensitive | unknown | 3.2 | 2 | 1.1 | PD | +29 |
| 9 | Adenocarcinoma | wild-type | NA | wild-type | 0.7 | 2 | 0.7 | withdrew | +20 |
| 10 | Squamous cell carcinoma | wild-type | NA | unknown | NA | 2 | 13.7+ | SD | +0 |
| 11 | Adenocarcinoma | wild-type | NA | wild-type | 2.1 | 2 | 0.0 | taken off study | +20 |
| 12 | Adenocarcinoma | unknown | NA | wild-type | 6.5 | 2 | 4.3 | SD | -14 |
| 13 | Adenocarcinoma | wild-type | NA | G12D | 15.5 | 2 | 0.4 | withdrew | +20 |
| 14 | Adenocarcinoma | wild-type | NA | G12D | 2.1 | 2 | 0.8 | PD** | +20 |
| 15 | Squamous cell carcinoma | wild-type | NA | wild-type | NA | 2 | 7.4 | PR | -38 |
| 16 | Adenocarcinoma | insertion/deletion (exon 19) | sensitive | unknown | 7.5 | 2 | 1.9 | PD | +27 |
| 17 | Adenocarcinoma | L858R (exon 21) | sensitive | wild-type | 6.1 | 1 | 7.7+ | SD | -23 |
| 18 | Adenocarcinoma | L858R (exon 21) | sensitive | unknown | NA | 2 | 6.3+ | SD | +0 |
| 19 | Adenocarcinoma | deletion (exon 19) | sensitive | wild-type | 8.0 | 2 | 2.1 | PD** | +20 |
| 20 | Squamous cell carcinoma | wild-type | NA | wild-type | 12.1 | 2 | 1.6 | PD | +24 |
”+” = did not progress at the time of analysis
“*” = clinical progression;
= new metastasis
Abbreviations: EGFR, Epidermal Growth Factor Receptor; KRAS, V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog; NA, Not applicable; PR, Partial response; PD, Progressive disease; SD, Stable disease; TTF, Time to treatment failure; TKI, tyrosine kinase inhibitor
Whenever possible, mutation testing was also performed on other genes. Two of 13 patients assessed for KRAS had a G12D mutation in codon 12; and the only patient assessed for P53 mutation had a V157F mutation. Three of 5 patients evaluated for expression of PTEN by immunohistochemistry had either partial or complete PTEN loss. Ten patients assessed for NRAS mutation, 10 for PIK3CA mutation, and 5 for AKT1 mutation were all wild-type.
Toxicities
All 20 patients were evaluated for safety (Table 4). The most common toxicities considered at least possibly related to study drug were rash (n=9, 45%); diarrhea (n=7, 35%); hypomagnesemia (n=6, 30%); fatigue (n=6, 30%); nausea (n=4, 20%); and, anorexia (n=3, 15%). Most of the toxicities (84%) were either grade 1 or 2 and in most instances (41 of 46 grade 1 or 2 events) were reported in patients treated at dose level 2. Serious grade 3 toxicities that were at least possibly related to study drug are rash (n=5); acute infusion reaction (n=2); and, hand-foot skin reaction (n=2). All of these were reported at dose level 2; except for one patient with rash. There were no drug-related grade 4 toxicities or deaths reported.
Table 4. Adverse eventsa at least possibly related to study drug.
| Dose level | 1 (n=2) | 2 (n=18) |
|---|---|---|
| Dose | ||
| erlotinib PO: daily (mg) | 100 | 150 |
| cetuximab IV: days 1, 8, 15, and 22 | ||
| loading (mg/m2) | 200 | 400 |
| maintenance (mg/m2) | 125 | 250 |
| Adverse event | Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | Total (n=55) |
|---|---|---|---|---|---|---|---|
| Hematologic | |||||||
| Anemia | 1 | 1 | |||||
| Nonhematologic | |||||||
| Rash | 1 | 4 | 4 (1DLT) | 9 | |||
| Diarrhea | 1 | 5 | 1 | 7 | |||
| Hypomagnesemia | 4 | 2 | 6 | ||||
| Fatigue | 1 | 1 | 4 | 6 | |||
| Nausea | 4 | 4 | |||||
| Anorexia | 1 | 2 | 3 | ||||
| Acute infusion reaction | 2 (DLTs) | 2 | |||||
| Hand-foot skin reaction | 2 | 2 | |||||
| Constipation | 2 | 2 | |||||
| Hyperbilirubinemia | 1 | 1 | |||||
| Hyperkalemia | 1 | 1 | |||||
| Dermatitis | 1 | 1 | |||||
| Vomiting | 1 | 1 | |||||
| Esophagitis | 1 | 1 | |||||
| Folliculitis | 1 | 1 | |||||
| Hypotension | 1 | 1 | |||||
| Mucositis | 1 | 1 | |||||
| Abdominal pain | 1 | 1 | |||||
| Weight loss | 1 | 1 | |||||
| Edema | 1 | 1 | |||||
| Fever | 1 | 1 | |||||
| Paronychia | 1 | 1 |
Adverse events were considered by worst severity for each patient
Abbreviation: DLT, dose limiting toxicity; PO, per os (by mouth); IV, intravenous
There were three DLT's, all at dose level 2. One patient (case #11, Table 3) had an anaphylactic reaction during the first infusion of cetuximab. Subsequently, the patient had a myocardial infarction with elevated troponins and was taken off study. A second patient (case #4, Table 3) had developed an acute hypersensitivity reaction during the first infusion of cetuximab and was subsequently continued on erlotinib alone. A third patient (case #7, Table 3) had a grade 3 rash that resolved with antibiotics. During the phase I study, dose level 2 was established as MTD (erlotinib 150 mg oral daily and cetuximab 250 mg/m2 IV on days 1, 8, 15, and 22 after a loading dose of 400 mg/m2 IV)(19). Therefore, the recommended phase II dose was erlotinib 150 mg oral daily and cetuximab 250 mg/m2 IV on days 1, 8, 15, and 22 after a loading dose of 400 mg/m2 IV.
Antitumor activity
All 20 treated patients were included in the efficacy evaluation. Fourteen of the 20 patients had at least one post-treatment imaging evaluation, and three patients came off study prior to post-treatment imaging evaluation due to clinical progression. The remaining three patients were taken off study for the following reasons: withdrawal of consent (n=2) and adverse event (acute infusion reaction, n=1). These patients were considered as treatment failures.
The best overall responses (n=20) are illustrated in Figure 1. Of the 20 patients, two patients (10%) attained PR for 24.2+ and 7.4 months. In addition, three patients (15%) attained SD≥6 months (13.7+, 7.7+ and 6.3+ months).
Figure 1.
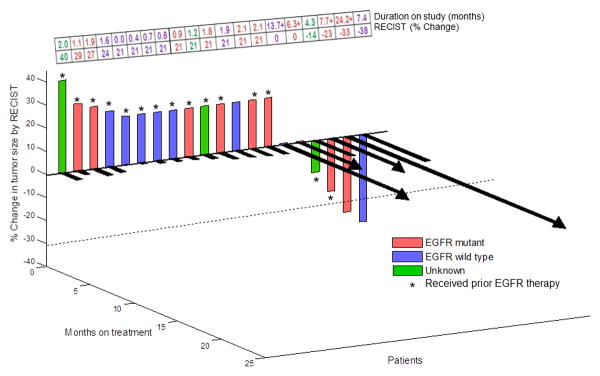
3-D waterfall plot. Best response by RECIST of 20 patients with NSCLC. Time to treatment failure in months is represented by solid black lines and the arrow indicates that the patient was still on study when the data was censored. Patients with clinical progression or with new metastasis and those who did not reach the first restaging scan for any reason were graphed as 20% progression.
Responses in patients who had received prior EGFR inhibitors
Fifteen of the 20 patients (75%) had received prior EGFR inhibitors (Table 3). Of 15 patients who had progressed previously on single-agent erlotinib, one patient (6.7%; case #17, Table 3) attained SD≥6 months on this study. The duration of treatment was longer (7.7+ months) on this combination study with dual EGFR inhibitors than on prior single-agent erlotinib (6.1 months).
Responses in NSCLC patients with mutant EGFR
Of the nine patients with EGFR-mutant NSCLC, one patient achieved PR and two patients attained SD≥6months. One patient (case #2, Table 3; Figure 2) had a known EGFR TKI-resistant mutation (insertion in exon 20, D770>GY) and achieved a PR (-33%; duration=24.2+ months). This patient had previously received two lines of standard chemotherapy but had not received prior EGFR inhibitor therapy. A second patient (case #17, Table 3) had a known EGFR TKI-sensitive mutation (L858R) in exon 21 and has ongoing SD≥6 months (-23%; duration=7.7+ months). This patient had received seven lines of prior therapy including single-agent erlotinib (TTF=6.1 months). A third patient (case #18, Table 3) with a known EGFR TKI-sensitive mutation (L858R) in exon 21 has SD ongoing for 6.3+ months. This patient had received two lines of prior therapy (with TTF of 4.2 months on the chemotherapy prior to this phase I therapy), but had not received prior erlotinib.
Figure 2.
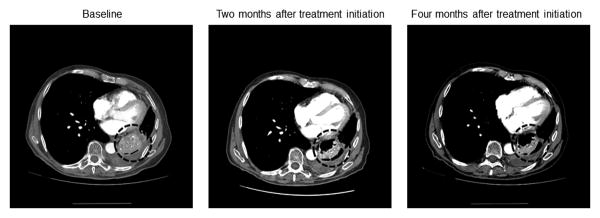
Computed tomography scans at baseline, 2 months, and, 4 months after initiating treatment on this study, of a NSCLC patient with a TKI-resistant EGFR mutation in exon 20, who has a partial response (-33%; duration=24.2+ months).
Responses in NSCLC patients with EGFR wild-type disease
Of the eight NSCLC patients with EGFR wild-type disease one patient had PR and one patient attained SD≥6 months. Both of these patients (cases #15 and 10, Table 3) had squamous cell histology. A total of 4 of 20 patients treated had squamous cell histology. One patient (case #15, Table 3) attained a PR (-38%; duration=7.4 months). This patient had two lines of prior standard therapy with TTF on therapy prior to this study of 0.7 months. A second patient (case #10, Table 3) with SD for 13.7+ months also had two lines of prior standard therapy with TTF of 8.1 months on the last therapy prior to this study.
Smoking status
Ten of the 20 patients had a history of smoking. These included six patients with adenocarcinoma histology versus four patients with squamous cell carcinoma. Mutation status was EGFR wild-type in seven patients, EGFR-mutant in two patients (exon 19 deletion, n=1; exon 20 insertion, n=1) and unknown in one patient. Of these, two patients achieved PR (cases #2 and 15, Table 3) and one patient (case #10, Table 3) attained SD≥6 months (EGFR-mutant adenocarcinoma, n=1; EGFR wild-type squamous cell carcinoma, n=2).
Discussion
Patients with known EGFR TKI-sensitive mutations in exon 19 and 21 respond well to matched therapy with EGFR inhibitors, but often quickly develop resistance. Preclinical studies suggest that dual agent molecular targeting of EGFR with a combination of a TKI (erlotinib/gefitinib) and an anti-EGFR antibody (cetuximab) may effectively overcome resistance(15, 16, 25). We conducted a phase I trial combining erlotinib and cetuximab in patients with advanced cancer(19). Herein, we report that 5 of 20 patients with NSCLC treated on this study achieved PR (n=2) or SD≥6 months (n=3).
The combination of erlotinib and cetuximab was well tolerated. The most frequently observed toxicities that were at least possibly related to study drug were rash (n=9); diarrhea (n=7); hypomagnesemia (n=6); fatigue (n=6); nausea (n=4); and, anorexia (n=3) (Table 4). The safety profile for the combination was consistent with the individual safety profile of each drug. These findings are similar to those reported in another phase I study of gefitinib and cetuximab in patients with refractory NSCLC, in which escalating doses of cetuximab were combined with fixed dose of gefitinib(17). We defined the recommended phase II dose of erlotinib 150 mg oral daily and cetuximab 250 mg/m2 IV on days 1, 8, 15, and 22 after a loading dose of 400 mg/m2 IV (dose level 2), with the main side effect being rash.
Among the five patients who demonstrated antitumor activity (PR or SD≥6 months), two had EGFR wild-type (of the eight total with EGFR wild-type); both had squamous histology (of a total of four with this histology) and achieved SD for 13.7+ months and a PR for 7.4 months. The third patient had an EGFR TKI-resistant mutation in exon 20 (D770>GY insertion; of a total of two with EGFR TKI-resistant mutation). Contrary to the fact that insertions beyond the C-helix (beyond Tyr 764) of the EGFR kinase domain do not respond to usual doses of erlotinib or gefitinib (26, 27), this patient achieved a PR for 24.2+ months. Two other patients had an EGFR TKI-sensitive mutation (L858R) in exon 21 and demonstrated SD for 7.7+ and 6.3+ months (the former had failed prior erlotinib after initial response and the latter had not received prior EGFR therapy). Three of five patients with PR/SD≥6 months had adenocarcinoma and two patients had squamous cell carcinoma.
There are two prior clinical studies evaluating a combination of EGFR inhibitors in NSCLC(17, 18). Significant response was not noted in patients with acquired resistance to erlotinib. Though 11 of 13 patients had SD (median PFS=3 months), including patients with T790M mutation, prolonged stabilization of disease was not reported (18). In another study, stable disease was observed in 4 of 13 NSCLC patients with wild-type EGFR disease (17); no PRs were seen. The difference in efficacy observed between these studies and our study is not entirely clear, but it seems possibly due to the small number of patients enrolled on each study.
Interestingly, we observed responses in two of four patients (50%) with EGFR wild-type, squamous cell histology. Patients with squamous cell carcinoma of the lung have EGFR wild-type disease (28) and are therefore not generally treated with EGFR inhibitors. Currently treatment options are limited for patients with squamous cell carcinoma of the lung. In a prior study of 121 patients with squamous cell carcinoma of the lung treated with single-agent erlotinib (29), partial responses were seen in only about 7.5% of the 69 evaluable patients. In another study (30), 79 patients with advanced squamous cell carcinoma of the lung were treated with EGFR TKIs. Though the median progression-free survival (PFS) or OS was not statistically different between patients treated with erlotinib or gefitinib, EGFR mutation-positive patients had significantly improved disease control rate, and prolonged median PFS and OS than patients with EGFR wild-type disease. A Phase III study (FLEX) (31) evaluating the survival benefit in advanced EGFR expressing NSCLC patients treated with cetuximab plus chemotherapy versus chemotherapy alone, included a significant number of patients with squamous cell histology (n=377; 34% of patients on study). A survival benefit of 10.2 versus 8.9 months (median survival) was seen with the addition of cetuximab in this subset of patients. However, no molecular profiling was performed, and response rates were not correlated with histology. On the other hand, Fiala et al (32) have concluded that the molecular profile of the tumor may not be predictive of the efficacy of the TKIs in patients with squamous cell carcinoma versus patients with adenocarcinoma. The median PFS and OS were not significantly different in 16 of the 179 patients with EGFR-mutant squamous cell NSCLC treated with EGFR TKI's versus 163 patients with wild-type disease. At present, response to EGFR inhibition is unclear in this subset of NSCLC patients.
Importantly, our results suggest that dual EGFR therapy may help to overcome some cases of primary EGFR TKI resistance. Indeed, one patient (case #2, Table 3) with a known EGFR TKI-resistant mutation (insertion in exon 20, D770>GY), who had not received prior EGFR therapy, has an ongoing PR at 24.2+ months (Figure 2). There is a lack of understanding of the molecular mechanisms that underlie the resistance patterns of these mutations (33). It has been reported that EGFR, through its kinase-independent activity is able to maintain basal intracellular glucose levels that enhances the survival capacity of tumor cells even in the presence of EGFR TKI's (25). It is therefore conceivable that the effect of an antibody such as cetuximab may help to overcome this pathway of resistance. In preclinical models of EGFR TKI-resistant tumors (exon 20 insertions), exposure to dual EGFR inhibitors resulted in much more substantial levels of apoptosis than that seen with single types of EGFR inhibitors (15, 16, 34), suggesting synergy. This may possibly explain the response seen in some of our patients such as those with primary resistance to EGFR TKI's. We also observed a response in a patient (case #17, Table 2; EGFR TKI-sensitive mutation (L858R) in codon 21) who had progressed on prior erlotinib (35). This patient now has SD for 7.7+ months (prior TTF = 6.1 months). Whether synergy with cetuximab or retreatment with erlotinib led to response is unclear (36, 37), but the fact that the TTF on the combination is longer than the prior TTF on single-agent erlotinib suggests that the cetuximab plays a role in the activity observed.
There are several clinical studies that are underway targeting other pathways of EGFR resistance including HER2/ERBB2 amplifications or mutations, MET amplifications, and, notch dysregulation in NSCLC patients (38, 39). Encouraging clinical results have also been reported with use of irreversible EGFR tyrosine kinases in NSCLC patients. Recently, Janjigian et al had reported of confirmed objective response in 40% of the 60 evaluable EGFR-mutant NSCLC patients with acquired resistance to erlotinib or gefitinib (including patients with T790M mutation) when treated on a combination with cetuximab and afatinib(40).
This study is not without limitations. The sample size is small (20 patients) and more so when we consider each specific subtype. Additionally, patients were treated at two different dose levels. Furthermore, it is unclear if the antitumor activity (SD for 7.7+ months) seen in a patient who had progressed on prior treatment with erlotinib (case #17, Table 3) is due to the re-treatment effect that occurs with reintroduction of an EGFR TKI after a drug holiday (41).
In conclusion, this study demonstrated that treatment with erlotinib plus cetuximab is feasible in NSCLC patients. It is a safe combination with the main toxicity being rash. While not conclusive due to the small sample size in this study, it is noteworthy that SD≥6 months/PR was observed in two of three patients (66%) with EGFR wild-type squamous cell carcinoma; one patient with an EGFR TKI-resistant mutation; and, two of eight patients with EGFR TKI-sensitive mutations including one patient who had progressed on prior erlotinib therapy after initial response. The combination of erlotinib plus cetuximab, either alone or with chemotherapy, warrants further exploration in select populations of NSCLC.
Acknowledgments
The authors thank Saady Kohanim in the Department of Investigational Cancer Therapeutics at MD Anderson Cancer Center for his role in data collection and help in preparing our manuscript.
Disclosure: R. Kurzrock received honoraria and research funding from Genetech.
Footnotes
The remaining authors declare no conflicts of interest.
Presented in part at the 2011 ASCO Annual Meeting, Chicago, Illinois
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 3.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–80. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riely GJ, Pao W, Pham D, Li AR, Rizvi N, Venkatraman ES, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:839–44. doi: 10.1158/1078-0432.CCR-05-1846. [DOI] [PubMed] [Google Scholar]
- 5.Mitsudomi T, Kosaka T, Endoh H, Horio Y, Hida T, Mori S, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005;23:2513–20. doi: 10.1200/JCO.2005.00.992. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto I, Araki J, Suto R, Shimada M, Nakagawa K, Fukuoka M. EGFR mutation in gefitinib-responsive small-cell lung cancer. Ann Oncol. 2006;17:1028–9. doi: 10.1093/annonc/mdj114. [DOI] [PubMed] [Google Scholar]
- 7.Jackman DM, Yeap BY, Sequist LV, Lindeman N, Holmes AJ, Joshi VA, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:3908–14. doi: 10.1158/1078-0432.CCR-06-0462. [DOI] [PubMed] [Google Scholar]
- 8.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787–99. doi: 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J. Why the epidermal growth factor receptor? The rationale for cancer therapy. Oncologist. 2002;7(Suppl 4):2–8. doi: 10.1634/theoncologist.7-suppl_4-2. [DOI] [PubMed] [Google Scholar]
- 10.Hammerman PS, Janne PA, Johnson BE. Resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2009;15:7502–9. doi: 10.1158/1078-0432.CCR-09-0189. [DOI] [PubMed] [Google Scholar]
- 11.Mumenthaler SM, Foo J, Leder K, Choi NC, Agus DB, Pao W, et al. Evolutionary modeling of combination treatment strategies to overcome resistance to tyrosine kinase inhibitors in non-small cell lung cancer. Mol Pharm. 2011;8:2069–79. doi: 10.1021/mp200270v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25:587–95. doi: 10.1200/JCO.2006.07.3585. [DOI] [PubMed] [Google Scholar]
- 13.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. Plos Medicine. 2005;2:225–35. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 15.Huang S, Armstrong EA, Benavente S, Chinnaiyan P, Harari PM. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res. 2004;64:5355–62. doi: 10.1158/0008-5472.CAN-04-0562. [DOI] [PubMed] [Google Scholar]
- 16.Matar P, Rojo F, Cassia R, Moreno-Bueno G, Di Cosimo S, Tabernero J, et al. Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): superiority over single-agent receptor targeting. Clin Cancer Res. 2004;10:6487–501. doi: 10.1158/1078-0432.CCR-04-0870. [DOI] [PubMed] [Google Scholar]
- 17.Ramalingam S, Forster J, Naret C, Evans T, Sulecki M, Lu H, et al. Dual inhibition of the epidermal growth factor receptor with cetuximab, an IgG1 monoclonal antibody, and gefitinib, a tyrosine kinase inhibitor, in patients with refractory non-small cell lung cancer (NSCLC): a phase I study. J Thorac Oncol. 2008;3:258–64. doi: 10.1097/JTO.0b013e3181653d1b. [DOI] [PubMed] [Google Scholar]
- 18.Janjigian YY, Azzoli CG, Krug LM, Pereira LK, Rizvi NA, Pietanza MC, et al. Phase I/II trial of cetuximab and erlotinib in patients with lung adenocarcinoma and acquired resistance to erlotinib. Clin Cancer Res. 2011;17:2521–7. doi: 10.1158/1078-0432.CCR-10-2662. [DOI] [PubMed] [Google Scholar]
- 19.Fok J, Kurzrock R, Tsimberidou AM, Wen S, Naing A, Hong DS, et al. An umbrella protocol for histology-independent, phase I modular study based on EGFR mutation status: Using erlotinib alone or in combination with cetuximab, bortezomib, or dasatinib to overcome resistance. J Clin Oncol. 2011;29(suppl; abstr 2536) 2011. [Google Scholar]
- 20.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 21.NationalCancerInstitute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE) 2006 3/1/2012]; Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 22.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–12. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 24.Sakr RA, Barbashina V, Morrogh M, Chandarlapaty S, Andrade VP, Arroyo CD, et al. Protocol for PTEN expression by immunohistochemistry in formalin-fixed paraffin-embedded human breast carcinoma. Appl Immunohistochem Mol Morphol. 2010;18:371–4. doi: 10.1097/PAI.0b013e3181d50bd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weihua Z, Tsan R, Huang WC, Wu Q, Chiu CH, Fidler IJ, et al. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell. 2008;13:385–93. doi: 10.1016/j.ccr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa DB, Yasuda H, Sng NJ, Yeo WL, de Figueiredo Pontes LL, Tenen DG, et al. Sensitivity to EGFR inhibitors based on location of EGFR exon 20 insertion mutations within the tyrosine kinase domain of EGFR. J Clin Oncol. 2012;30(suppl; abstr 7523) 2012. [Google Scholar]
- 27.Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol. 2012;13:e23–31. doi: 10.1016/S1470-2045(11)70129-2. [DOI] [PubMed] [Google Scholar]
- 28.Rekhtman N, Paik PK, Arcila ME, Tafe LJ, Oxnard GR, Moreira AL, et al. Clarifying the spectrum of driver oncogene mutations in biomarker-verified squamous carcinoma of lung: lack of EGFR/KRAS and presence of PIK3CA/AKT1 mutations. Clin Cancer Res. 2012;18:1167–76. doi: 10.1158/1078-0432.CCR-11-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurpide A, Massuti B, Pallares C, Salinas P, Montes A, Lopez-Vivanco G, et al. Erlotinib in patients with advanced squamous cell carcinoma of the lung. J Clin Oncol. 2006;24:407s-s. [Google Scholar]
- 30.Duan JC, An TT, Wu MN, Yang L, Bai H, Wang ZJ, et al. Correlation between the efficacy of epidermal growth factor receptor tyrosine kinase inhibitors and EGFR mutations in advanced squamous cell lung cancer. Zhonghua Jie He He Hu Xi Za Zhi. 2012;35:323–8. [PubMed] [Google Scholar]
- 31.Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525–31. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 32.Fiala O, Pesek M, Finek J, Benesova L, Bortlicek Z, Minarik M. Gene mutations in squamous cell NSCLC: insignificance of EGFR, KRAS and PIK3CA mutations in prediction of EGFR-TKI treatment efficacy. Anticancer Res. 2013;33:1705–11. [PubMed] [Google Scholar]
- 33.Wu JY, Wu SG, Yang CH, Gow CH, Chang YL, Yu CJ, et al. Lung cancer with epidermal growth factor receptor exon 20 mutations is associated with poor gefitinib treatment response. Clin Cancer Res. 2008;14:4877–82. doi: 10.1158/1078-0432.CCR-07-5123. [DOI] [PubMed] [Google Scholar]
- 34.Cho J, Chen L, Sangji N, Du J, Okabe T, Yonesaka K, et al. Cetuximab resistance associated with dimerization-independence of oncogenic EGFR mutants. Mol Cancer Ther. 2009;8(12 Suppl):A177. 2009. [Google Scholar]
- 35.Bamford S, Dawson E, Forbes S, Clements J, Pettett R, Dogan A, et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br J Cancer. 2004;91:355–8. doi: 10.1038/sj.bjc.6601894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Becker A, Crombag L, Heideman DA, Thunnissen FB, van Wijk AW, Postmus PE, et al. Retreatment with erlotinib: Regain of TKI sensitivity following a drug holiday for patients with NSCLC who initially responded to EGFR-TKI treatment. Eur J Cancer. 2011;47:2603–6. doi: 10.1016/j.ejca.2011.06.046. [DOI] [PubMed] [Google Scholar]
- 37.Naing A, Kurzrock R. Dodging a dogma: is treating beyond progression beneficial? Cancer Chemotherapy and Pharmacology. 2013;71:1385–6. doi: 10.1007/s00280-013-2123-z. [DOI] [PubMed] [Google Scholar]
- 38.Janku F, Garrido-Laguna I, Petruzelka LB, Stewart DJ, Kurzrock R. Novel therapeutic targets in non-small cell lung cancer. J Thorac Oncol. 2011;6:1601–12. doi: 10.1097/JTO.0b013e31822944b3. [DOI] [PubMed] [Google Scholar]
- 39.Maraver A, Fernandez-Marcos PJ, Herranz D, Canamero M, Munoz-Martin M, Gomez-Lopez G, et al. Therapeutic effect of gamma-secretase inhibition in KRAS G12V-driven non-small cell lung carcinoma by derepression of DUSP1 and inhibition of ERK. Cancer Cell. 2012;22:222–34. doi: 10.1016/j.ccr.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janjigian YY, Smit EF, Horn L, Groen HJM, Camidge DR, Gettinger S, et al. Activity of afatinib/cetuximab in patients (Pts) with EGFR-mutant non-small cell lung cancer (NSCLC) and acquired resistance (AR) to EGFR inhibitors. Annals of Oncology. 2012;23:401. [Google Scholar]
- 41.Jackman D, Pao W, Riely GJ, Engelman JA, Kris MG, Janne PA, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2010;28:357–60. doi: 10.1200/JCO.2009.24.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]


