Abstract
Adenine phosphoribosyltransferase (APRT) deficiency, cystinuria, Dent disease, familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC) and primary hyperoxaluria (PH) are rare but important causes of severe kidney stone disease and/or chronic kidney disease in children. Recurrent kidney stone disease and nephrocalcinosis, particularly in pre-pubertal children, should alert the physician to the possibility of an inborn error of metabolism as the underlying cause. Unfortunately, the lack of recognition and knowledge of the five disorders has frequently resulted in an unacceptable delay in diagnosis and treatment, sometimes with grave consequences. A high index of suspicion coupled with early diagnosis may reduce or even prevent the serious long-term complications of these diseases. In this paper, we review the epidemiology, clinical features, diagnosis, treatment and outcome of patients with APRT deficiency, cystinuria, Dent disease, FHHNC and PH with emphasis on childhood manifestations.
Keywords: Nephrolithiasis; nephrocalcinosis; kidney failure; crystalline nephropathy; hereditary disorders; adenine phosphoribosyltransferase deficiency; 2,8-dihydroxyadeninuria; cystinuria; Dent disease; familial hypomagnesemia with hypercalciuria and nephrocalcinosis; primary hyperoxaluria
Introduction
Recent epidemiological studies have suggested an increased frequency of kidney stone disease in all age groups during the last decades [1, 2]. Although this increase is mostly driven by more frequent diagnosis of idiopathic calcium kidney stone disease, inherited metabolic disorders such as adenine phosphoribosyltransferase (APRT) deficiency, cystinuria, Dent disease, familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC), and primary hyperoxaluria (PH), should always be considered in the differential diagnosis of pediatric stone disease. All of the above hereditary disorders cause urinary hyperexcretion of insoluble mineral salts which can lead to recurrent kidney stones or nephrocalcinosis. All of these disorders except cystinuria frequently cause chronic kidney disease (CKD) and can progress to end-stage renal disease (ESRD) [3]. Unfortunately, the lack of recognition and knowledge of these diseases has frequently resulted in unacceptable delay in diagnosis and treatment, sometimes with grave consequences.
Patients with each of the five aforementioned disorders often have their initial kidney stone episode in the first decade of life. Recurrent stones, particularly in pre-pubertal children, should alert the physician to the possibility of an inborn error of metabolism as the underlying cause. Nephrocalcinosis, with or without a history of stone disease, is a common feature of Dent disease, FHHNC and PH, but not APRT deficiency or cystinuria. Children with APRT deficiency [4], FHHNC [5] and PH [6] may develop crystalline nephropathy and CKD, while patients with Dent disease and cystinuria usually do not suffer renal impairment until adulthood [7]. All children with reduced renal function and/or stone disease should undergo a thorough metabolic evaluation which includes screening for APRT deficiency, cystinuria, Dent disease, FHHNC and PH .
Kidney stones are common in the general population, affecting approximately 1 in 10 individuals in their lifetimes. However, it is important to remember that stone disease is different in children than in adults. In particular, it has been suggested that a metabolic workup is not necessary in all adults after a single stone event. However, all children that present with stones deserve a complete evaluation since these rare forms of stone disease are associated with a distinct risk of renal failure. A key part of the pediatric kidney stone workup is the stone analysis. Cystine and 2,8-dihydroxyadenine (DHA) will be easily recognized by infrared spectroscopy. Stone composition is not definitive for PH (calcium oxalate) and Dent disease (calcium oxalate or calcium phosphate), but still narrows the differential diagnosis. Urine chemistries are the next important test. Twenty-four hour collections are most helpful, though random urine specimens can be employed in younger children. Interpretation of random values is difficult, especially for children under 1 year of age, and often several collections will be needed to confirm a normal or abnormal result. Hypomagnesemia and hypercalciuria in patients with kidney stones or nephrocalcinosis is strongly suggestive of FHHNC. In the absence of fat malabsorption and enteric hyperoxaluria, high urine oxalate levels (> 0.7 mmol/1.73 m2 per 24 hours) are highly suggestive of PH and should lead to further testing. Cystinuria should be excluded with a qualitative (nitroprusside test) or quantitative urinary cystine study in a patient with radiopaque stones in whom stone analysis has not been performed, particularly if other urine chemistries are unremarkable. Round, brown DHA crystals (Figure 1A) detected by microscopic examination of the urine are pathognomonic of dihydroxyadeninuria and the typical hexagonal cystine crystals (Figure 1C) are diagnostic of cystinuria. Patients with PH often have calcium oxalate crystals (Figure 1D), while calcium oxalate or calcium phosphate crystals (Figure 1E) may be detected in patients with Dent diease. Finally, all boys with unexplained stones, nephrocalcinosis, and or proteinuria should have a quantitative test for low molecular weight (LMW) proteinuria to exclude the possibility of Dent disease. A high index of suspicion coupled with early diagnosis and treatment may reduce or even prevent the serious long-term complications of these well defined hereditary causes of severe stone disease and/or CKD.
Figure 1.
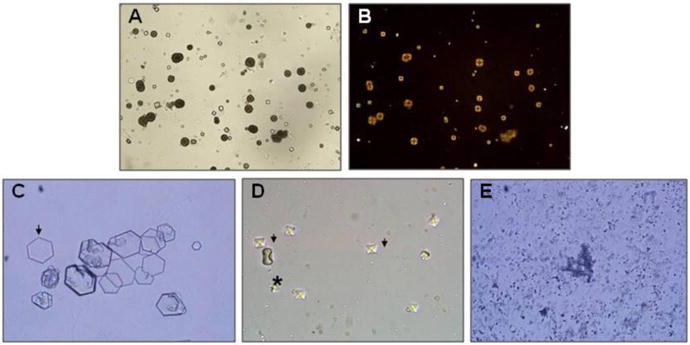
Urinary crystals.
A. Typical small and medium sized 2,8-dihydroxyadenine crystals. The medium sized cystals are brown with dark outline and central spicules. (Original magnification × 400).
B. The same field viewed with polarized light microscopy. The small and medium sized crystals appear yellow in colour and produce a central Maltese cross pattern. (Original magnification × 400).
C. Urinary cystine crystals. The typical 6-sided crystal is diagnostic of cystinuria. A good example can be seen on the left side of the Figure (arrow).
D. Urinary calcium oxalate crystals. The typical bipyramidal calcium oxalate dihydrate crystals (arrows) and a large dumbbell calcium oxalate monohydrate crystal (asterisk) are both seen.
E. Amorphous calcium phosphate crystals. Although their appearance is not as distinctive, amorphous calcium phosphate crystals should be suspected if the urine is alkaline (pH > 6.0).
In this paper, we review the epidemiology and genetics, clinical features, diagnosis, treatment and outcome of APRT deficiency, cystinuria, Dent disease, FHHNC and PH. Emphasis will be placed on childhood manifestations and features suggestive of these rare causes of kidney stone disease, nephrocalcinosis and CKD (Table 1). Diagnostic algorithms will be presented. Other rare hereditary causes of kidney stones or nephrocalcinosis, including xanthinuria and hypoxanthine-guanine phosphoribosyltransferase deficiency (Lech-Nyhan syndrome), will not be discussed in this review.
Table 1. When to suspect rare causes of kidney stones.
| History |
|
| Laboratory testing | |
| Imaging |
|
Suggestive of adenine phosphoribosyltransferase deficiency.
Diagnostic of cystinuria.
Low molecular weight proteinuria is suggestive of Dent disease.
Highly suggestive of familial hypomagnesemia with hypercalciuria and nephrocalcinosis.
May suggest primary hyperoxaluria.
Suggestive of cystinuria in children 2 years and older.
Abbreviation: GFR, glomerular filtration rate.
Adenine phosphoribosyltransferase deficiency and 2,8-dihydroxyadeninuria
Background
APRT deficiency (OMIM 102600) is a rare autosomal recessive inborn error of adenine metabolism resulting in the generation of large amounts of poorly soluble DHA, which leads to stone formation in the urinary tract and crystalline nephropathy [8, 9]. APRT deficiency was first described in 1968 by Kelley and coworkers [10] who reported partial deficiency of the enzyme in 4 asymptomatic subjects. The disorder has since been described in all ethnic groups, although the majority of reported cases have come from Japan, France and Iceland [8].
APRT is a cytoplasmic enzyme that catalyzes the synthesis of 5′-adenosine monophosphate from adenine and 5-phosphoribosyl-1-pyrophosphate (PRPP) leading to effective recycling of adenine [11]. Because other metabolic pathways for adenine are lacking in humans, the absence of functional APRT leads to the conversion of the 8-hydroxy intermediate metabolite by xanthine dehydrogenase (XDH) to DHA. A simplified overview of adenine metabolism is depicted in Figure 2.
Figure 2.
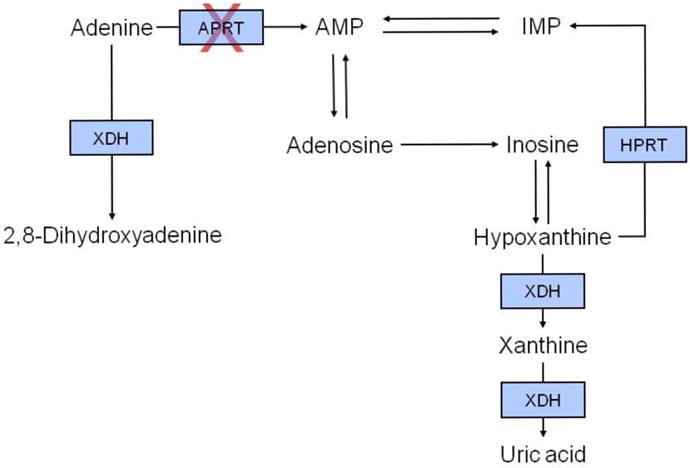
Schematic overview of adenine metabolism. In adenine phosphoribosyltransferase deficiency, adenine cannot be converted to adenosine monophsophate and is instead converted by xanthine dehydrogenase to 2,8-dihydroxyadenine. Abbreviations: APRT, adenine phosphoribosyltransferase; AMP, adenosine monophsophate; HPRT, hypoxanthine-guanine phosphoribosyltransferase; IMP, inosine monophosphate; XDH, xanthine dehydrogenase.
The human APRT gene, located on chromosome 16q24, is 2466 base pair long and contains five exons encoding a 180 amino acid protein [12]. More than 40 functionally significant human APRT mutations that completely abolish the function of the enzyme in vivo have been reported to date [13]. Most of these mutations are single base changes and small deletions. In the Japanese population a missense mutation in exon 5 p. Thr136Met, is most common, affecting approximately 70% of patients [14]. The most common mutations in other ethnic groups are a T insertion at the splice donor site of intron 4 (c.IVS4 + 2insT) [9] and a missense mutation in exon 3 p. Asp65Val, which interestingly accounts for all cases of APRT deficiency in Iceland [8].
There is suggestive evidence that APRT deficiency may be a seriously under recognized cause of kidney stones and CKD that progresses to ESRD in a significant proportion of untreated patients [15]. However, early recognition of the disease and prompt institution of effective pharmacologic therapy effectively prevents the development of kidney failure [8, 9].
The estimated heterozygote frequency in different populations ranges from 0.4 to 1.2% [16, 17], suggesting that the prevalence of a homozygous state is at least 1:50,000-100,000. If this holds true, there should be at least 70-80,000 cases worldwide of which 40,000 would be expected to be in Asia, 9,000 in Europe, 8,000 in the Americas, including at least 3000 cases in the U.S. alone. The disease is currently unrecognized in the majority of these patients who, therefore, are not receiving the benefits of medical therapy. Lack of familiarity with dihydroxyadeninuria among clinicians and pathologists is a major concern and undoubtedly contributes to the low number of reported cases worldwide. Other plausible explanations include inadequate evaluation of patients with kidney stones and confusion of DHA calculi with uric acid and/or xanthine stones.
Clinical features
Radiolucent kidney stones are by far the most common clinical manifestation of APRT deficiency in the pediatric population [8, 18]. An initial presentation of acute kidney injury due to bilateral DHA calculi and urinary tract obstruction has also been well documented in children [4, 8, 19, 20]. Other common clinical features include recurrent urinary tract infections, hematuria and reddish-brown diaper stains [8, 9, 20]. However, many children remain asymptomatic throughout childhood and, not uncommonly, the disease is diagnosed by the detection of DHA crystals on routine urine microscopy or through screening of siblings of index cases [8, 18] Although CKD with reduced glomerular filtration rate has been reported in a number of children with APRT deficiency [18]. In contrast, ESRD secondary to crystalline nephropathy is a much more common presenting feature of APRT deficiency in adults [8, 9], which in a number of cases has not been recognized until after kidney transplantation [15, 21, 22]. This point is well demonstrated in a recent report from the Mayo Clinic of 3 adult patients with ESRD due to dihydroxyadeninuria [15]. While all these patients were found to have crystalline nephropathy, two did not have a past history of kidney stones and the third one had only experienced a single stone episode 36 years prior to diagnosis. Early DHA crystalline nephropathy in a child was reported by Greenwood and colleagues [4] who performed a kidney biopsy in a 4 year old girl with bilateral stone disease and acute kidney injury. Fifteen of 32 Icelandic patient with data in the APRT Deficiency Registry of the Rare Kidney Stone Consortium presented before the age of 18 years. The major presenting features included kidney stones in 6 patients, reddish-brown diaper stain in 5, renal colic in 1 and 3 were asymptomatic (unpublished observation).
The kidney and urinary tract appear to be the only organ system affected in APRT deficiency. However, we have encountered occasional adults with this disorder who have complained of eye discomfort, though it is unclear if this is related to DHA crystal deposition in ocular tissues.
Diagnosis
The diagnosis of APRT deficiency should be considered in all children presenting with renal colic, radiolucent kidney stones or acute kidney injury and in infants with a history of reddish-brown diaper stain [8, 18]. It is particularly important to consider APRT deficiency in children with radiolucent stones because uric acid stones are uncommon in this age group. A high urine pH in patients who present with radiolucent stones provides an additional clue, because uric acid stones develop in acidic urine. In addition, APRT deficiency should always be included in the differential diagnosis of childhood acute kidney injury, especially when associated with radiolucent kidney stones. The diagnosis of kidney stones requires imaging techniques that are capable of detecting radiolucent stones, such as ultrasound or computed tomography. A diagnostic algorithm for APRT deficiency is presented in Figure 3.
Figure 3.
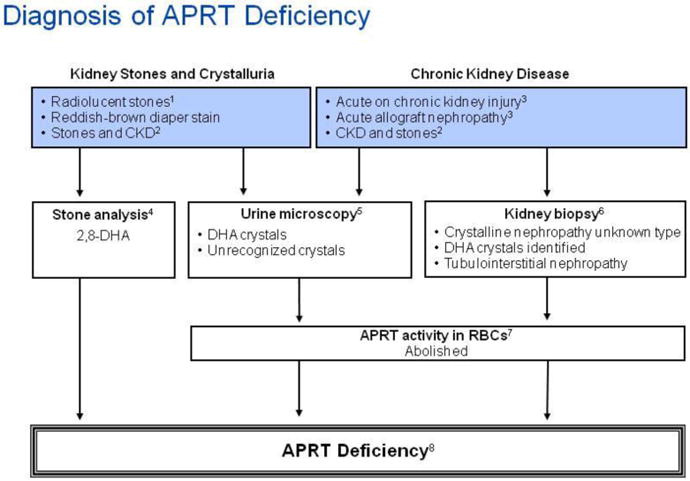
Algorithm for diagnostic evaluation of adenine phosphoribosyltransferase (APRT) deficiency and 2,8-dihydroxyadeninuria.
Annotations to Figure 3
12,8-dihydroxyadeninuria, hyperuricosuria and xanthinuria should always be considered in the differential diagnosis of radiolucent kidney stones in childhood.
2 Children with radiolucent kidney stones and chronic kidney disease (CKD) should be evaluated for adenine phosphoribosyltransferase (APRT) deficiency.
3,6Patients with APRT deficiency may present with acute kidney injury (AKI), CKD or acute allograft nephropathy, even in the absence of previous history of kidney stones. Kidney biopsy shows variable degree of tubulointerstitial injury and features consistent with crystalline nephropathy.
4Ultraviolet spectrophotometry and/or x-ray crystallography easily differentiates 2,8-dihydroxyadenine (DHA) from uric acid and xanthine.
5The pathognomonic round, brown urinary DHA crystals (Figure 1) are seen in almost all patients with the disorder. The crystals may, however, be hard to identify in patients with markedly decreased renal function due to reduced crystal clearance.
7APRT activity in red blood cell lysates ranges from 16-32 nmol/h/mg hemoglobin in healthy subjects [118]; homozygotes and compound heterozygotes have no measurable enzyme activity and in heterozygotes the activity is approximately 25%. Recent blood transfusion may falsely elevate APRT activity.
8 Genetic testing, which confirms the diagnosis when functionally significant mutations are found in both copies of the APRT gene, is not clinically indicated in patients with abolished APRT activity.
The pathognomonic round and brown DHA crystals can usually be detected by urine microscopy in nonoliguric patients (Figure 1A). The crystals may, however, be difficult to identify in urine samples from patients with advanced CKD, possibly due to reduced renal crystal clearance [8, 9]. Another clue for the identification of DHA crystals is a central Maltese cross pattern that can be observed when small or medium sized crystals are viewed by polarized light microscopy (Figure 1B). Furthermore, analysis of DHA crystals and stone material using infrared and ultraviolet spectrophotometry and/or x-ray crystallography easily differentiates DHA from uric acid and xanthine. In contrast, biochemical analysis of stones does not distinguish DHA from uric acid and is no longer recommended.
Absence of APRT activity in red cell lysates is diagnostic of APRT deficiency. Enzyme activity measurements are also needed to determine the functional significance of novel mutations. Identification of functionally significant mutations in both copies of the APRT gene confirms the diagnosis. Renal histological examination will reveal DHA crystalline nephropathy in patients with APRT deficiency and CKD or acute allograft dysfunction, even in the absence of a kidney stone history [15]. Greenwood and coworkers [4] described intratubular, intracellular and interstitial DHA crystal deposition and widespread tubular atrophy in a kidney biopsy specimen from a 4-year-old female with bilateral stone disease and acute kidney injury. It is important, however, not to confuse the histopathologic manifestations of DHA nephropathy with features of other types of crystalline nephropathy, particularly oxalate- and uric acid-induced kidney damage [15]. Once suspected, the diagnosis of APRT deficiency can readily be made using the strategies outlined above. The alertness of practicing pediatricians is of paramount importance when children present with clinical manifestations of the disorder, to facilitate timely therapeutic intervention.
Treatment and outcome
Treatment with the XDH inhibitor allopurinol is effective and generally well-tolerated in patients with APRT deficiency. Allopurinol 5-10 mg/kg/day, taken either as a single dose or divided into two doses, prevents stone formation, renal crystal deposition and the development of kidney failure [8, 9]. Moreover, treatment with allopurinol can even result in a resolution of kidney stones and improvement of kidney function in patients with advanced renal failure [8, 9, 18]. The recently introduced XDH inhibitor febuxostat provides an alternative treatment option for patients allergic to or intolerant of allopurinol [23]. No data have been published on the use of febuxostat in patients with dihydroxyadeninuria. However, we have achieved a significant reduction in DHA crystalluria in one of our adult patients using a daily febuxostat dose of 80 mg (unpublished observation). Low purine diet and ample fluid intake provide adjunctive benefits to pharmacologic therapy.
Cystinuria
Background
Though it meets the definition of a rare disease, cystinuria (OMIM 220100) is the most common cause of inherited kidney stones addressed in this article. It is said to account for about 1% of all kidney stones and up to 25% of pediatric stones [24]. The worldwide prevalence of cystinuria is estimated at 1:7,000, ranging from 1:2,500 among a population of Libyan Jews to 1:100,000 persons in Sweden. In the United States, one estimate is that cystinuria occurs in approximately 1:15,000 adults. However, the true prevalence may be higher as some affected individuals never make a stone or make them infrequently and never have the diagnosis made.
Cystinuria is the result of a defect in proximal tubular reabsorption of filtered cystine, a homodimer of the amino acid cysteine [25]. Normally, 100% of filtered cystine is reabsorbed so that normal urine cystine excretion is 0-100 μmoles of cystine per gram of creatinine. The disorder is the result of autosomal recessive inheritance of a genetic defect in one of two genes whose protein products are required for tubular cystine reabsorption [26]. The transport protein is encoded by SLC7A9 and is called b0,+AT (amino acid transporter of positively charged or neutral amino acids). It forms a heterodimer in the apical membrane of proximal tubular epithelial cells with the protein product of SLC3A1, called rBAT (related to b0,+AT amino acid transporter). The rBAT protein is required to correctly direct b0,+AT to the apical membrane. Mutation of both alleles of SLC3A1 (type A genotype) or SLC7A9 (type B genotype) will lead to failure to reabsorb cystine filtered at the glomerulus and excretion of abnormal amounts of the relatively insoluble cystine. The result is formation of recurrent renal stones. Defective cystine transport physiology is also associated with concomitant loss of the dibasic amino acids ornithine, arginine, and lysine, but their loss is not clinically significant. People heterozygous for SLC3A1 mutations will generally not have cystine in the urine, while heterozygotes for abnormal SLC7A9 sequences will have abnormal amounts that are very rarely sufficient to cause stones, perhaps if other factors such as low urine volume or low urine pH are also present. SLC7A9 mutations can therefore be considered as incompletely dominant mutations. The same cystine transporter is expressed in the small intestine, responsible for absorption of cystine from the intestinal lumen, and is defective in cystinuria. However, cystine can also be absorbed as a component of oligopeptides and, therefore, the intestinal defect does not lead to any known clinical consequence.
Clinical features
Recurrent urinary tract stone disease is the only clinical manifestation of cystinuria in childhood. The hypotonia-cystinuria syndrome (OMIM 606407) arises when mutations occur both in SLC3A1 and the contiguous gene PREPL (prolyl endopeptidase-like protein). Besides cystinuria, affected individuals have severe hypotonia at birth, delayed growth and hyperphagia and excessive weight gain later in childhood [27]. Cystinuria can also result in CKD due to recurrent stones, obstructive uropathy and repeated urologic interventions. Most patients with cystinuria present in childhood with stone formation. Average age of detection of a first renal stone is about 12-13 years [28], with 50% forming a first stone in the first decade of life and another 25% in teenage years. Males and females have a similar age of onset but more male patients than female patients present in the first 3 years of life and males tend to have new stones more frequently than females. On the other hand, some patients surprisingly have their first stone between 40 and 80 years of age [29]. Type A and B genotypes apparently have similar phenotypes with similar rates of stone recurrence. A significant proportion of adults with cystinuria develop CKD and the renal pathologic findings correlate with the glomerular filtration rate reduction. One study demonstrated that 52 adult patients with cystinuria had a creatinine clearance of 63.2 ml/min compared with 3215 patients without cystinuria whose creatinine clearance was 111.1 ml/min [30]. However, more open surgical stone removing procedures and nephrectomy are variables correlated with an increased serum creatinine, with 14.1% of cystinuric patients compared to 2.9% of calcium oxalate stone formers in one cohort having undergone nephrectomy [31].
Diagnosis
Diagnosis of cystinuria is outlined in the diagnostic algorithm shown in Figure 4. Demonstration of hexagonal crystals on urinalysis is pathognomonic of cystinuria (Figure 1C). Some clinical laboratories screen all 24 hour urine collections from new clients with the sensitive and specific nitroprusside test [32]. Demonstrating increased urine content of lysine, ornithine and arginine is merely confirmatory and not necessary if cystine stones or crystals are identified. At present, genotyping patients with cystinuria is not required for diagnostic purposes and yields no clinically useful information.
Figure 4.
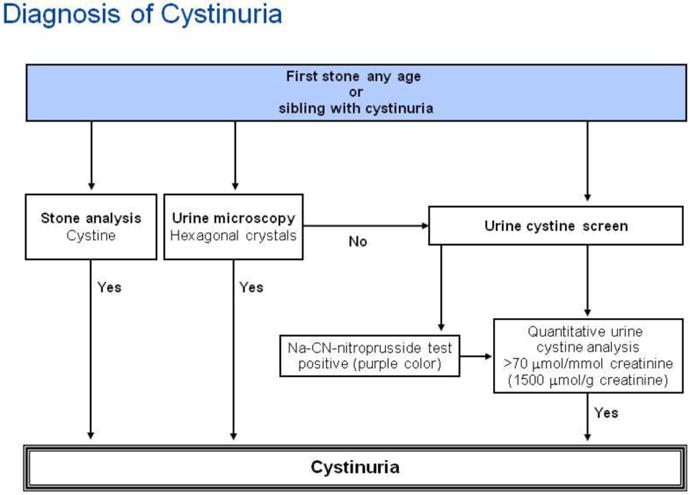
Algorithm for diagnostic evaluation of cystinuria.
Annotations to Figure 4
Demonstration of aminoaciduria with excessive levels of ornithine, arginine or lysine is not required for diagnosis.
All patients with stones prior to age 30 years should be screened. Sodium-cyanide-nitroprusside test can yield false positive result in heterozygotes below the age of 2 years. A positive sodium-cyanide-nitroprusside test should be confirmed with 24 hour urine collection. Homozygotes have more than 170 μmol cystine/mmol creatinine (1500 μmol cystine/g creatinine). Heterozygotes (with SLC7A9 mutations) will have between 11 and 110 μmol cystine/mmol creatinine (100-1000 μmol cystine/g creatinine), while other heterozygotes (with SLC3A1 mutations) have normal cystine excretion (≤11 μmol cystine/mmol creatinine). Adult homozygotes excrete more than 1.3 mmol cystine per day (300 mg/day); heterozygotes (with SLC7A9 mutations) will excrete more than 0.13 mmol/day (30 mg/day) while other heterozygotes (with SLC3A1 mutations) have normal cystine excretion.
Although data regarding the renal histopathology of cystinuria are relatively sparse, affected patients may develop crystalline nephropathy. One paper stands out in this regard, presenting the results of renal papillary biopsy of 7 patients who underwent percutaneous nephrolithotomy [33]. The ducts of Bellini and inner medullary collecting ducts (IMCD) were frequently plugged with cystine crystals, had injured or absent lining cells with nearby inflammation or fibrosis of the interstitium. In addition, hydroxyapatite (calcium phosphate) crystals were noted in the lumens of loops of Henle and IMCD. Cortical glomerular obsolescence and interstitial fibrosis were also present.
A newborn screening program in Montreal demonstrated that heterozygous children have transiently increased levels of urine cystine at 1-2 months after birth which is significantly reduced by 4 years of age [34]. This maturational effect was present in heterozygotes whether of type A or B genotype, though it was more dramatic in the latter group. One conclusion is that screening urine for cystine can be delayed for 1-2 years in asymptomatic siblings of known cystinuric patients.
Treatment and outcome
Treatment focuses on reducing the supersaturation of urinary cystine to limit the potential of crystallization. The variables that can be addressed include cystine concentration, cystine amount and urine pH. Oral fluid intake of 1.5-4.5 L/day, as tolerated, should be prescribed to decrease urine cystine concentration to less than 250 mg/L (about 1 mmol/L), aiming for urine output of at least > 750 ml/24 hours in infants, >1000 ml/24 hours in young children up to 5 years of age, > 1500 ml/24 hours up to 10 years of age, > 2,000 ml/24 hours in older children and adolescents and > 3,000 ml/24 hours in older adolescents and adults may be needed. Gastric tube placement should be considered in younger children to facilitate fluid intake.
The amount of excreted cystine can be reduced by limiting animal protein intake and salt ingestion [35]. Modest animal protein restriction (1g/kg/day) can lead to reduced ingestion of methionine, a cystine precursor, and reduced cystine excretion [36]. Similarly, reduction of sodium intake leads to reduced cystine excretion, though the mechanism of this effect is not known. Neither of these dietary manipulations have, however, been shown to actually reduce stone formation in randomized controlled trials.
Cystine supersaturation is significantly reduced at higher urine pH. The goal of therapy is to alkalinize the urine to pH of 7.5 with potassium citrate. Sodium citrate should be avoided because of the effect of increased urine sodium on urine cystine excretion. Usual doses might be 0.5-1 mmol/kg/day given 2 or 3 times a day in children, with urine pH testing useful to document the effect. Round-the-clock alkalinization is desirable for most significantly affected individuals and can be monitored by measuring urine pH once a day at varying times. For teenagers or adults this might require 30 mmol or more 2 or 3 times a day. Alkalinization may occasionally cause calcium phosphate stones to form, particularly if urine calcium is elevated or urine volume is not increased.
When fluid intake and alkalinization fail to halt stone formation, the thiol drugs d-penicillamine and tiopronin are both efficacious at breaking the disulfide bridge of cystine and forming soluble drug-cysteine complexes [37]. Both drugs reduce stone formation but have significant rates of discontinuation due to a variety of side effects, including rash, allergy, hematologic and liver function abnormalities and rarely nephrotic syndrome due to membranous nephropathy.
Collection of urine for 24 hours can be useful in monitoring therapy. Measurement of urine pH, sodium and surrogates of protein ingestion such as urea can help monitor adherence to dietary therapy and judge the adequacy of citrate supplementation. Measurement of cystine is useful but can be artifactually lowered by precipitation of the amino acid after voiding; alkalinization of the collected urine ensures that cystine is in a soluble and measureable state [38]. Thiol therapy may also interfere with cystine measurement and can be overcome by use of a solid phase assay to directly measure urinary cystine capacity or supersaturation [35, 39]. Such an assay may eventually prove to be useful in prescribing thiol drugs and judging metabolic stone-forming activity.
Cystinuria has a better prognosis today in the era of effective medical therapy and modern endourology. Although no randomized controlled trials have been performed in cystinuria, some small, retrospective cohorts suggest that the outcome of prophylactic regimens can reduce stone recurrence [40]. Cystine stones are often less amenable to being fractured by shockwave lithotripsy, but can be effectively turned to dust by ureteroscopy and the holmium laser [41]. The result is the virtual elimination of open surgery and nephrectomy. The expectation then is that less significant reductions in glomerular filtration rate should be seen in the coming years, though the impact of recurrent stones, crystalline nephropathy and nephronal obstruction may persist.
Dent disease
Background
Dent Disease (OMIM 300009) is a rare renal tubular disorder, with about 250 affected families having been reported around the world to date. The term Dent disease was first introduced in 1990 [42] and identifies a group of X-linked renal disorders characterized by LMW proteinuria, and variable presence of hypercalciuria, nephrocalcinosis and/or nephrolithiasis. This triad of symptoms has been variably named X-linked recessive nephrolithiasis with renal failure (XRN; OMIM 310468), X-linked recessive hypophosphatemic rickets (XLRH; OMIM 300554) or idiopathic LMW proteinuria of Japanese children (JILMWP; OMIM 308990) [43, 44]. The disease usually presents in childhood or early adult life with males much more severely affected than females [42, 45]. Progression to CKD occurs between the 3rd and 5th decades of life in 30-80% of affected males [42, 43]. It is hoped that early diagnosis can delay or prevent CKD. However, effective treatments have not yet been clearly established. LMW proteinuria is universal and hypercalciuria is common among affected Dent disease patients, but both conditions are rarely screened for. Therefore, many cases go undetected until more significant sequelae, such as progressive renal failure, develop.
Most diagnosed cases of Dent disease have been caused by mutations in the CLCN5 gene, located on the X chromosome (Xp11.22). This gene encodes a 746-amino-acid ClC-5 chloride channel that belongs to a voltage-gated chloride channel family [46-48]. In the human kidney, ClC-5 is primarily expressed in proximal tubular cells, alpha- and beta-intercalated cells of the cortical collecting duct, and in the thick ascending limb of Henle's loop [49]. In proximal tubular cells it is predominantly located in intracellular subapical endosomes, which are involved in the endocytic reabsorption of LMW proteins that have passed the glomerular filter. Specifically, ClC-5 has been proposed to function by providing a shunt conductance in early endosomes, thus permitting their efficient intraluminal acidification by a V-type H+-ATPase [49, 50]. Recently, however, two independent groups demonstrated that ClC-5 functions as a 2Cl−/H+ antiporter when activated by positive voltages [51, 52]. Interestingly, it appears that endosomal chloride concentration is at least as important as pH for normal endosomal function [53]. A small portion of ClC-5 channels are also located on the cell surface, where they are thought to mediate plasma membrane chloride currents [54], or as recently demonstrated, join macromolecular complexes at the plasma membrane believed to mediate LMW protein and albumin endocytosis [55].
ClC-5 knock-out mice demonstrate severe impairment of receptor-mediated endocytosis [56], including the endocytotic retrieval of the plasma membrane multiligand megalin and cubilin receptors [57, 58]. Like humans, knock-out mouse models have LMW proteinuria, but not all of them develop hypercalciuria and nephrocalcinosis [48, 59]. Indeed, the relationship between impaired endocytosis and renal calcium handling remains unclear. It has been suggested that the hypercalciuria seen in many affected patients stems from secondary changes in the regulation of calciotropic hormone caused by urinary loss of key hormone-binding proteins [59, 60].
To date, more than 100 different nonsense or missense mutations, insertions or deletions and splicing mutations in the CLCN5 gene have been reported [61, 62], meaning that the spectrum of CLCN5 mutations is highly varied and de novo mutations are frequent. Genotype-phenotype correlations have yet to be established. CLCN5 mutations are scattered throughout the gene's coding sequence and generate truncated or absent ClC-5 channels in approximately 70% of cases. In approximately 40% of patients with classic symptoms of Dent disease, no CLCN5 mutations were detected, suggesting locus heterogeneity [63, 64]. Subsequently, Hoopes and colleagues [64] demonstrated that OCRL1 mutations, initially associated with Lowe syndrome, account for 15% of patients with Dent disease, which is now termed Dent 2 disease (OMIM 300555). OCRL1 encodes a phosphatidylinositol 4,5-biphosphate 5-phosphatase localized at the Golgi apparatus. The mechanism by which loss of OCRL1 protein function leads to disease has not yet been elucidated. Very recently, however, the OCRL1 protein was localized to early endosomes and the trans-Golgi network (TGN), and clathrin coated transport intermediates [65, 66]. Depletion of OCRL1 perturbs trafficking at the TGN/endosome interface, suggesting a role in regulating transport between these compartments. Renal tubulopathy in Lowe syndrome is quite similar to that of Dent disease, and is mainly characterized by altered protein reabsorption. It is interesting to note, however, that OCRL1 mutations associated with Dent 2 disease do not overlap those causing Lowe syndrome [67, 68].
Clinical features
The presentation of patients with Dent disease appears quite variable. Most patients are recognized due to the presence of nephrocalcinosis or unexplained CKD. Hypophosphatemia and bone disease can also be observed, but appear to be a less prominent part of the phenotype than first believed. A universal feature is proteinuria, which can be detected in typical urinary total protein screens, including dipstick (Figure 5). A large proportion of this is LMW proteins which must be specifically screened for. More recently, patients have been recognized with nephrotic range proteinuria and glomerular changes of focal segmental glomerulosclerosis (FSGS) on renal biopsy [69, 70]. It is not clear what percentage of FSGS patients might have unrecognized Dent disease, or what role glomerular dysfunction might play in the loss of renal function in the majority of Dent patients. Other presenting features in children and adolescents often include nephrocalcinosis and hypercalciuria, with or without kidney stones, although the prevalence of stones versus nephrocalcinosis remains unclear. Hematuria may also be seen, presumably due to the stones and nephrocalcinosis. It must be stressed that LMW proteinuria is the pathognomonic feature of Dent disease, and can occur in isolation [69, 70]. The prevalence of other manifestations such as nephrocalcinosis or stones among those with CLCN5 and/or OCRL1 mutations is currently unknown.
Figure 5.
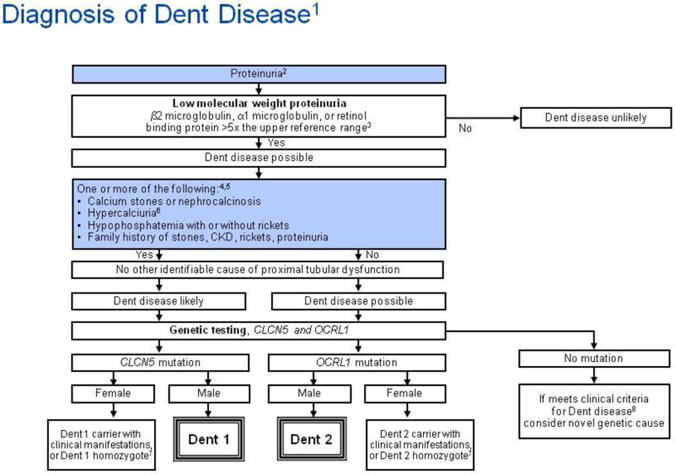
Algorithm for diagnostic evaluation of Dent disease.
Annotations to Figure 5
1The guideline does not address prenatal diagnosis.
2Trace or greater by dipstick; or >0.2 mg protein/mg creatinine; or >100 mg protein/day.
3Low molecular weight (LMW) proteinuria may be reduced in advanced renal insufficiency, or be present in renal failure from other causes, but remains helpful when markedly increased.
4In the presence of kidney failure, defined as a measured or estimated glomerular filtration rate (GFR) less than 15 ml/min/1.73 m2, calcium stones, nephrocalcinosis, hypercalciuria, hypophosphatemia with or without rickets, and family history of stones, CKD, rickets or proteinuria may be sufficient to consider Dent disease, even in the absence of documented LMW proteinuria, and genetic testing could be considered.
5Dent disease mutations have also been described in patients with histologic features of focal segmental glomerulosclerosis.
| Random urine calcium-to-creatinine ratio (Ca/Cr) by age [119] | ||
| Age (yr) | Ca/Cr ratio Upper limit of normal | |
| (mmol/mmol) | (mg/mg) | |
| 0-1 | 2.29 | 0.81 |
| 1-2 | 1.58 | 0.56 |
| 2-3 | 1.41 | 0.50 |
| 3-5 | 1.16 | 0.41 |
| 5-7 | 0.85 | 0.30 |
| 7-10 | 0.71 | 0.25 |
| 10-14 | 0.68 | 0.24 |
| 14-17 | 0.68 | 0.24 |
7Possibly homozygous for mutation or with inactivation of normal X-chromosome.
8Clinical criteria include low molecular weight (LMW) proteinuria plus one of the findings in Box A.
The initial patients described with Dent 2 disease caused by OCRL1 mutations exhibited none of the classic extrarenal symptoms of Lowe syndrome such as mental retardation, bone disease, growth retardation, congenital cataracts, or delayed motor milestones [64]. This milder phenotype could not be attributed to lesser protein expression or enzyme activity. Interestingly, patients with classic Lowe syndrome have a renal phenotype very similar to those with Dent disease, with some subtle differences. Renal tubular acidosis is one of the cardinal signs of Lowe syndrome, whereas in Dent disease it is rare. Features of Fanconi syndrome are also observed more frequently in patients with Lowe syndrome than in patients with Dent disease, whereas hypercalciuria, nephrocalcinosis and nephrolithiasis are common for Dent disease but rare in Lowe syndrome. However, more recently it has been suggested that a continuum exists since patients with OCRL1 mutations may present with clinical phenotypes ranging from a severe Lowe syndrome with the typical ocular, neurological and renal features to Dent 2 disease with only renal impairment [71]. To date, around 25 patients with Dent 2 disease have been reported.
Many affected males (30-80%) will develop ESRD between the ages of 30-50 years, although some may be delayed until later in life [42, 43]. Deterioration of renal function can be observed even in the absence of nephrocalcinosis [69, 70].
Diagnosis
Diagnostic features of Dent disease include LMW proteinuria, hypercalciuria, nephrolithiasis and/or nephrocalcinosis and renal insufficiency. Since proteinuria is often screened for, especially in patients with CKD, it is stressed as an entry point in the suggested diagnostic algorithm (Figure 5). The important clue for a diagnosis of Dent disease is markedly increased levels of LMW proteins in the urine (5-10 times above normal or greater), in the absence of other causes of proximal tubular dysfunction. Commonly screened LMW proteins are retinol-binding protein and α1 microglobulin. β2-microglobulin is also often measured to screen for LMW proteinuria but we do not recommend it since it is not very stable in even minimally acidic urine [72]. It has been reported that proximal tubular uptake of 99Tc-DMSA is abnormally low in patients with proximal tubular dysfunction, including those with CLCN5 mutations [73]. Therefore, abnormal 99Tc-DMSA in the setting of normal glomerular filtration rate (GFR) can be another clue to the diagnosis of Dent disease [70]. Although genetically-confirmed Dent disease cases have been reported with LMW proteinuria alone, one or more of the following additional features is sufficient to make a clinical diagnosis of Dent disease when LMW proteinuria is present: stones or nephrocalcinosis, hypercalciuria, hypophosphatemia with or without rickets, or a family history of Dent disease (Figure 5). Genetic screening of the two implicated genes (CLCN5 and OCRL1) can be confirmatory. Findings on renal biopsy consistent with Dent disease include nephrocalcinosis, interstitial fibrosis, and FSGS [69, 70]. Stones, when present, are composed of calcium oxalate and/or calcium phosphate.
Treatment and outcome
There is no treatment that targets the specific defect causing Dent disease. The primary goal of treatment, therefore, is delaying the progression of CKD. Available interventions are primarily aimed at decreasing hypercalciuria and preventing kidney stones and nephrocalcinosis. No treatment has been tested for that outcome in randomized controlled trials. Thiazide diuretics are often used to treat kidney stone disease as they decrease urinary calcium excretion. However, thiazides are often not well tolerated in children with Dent disease, who frequently develop severe hypokalemia and/or volume depletion. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB) have been used in children with proteinuria to prevent or delay further loss of kidney function, with unclear effect. Since some patients do have FSGS on kidney biopsy, treatment with ACE inhibitor or ARB might prove to be somewhat beneficial, at least in a selected population with glomerular changes, as it is not thought to significantly affect LMW proteinuria. A high citrate diet has been shown to slow progression of CKD in ClC-5 KO mice [74] and it has been used by clinicians and patients, but no human trials have proven its effectiveness.
Familial hypomagnesemia with hypercalciuria and nephrocalcinosis
Background
Familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC) is a rare autosomal recessive disorder characterized by renal magnesium wasting, hypercalciuria, nephrocalcinosis and kidney failure. The disease was first described in 1972 [75] and approximately 110 cases have since been reported in the literature [5, 76-78]. Hypomagnesemia is invariably present and is a characteristic feature of the disease which usually presents in early childhood [76]. Progressive CKD commonly occurs in children with most patients developing ESRD in adolescence or early adult life. Clearance studies in patients with FHHNC reported 25 years ago suggested impairment in the reabsorption of magnesium and calcium in the thick ascending limb (TAL) of the loop of Henle as the primary tubular abnormality [79]. The molecular defect has been characterized and was found to be caused by mutations in two genes, CLDN16 located on the long arm of chromosome 3 (3q27) [80] and CLDN19 on the short arm of chromosome 1 (1p34.2) [80, 81]. The proteins encoded by these two genes, claudin-16 (previously known as paracellin 1) and claudin-19, belong to the claudin family of integral membrane proteins that are important components of tight junctions in many tissues [82]. Both proteins are expressed in the TAL where they are involved in paracellular reabsorption of calcium and magnesium, a process driven by lumen-positive transepithelial potential [83, 84]. Claudin-16 is exclusively expressed in the TAL [85], whereas claudin-19 is also expressed in tight junctions of the retina [81]. Mutational analysis has identified a locus of negatively charged amino acids in the first extracellular loop of claudin-16 as critical for its cation selectivity [86]. Moreover, in vitro studies have shown that claudin-16 interacts with claudin-19 to form heteromultimers causing a dramatic upregulation of PNa and down-regulation of PCl, and thereby generating a cation-selective paracellular channel in the tight junctions of the TAL that regulates magnesium and calcium permeability [87]. The interaction between these two proteins is required for their stable integration into tight junctions [88]. Confirmation that FHHNC is caused by loss-of-function mutations in CLDN16 and CLDN19 was provided by siRNA-mediated knockdown of the two genes in mice, yielding a phenotype characterized by severe renal wasting of magnesium and calcium [88, 89].
In FHHNC, hypercalciuria leads to nephrocalcinosis and stone formation. Renal failure is caused by a chronic tubulointerstitial nephropathy, the pathogenesis of which remains poorly understood. The deposition of calcium crystals in the renal tissue seems unlikely to be the sole cause since other disorders associated with nephrocalcinosis do not generally result in progressive deterioration of renal function [5]. Inactivating mutations of CLDN16 or CLDN19 result in urinary loss of calcium and magnesium. The renal phenotype is identical, while severe ocular involvement occurs in patients with the CLDN19 mutation [5, 78, 81]. The majority of reported FHHNC cases harbor mutations in CLDN16, which predominantly are missense mutations found in the sequence encoding the first extracellular loop of the protein. More than 40 mutations in CLDN16 have been reported, the most common of which is p. Leu151Phe, detected in patients from Germany and Eastern Europe [76]. An analysis of microsatellite markers has provided support for a common founder effect. Another missense mutation, p. Ala139Val, has been observed in North African families, also with an apparent common founder effect [78]. Less than 10 mutations in CLDN19 have been characterized. The most commonly observed is a missense mutation, p. Gly20Asp, located in the first transmembrane domain, which has been detected in patients of Spanish, Hispanic or French origin with haplotype analysis suggesting a common ancestor [77, 78, 81]. Functional characterization of mutant claudin-16 by heterologous expression in MDCK and LLC-PK1 cells showed significant residual function in several missense mutants [86]. While loss-of-function mutations, caused by a stop codon, frameshift or splice-site mutations, have been shown to interfere with intracellular trafficking of claudin-16, mutants with residual claudin-16 function were correctly targeted to tight junctions [5, 90]. Substantial interfamilial variability and intrafamilial concordance has been noted in FHHNC, suggesting a genotype-phenotype correlation. In patients with a CLDN16 mutation, age at onset and severity of the renal disease appears to be predicted by the genotype [5]. In a recently published study from France [78], progression of CKD was more frequently observed in patients harboring CLD1 9 mutations, with 61% of patients having developed ESRD at last follow-up compared with 33% of patients with CLDN16 mutations. The genetic heterogeneity in FHHNC is further highlighted by the phenotypic difference caused by mutations in CLDN19, which is characterized by severe ocular involvement [78, 81]. Finally, the phenotype of children with complete loss of claudin-16 function appears to be more severe, with the disease presenting earlier and often progressing to kidney failure at a significantly younger age compared than in those with residual claudin-16 function [5, 91].
Clinical features
The reported median age at onset of symptoms ranges from 3.5 to 7 years [76, 78]. The most common presenting features are recurrent urinary tract infection, polyuria and polydipsia, hematuria and pyuria [76]. Additional manifestations at onset include kidney stones, failure to thrive, seizures, abdominal pain and muscular tetany due to symptomatic hypocalcemia or hypomagnesemia. All affected patients have hypomagnesemia, hypercalciuria and nephrocalcinosis at the time of diagnosis. Other notable biochemical abnormalities observed during the course of the disease are hypocalcemia, incomplete distal renal tubular acidosis, hypocitraturia, increased parathyroid hormone levels independent of GFR and hyperuricemia. Approximately 30% of patients experience episodes of kidney stones [76, 78, 81]. CKD is frequently present in childhood with one-third of patients reaching ESRD during adolescence [5, 78]. Significant proteinuria has not been reported in patients with FHHNC. Ocular findings in patients with CLDN19 mutations include myopia, pigmentary retinitis, macular coloboma, strabismus, astigmatism and nystagmus [78].
Diagnosis
The diagnosis of FHHNC should be considered in all children with nephrocalcinosis, history of kidney stones, and/or reduced kidney function. The diagnosis is based on the triad of hypomagnesemia, hypercalciuria and nephrocalcinosis. Hypocalcemia, incomplete distal renal tubular acidosis and hypocitraturia are supportive features [5, 92]. Unfortunately, a delay in the diagnosis is common, reflecting lack of awareness among physicians [78]. Levels of serum magnesium and urinary calcium should be measured in all patients with nephrocalcinosis and/or CKD. The average serum magnesium concentration was 0.4 and 0.5 mmol/L, respectively, in two different reports [76, 78]. High fractional urinary excretion of magnesium is found while serum magnesium is inappropriately low [93]. Urinary calcium-to-creatinine ratio can be used to estimate the urinary calcium excretion which is generally in the range of 0.8-2.5 mmol/mmol (see annotations to Figure 5) [5, 78]. Renal histologic findings are not specific and include calcium deposits, glomerulosclerosis, tubular atrophy and interstitial fibrosis, consistent with a chronic tubulointerstitial nephropathy. In the differential diagnosis, the presence of CKD distinguishes FHHNC from other magnesium wasting disorders. Excluding hypokalemic metabolic alkalosis is important in the diagnostic process to avoid confusion with Barrter syndrome. The diagnosis of FHHNC can be confirmed by demonstrating mutations in both copies of CLDN16 or CLDN19.
Treatment and outcome
No effective therapeutic interventions for FHHNC are available. Medical management has included administration of magnesium supplements in high doses and thiazide diuretics to reduce urinary calcium excretion and the progression of nephrocalcinosis. Unfortunately, thiazides neither yield a meaningful reduction in urinary calcium excretion nor delay the progression of CKD [76, 78, 93]. Moreover, thiazides can aggravate renal magnesium wasting, often leading to their discontinuation [5, 92, 94]. The serum magnesium level tends to remain low despite high-dose magnesium supplementation [78]. Indomethacin has been used for treatment of FHHNC as it decreases sodium reabsorption in the proximal tubule and, thus, may theoretically increase the paracellular reabsorption of calcium. Limited experience suggests that indomethacin does not affect the serum magnesium concentration or urinary calcium excretion [78].
Therapies aimed at delaying the progression of CKD should be provided as well as conventional management strategies for kidney stones. Renal transplantation is the optimal treatment for ESRD in most cases and corrects the defect in renal handling of magnesium and calcium [76, 78, 93].
Primary hyperoxaluria
Background
The primary hyperoxalurias are inherited disorders of metabolism in which hepatic enzyme deficiencies result in overproduction of oxalate. The excess oxalate cannot be degraded in humans and is excreted largely by the kidneys, resulting in high urinary concentrations. Calcium oxalate crystals form in renal tubules acting as a nidus for crystal aggregation and growth, thus initiating stone formation. Crystals attach to renal tubular epithelial cells causing degenerative change and necrosis and they also can undergo transcytosis into the interstitium of the kidney where they incite an inflammatory reaction with resulting renal injury and scarring [95, 96]. Health consequences include frequent formation of calcium oxalate kidney stones as well as renal injury that leads to kidney failure in a significant proportion of affected patients [6].
Primary hyperoxaluria (PH) is worldwide in its distribution. The incidence and prevalence are unknown, though surveys of physicians in central Europe suggest an incidence of 1:120,000 live births and prevalence of 1-3 per million population [97, 98]. The prevalence of PH appears to be higher in certain regions including the Canary Islands, Tunisia, and parts of the Middle East [99].
Three types of PH have been described to date, each involving a different enzyme of the oxalate metabolic pathways. Type 1 PH (PH1, OMIM 259900), due to mutations in AGXT is found in approximately 80% of PH patients. When the activity of alanine-glyoxylate aminotransferase (AGT) is reduced or absent, glyoxylate accumulates and is shunted to oxalate and glycolate. For effective disposition of glyoxylate, AGT must be present in the peroxisome of the hepatocyte. Mistargeting of AGT to mitochondria, as results from certain mutations in AGXT, also results in hyperoxaluria even though enzyme activity is measurable [100].
Type 2 PH (PH2, OMIM 260000) accounts for approximately 10% of patients with known types of PH, is caused by mutations in GRHPR, and results from deficiency or absence of the enzyme glyoxylate reductase/hydroxy pyruvate reductase (GRHPR). Increased glycerate in the urine along with hyperoxaluria is indicative of type 2 disease [101]. A third type of PH (PH3, OMIM 613616) was recently shown to be due to mutations of the HOGA1 (formerly DHDPSL) [102]. A founder mutation was detected among Ashkenazi-Jewish patients [102], though PH type 3 has been confirmed in other populations [103]. Based on what is known of the gene product, PH type 3 is thought to be due to abnormalities of the enzyme 4-hydroxy-2-oxaloglutarate aldolase that is found in hepatic mitochondria [102, 104]. Data from the International Primary Hyperoxaluria Registry (IPHR) suggests that PH3 accounts for approximately 10% of patients with PH of known type. In addition, there are patients who have marked hyperoxaluria and clinical manifestations indistinguishable from those of PH, but who have no demonstrable abnormalities of the enzymes or the genes implicated in types 1, 2, and 3 PH. It is expected that additional PH types will be identified.
PH1 is an autosomal recessive disease. To date, more than 140 mutations of AGXT have been implicated (www.hgmd.cf.ac.uk). Since patients with mistargeting of enzyme to mitochondria have been shown to respond to treatment with a pharmacologic dose of pyridoxine (vitamin B6), identification of the genotype in individual patients has important implications for clinical management. Urine oxalate excretion rates in patients homozygous for mutations causing mistargeted AGT can correct into the normal range during pyridoxine treatment, while those heterozygous for these mutations demonstrate partial correction [105]. One of the mutations responsible for mistargeting, p. Gly170Arg, is the most common mutation in North American and Northern European patients with PH1 [106].
PH2 is autosomal recessive. Though in PH3 autosomal recessive inheritance is suggested by family studies, there also appear to be variable and intermittent elevations of urine oxalate in some heterozygotes [103].
Clinical features
Most patients present with signs or symptoms related to kidney stones. Among 300 patients in the IPHR as of May 2011, median age at onset of symptoms was 5 years. Symptoms were present in 65% of patients with PH before 10 years of age and in 85% before 20 years of age. A small minority of patients initially present with failure to thrive, nausea, pallor or fatigue of ESRD with no history of stones. Such patients may have diffuse calcification of kidney tissue visible by ultrasound or other imaging studies (nephrocalcinosis) without discrete stones. Extensive calcium oxalate crystal deposition is observed on renal biopsy. This mode of presentation is seen especially in infants. Irreversible kidney failure can be evident as early as 4 months of age.
Recurring stones throughout childhood, adolescence, and adulthood are characteristic. Nephrocalcinosis may also be observed. Growth and development are normal unless compromised by CKD or other unrelated medical problems. Over time, however, progressive renal damage leads to reduced kidney function. Effects on kidney function vary by type, with PH1 the most severe. Data from the IPHR in patients with PH1 showed that median age at progression to kidney failure was 33 years [107]. Recent analysis of a larger IPHR cohort of 222 PH1 patients by the same method showed a renal survival of 89% at 10 years of age and 75% at 20 years of age (unpublished data). There was no change in the median age at kidney failure in the larger cohort. Data from the OxalEurope registry [108] show similar findings. Whether these findings hold true in other populations remains to be established. Observations in Israeli patients with PH type 1 suggest early onset of symptoms with progression to kidney failure within the first decade of life as a prevalent phenotype [109]. Patients with PH2 and PH3 appear to have better kidney outcomes. In the IPHR, preservation of renal function has been found in 100% of PH2 and PH3 patients at 20 years of age. However, renal outcome in PH2 and PH3 must be interpreted with caution due to the small number of patients studied.
Diagnosis
The diagnosis of PH should be suspected in any child or adolescent who presents with radiopaque stones consistent with calcium oxalate, especially if stones are bilateral or present before a child is of school age. An algorithm is provided to assist in diagnostic assessment (Figure 6). Stones should be analyzed whenever possible. Those of PH patients are typically calcium oxalate monohydrate (COM), though they can be of mixed COM and calcium oxalate dihydrate composition. Measurement of urine chemistries is essential to the metabolic work-up. A timed 12 or 24 hour urine collection is the most informative. Oxalate excretion must be normalized to body surface area for accurate interpretation in children. In young children who have not yet become toilet trained or those who are developmentally delayed a random urine sample will provide useful information. A full supersaturation profile should be obtained to optimally guide treatment.
Figure 6.
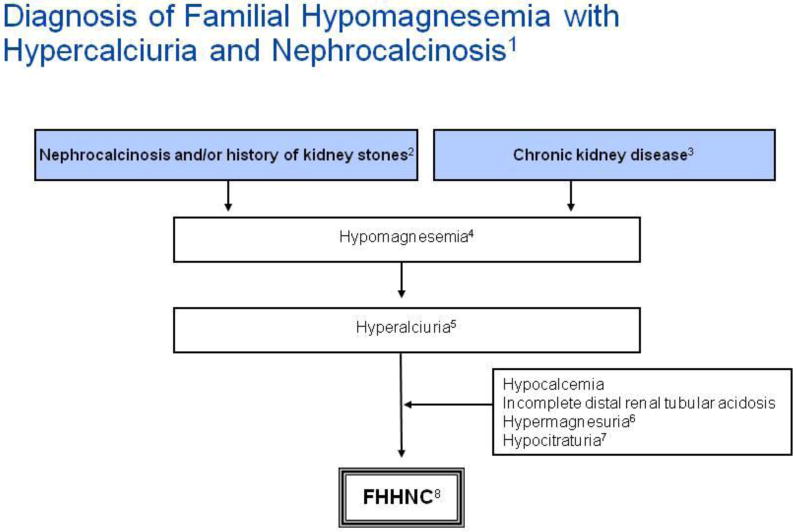
Algorithm for diagnostic evaluation of familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC).
Annotations to Figure 6
1The diagnosis of familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC) is based on the triad of hypomagnesemia, hypercalciuria and nephrocalcinosis [5]. Tables of normal values should be consulted in the interpretation of any random urine solute-to-creatinine ratios.
2All patients have nephrocalcinosis early in the course of the disease and 30% of patients eventually develop kidney stones.
3Progressive chronic kidney disease (CKD) is apparent during childhood and adolescence, with half of patients reaching end stage renal disease (ESRD) at 20 years of age [5]. Renal histological findings are not specific and include calcium deposits, glomerular sclerosis, tubular atrophy and interstitial fibrosis consistent with a tubulointerstitial nephropathy.
4The serum magnesium concentration at presentation has been shown to range from 0.23 to 0.61 mmol/L with a median concentration of 0.40 mmol/L [76].
5Normal limits for urinary calcium excretion is presented above in the annotations to Figure 5.
| Random urine magnesium-to-creatinine (Mg/Cr) ratio by age [119] | ||
| Age (yr) | Mg/Cr ratioUpper limit of normal | |
| mmo/mmol | mg/mg | |
| 0-1 | 2.2 | 0.48 |
| 1-2 | 1.7 | 0.37 |
| 2-3 | 1.6 | 0.34 |
| 3-5 | 1.3 | 0.29 |
| 5-7 | 1.0 | 0.21 |
| 7-10 | 0.9 | 0.18 |
| 10-14 | 0.7 | 0.15 |
| 14-17 | 0.6 | 0.13 |
| Random urine citrate-to-creatinine (Cit/Cr) ratio by age [120] | ||
| Age (yr) | Cit/Cr ratio Lower limit of normal | |
| mmol/mmol | mg/mg | |
| 0-5 | 0.25 | 0.42 |
| >5 | 0.15 | 0.25 |
8Familial hypomagnesemia with hypercalciuria and nephrocalcinosis (HHNC) is caused by mutations in the CLDN16 or CLDN19 genes. Genetic testing may be available at research institutions. Ocular abnormalities are indicative of a CLDN19 defect.
The diagnosis of PH can now be confirmed by DNA testing in most patients. Yet due to lack of familiarity with this rare disease, diagnosis is often delayed for years after onset of symptoms. In a survey of U.S. physicians, a significant delay in diagnosis was noted in 42% of cases and 30% were first tested for PH only after reaching ESRD [110]. Delays in diagnosis delay effective therapy, place renal function at risk, may result in systemic oxalosis, and entail risk of allograft loss after kidney transplantation.
Treatment and outcome
Maintenance of sufficient oral or gastric tube fluid intake to maintain high urine volume and use of orally administered inhibitors of calcium oxalate crystal formation are key to minimizing stone formation and preserving kidney function. Both neutral phosphates [111] and citrate [112] reduce calcium oxalate crystal formation in PH. Since kidney outcome in PH has been shown to correlate with the degree of hyperoxaluria [111], reduction in urine oxalate excretion is the most effective treatment strategy. Restriction of dietary oxalate is of limited benefit since the overwhelming majority of oxalate in the urine of PH patients comes from hepatic production. The role of enteric elimination of oxalate has received considerable attention in recent years. There was optimism based on preliminary studies [111] that treatment with oral Oxalobacter formigenes might reduce oxalate absorption and/or promote its secretion into the intestinal lumen. However, a recent double blind, placebo-controlled clinical trial conducted in the U.S. and Europe failed to show benefit [113].
Pyridoxine is the only pharmacologic agent that has been shown to reduce oxalate excretion, and is effective in some, but not all PH1 patients [105, 114]. Every patient with PH type 1 deserves a trial of pyridoxine therapy of at least 3 months duration. Effectiveness of 7-9 mg/kg/day of pyridoxine given orally as a twice a day dose should be assessed in patients with good renal function by measurement of oxalate excretion in 2 or more timed urine collections before and repeated after 3 months of treatment. If there is even partial improvement in urine oxalate, the pyridoxine should be continued, though lower doses in the range of 5-7 mg/kg/day are often sufficient for ongoing treatment [105]. It is not unusual for pyridoxine-responsive patients to have progressed to ESRD before the diagnosis of PH has been considered. During ESRD, determination of pyridoxine responsiveness on clinical grounds is complicated by low GFR and tissue oxalate stores and is often not possible. For that reason, empiric treatment with pyridoxine should be considered. AGXT genotype can be helpful in this situation [105, 114], though details are beyond the scope of this review. Pyridoxine is not effective in PH2. Early observations in PH3 also suggest lack of benefit, though definitive information has not yet been published.
Once the GFR declines to less than 30-35 ml/min/1.73m2, the kidneys can no longer keep up with the large oxalate production and plasma levels begin to increase rapidly. When the threshold for supersaturation in plasma is exceeded, calcium oxalate crystals form in many organs. Systemic oxalosis can result in refractory anemia, osteodystrophy, cardiac arrhythmias, oxalate cardiomyopathy, painful ischemic ulcers, and other manifestations eventually resulting in death. In patients with stages IV or V CKD minimizing systemic oxalosis is also critically important for a favorable outcome of subsequent kidney transplantation [6, 98]. For most patients, hemodialysis 6 or 7 days per week is required for oxalate removal and still may not keep pace with oxalate production. Peritoneal dialysis alone is insufficient though it may be combined with daily hemodialysis for better oxalate removal [115]. Patients who are fully pyridoxine responsive and have no interruptions in pyridoxine administration may require less intensive dialysis. Since systemic oxalate poses high risk for a transplanted kidney, the shortest time possible between start of dialysis and kidney transplantation should be the goal.
With intensive dialysis, shortened times to kidney transplantation, and careful post-transplant management, transplantation outcomes in PH patients have improved over time and are now similar to those for patients with other forms of kidney disease [116, 117]. Most patients with PH type 1 should undergo combined kidney and liver transplantation due to the risk to the transplanted kidney of continued high oxalate production. Patients who are fully pyridoxine responsive, in whom urine oxalate can be normalized by administration of pharmacologic doses of pyridoxine, may be candidates for kidney transplant alone. It is important to recognize that marked hyperoxaluria due to gradual mobilization of tissue oxalate stores poses a risk to all PH patients undergoing kidney transplantation, including those who undergo liver transplantation. Hyperoxaluria often persists for years following successful kidney transplantation [116]. In order to protect the transplanted kidney from oxalate-induced damage, consistent attention for maintenance of high urine volume and use of crystal inhibitor medications until urine oxalate returns to normal are important factors.
Kidney transplantation only is the current recommendation for PH2 patients who progress to ESRD. Unlike AGT1 which is specific to the liver, the enzyme GRHPR is present in multiple tissues and there have been no reports to date of correction of the metabolic defect in type 2 disease by liver transplantation. No patients with ESRD due to PH type 3 have yet been reported. Given uncertainties regarding the metabolic pathway involved, liver transplantation cannot at this time be recommended for patients with PH type 3.
Summary and future directions
Early diagnosis of APRT deficiency, cystinuria, Dent disease, FHHNC and PH is very important as adverse outcomes can often be ameliorated or even prevented with prompt diagnosis and treatment. However, lack of familiarity with these diseases among clinicians and pathologists remains a major problem and strategies to improve outcomes are much needed. Ready availability of diagnostic algorithms and their use in routine clinical care of children with kidney stone disease may be of value.
To facilitate early recognition and treatment of rare causes of kidney stones and CKD and to advance scientific knowledge in this field, the authors of this paper recently formed the Rare Kidney Stone Consortium (rarekidneystones. org and rarediseasesnetwork.epi.usf.edu/RKSC/index.htm) with support from the National Institute of Diabetes and Digestive and Kidney Diseases and The Office of Rare Diseases Research at the National Institutes of Health. This approach is analogous to current strategies in other rare diseases and has been supported by healthcare authorities both in Europe and the US.
To increase awareness of the five disorders and further enhance the educational mission of this project, collaboration has been established with international patient support groups, including the Oxalosis and Hyperoxaluria Foundation (ohf.org), International Cystinuria Foundation (cystinuria.org), Cystinuria Support Network (cystinuria.com), and Lowe Syndrome Association (http://www.lowesyndrome.org/). The APRT Deficiency Support Network (aprtd.org) was recently founded. Partnership with advocacy groups will allow rapid dissemination of relevant information and engage patients, their families, and local physicians in learning more about the diseases. Finally, we believe that exchange of information between investigators, clinicians, and patients will improve the lives of patients with rare causes of kidney stone disease through better care and outcomes.
Acknowledgments
The authors gratefully acknowledge the support of the Rare Kidney Stone Consortium (U54KD083908), a part of the National Institutes of Health (NIH) Rare Diseases Clinical Research Network (RDCRN), funded by the NIDDK, and the NIH Office of Rare Diseases Research (ORDR) and the Mayo Clinic O'Brien Urology Research Center (P50 DK083007) funded by the NIDDK. We thank Rachel Miller of the Mayo Clinic Renal Function Laboratory for photomicrographs of cystine, calcium oxalate and calcium phosphate crystals and Hrafnhildur L. Runolfsdottir, Medical Student at the University of Iceland, Reykjavik, Iceland for generating the images of urinary DHA crystals. The authors also thank members of the Rare Kidney Stone Consortium for critical feedback during construction of the diagnostic algorithms (Figures 3, 4, 5, 6 and 7).
Figure 7.
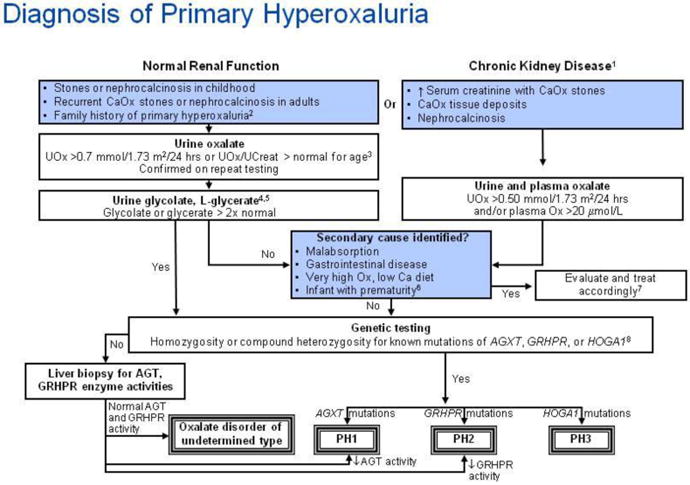
Algorithm for diagnostic evaluation of primary hyperoxaluria.
Annotations to Figure 7
1Chronic kidney disease is defined as a glomerular filtration rate of less than 50 ml/min/1.73 m2, or serum creatinine that is greater than or equal to two times normal for age.
2The guideline does not address prenatal diagnosis [121, 122].
Little data is available to guide interpretation of random urine oxalate-to-creatinine ratio in adolescents and adults. Upper limits of normal ratios declining to 0.04 by age 18-20 years and then remaining stable through adult age are suggested by available literature [125, 127]. In patients of all ages, confirmation of hyperoxaluria by a 24 hour urine collection with normalization of the oxalate excretion rate to 1.73 m2 body surface area, is strongly recommended. From 2 years of age through adulthood, normal urine oxalate is constant at <0.45 mmol/1.73 m2/24 hours [125].
4Urine and plasma oxalate and urine glycolate measurements for diagnostic testing should be obtained while the patient is not receiving pyridoxine or vitamin supplements.
5 Increased urine glycolate in the presence of hyperoxaluria is suggestive, but not diagnostic of primary hyperoxaluria (PH) type 1. Increased urine L-glycerate in a hyperoxaluric patient suggests PH type 2.
6Urine oxalate-to-creatinine ratios are higher in very premature infants than in term infants, especially when they are receiving parenteral nutrition containing amino acids. The ratio falls when premature infants are receiving only glucose and electrolyte solutions [128].
7When very high oxalate or low dietary calcium is suspected as the cause of the hyperoxaluria, the diet should be corrected and the urine oxalate remeasured for verification.
8In some cases with firm clinical diagnosis, only one mutation is found even after analysis for large rearrangements, suggesting that regulatory or deep intronic mutations may be the second, undetected mutation. In such cases, the finding of a single disease-associated mutation in the context of a typical phenotype supports the clinical diagnosis of PH.
Footnotes
Conflict of interest statement: Dr. Goldfarb is a consultant for Takeda.
References
- 1.Sas DJ. An update on the changing epidemiology and metabolic risk factors in pediatric kidney stone disease. Clin J Am Soc Nephrol. 2011;6:2062–2068. doi: 10.2215/CJN.11191210. [DOI] [PubMed] [Google Scholar]
- 2.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 3.Beara-Lasic L, Edvardsson V, Palsson R, Lieske J, Goldfarb D, Milliner D. Genetic causes of kidney stones and kidney failure. Clin Rev Bone Miner Metab. 2011;10(6):1–18. [Google Scholar]
- 4.Greenwood MC, Dillon MJ, Simmonds HA, Barratt TM, Pincott JR, Metreweli C. Renal failure due to 2,8-dihydroxyadenine urolithiasis. Eur J Pediatr. 1982;138:346–349. doi: 10.1007/BF00442515. [DOI] [PubMed] [Google Scholar]
- 5.Konrad M, Hou J, Weber S, Dotsch J, Kari JA, Seeman T, Kuwertz-Broking E, Peco-Antic A, Tasic V, Dittrich K, Alshaya HO, von Vigier RO, Gallati S, Goodenough DA, Schaller A. CLDN16 genotype predicts renal decline in familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol. 2008;19:171–181. doi: 10.1681/ASN.2007060709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoppe B, Beck BB, Milliner DS. The primary hyperoxalurias. Kidney Int. 2009;75:1264–1271. doi: 10.1038/ki.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rule AD, Krambeck AE, Lieske JC. Chronic kidney disease in kidney stone formers. Clin J Am Soc Nephrol. 2011;6:2069–2075. doi: 10.2215/CJN.10651110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edvardsson V, Palsson R, Olafsson I, Hjaltadottir G, Laxdal T. Clinical features and genotype of adenine phosphoribosyltransferase deficiency in Iceland. Am J Kidney Dis. 2001;38:473–480. doi: 10.1053/ajkd.2001.26826. [DOI] [PubMed] [Google Scholar]
- 9.Bollee G, Dollinger C, Boutaud L, Guillemot D, Bensman A, Harambat J, Deteix P, Daudon M, Knebelmann B, Ceballos-Picot I. Phenotype and genotype characterization of adenine phosphoribosyltransferase deficiency. J Am Soc Nephrol. 2010;21:679–688. doi: 10.1681/ASN.2009080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelley WN, Levy RI, Rosenbloom FM, Henderson JF, Seegmiller JE. Adenine phosphoribosyltransferase deficiency: a previously undescribed genetic defect in man. J Clin Invest. 1968;47:2281–2289. doi: 10.1172/JCI105913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahota AS, Tischfield AJ, Kamatani N, Simmonds HA. Adenine phosphoribosyltransferase deficiency and 2,8-dihydroxyadenine lithiasis. In: Scriver CR, B A, Sly WS, Valle D, Vogelstein B, Childs B, editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York, NY: 2001. pp. 2571–2584. [Google Scholar]
- 12.Broderick TP, Schaff DA, Bertino AM, Dush MK, Tischfield JA, Stambrook PJ. Comparative anatomy of the human APRT gene and enzyme: nucleotide sequence divergence and conservation of a nonrandom CpG dinucleotide arrangement. Proc Natl Acad Sci U S A. 1987;84:3349–3353. doi: 10.1073/pnas.84.10.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edvardsson VO, Palsson R, Sahota A. GeneReviews at GeneTests: Medical Genetics Information Resource [database online] Copyright, University of Washington; Seattle: 2012. Adenine Phosphoribosyltransferase Deficiency. 1997-2010. Available at http://www.genetests.org. [PubMed] [Google Scholar]
- 14.Kamatani N, Hakoda M, Otsuka S, Yoshikawa H, Kashiwazaki S. Only three mutations account for almost all defective alleles causing adenine phosphoribosyltransferase deficiency in Japanese patients. J Clin Invest. 1992;90:130–135. doi: 10.1172/JCI115825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasr SH, Sethi S, Cornell LD, Milliner DS, Boelkins M, Broviac J, Fidler ME. Crystalline nephropathy due to 2,8-dihydroxyadeninuria: an under-recognized cause of irreversible renal failure. Nephrol Dial Transplant. 2010;25:1909–1915. doi: 10.1093/ndt/gfp711. [DOI] [PubMed] [Google Scholar]
- 16.Hidaka Y, Tarle SA, O'Toole TE, Kelley WN, Palella TD. Nucleotide sequence of the human APRT gene. Nucleic Acids Res. 1987;15:9086. doi: 10.1093/nar/15.21.9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahota A, Chen J, Boyadjiev SA, Gault MH, Tischfield JA. Missense mutation in the adenine phosphoribosyltransferase gene causing 2,8-dihydroxyadenine urolithiasis. Hum Mol Genet. 1994;3:817–818. doi: 10.1093/hmg/3.5.817. [DOI] [PubMed] [Google Scholar]
- 18.Harambat J, Bollee G, Daudon M, Ceballos-Picot I, Bensman A. Adenine phosphoribosyltransferase deficiency in children. Pediatr Nephrol. 2012;27:571–579. doi: 10.1007/s00467-011-2037-0. [DOI] [PubMed] [Google Scholar]
- 19.Debray H, Cartier P, Temstet A, Cendron J. Child's urinary lithiasis revealing a complete deficit in adenine phosphoribosyl transferase. Pediatr Res. 1976;10:762–766. doi: 10.1203/00006450-197608000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Chiba P, Zwiauer K, Muller MM. Characterization of an adenine phosphoribosyltransferase deficiency. Clin Chim Acta. 1988;172:141–147. doi: 10.1016/0009-8981(88)90318-x. [DOI] [PubMed] [Google Scholar]
- 21.Cassidy MJ, McCulloch T, Fairbanks LD, Simmonds HA. Diagnosis of adenine phosphoribosyltransferase deficiency as the underlying cause of renal failure in a renal transplant recipient. Nephrol Dial Transplant. 2004;19:736–738. doi: 10.1093/ndt/gfg562. [DOI] [PubMed] [Google Scholar]
- 22.Benedetto B, Madden R, Kurbanov A, Braden G, Freeman J, Lipkowitz GS. Adenine phosphoribosyltransferase deficiency and renal allograft dysfunction. Am J Kidney Dis. 2001;37:E37. doi: 10.1016/s0272-6386(05)90001-2. [DOI] [PubMed] [Google Scholar]
- 23.Becker MA, Schumacher HR, Jr, Wortmann RL, MacDonald PA, Palo WA, Eustace D, Vernillet L, Joseph-Ridge N. Febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase: a twenty-eight-day, multicenter, phase II, randomized, double-blind, placebo-controlled, dose-response clinical trial examining safety and efficacy in patients with gout. Arthritis Rheum. 2005;52:916–923. doi: 10.1002/art.20935. [DOI] [PubMed] [Google Scholar]
- 24.Milliner DS, Murphy ME. Urolithiasis in pediatric patients. Mayo Clin Proc. 1993;68:241–248. doi: 10.1016/s0025-6196(12)60043-3. [DOI] [PubMed] [Google Scholar]
- 25.Chillaron J, Font-Llitjos M, Fort J, Zorzano A, Goldfarb DS, Nunes V, Palacin M. Pathophysiology and treatment of cystinuria. Nat Rev Nephrol. 2010;6:424–434. doi: 10.1038/nrneph.2010.69. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez E, Carrascal M, Rousaud F, Abian J, Zorzano A, Palacin M, Chillaron J. rBAT-b(0,+)AT heterodimer is the main apical reabsorption system for cystine in the kidney. Am J Physiol Renal Physiol. 2002;283:F540–548. doi: 10.1152/ajprenal.00071.2002. [DOI] [PubMed] [Google Scholar]
- 27.Martens K, Jaeken J, Matthijs G, Creemers JW. Multi-system disorder syndromes associated with cystinuria type I. Curr Mol Med. 2008;8:544–550. doi: 10.2174/156652408785747997. [DOI] [PubMed] [Google Scholar]
- 28.Dello Strologo L, Laurenzi C, Legato A, Pastore A. Cystinuria in children and young adults: success of monitoring free-cystine urine levels. Pediatr Nephrol. 2007;22:1869–1873. doi: 10.1007/s00467-007-0575-2. [DOI] [PubMed] [Google Scholar]
- 29.Lambert EH, Asplin JR, Herrell SD, Miller NL. Analysis of 24-hour urine parameters as it relates to age of onset of cystine stone formation. J Endourol. 2010;24:1179–1182. doi: 10.1089/end.2010.0133. [DOI] [PubMed] [Google Scholar]
- 30.Worcester EM, Coe FL, Evan AP, Parks JH. Reduced renal function and benefits of treatment in cystinuria vs other forms of nephrolithiasis. BJU Int. 2006;97:1285–1290. doi: 10.1111/j.1464-410X.2006.06169.x. [DOI] [PubMed] [Google Scholar]
- 31.Assimos DG, Leslie SW, Ng C, Streem SB, Hart LJ. The impact of cystinuria on renal function. J Urol. 2002;168:27–30. [PubMed] [Google Scholar]
- 32.Nakagawa Y, Coe FL. A modified cyanide-nitroprusside method for quantifying urinary cystine concentration that corrects for creatinine interference. Clin Chim Acta. 1999;289:57–68. doi: 10.1016/s0009-8981(99)00159-x. [DOI] [PubMed] [Google Scholar]
- 33.Evan AP, Coe FL, Lingeman JE, Shao Y, Matlaga BR, Kim SC, Bledsoe SB, Sommer AJ, Grynpas M, Phillips CL, Worcester EM. Renal crystal deposits and histopathology in patients with cystine stones. Kidney Int. 2006;69:2227–2235. doi: 10.1038/sj.ki.5000268. [DOI] [PubMed] [Google Scholar]
- 34.Boutros M, Vicanek C, Rozen R, Goodyer P. Transient neonatal cystinuria. Kidney Int. 2005;67:443–448. doi: 10.1111/j.1523-1755.2005.67100.x. [DOI] [PubMed] [Google Scholar]
- 35.Goldfarb DS, Coe FL, Asplin JR. Urinary cystine excretion and capacity in patients with cystinuria. Kidney Int. 2006;69:1041–1047. doi: 10.1038/sj.ki.5000104. [DOI] [PubMed] [Google Scholar]
- 36.Rodman JS, Blackburn P, Williams JJ, Brown A, Pospischil MA, Peterson CM. The effect of dietary protein on cystine excretion in patients with cystinuria. Clin Nephrol. 1984;22:273–278. [PubMed] [Google Scholar]
- 37.Chow GK, Streem SB. Medical treatment of cystinuria: results of contemporary clinical practice. J Urol. 1996;156:1576–1578. doi: 10.1016/s0022-5347(01)65451-x. [DOI] [PubMed] [Google Scholar]
- 38.Nakagawa Y, Asplin JR, Goldfarb DS, Parks JH, Coe FL. Clinical use of cystine supersaturation measurements. J Urol. 2000;164:1481–1485. [PubMed] [Google Scholar]
- 39.Dolin DJ, Asplin JR, Flagel L, Grasso M, Goldfarb DS. Effect of cystine-binding thiol drugs on urinary cystine capacity in patients with cystinuria. J Endourol. 2005;19:429–432. doi: 10.1089/end.2005.19.429. [DOI] [PubMed] [Google Scholar]
- 40.Pareek G, Steele TH, Nakada SY. Urological intervention in patients with cystinuria is decreased with medical compliance. J Urol. 2005;174:2250–2252. doi: 10.1097/01.ju.0000181817.89703.66. [DOI] [PubMed] [Google Scholar]
- 41.Trinchieri A, Montanari E, Zanetti G, Lizzano R. The impact of new technology in the treatment of cystine stones. Urol Res. 2007;35:129–132. doi: 10.1007/s00240-007-0089-1. [DOI] [PubMed] [Google Scholar]
- 42.Wrong OM, Norden AG, Feest TG. Dent's disease; a familial proximal renal tubular syndrome with low-molecular-weight proteinuria, hypercalciuria, nephrocalcinosis, metabolic bone disease, progressive renal failure and a marked male predominance. Q J Med. 1994;87:473–493. [PubMed] [Google Scholar]
- 43.Lloyd SE, Gunther W, Pearce SH, Thomson A, Bianchi ML, Bosio M, Craig IW, Fisher SE, Scheinman SJ, Wrong O, Jentsch TJ, Thakker RV. Characterisation of renal chloride channel, CLCN5, mutations in hypercalciuric nephrolithiasis (kidney stones) disorders. Hum Mol Genet. 1997;6:1233–1239. doi: 10.1093/hmg/6.8.1233. [DOI] [PubMed] [Google Scholar]
- 44.Thakker RV. Pathogenesis of Dent's disease and related syndromes of X-linked nephrolithiasis. Kidney Int. 2000;57:787–793. doi: 10.1046/j.1523-1755.2000.00916.x. [DOI] [PubMed] [Google Scholar]
- 45.Reinhart SC, Norden AG, Lapsley M, Thakker RV, Pang J, Moses AM, Frymoyer PA, Favus MJ, Hoepner JA, Scheinman SJ. Characterization of carrier females and affected males with X-linked recessive nephrolithiasis. J Am Soc Nephrol. 1995;5:1451–1461. doi: 10.1681/ASN.V571451. [DOI] [PubMed] [Google Scholar]
- 46.Waldegger S, Jentsch TJ. From tonus to tonicity: physiology of CLC chloride channels. J Am Soc Nephrol. 2000;11:1331–1339. doi: 10.1681/ASN.V1171331. [DOI] [PubMed] [Google Scholar]
- 47.Dutzler R. Structural basis for ion conduction and gating in ClC chloride channels. FEBS Lett. 2004;564:229–233. doi: 10.1016/S0014-5793(04)00210-8. [DOI] [PubMed] [Google Scholar]
- 48.Gunther W, Luchow A, Cluzeaud F, Vandewalle A, Jentsch TJ. ClC-5, the chloride channel mutated in Dent's disease, colocalizes with the proton pump in endocytotically active kidney cells. Proc Natl Acad Sci U S A. 1998;95:8075–8080. doi: 10.1073/pnas.95.14.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devuyst O, Christie PT, Courtoy PJ, Beauwens R, Thakker RV. Intra-renal and subcellular distribution of the human chloride channel, CLC-5, reveals a pathophysiological basis for Dent's disease. Hum Mol Genet. 1999;8:247–257. doi: 10.1093/hmg/8.2.247. [DOI] [PubMed] [Google Scholar]
- 50.Hara-Chikuma M, Wang Y, Guggino SE, Guggino WB, Verkman AS. Impaired acidification in early endosomes of ClC-5 deficient proximal tubule. Biochem Biophys Res Commun. 2005;329:941–946. doi: 10.1016/j.bbrc.2005.02.060. [DOI] [PubMed] [Google Scholar]
- 51.Picollo A, Pusch M. Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature. 2005;436:420–423. doi: 10.1038/nature03720. [DOI] [PubMed] [Google Scholar]
- 52.Scheel O, Zdebik AA, Lourdel S, Jentsch TJ. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature. 2005;436:424–427. doi: 10.1038/nature03860. [DOI] [PubMed] [Google Scholar]
- 53.Novarino G, Weinert S, Rickheit G, Jentsch TJ. Endosomal chloride-proton exchange rather than chloride conductance is crucial for renal endocytosis. Science. 2010;328:1398–1401. doi: 10.1126/science.1188070. [DOI] [PubMed] [Google Scholar]
- 54.Friedrich T, Breiderhoff T, Jentsch TJ. Mutational analysis demonstrates that ClC-4 and ClC-5 directly mediate plasma membrane currents. J Biol Chem. 1999;274:896–902. doi: 10.1074/jbc.274.2.896. [DOI] [PubMed] [Google Scholar]
- 55.Hryciw DH, Ekberg J, Pollock CA, Poronnik P. ClC-5: a chloride channel with multiple roles in renal tubular albumin uptake. Int J Biochem Cell Biol. 2006;38:1036–1042. doi: 10.1016/j.biocel.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 56.Piwon N, Gunther W, Schwake M, Bosl MR, Jentsch TJ. ClC-5 Cl- -channel disruption impairs endocytosis in a mouse model for Dent's disease. Nature. 2000;408:369–373. doi: 10.1038/35042597. [DOI] [PubMed] [Google Scholar]
- 57.Wang SS, Devuyst O, Courtoy PJ, Wang XT, Wang H, Wang Y, Thakker RV, Guggino S, Guggino WB. Mice lacking renal chloride channel, CLC-5, are a model for Dent's disease, a nephrolithiasis disorder associated with defective receptor-mediated endocytosis. Hum Mol Genet. 2000;9:2937–2945. doi: 10.1093/hmg/9.20.2937. [DOI] [PubMed] [Google Scholar]
- 58.Christensen EI, Devuyst O, Dom G, Nielsen R, Van der Smissen P, Verroust P, Leruth M, Guggino WB, Courtoy PJ. Loss of chloride channel ClC-5 impairs endocytosis by defective trafficking of megalin and cubilin in kidney proximal tubules. Proc Natl Acad Sci U S A. 2003;100:8472–8477. doi: 10.1073/pnas.1432873100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silva IV, Cebotaru V, Wang H, Wang XT, Wang SS, Guo G, Devuyst O, Thakker RV, Guggino WB, Guggino SE. The ClC-5 knockout mouse model of Dent's disease has renal hypercalciuria and increased bone turnover. J Bone Miner Res. 2003;18:615–623. doi: 10.1359/jbmr.2003.18.4.615. [DOI] [PubMed] [Google Scholar]
- 60.Leheste JR, Melsen F, Wellner M, Jansen P, Schlichting U, Renner-Muller I, Andreassen TT, Wolf E, Bachmann S, Nykjaer A, Willnow TE. Hypocalcemia and osteopathy in mice with kidney-specific megalin gene defect. FASEB J. 2003;17:247–249. doi: 10.1096/fj.02-0578fje. [DOI] [PubMed] [Google Scholar]
- 61.Devuyst O, Thakker RV. Dent's disease. Orphanet J Rare Dis. 2010;5:28. doi: 10.1186/1750-1172-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Claverie-Martin F, Ramos-Trujillo E, Garcia-Nieto V. Dent's disease: clinical features and molecular basis. Pediatr Nephrol. 2011;26:693–704. doi: 10.1007/s00467-010-1657-0. [DOI] [PubMed] [Google Scholar]
- 63.Hoopes RR, Jr, Raja KM, Koich A, Hueber P, Reid R, Knohl SJ, Scheinman SJ. Evidence for genetic heterogeneity in Dent's disease. Kidney Int. 2004;65:1615–1620. doi: 10.1111/j.1523-1755.2004.00571.x. [DOI] [PubMed] [Google Scholar]
- 64.Hoopes RR, Jr, Shrimpton AE, Knohl SJ, Hueber P, Hoppe B, Matyus J, Simckes A, Tasic V, Toenshoff B, Suchy SF, Nussbaum RL, Scheinman SJ. Dent Disease with mutations in OCRL1. Am J Hum Genet. 2005;76:260–267. doi: 10.1086/427887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghanekar Y, Lowe M. Signalling for secretion. Nat Cell Biol. 2005;7:851–853. doi: 10.1038/ncb0905-851. [DOI] [PubMed] [Google Scholar]
- 66.Choudhury R, Diao A, Zhang F, Eisenberg E, Saint-Pol A, Williams C, Konstantakopoulos A, Lucocq J, Johannes L, Rabouille C, Greene LE, Lowe M. Lowe syndrome protein OCRL1 interacts with clathrin and regulates protein trafficking between endosomes and the trans-Golgi network. Mol Biol Cell. 2005;16:3467–3479. doi: 10.1091/mbc.E05-02-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tosetto E, Addis M, Caridi G, Meloni C, Emma F, Vergine G, Stringini G, Papalia T, Barbano G, Ghiggeri GM, Ruggeri L, Miglietti N, A DA, Melis MA, Anglani F. Locus heterogeneity of Dent's disease: OCRL1 and TMEM27 genes in patients with no CLCN5 mutations. Pediatr Nephrol. 2009;24:1967–1973. doi: 10.1007/s00467-009-1228-4. [DOI] [PubMed] [Google Scholar]
- 68.Hichri H, Rendu J, Monnier N, Coutton C, Dorseuil O, Poussou RV, Baujat G, Blanchard A, Nobili F, Ranchin B, Remesy M, Salomon R, Satre V, Lunardi J. From Lowe syndrome to Dent disease: correlations between mutations of the OCRL1 gene and clinical and biochemical phenotypes. Hum Mutat. 2011;32:379–388. doi: 10.1002/humu.21391. [DOI] [PubMed] [Google Scholar]
- 69.Copelovitch L, Nash MA, Kaplan BS. Hypothesis: Dent disease is an underrecognized cause of focal glomerulosclerosis. Clin J Am Soc Nephrol. 2007;2:914–918. doi: 10.2215/CJN.00900207. [DOI] [PubMed] [Google Scholar]
- 70.Frishberg Y, Dinour D, Belostotsky R, Becker-Cohen R, Rinat C, Feinstein S, Navon-Elkan P, Ben-Shalom E. Dent's disease manifesting as focal glomerulosclerosis: Is it the tip of the iceberg? Pediatr Nephrol. 2009;24:2369–2373. doi: 10.1007/s00467-009-1299-2. [DOI] [PubMed] [Google Scholar]
- 71.Bokenkamp A, Bockenhauer D, Cheong HI, Hoppe B, Tasic V, Unwin R, Ludwig M. Dent-2 disease: a mild variant of Lowe syndrome. J Pediatr. 2009;155:94–99. doi: 10.1016/j.jpeds.2009.01.049. [DOI] [PubMed] [Google Scholar]
- 72.Davey PG, Gosling P. Beta 2-microglobulin instability in pathological urine. Clin Chem. 1982;28:1330–1333. [PubMed] [Google Scholar]
- 73.Suzuki S, Suzuki J, Kume K, Yoshida K, Suyama H, Kawasaki Y, Nozawa R, Suzuki H, Fujiki T, Kamiyama S, Suzuki A. Poor renal accumulation of 99mTc-DMSA in idiopathic tubular proteinuria. Nephron. 1999;81:49–54. doi: 10.1159/000045245. [DOI] [PubMed] [Google Scholar]
- 74.Cebotaru V, Kaul S, Devuyst O, Cai H, Racusen L, Guggino WB, Guggino SE. High citrate diet delays progression of renal insufficiency in the ClC-5 knockout mouse model of Dent's disease. Kidney Int. 2005;68:642–652. doi: 10.1111/j.1523-1755.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 75.Michelis MF, Drash AL, Linarelli LG, De Rubertis FR, Davis BB. Decreased bicarbonate threshold and renal magnesium wasting in a sibship with distal renal tubular acidosis. (Evaluation of the pathophysiological role of parathyroid hormone) Metabolism. 1972;21:905–920. doi: 10.1016/0026-0495(72)90025-x. [DOI] [PubMed] [Google Scholar]
- 76.Weber S, Schneider L, Peters M, Misselwitz J, Ronnefarth G, Boswald M, Bonzel KE, Seeman T, Sulakova T, Kuwertz-Broking E, Gregoric A, Palcoux JB, Tasic V, Manz F, Scharer K, Seyberth HW, Konrad M. Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol. 2001;12:1872–1881. doi: 10.1681/ASN.V1291872. [DOI] [PubMed] [Google Scholar]
- 77.Faguer S, Chauveau D, Cintas P, Tack I, Cointault O, Rostaing L, Vargas-Poussou R, Ribes D. Renal, ocular, and neuromuscular involvements in patients with CLDN19 mutations. Clin J Am Soc Nephrol. 2011;6:355–360. doi: 10.2215/CJN.02870310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Godron A, Harambat J, Boccio V, Mensire A, May A, Rigothier C, Couzi L, Barrou B, Godin M, Chauveau D, Faguer S, Vallet M, Cochat P, Eckart P, Guest G, Guigonis V, Houillier P, Blanchard A, Jeunemaitre X, Vargas-Poussou R. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis: phenotype-genotype correlation and outcome in 32 patients with CLDN16 or CLDN19 mutations. Clin J Am Soc Nephrol. 2012;7:801–809. doi: 10.2215/CJN.12841211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodriguez-Soriano J, Vallo A, Garcia-Fuentes M. Hypomagnesaemia of hereditary renal origin. Pediatr Nephrol. 1987;1:465–472. doi: 10.1007/BF00849255. [DOI] [PubMed] [Google Scholar]
- 80.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 81.Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C, Schlingmann KP, Schmid M, Rodriguez-Soriano J, Ariceta G, Cano F, Enriquez R, Juppner H, Bakkaloglu SA, Hediger MA, Gallati S, Neuhauss SC, Nurnberg P, Weber S. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet. 2006;79:949–957. doi: 10.1086/508617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li J, Ananthapanyasut W, Yu AS. Claudins in renal physiology and disease. Pediatr Nephrol. 2011;26:2133–2142. doi: 10.1007/s00467-011-1824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blanchard A, Jeunemaitre X, Coudol P, Dechaux M, Froissart M, May A, Demontis R, Fournier A, Paillard M, Houillier P. Paracellin-1 is critical for magnesium and calcium reabsorption in the human thick ascending limb of Henle. Kidney Int. 2001;59:2206–2215. doi: 10.1046/j.1523-1755.2001.00736.x. [DOI] [PubMed] [Google Scholar]
- 84.Haisch L, Almeida JR, Abreu da Silva PR, Schlingmann KP, Konrad M. The role of tight junctions in paracellular ion transport in the renal tubule: lessons learned from a rare inherited tubular disorder. Am J Kidney Dis. 2011;57:320–330. doi: 10.1053/j.ajkd.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 85.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002;13:875–886. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- 86.Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci. 2005;118:5109–5118. doi: 10.1242/jcs.02631. [DOI] [PubMed] [Google Scholar]
- 87.Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest. 2008;118:619–628. doi: 10.1172/JCI33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci U S A. 2009;106:15350–15355. doi: 10.1073/pnas.0907724106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hou J, Shan Q, Wang T, Gomes AS, Yan Q, Paul DL, Bleich M, Goodenough DA. Transgenic RNAi depletion of claudin-16 and the renal handling of magnesium. J Biol Chem. 2007;282:17114–17122. doi: 10.1074/jbc.M700632200. [DOI] [PubMed] [Google Scholar]
- 90.Kausalya PJ, Amasheh S, Gunzel D, Wurps H, Muller D, Fromm M, Hunziker W. Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of Claudin-16. J Clin Invest. 2006;116:878–891. doi: 10.1172/JCI26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hampson G, Konrad MA, Scoble J. Familial hypomagnesaemia with hypercalciuria and nephrocalcinosis (FHHNC): compound heterozygous mutation in the claudin 16 (CLDN16) gene. BMC nephrology. 2008;9:12. doi: 10.1186/1471-2369-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stechman MJ, Loh NY, Thakker RV. Genetic causes of hypercalciuric nephrolithiasis. Pediatr Nephrol. 2009;24:2321–2332. doi: 10.1007/s00467-008-0807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Praga M, Vara J, Gonzalez-Parra E, Andres A, Alamo C, Araque A, Ortiz A, Rodicio JL. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis. Kidney Int. 1995;47:1419–1425. doi: 10.1038/ki.1995.199. [DOI] [PubMed] [Google Scholar]
- 94.Zimmermann B, Plank C, Konrad M, Stohr W, Gravou-Apostolatou C, Rascher W, Dotsch J. Hydrochlorothiazide in CLDN16 mutation. Nephrol Dial Transplant. 2006;21:2127–2132. doi: 10.1093/ndt/gfl144. [DOI] [PubMed] [Google Scholar]
- 95.Lieske JC, Spargo BH, Toback FG. Endocytosis of calcium oxalate crystals and proliferation of renal tubular epithelial cells in a patient with type 1 primary hyperoxaluria. J Urol. 1992;148:1517–1519. doi: 10.1016/s0022-5347(17)36954-9. [DOI] [PubMed] [Google Scholar]
- 96.Muda AO, Barsotti P, Rinaldi S, Rizzoni G, Faraggiana T. Renal pathology in hyperoxaluria. In: Sessa A, Conte F, Meroni M, Battini G, editors. Hereditary Kidney Diseases. Karger; Basel: 1997. pp. 160–166. [Google Scholar]
- 97.van Woerden CS, Groothoff JW, Wanders RJ, Davin JC, Wijburg FA. Primary hyperoxaluria type 1 in The Netherlands: prevalence and outcome. Nephrol Dial Transplant. 2003;18:273–279. doi: 10.1093/ndt/18.2.273. [DOI] [PubMed] [Google Scholar]
- 98.Cochat P, Liutkus A, Fargue S, Basmaison O, Ranchin B, Rolland MO. Primary hyperoxaluria type 1: still challenging. Pediatr Nephrol. 2006;21:1075–1081. doi: 10.1007/s00467-006-0124-4. [DOI] [PubMed] [Google Scholar]
- 99.Coulter-Mackie MB. Preliminary evidence for ethnic differences in primary hyperoxaluria type 1 genotype. Am J Nephrol. 2005;25:264–268. doi: 10.1159/000086356. [DOI] [PubMed] [Google Scholar]
- 100.Danpure CJ. Molecular etiology of primary hyperoxaluria type 1: new directions for treatment. Am J Nephrol. 2005;25:303–310. doi: 10.1159/000086362. [DOI] [PubMed] [Google Scholar]
- 101.Milliner DS. The primary hyperoxalurias: an algorithm for diagnosis. Am J Nephrol. 2005;25:154–160. doi: 10.1159/000085407. [DOI] [PubMed] [Google Scholar]
- 102.Belostotsky R, Seboun E, Idelson GH, Milliner DS, Becker-Cohen R, Rinat C, Monico CG, Feinstein S, Ben-Shalom E, Magen D, Weissman I, Charon C, Frishberg Y. Mutations in DHDPSL are responsible for primary hyperoxaluria type III. Am J Hum Genet. 2010;87:392–399. doi: 10.1016/j.ajhg.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Monico CG, Rossetti S, Belostotsky R, Cogal AG, Herges RM, Seide BM, Olson JB, Bergstrahl EJ, Williams HJ, Haley WE, Frishberg Y, Milliner DS. Primary hyperoxaluria type III gene HOGA1 (formerly DHDPSL) as a possible risk factor for idiopathic calcium oxalate urolithiasis. Clin J Am Soc Nephrol. 2011;6:2289–2295. doi: 10.2215/CJN.02760311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Riedel TJ, Johnson LC, Knight J, Hantgan RR, Holmes RP, Lowther WT. Structural and biochemical studies of human 4-hydroxy-2-oxoglutarate aldolase: implications for hydroxyproline metabolism in primary hyperoxaluria. PLoS One. 2011;6:e26021. doi: 10.1371/journal.pone.0026021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Monico CG, Rossetti S, Olson JB, Milliner DS. Pyridoxine effect in type I primary hyperoxaluria is associated with the most common mutant allele. Kidney Int. 2005;67:1704–1709. doi: 10.1111/j.1523-1755.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 106.Monico CG, Rossetti S, Schwanz HA, Olson JB, Lundquist PA, Dawson DB, Harris PC, Milliner DS. Comprehensive mutation screening in 55 probands with type 1 primary hyperoxaluria shows feasibility of a gene-based diagnosis. J Amer Soc Nephrol. 2007;18:1905–1914. doi: 10.1681/ASN.2006111230. [DOI] [PubMed] [Google Scholar]
- 107.Lieske JC, Monico CG, Holmes WS, Bergstralh EJ, Slezak JM, Rohlinger AL, Olson JB, Milliner DS. International registry for primary hyperoxaluria. Am J Nephrol. 2005;25:290–296. doi: 10.1159/000086360. [DOI] [PubMed] [Google Scholar]
- 108.Harambat J, Fargue S, Acquaviva C, Gagnadoux MF, Janssen F, Liutkus A, Mourani C, Macher MA, Abramowicz D, Legendre C, Durrbach A, Tsimaratos M, Nivet H, Girardin E, Schott AM, Rolland MO, Cochat P. Genotype-phenotype correlation in primary hyperoxaluria type 1: the p.Gly170Arg AGXT mutation is associated with a better outcome. Kidney Int. 2010;77:443–449. doi: 10.1038/ki.2009.435. [DOI] [PubMed] [Google Scholar]
- 109.Frishberg Y, Rinat C, Shalata A, Khatib I, Feinstein S, Becker-Cohen R, Weismann I, Wanders RJ, Rumsby G, Roels F, Mandel H. Intra-familial clinical heterogeneity: absence of genotype-phenotype correlation in primary hyperoxaluria type 1 in Israel. Am J Nephrol. 2005;25:269–275. doi: 10.1159/000086357. [DOI] [PubMed] [Google Scholar]
- 110.Hoppe B, Langman CB. A United States survey on diagnosis, treatment, and outcome of primary hyperoxaluria. Pediatr Nephrol. 2003;18:986–991. doi: 10.1007/s00467-003-1234-x. [DOI] [PubMed] [Google Scholar]
- 111.Milliner D. Treatment of the primary hyperoxalurias: a new chapter. Kidney Int. 2006;70:1198–1200. doi: 10.1038/sj.ki.5001821. [DOI] [PubMed] [Google Scholar]
- 112.Leumann E, Hoppe B, Neuhaus T. Management of primary hyperoxaluria: efficacy of oral citrate administration. Pediatr Nephrol. 1993;7:207–211. doi: 10.1007/BF00864405. [DOI] [PubMed] [Google Scholar]
- 113.Hoppe B, Groothoff JW, Hulton SA, Cochat P, Niaudet P, Kemper MJ, Deschenes G, Unwin R, Milliner D. Efficacy and safety of Oxalobacter formigenes to reduce urinary oxalate in primary hyperoxaluria. Nephrol Dial Transplant. 2011;26:3609–3615. doi: 10.1093/ndt/gfr107. [DOI] [PubMed] [Google Scholar]
- 114.Monico CG, Olson JB, Milliner DS. Implications of genotype and enzyme phenotype in pyridoxine response of patients with type I primary hyperoxaluria. Am J Nephrol. 2005;25:183–188. doi: 10.1159/000085411. [DOI] [PubMed] [Google Scholar]
- 115.Illies F, Bonzel KE, Wingen AM, Latta K, Hoyer PF. Clearance and removal of oxalate in children on intensified dialysis for primary hyperoxaluria type 1. Kidney Int. 2006;70:1642–1648. doi: 10.1038/sj.ki.5001806. [DOI] [PubMed] [Google Scholar]
- 116.Bergstralh EJ, Monico CG, Lieske JC, Herges RM, Langman CB, Hoppe B, Milliner DS. Transplantation outcomes in primary hyperoxaluria. Am J Transplant. 2010;10:2493–2501. doi: 10.1111/j.1600-6143.2010.03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Millan MT, Berquist WE, So SK, Sarwal MM, Wayman KI, Cox KL, Filler G, Salvatierra O, Jr, Esquivel CO. One hundred percent patient and kidney allograft survival with simultaneous liver and kidney transplantation in infants with primary hyperoxaluria: a single-center experience. Transplantation. 2003;76:1458–1463. doi: 10.1097/01.TP.0000084203.76110.AC. [DOI] [PubMed] [Google Scholar]
- 118.Silva M, Silva CH, Iulek J, Thiemann OH. Three-dimensional structure of human adenine phosphoribosyltransferase and its relation to DHA-urolithiasis. Biochemistry. 2004;43:7663–7671. doi: 10.1021/bi0360758. [DOI] [PubMed] [Google Scholar]
- 119.Matos V, van Melle G, Boulat O, Markert M, Bachmann C, Guignard JP. Urinary phosphate/creatinine, calcium/creatinine, and magnesium/creatinine ratios in a healthy pediatric population. J Pediatr. 1997;131:252–257. doi: 10.1016/s0022-3476(97)70162-8. [DOI] [PubMed] [Google Scholar]
- 120.Hoppe B, Kemper MJ. Diagnostic examination of the child with urolithiasis or nephrocalcinosis. Pediatr Nephrol. 2010;25:403–413. doi: 10.1007/s00467-008-1073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rumsby G. Experience in prenatal diagnosis of primary hyperoxaluria type 1. J Nephrol. 1998;11(1):13–14. [PubMed] [Google Scholar]
- 122.Barratt TM, Kasidas GP, Murdoch I, Rose GA. Urinary oxalate and glycolate excretion and plasma oxalate concentration. Arch Dis Child. 1991;66:501–503. doi: 10.1136/adc.66.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.von Schnakenburg C, Byrd DJ, Latta K, Reusz GS, Graf D, Brodehl J. Determination of oxalate excretion in spot urines of healthy children by ion chromatography. Eur J Clin Chem Clin Biochem. 1994;32:27–29. doi: 10.1515/cclm.1994.32.1.27. [DOI] [PubMed] [Google Scholar]
- 124.Gibbs DA, Watts RW. The variation of urinary oxalate excretion with age. J Lab Clin Med. 1969;73:901–908. [PubMed] [Google Scholar]
- 125.Morgenstern BZ, Milliner DS, Murphy ME, Simmons PS, Moyer TP, Wilson DM, Smith LH. Urinary oxalate and glycolate excretion patterns in the first year of life: a longitudinal study. J Pediatr. 1993;123:248–251. doi: 10.1016/s0022-3476(05)81696-8. [DOI] [PubMed] [Google Scholar]
- 126.Campfield T, Braden G. Urinary oxalate excretion by very low birth weight infants receiving parenteral nutrition. Pediatrics. 1989;84:860–863. [PubMed] [Google Scholar]
- 127.Milliner DS, Eickholt JT, Bergstralh EJ, Wilson DM, Smith LH. Results of long-term treatment with orthophosphate and pyridoxine in patients with primary hyperoxaluria. N Engl J Med. 1994;331:1553–1558. doi: 10.1056/NEJM199412083312304. [DOI] [PubMed] [Google Scholar]
- 128.Morgan SH, Purkiss P, Watts RW, Mansell MA. Oxalate dynamics in chronic renal failure. Comparison with normal subjects and patients with primary hyperoxaluria. Nephron. 1987;46:253–257. doi: 10.1159/000184364. [DOI] [PubMed] [Google Scholar]


