Abstract
There are several clinical trials worldwide utilizing BMSCs as a cellular therapy to modulate immune responses in patients suffering from various inflammatory conditions. A deeper understanding of the molecular mechanisms involved in this modulatory effect could help us design better, more effective protocols to treat immune mediated diseases.
In our present study we demonstrated that human BMSCs express H1, H2 and H4 histamine receptors and they respond to histamine stimulation with an increased IL-6 production both in vitro and in vivo. Using different receptor antagonists we pinpointed the importance of the H1 histamine receptor, while Western blot analysis and application of various MAP kinase inhibitors highlighted the role of p38, ERK and JNK kinases in the observed effect. When BMSCs were pretreated with either histamine, or degranulated human mast cells they exhibited an enhanced IL-6 dependent anti-apoptotic effect on neutrophil granulocytes. Based on these observations it is likely that introduction of BMSCs into a histamine rich environment (such as any allergic setting) or pretreatment of these cells with synthetic histamine could have a significant modulatory effect on the therapeutic potential of BMSCs.
Introduction
Bone marrow stromal cells (BMSCs also called mesenchymal stem cells or MSCs) are known to generate osteogeneic, adipogeneic, and chondrogeneic lineages and help maintain the microenvironment necessary for hematopoiesis. In the last five years it became evident that BMSCs - in addition to nursing hematopoietic stem cells - also have potent immunoregulatory functions; they appear to regulate the differentiation, survival and activation of a wide variety of immune cells1–4. In recent studies, we have shown that when BMSCs are introduced into a pathological millieu they can detect soluble disease-specific mediators (e.g., LPS and TNF-α in sepsis, or IL-4 and IL-13 in asthma) and respond to them in ways that are optimal for the host 5, 6.
Histamine is a biogenic amine that plays an essential role in several physiological and pathophysiological processes. It acts as a neurotransmitter in the CNS, regulates HCl synthesis in the stomach, and mediates anaphylaxis in allergic conditions. Released from mast cells and basophil granulocytes it also contributes to the pathological changes seen in sepsis and several auto- and alloimmune disorders7, 8 and plays an important role in immunomodulation 9 through its affect on cytokines. Histamine appears to stimulate the production and release of cytokines including IL-1a and IL-6 in different cells, both known to be produced by BMSCs 10–12 and has pro- and anti-inflammatory and immunoregulatory functions13, 14. Since in our previously published study 15 a GPCR array based on multiplex PCR suggested that BMSCs might express mRNAs encoding at least two of the histamine receptors, H1 and H2, we wondered if BMSC-derived IL-6 production might be regulated by histamine in vitro as well as in vivo.
Materials and Methods
BMSCs were isolated from bone marrow aspirates donated by healthy volunteers. Cells were grown in complete MEM-alpha medium (20% FBS, 1% Pen/Strep, 1% Glutamine). Characterization of the cells showed osteogeneic and adipogeneic differentiation potential in vitro, and expression of various BMSC specific cell surface markers (CD73, CD90, CD105, CD146) but lack of hematopoietic markers (CD45, CD14, CD34).
Immunohistochemistry
For immunostaining, human BMSCs in chamber slides (8-well chambers from Lab-Tek) were used (5000 cells/ well fixed with 4% paraformaldehyde). The slides were blocked with 1× Universal Blocking Reagent (Biogenex, San Ramon, CA, USA) for 10 min at RT. Primary antibodies (rabbit anti-human H1R, H2R, H3R, H4R from Alpha Diagnostic) were applied overnight at 4° C, diluted 1:100 in 1% BSA containing 0.25% triton X100. After washing 3 times in PBS, Alexa-488 conjugated anti-rabbit secondary antibody was used at a 1:1000 dilution for 1 hour at RT. DAPI was used to visualize cell nuclei. The fluorescent staining was observed with a Leica DMI600 inverted fluorescent microscope using the FITC filter set.
Western blots
BMSCs were collected following trypsin digestion and total protein concentrations were determined using the Micro BCA kit (Pierce). Twenty micrograms of the total protein lysates were run on 4-12% Bis Tris gels and blotted to nitrocellulose membranes (Invitrogen). The membranes were blocked with 4% nonfat dry milk in TBS (1x TBS, 0.01% Tween 20) and incubated overnight at 4 °C with antibodies against phosphorylated p38 MAP kinase (p38), phosphorylated p44/42 MAP kinase (ERK), and phosphorylated c-jun terminal NH2 kinase at 1:500, 1:1000, and 1:500 dilution, respectively or as a control with antibodies against total p38, total ERK, and total JNK at 1:1000 dilution each. All antibodies were from Cell Signaling. Anti rabbit HRP (Jackson Immunoresearch) was used as a secondary antibody for 1 hour at RT. The blots were visualized with Western lightning enhanced chemiluminescence reagents (Perkin Elmer).
Cytosolic Ca2+ measurements
For cytosolic Ca2+ measurements, cells were loaded with 3 µM fura-2/AM (45 min, room temperature). Ca2+ measurements were performed at room temperature in a modified Krebs-Ringer buffer containing 120 mM NaCl, 4.7 mM KCl, 1.2 mM CaCl2, 0.7 mM MgSO4, 10 mM glucose, and 10 mM Na-Hepes, pH 7.4. An inverted microscope (IX70; Olympus) equipped with an illuminator (Lambda-DG4; Sutter Instrument Co.) and a digital camera (MicroMAX-1024BFT; Roper Scientific) and the appropriate filter sets were used for Ca2+ analysis. Data acquisition and processing was performed by the MetaFluor software (Molecular Devices).
Histamine stimulation with or without antagonists, inhibitors
For in vitro IL-6 measurements BMSCs were cultured in 96 well flat bottom cell culture plates (Corning) at a density of 20 thousand cells/well/200uL in complete MEM-alpha medium. Histamine was applied at a concentration of 10-4, 10-5, 10-6, 10-7 or 10-8 M, histamine receptor antagonists at 10−5 M (Diphenhydramine hydrochloride (H1 antagonist), Cimetidine (H2 receptor antagonist), Ciproxifan hydrochloride (H3 antagonist), JNJ7777120 (H4 antagonist). MAP kinase pathway inhibitors (SP203580 a specific inhibitor of p38, PD98059 a specific inhibitor of ERK phosphorylation, SP600125 a specific inhibitor of JNK) and the PLC inhibitor U73122 were used at 10-5 M or 10-6 M concentrations. Antagonists or inhibitors were added 1 hour prior to addition of histamine. IL-6 was measured from the supernatants after 6, 12 or 24 hour histamine stimulation using DuoSet ELISA kit following the manufacturer’s instructions (R&D Systems).
Stimulation of BMSCs with additional MC derived factors
To test the hypothesis of additional MC derived factors affecting the BMSCs IL-6 production we repeated the in vitro experiments and stimulated the BMSCs with additional factors released by MCs 16, such as leukotriene C4 (LC4), platelet-activating factor (PAF), serotonin (5HT), leukotriene B4 (LB4) and prostaglandin D2 (PGD2). We chose these compounds, because based on our GPCR array 15 BMSCs bear the appropriate receptors to respond (Supplemental Table 1). Ten thousand BMSCs /well were plated in 96 well plates in MEM-alpha medium with 5% FBS. After overnight incubation, the medium was changed and the cells were stimulated with prostaglandin D2 (PGD2) at a concentration of 10−5 M, 10−6 M and 10−7 M for 6, 12 or 24 hrs. Control wells were added medium and a same volume of vehicle (ethanol) as by the PDG2 stimulation. Other mediators we used to stimulate BMSCs for 6 hrs in these experiments were: serotonin (concentration range: 10−4 M – 10−10 M), platelet activating factor (10−6 M – 10−12 M), leukotriene B4 (10−6 M – 10−10 M) and leukotriene C4 (10−6 M – 10−10 M). All of the mediators were purchased from Cayman Chemical (Ann Arbor, MI), except serotonin that was from Tocris Bioscience (Ellisville, MO). Cell culture supernatants were measured in quadruplicates using the human IL-6 DuoSet ELISA kit (R&D Systems). Non-stimulated cells (medium alone) and histamine-stimulated cells were included in each assay (data not shown). Experiments were done using BMSCs derived from 4 different donors.
Culture and assays involving human mast cells
Human derived mast cells (HuMC) were cultured as described. In brief, peripheral blood CD34+ progenitor cells were collected from healthy donors after informed consent and affinity column apheresis. CD34+ cells were cultured in StemPro-34 SFM (Life Technologies, Grand Island, NY) in the presence of recombinant IL-3 (first week only), recombinant IL-6 and recombinant human stem cell factor (rhSCF) (Peprotech). HuMC cultures were maintained up to 10 weeks at 37°C and 5 % CO2.
HuMC were sensitized overnight with 100 ng/ml biotinylated IgE (Sigma-Aldrich). The next day 50 thousand HuMCs were added to the upper chamber of a 96-well transwell system (Corning) in complete MEM-alpha medium and degranulated by the addition of 100 ng/ml streptavidin (Sigma-Aldrich). After 6 hours the transwell inserts were removed and the lower chamber gently washed with cell culture medium twice. After the last wash 200 uL fresh medium was added to each wells. 6 hours thereafter supernatants were collected and assayed for IL-6.
Isolation of granulocytes from whole blood
Granulocytes were isolated using Lymphocyte Poly(R) density gradient (Cedarlane Labs) following the manufacturers instructions. Briefly, anticoagulated whole blood is layered on top of the density solution. After centrifugation the granulocyte layer was removed, and contaminating red blood cells were lysed using ACK lysing buffer. Granulocyte purity was routinely over 90% as confirmed by FACS using CD66b-FITC antibody (BD biosciences). For the superoxide production experiments granulocytes were isolated using a different method. In these experiments anticoagulated (heparin) blood was layered on top of Histopaque 1119 (Sigma, St. Louis, MO, USA) and centrifuged (800g 30min RT). Upper serum layer was aspirated, the middle phase containing white blood cells was collected. Pellets were resuspended in PBS and layered on top of a Percoll gradient (65, 70, 75, 80 and 85%, Sigma, St. Louis, MO, USA). After centrifugation (800g 20 min RT) the 70-75-80% Percoll layers containing neutrophils were collected, washed and resuspended in PBS. Viability of the cells was determined by Trypan Blue dye extrusion and resulted in >99% viable neutrophils. The purity of the preparations was determined by Wright-Giemsa staining and yielded >95% neutrophil granulocytes.
Granulocyte/BMSC cocultures and assay for apoptosis
200 thousand granulocytes were added in 200 uL complete medium to 96-well U-bottom plates either alone, or together with 20 thousand BMSCs. After 18 hours of culture, percentage of apoptotic granulocytes were assessed by FACS using Annexin-V-APC and 7-AAD (both from BD biosciences). Annexin-V single positive cells were considered early apoptotic, whereas Annexin-V/7-AAD double positive cells were late apoptotic.
In case of histamine or HuMC prestimulation of stem cells, BMSCs were pretreated for 24h with 10−5 M histamine, or cocultured for 6 hours with 50 thousand degranulated HuMCs. Granulocytes were given thereafter.
Granulocyte/BSMC cocultures and measurement of neutrophil superoxide production
Granulocyte were cultured with or without BMSCs in RPMI1640 complete medium on 6-well plates at a 500:1 granulocyte:BMSC ratio for 24 hours. Neutrophils were collected, washed, resuspended in HBSS and transferred to 96-well white opaque plates. Cells were stimulated in triplicates at a final concentration of 1.2x106/mL with 1 µM formyl-methionyl-leucyl-phenylalanine (fMLP; Sigma, St. Louis, MO, USA) or 10 nM phorbol myristate acetate (PMA; Sigma, St. Louis, MO, USA). Superoxide production was measured by the Diogenes cellular luminescence enhancement system (National Diagnostics, Atlanta, GA, USA). Luminescence was followed for 15 minutes on a Luminoskan Ascent microplate luminometer (ThermoScientific, Hudson, NH, USA). Cumulative superoxide production was calculated as the area under the measured curve and it is presented as integrated relative luminescence units (RLU).
In vivo experiments
1. Assaying the specificity of Histamine in the effect
6–8 weeks old male WT C57BL/6 mice (JAX), WT Balb/C and HDC (histidine-decarboxylase) KO mice (provided by Dr. Andras Falus, Department of Genetics, Cell- and Immunobiology, Semmelweis University, Hungary) or Mast cell-deficient KitW-sh/W-sh mice (provided by Dr. David M Segal, NCI, NIH) were sensitized i.p. with 1 µg monoclonal IgE anti-DNP (Sigma) in 200 uL PBS followed 24 hours later by i.p. challenge with 0.5 mg/ml DNP-HSA in 200 µL PBS. Immediately following challenge, 4 million human BMSCs were injected into the peritoneal cavity. 6 hours later peritoneal cavity was washed with 2 ml PBS. After centrifugation of the lavage fluid to remove cellular components, the supernatants were frozen and stored at −80 degrees until assaying for human IL-6.
2. Testing Histamine prestimulation on BMSCs effect in Zymosan induced peritonitis
500 µl of 2mg/ml Zymosan A (Sigma) was injected intraperitoneally into 10 week old male C57/B6 mice (n=4 per group). Human BMSCs (0.5 million in 200 µ PBS) were injected 30 minutes later intraperitoneally. In some cases BMSCs were prestimulated overnight with 10-4 histamine or cocultured with degranulated human mast cells with transwell separation for 6 hours. Six hours later peritoneal cavity was washed with 5 ml PBS. Peritoneal cells were analysed using FACS. To rule out possible contamination of human BMSCs, first we gated on mouse CD45 positive cells (CD45-PerCP from BD Biosciences). Neutrophil granulocytes were identified as Gr-1 (high) CD11b (high) double positive cells (Gr-1 FITC, CD11b-PE both from BD Bio-sciences). Apoptotic cells were detected using APC conjugated Annexin-V (eBioscience). The readout of the experiment was the absolute number of living (functional) peritoneal neutrophils.
Results
The actions of histamine are mediated by 4 G-protein coupled receptor subtypes (H1–H4), each of which has a tissue and cell specific distribution in the human body. In order to find out which histamine receptors are present in BMSCs we used RT-PCR and immunostaining. We observed a relatively strong expression of the H1 receptor, a less pronounced expression of the H2 and H4 receptors, and a complete lack of H3 receptors (Fig 1.).
Fig. 1.
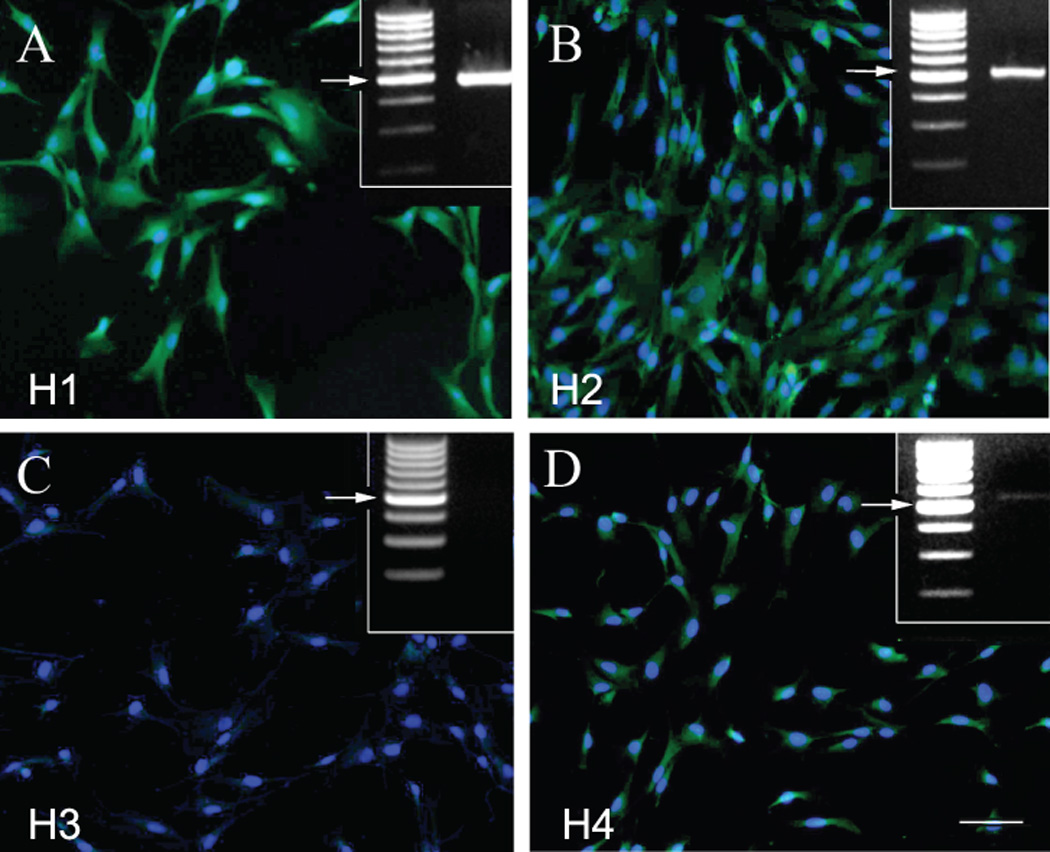
Human BMSCs express histamine receptors
Immunostaining of cultured human BMSCs using specific antibodies to recognize the H1(A), H2 (B), H3 (C) and H4 (D) receptors. The green fluorescence is due to the Alexafluor-488 secondary antibody. The bar represents 25 µm. The upper right insets demonstrate RT-PCR results using receptor specific primers. The arrows point at the expected size of the receptor proteins (all around 500 bases).
The H1, H2 and H4 receptors are present with both techniques, while the H3 does not seem to be expressed.
Next we measured the amount of IL-6 in the media of human BMSC cultures after exposing the cells to different concentrations of histamine, and found a time-and dose-dependent increase in the IL-6 output. The dose/response curve was bell shaped with a maximum IL-6 response (a 4-5 fold increase over the baseline) at 10−5 M histamine. This trend can be seen at all time points examined -- 6, 12, and 24 hours after introduction of histamine into the culture medium (Fig 2.A). This effect of histamine could be completely blocked using a specific H1 receptor antagonist, but was not affected by H2-H4 receptor antagonists (Fig.2.B).
Fig. 2.
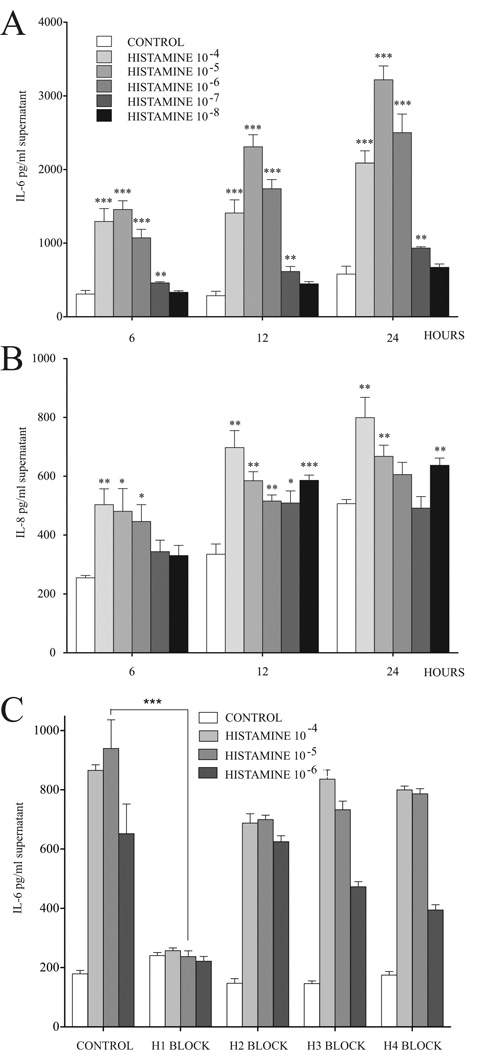
Histamine stimulates IL-6 and IL-8 secretion in BMSCs
The IL-6 and IL-8 concentrations of the BMSCs supernatants are dependent on the length of the treatment and concentration of histamine used, 10−5 M resulting in the largest effect when IL-6 is measured (A) and 10−4 M being the strongest stimulator when IL-8 release is studied (B). Using specific antagonists to block the effect on the individual histamine receptors demonstrates that H1 receptor block eliminates the increase in IL-6 at all concentrations (C).
To see if histamine released from human MCs could also stimulate IL-6 production and secretion by BMSCs, we cocultured the two cell types in a transwell system. Preincubation of BMSCs with activated (degranulated) MCs resulted in a significant increase in their IL-6 secretion. Unlike when only histamine was used, this effect was blunted but not eliminated by the addition of a histamine H1 antagonist to the culture medium. The effect of H1 antagonist studied on IL-6 secretion (Fig.3A) was incomplete suggesting that MC-derived factors in addition to histamine might mediate the effect on cytokine release. When we tested additional factors released by MCs that had the appropriate receptors present in BMSCs (Suppl. Table 1) we found that prostaglandin D2 (PGD2) was the only compound that also induced BMSC derived IL-6 release (Fig.3B).
Fig. 3.
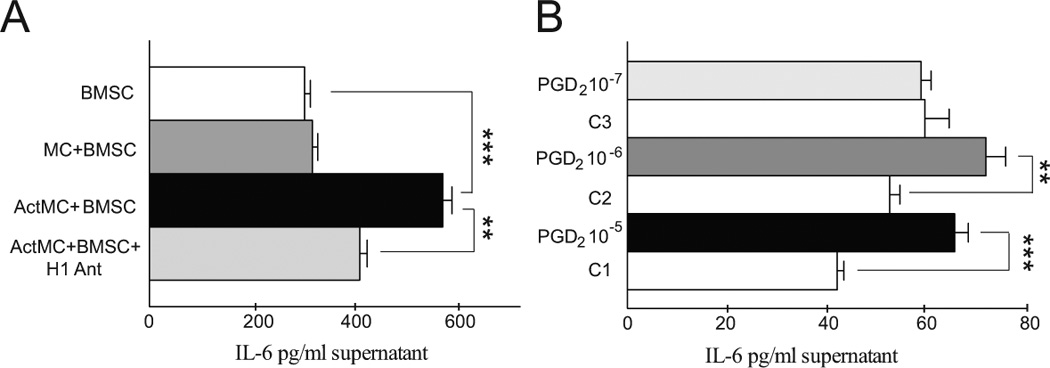
Mast cell derived factors increase IL-6 production of BMSCs in vitro. A: IL-6 production of BMSCs is not effected by co-culturing them with human MCs at a 1:1 ratio. When the MCs are first degranulated (ActMC) and then co-cultured with the BMSCs, there is a significant increase in the IL-6 production (p<0.001). Adding a H1 receptor antagonist to the media significantly decreases the production of IL-6 by BMSCs. B: prostaglandin D2, another major MC mediator also induces BMSC derived IL-6 secretion. Since PGD2 was dissolved in ethanol, the individual controls (C1-3) contain the same EtOH concentration. In 10−6 and 10−5 Molar concentrations PDG2 significantly increases the IL-6 production of BMSCs after 6 hours of treatment.
Next, after inducing mast cell degranulation we introduced human BMSCs into the peritoneal cavities of control and histamine deficient mice - animals lacking HDC, an enzyme required for histamine biosynthesis 17. Six hours later IL-6 increased in peritoneal lavage fluid from control animals but significantly reduced in fluid from the histamine deficient mice. It was not absent, however, suggesting that IL-6 production by the BMSCs in the presence of activated MCs is due not only to histamine but also to other factors. When BMSCs were introduced into MC deficient mice the same sensitisation/challenge protocol resulted in no IL-6 increase (Fig 4.) suggesting that the MCs are indeed responsible for driving the release of IL-6.
Fig. 4.
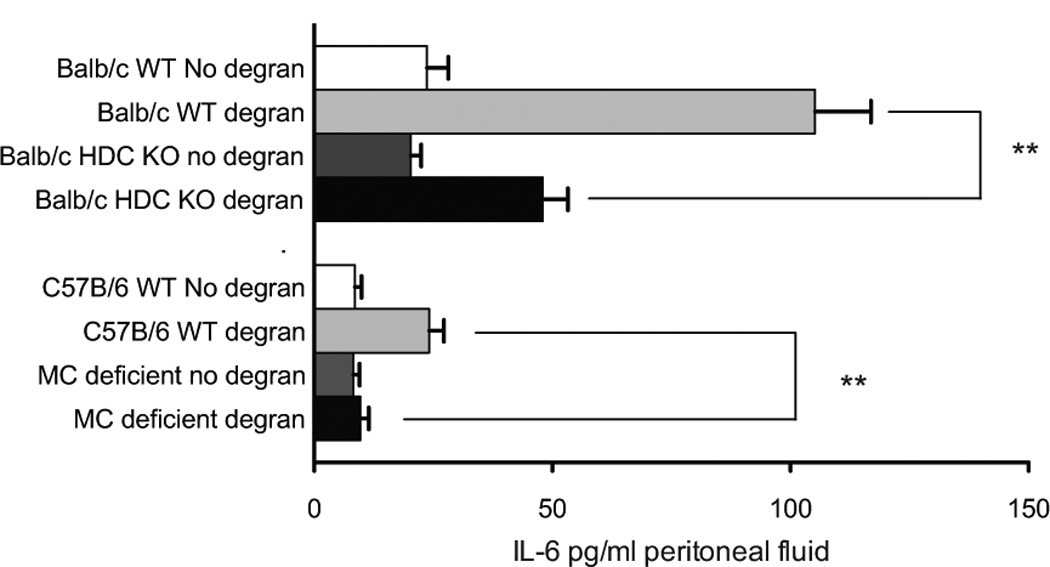
Human BMSCs produce increased amounts of IL-6 in vivo in a HDC-and mast cell- dependent manner. Human BMSCs were placed into the peritoneal cavity of control and histamine-deficient (lacking histidine decarboxylase or HDC, a vital enzyme in histamine biosynthesis) mice afer inducing MC degranulation. Six hours later the increase in IL-6 content of the cell-free peritoneal wash was significantly reduced, but still present, in the histamine-deficient mice. Once again this suggests that increased IL-6 production is due to not only histamine but also other MC derived factors. When BMSCs were introduced into MC-deficient mice the same sensitisation/challenge protocol resulted in no IL-6 suggesting the MCs to be responsible for the effect.
Histamine mobilizes calcium in several cell types8, 9. To test if it has the same effect on BMSCs we measured calcium concentrations in individual cells after addition of histamine at a dose of 10−5 M. Immediately after the amine was added, cytoplasmatic calcium concentrations started to increase and peaked after 30 seconds and reached a plateau thereafter (Fig 5.A).
Fig. 5.
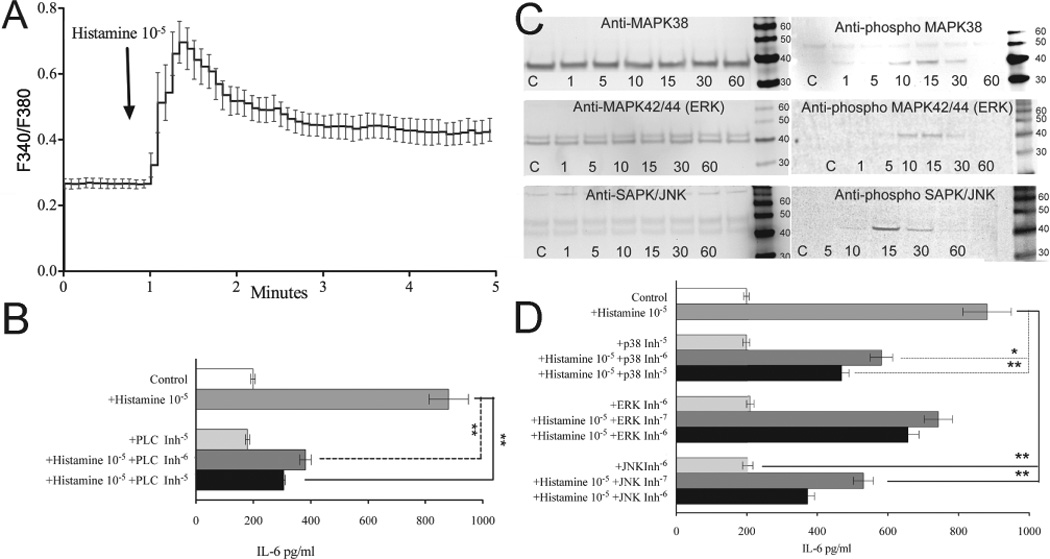
Histamine stimulation of BMSCs regulates IL-6 production via the MAP Kinase Pathway
(A) We measured cytosolic Ca2+ concentrations with Fura2 in individual cells. Addition of histamine (10−5 M) evoked a rapid increase in cytosolic Ca2+ peaking at 30 seconds after stimulation, followed by a sustained elevation.
(B) IL-6 increase in the presence of specific PLC inhibitor
(C) Western blot analysis shows the presence of p38, ERK and JNK. Addition of histamine elicited the activation of p38, ERK, and JNK after 15 minutes and declined to basal level by 60 minutes.
(D) IL-6 increase in the presence of specific MAP kinase inhibitors. SB203580, a specific p38 inhibitor, PD98059, a specific ERK inhibitor and SP600125, a specific JNK inhibitor all significantly impaired histamine's ability to induce BMSCs IL-6 production.
Phospholipase C (PLC) plays a critical role in mobilization of intracellular calcium. Upon cleavage of PIP2, phospholipase C releases IP3 which triggers calcium release from the smooth endoplasmic reticulum. PLC inhibitors can prevent cells from mobilizing calcium in response to histamine, and we used U73122, a specific inhibitor of PLC, to determine whether this enzyme mediated the effect of histamine on BMSCs described above. When the cells were pretreated with this agent, histamine could no longer elicit the same level of IL-6 increase seen earlier, pointing to the importance PLC triggered cellular events (Fig 5.B)
Histamine has been shown to trigger the activation of MAP kinase pathways in different cell types 18. To determine whether histamine can stimulate MAP kinases in BMSCs we performed Western blot analysis measuring the amount of phosphorylated p38, ERK and JNK. Addition of histamine elicited a time-dependent activation of all of the kinases examined. Maximal phosphorylation of p38, ERK, and JNK occurred after 15 minutes and declined to basal level by 60 minutes (Fig 5.C). Levels of the non-phosphorylated MAP kinases studied remained unchanged at all time points.
To explore how much individual MAP kinases contribute to the histamine induced IL-6 increase we looked at the effects of specific MAP kinase inhibitors. SB203580, a p38 inhibitor, PD98059, an ERK inhibitor, and SP600125, a JNK inhibitor all significantly impaired histamine's ability to induce IL-6 production by the stem cells (Fig 5.D).
Neutrophil granulocytes (PMNs) are professional phagocytes that rapidly engulf invading organisms that are coated with antibodies and complement. PMNs are inherently short-lived cells, with a half-life of about 6 –12 h in the circulation. At the end of their lives, they undergo spontaneous apoptosis. Recently Pistoya’s group reported that BMSCs can significantly prolong survival of PMNs in vitro in an IL-6 dependent manner 12. Since histamine seemed to increase IL-6 production by BMSCs we asked whether histamine pretreatment could enhance the antiapoptotic effect of BMSCs on PMNs. As expected, BMSCs suppressed spontaneous apoptosis of neutrophils as measured by Annexin-V binding and concurrent 7-AAD staining using FACS. Histamine prestimulation of the BMSCs increased their efficacy; the number of apoptotic PMNs was even smaller than it had been when unstimulated BMSCs were cocultured with neutrophils (Fig 6 A-C). To find out if histamine prestimulation of BMSCs can also help preserve the ROS producing activity of neutrophils, we measured superoxide production of neutrophil granulocytes following coculture with unstimulated or histamine pre-treated BMSCs. Although histamine prestimulation of BMSCs didn’t cause a measurable effect on the spontaneous superoxide production of neutrophils, we were able to detect a significant increase in fMLP triggered superoxide synthesis (Fig 6 D). To test if histamine pretreatment of BMSCs will result in an increase of neutrophil survival in vivo in an infectious environment we used a model of zymosan induced peritonitis 19. When the mice were injected with histamine pretreated BMSCs intraperitoneally, we found that the peritoneal wash contained significantly higher numbers of live neutrophils than if the BMSCs were not pretreated or were cultured with activated MCs (Fig.7).
Fig. 6.
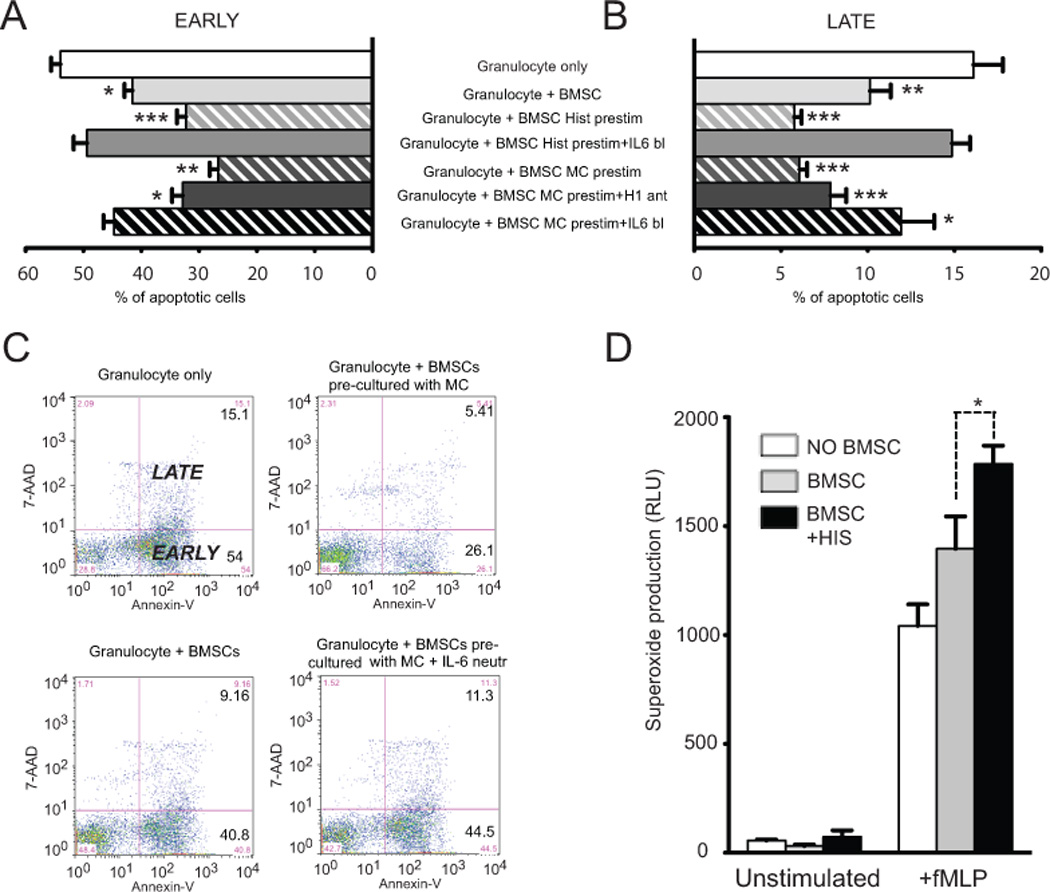
Histamine prestimulation increases the IL-6 driven antiapoptotic effect of BMSCs on peripherial blood derived granulocytes.
When placed in co-culture BMSCs effectively suppressed spontaneous apoptosis of neutrophils as measured by Annexin-V binding and concurrent 7-AAD staining using FACS (A-C). Histamine prestimuation of BMSCs was able to even further decrease the number of apoptotic PMNs. This potentiation of antiapoptotic function was eliminiated in the presence of neutralizing anti-IL-6 antibodies in the media. In another set of experiments instead of adding histamine itself we used degranulated human mast cells as a source of histamine to prestimulate the stromal cells. These pretreated BMSCs exhibited a significantly greater antiapoptotic effect as compared to histamine stimulation alone. Moreover this increased pro-survival phenotype was only partially suspended in the presence of H1 receptor blockade. IL-6 neutralization eliminated the anti-apoptotic effect. Neutrophil granulocytes significantly increased their fMLP induced superoxide production when they were co-cultured with BMSCs. Histamine prestimulation of BMSCs further increased the superoxide production of neutrophil granulocytes (D).
Fig. 7.
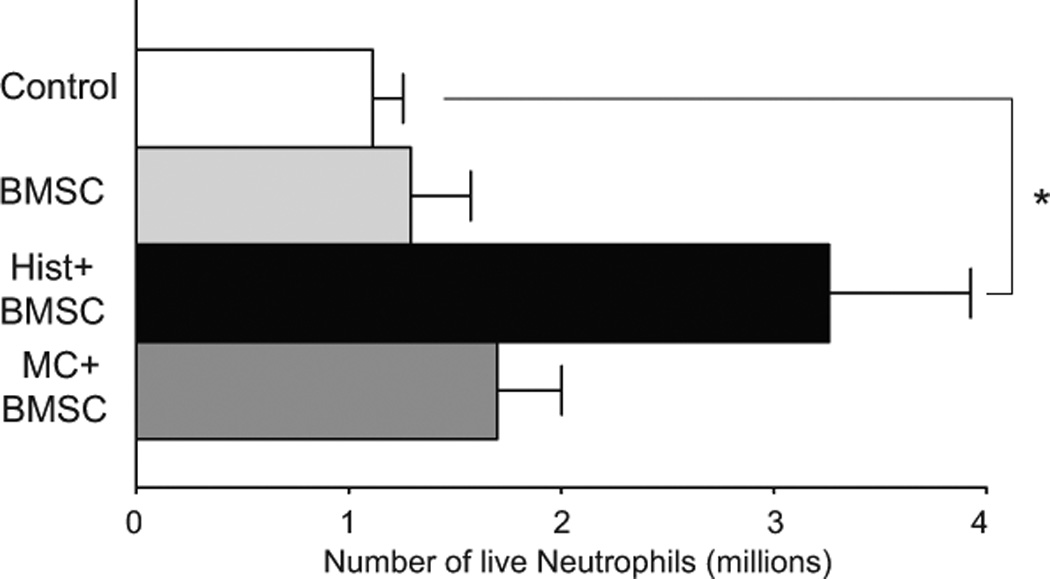
Number of live neutrophils in the peritoneal wash of mice with zymosan induced peritonitis. When the intraperitoneally injected BMSCs were pretreated with histamine there were almost three times more live neutrophils, than without pretreatment.
To explore the molecular mechanisms through which BMSCs acted we repeated the apoptotis experiments in the presence of an IL-6 blocking antibody or using an H1 receptor inhibitor. Potentiation of the antiapoptotic effect of BMSCs was eliminated when neutralizing anti-IL-6 antibodies were added to the culture medium, suggesting that IL-6 mediates the phenomenon. In another set of experiments instead of adding histamine we used degranulated human mast cells to prestimulate the stromal cells. BMSCs treated this way exhibited a significantly greater antiapoptotic effect than those treated with histamine alone, and the increase in PMN survival in response to degranulated mast cells was only partly countered by a histamine H1 receptor antagonist. As stated earlier, the most likely reason for this, is that factors in addition to histamine are secreted by mast cells to modulated BMSCs. Based on our findings, these other factors are likely to contribute to the increased production and secretion of IL-6, which appears to be the principal mediator of the anti-apoptotic effect of the BMSCs on PMNs.
Discussion
Histamine was discovered a century ago by Dale and Laindlaw 20, and it soon became obvious that the amine has a variety of biological functions. With regard to BMSCs, histamine has been suggested to play a role in osteogenesis, and the H1 receptor was identified as one of the downstream targets for the vitamin D receptor and was suggested to play a role in osteogenic differentiation of BMSCs 21. It soon became clear that histamine was also a very important proinflammatory mediator. Its role in the immediate type allergic reactions was quickly recognized, but its other immunomodulatory effects were only appreciated in the last decade. Histamine is now known to alter cytokine and chemokine production by a variety of cells including mast cells, eosinophils, dendritic cells and T cells9, 22–24. We wondered if and how histamine might affect the immunomodulatory functions of human BMSCs 8, 11, 25–28. Histamine was described to induce mouse bone marrow cells to produce IL-1a, a proinflammatory cytokine that is needed in small amounts to combat infection and initiate healing processes 29, 30 but one that can be harmful when its levels are excessive. A human disease, mastocytosis is a condition characterized by infiltration of mast cells into the tissues of the body, including the bone marrow. Mast cells release chemicals including large amounts of histamine during a variety of inflammatory and allergic conditions. In patients with mastocytosis the plasma histamine leves are usually high and increased IL-6 levels were reported 31. In patients with elevated IL-6 levels a significant increase in circulating neutrophil counts was also observed 31. We hypothesized that human BMSCs might respond to histamine stimulation by increasing their IL-6 production just as other immune cells do. Using cultured human BMSCs we found that histamine stimulation results in an increase of BMSC derived IL-6 secretion in a dose-and time-dependent manner. Next, we asked which of the four histamine receptors might be responsible for this effect and found, that in the in vitro conditions it is the H1 receptor that is responsible for the majority of the increase in BMSC IL-6 production in response to histamine. Histamine is a major mediator of acute inflammatory and immediate hypersensitivity responses and mast cells are known to release large amounts of histamine during degranulation, thus they might be a natural source of histamine when interacting with BMSCs in vivo. This is why we used a co-culture of human BMSCs and human MCs as the source of histamine, and found that – unlike when histamine alone was used - the H1 receptor blockade only partially eliminated the IL-6 increase in the medium. To determine the contribution of MC-derived histamine to the effect seen, we performed an in vivo experiment when we introduced human BMSCs into the peritoneal cavity of control and histamine-deficient mice 17 after inducing mast cell degranulation. Although the increase in IL-6 content of the cell-free peritoneal wash was significantly reduced in the histamine-deficient mice, the IL-6 content of the peritoneal wash was still significantly higher than without inducing mast cell degranulation. The most likely explanation for this result is that degranulating MCs release a number of mediators in addition to histamine, such as monoamines, leukotrienes, proteases, SCF etc 7, 16. We focused on a few MC mediators that affect receptors that we have found to be present in human BMSCs based on our GPCR array 15. These included LTD4, LB4, PAF, serotonin and PGD2. We found that in our experimental conditions only prostaglandin D2 was able to induce IL-6 release from BMSCs at the 6 hour timepoint. In fact, since PGD2 is one of the major lipid mediators in MCs 16, it is possible that the IL-6 increase that we could not block using H1 blockers is due - at least in part - to released PGD2.
Using kinase inhibitors, we established that the signaling molecules responsible for IL-6 induction belong to the MAP kinase family; p38, ERK, and JNK are all phosphorylated in BMSCs in response to histamine. This result is in agreement with data showing that histamine receptors trigger the MAPK pathways in other cell types 18. In addition to MAP kinases, Ca signaling has also been suggested to play a role in histamine receptor-induced cellular effects 8 and we indeed found that addition of histamine induces intracellular calcium concentrations and blocking calcium mobilization eliminates the IL-6 increase.
After we established that both histamine and MCs induce human BMSCs to produce and release IL-6, we wondered about the physiological significance of this effect. A number of studies suggested that IL-6 can block apoptosis of a variety of cell types including immune cells and neural stem cells11, 12, 27, 32, 33. Since our previous study in septic mice 6 suggested that increasing the number of circulating PMNs has a significant effect in fighting infection, we wanted to see whether BMSCs might release IL-6 to decrease PMN apoptosis. Our results showed that in a co-culture system of human PMNs and BMSCs the BMSCs effectively suppressed spontaneous apoptosis of neutrophils. Prestimulation of BMSCs with histamine further decreased the number of apoptotic PMNs while increasing fMLP induced superoxide production of these phagocytic cells. Co-culturing the BMSCs with degranulated human MCs before they were added to PMN cultures significantly increased the anti-apoptotic effect. This effect that could only be partially blocked by H1 blockade, is suggesting a role for other MC derived factors in the process. To examine the significance of IL-6 in such effect we also used neutralizing antibodies to IL-6 in the media, and this treatment eliminated the anti-apoptotic function. We set up an in vivo experiment utilizing a mouse model to test if histamine pretreatment would increase the efficiency of BMSCs in improving PMN survival. In this model, zymosan, a polysaccharide cell wall component derived from Saccharomyces cerevisiae, causes an acute inflammation and the zymosan-induced peritonitis model is extensively used to quantify the recruitment of monocytes and neutrophils into the peritoneal cavity 19. We found that the BMSCs were significantly more efficient if they were pretreated with histamine prior to their intraperitoneal injection. In the peritoneal wash we found a significant increase in the number of live neutrophils if these histamine pretreated BMSCs were applied. Interestingly though, if instead of pretreatment with histamine, the BMSCs were co-cultured with human MCs prior to injection, we did not find an increased number of PMNs in the peritoneal wash. This might be due to a variety of factors, such as using human MCs for pretreatment (unlike our previous in vivo experiment, when we inject the human BMSCs and the responding cells are naturally mouse MCs) or the likelihood that MCs release many additional moderators other than histamine, and some of these might have opposing effects on neutrophil survival.
Most of the in vivo studies suggesting the immunomodulatory actions of BMSCs are based on intravenous injection of BMSCs. After introducing the BMSCs into the circulation the cells will end up in small vessels (arterioles, capillaries, post-capillary venules) by following the natural path of blood circulation. In these vascular beds some of the BMSCs might participate in clot formation which attracts large number of platelets (that might also release histamine) while others might engage in direct interactions with endothelial cells and eventually enter the perivascular space. During these processes the BMSCs are exposed to platelet and endothelial cell derived factors both of which might release histamine22, 34 which will activate the BMSCs. This activation can be further enhanced when BMSCs encounter mast cells in the perivascular space (where most of the MCs reside), especially when administered in an allergic setting or in a tissue that previously recruited large number of MCs (as seen in mastocytosis or in tolerated allogeneic organ grafts). In these in vivo settings the BMSCs could be bathed in histamine that would cause them (through their histamine receptors) to make and release large amounts of IL-8 a potent neutrophil chemoattractant as well as IL-6 a known anti-apoptotic cytokine. From the 4 receptors examined, the H1 receptor had the highest relative expression in BMSCs and using receptor specific antagonists we also found that histamine induced IL-6 production in vitro is solely dependent on H1 binding. Our experiments using human BMSCs and histamine deficient mice confirmed that MC derived histamine works in concert with other MC specific factors to induce the observed IL-6 increase.
Our studies revealed another new mechanism underlying the anti-inflammatory/anti-infectious effects of human BMSCs. We established the presence of three of the histamine receptors on these cells and pinpointed the H1 receptor as the major target receptor for the effect. Since H1 receptor antagonists are available and widely used, their effects on the BMSCs function could be significant and this possible side effect should be in consideration. On the other hand, since histamine treatment seems to induce the ability of BMSCs to attract PMNs and block their apoptosis, one could also imagine an advantage of using pretreatment of donor BMSCs before their therapeutic use to enhance their efficacy in improving survival in septic settings or in other inflammatory disorders in patients.
Supplementary Material
Fig. 8.
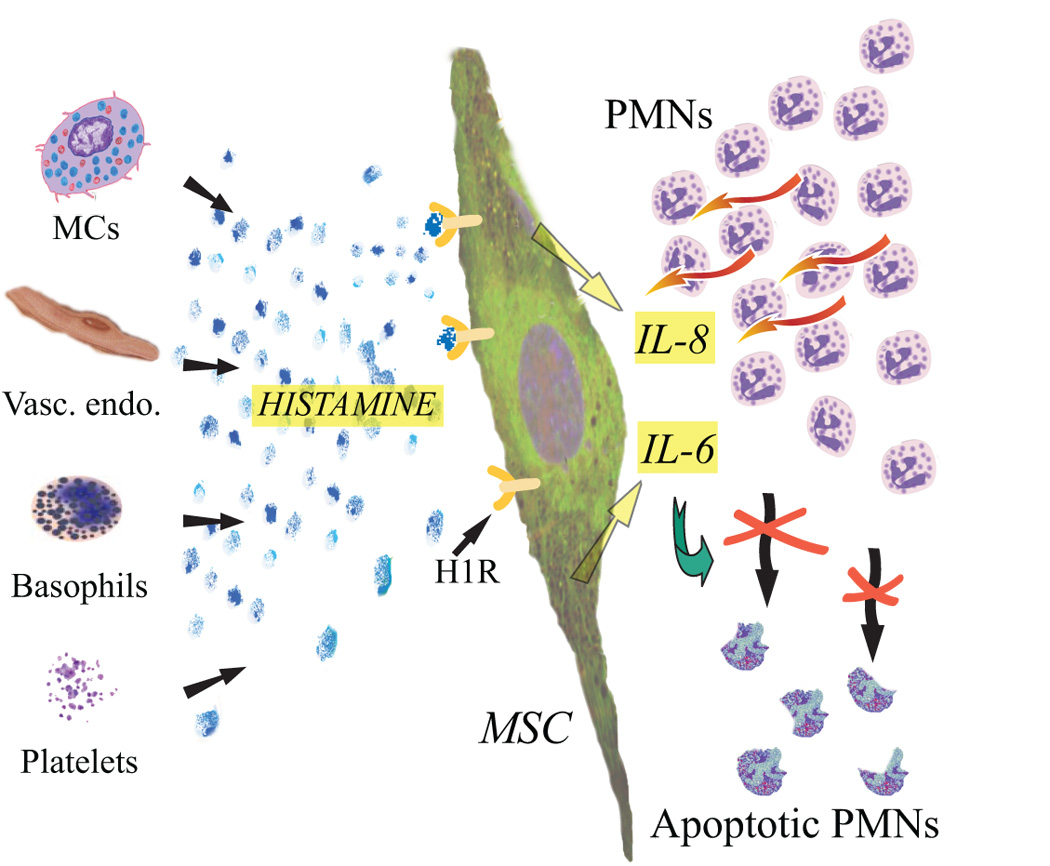
A schematic drawing summarizing the hypothesized effect of histamine stimulation on BMSCs. The histamine might derive from a variety of cellular sources (or might be added as a prestimulation before delivering the cells) and effects the H1 receptor of the BMSC. In response the BMSCs will increase their IL-8 production, a cytokine that is a strong chemoattractant for neutrophil granulocytes. At the same time, the increased release of IL-6 will ensure the better survival of these attracted cells by its strong anti-apoptotic function. Thus the end-result is a larger number of live, functioning neutrophil granulocytes that will normalize an inflammatory /infectious environment.
Acknowledgments
The authors would like to thank Dr. Dean Metcalfe for his help with the human mast cells; Dr.Tamas Balla for his guidance and help with calcium measurements.
This work was supported by the DIR, NIDCR, of the IRP, NIH, DHHS.
References
- 1.Mezey E, Mayer B, Nemeth K. Unexpected roles for bone marrow stromal cells (or MSCs): a real promise for cellular, but not replacement, therapy. Oral Dis. 2010;16:129–135. doi: 10.1111/j.1601-0825.2009.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nemeth K, Mayer B, Mezey E. Modulation of bone marrow stromal cell functions in infectious diseases by toll-like receptor ligands. J Mol Med. 2010;88:5–10. doi: 10.1007/s00109-009-0523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tasso R, Pennesi G. When stem cells meet immunoregulation. Int Immunopharmacol. 2009;9:596–598. doi: 10.1016/j.intimp.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 5.Nemeth K, Keane-Myers A, Brown JM, et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci U S A. 2010;107:5652–5657. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundequist A, Pejler G. Biological implications of preformed mast cell mediators. Cell Mol Life Sci. 2010 doi: 10.1007/s00018-010-0587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacGlashan D., Jr Histamine: A mediator of inflammation. J Allergy Clin Immunol. 2003;112:S53–S59. doi: 10.1016/s0091-6749(03)01877-3. [DOI] [PubMed] [Google Scholar]
- 9.Shahid M, Tripathi T, Sobia1 F, et al. Histamine, Histamine Receptors, and their Role in Immunomodulation: An Updated Systematic Review. The Open Immunology Journal. 2009;2:9–41. [Google Scholar]
- 10.Chen L, Tredget EE, Wu PY, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PloS one. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pricola KL, Kuhn NZ, Haleem-Smith H, et al. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J Cell Biochem. 2009;108:577–588. doi: 10.1002/jcb.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raffaghello L, Bianchi G, Bertolotto M, et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 2008;26:151–162. doi: 10.1634/stemcells.2007-0416. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen BK, Steensberg A, Keller P, et al. Muscle-derived interleukin-6: lipolytic, anti-inflammatory and immune regulatory effects. Pflugers Arch. 2003;446:9–16. doi: 10.1007/s00424-002-0981-z. [DOI] [PubMed] [Google Scholar]
- 14.Woods JA, Vieira VJ, Keylock KT. Exercise, inflammation, and innate immunity. Immunol Allergy Clin North Am. 2009;29:381–393. doi: 10.1016/j.iac.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Hansen A, Chen Y, Inman JM, et al. Sensitive and specific method for detecting G protein-coupled receptor mRNAs. Nat Methods. 2007;4:35–37. doi: 10.1038/nmeth977. [DOI] [PubMed] [Google Scholar]
- 16.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125:S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohtsu H, Tanaka S, Terui T, et al. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 2001;502:53–56. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- 18.Lipnik-Stangelj M. Multiple role of histamine H1-receptor-PKC-MAPK signalling pathway in histamine-stimulated nerve growth factor synthesis and secretion. Biochem Pharmacol. 2006;72:1375–1381. doi: 10.1016/j.bcp.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 19.Cash JL, White GE, Greaves DR. Chapter 17. Zymosan-induced peritonitis as a simple experimental system for the study of inflammation. Methods Enzymol. 2009;461:379–396. doi: 10.1016/S0076-6879(09)05417-2. [DOI] [PubMed] [Google Scholar]
- 20.Dale HH, Laidlaw PP. The physiological action of β-iminazolylethylamine. J. Physiol. 1910;41:318–344. doi: 10.1113/jphysiol.1910.sp001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pochampally RR, Ylostalo J, Penfornis P, et al. Histamine receptor H1 and dermatopontin: new downstream targets of the vitamin D receptor. J Bone Miner Res. 2007;22:1338–1349. doi: 10.1359/jbmr.070605. [DOI] [PubMed] [Google Scholar]
- 22.Jutel M, Blaser K, Akdis CA. The role of histamine in regulation of immune responses. Chem Immunol Allergy. 2006;91:174–187. doi: 10.1159/000090280. [DOI] [PubMed] [Google Scholar]
- 23.Oda T, Morikawa N, Saito Y, et al. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J Biol Chem. 2000;275:36781–36786. doi: 10.1074/jbc.M006480200. [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Thurmond RL, Dunford PJ. The histamine H(4) receptor: a novel modulator of inflammatory and immune disorders. Pharmacol Ther. 2007;113:594–606. doi: 10.1016/j.pharmthera.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Triggiani M, Gentile M, Secondo A, et al. Histamine induces exocytosis and IL-6 production from human lung macrophages through interaction with H1 receptors. J Immunol. 2001;166:4083–4091. doi: 10.4049/jimmunol.166.6.4083. [DOI] [PubMed] [Google Scholar]
- 26.Jutel M, Blaser K, Akdis CA. Histamine in allergic inflammation and immune modulation. Int Arch Allergy Immunol. 2005;137:82–92. doi: 10.1159/000085108. [DOI] [PubMed] [Google Scholar]
- 27.Parekkadan B, van Poll D, Megeed Z, et al. Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochem Biophys Res Commun. 2007;363:247–252. doi: 10.1016/j.bbrc.2007.05.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vena GA, Cassano N, Buquicchio R, et al. Antiinflammatory effects of H1-antihistamines: clinical and immunological relevance. Curr Pharm Des. 2008;14:2902–2911. doi: 10.2174/138161208786369777. [DOI] [PubMed] [Google Scholar]
- 29.Dinarello CA. The interleukin-1 family: 10 years of discovery. FASEB J. 1994;8:1314–1325. [PubMed] [Google Scholar]
- 30.Tasaka K, Mio M, Shimazawa M, et al. Histamine-induced production of interleukin-1 alpha from murine bone marrow stromal cells and its inhibition by H2 blockers. Mol Pharmacol. 1993;43:365–371. [PubMed] [Google Scholar]
- 31.Brockow K, Akin C, Huber M, et al. IL-6 levels predict disease variant and extent of organ involvement in patients with mastocytosis. Clin Immunol. 2005;115:216–223. doi: 10.1016/j.clim.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J Leukoc Biol. 2008;83:804–816. doi: 10.1189/jlb.0807581. [DOI] [PubMed] [Google Scholar]
- 33.Walker PA, Harting MT, Jimenez F, et al. Direct intrathecal implantation of mesenchymal stromal cells leads to enhanced neuroprotection via an NFkappaB-mediated increase in interleukin-6 production. Stem Cells Dev. 2010;19:867–876. doi: 10.1089/scd.2009.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mannaioni PF, Di Bello MG, Raspanti S, et al. Storage and release of histamine in human platelets. Inflamm Res. 1995;44(Suppl 1):S16–S17. doi: 10.1007/BF01674374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


