Abstract
Background
Urotensin II is a vasoactive polypeptide. It is known that some vasoactive polypeptides are produced and secreted by tumor cells, and act as a paracrine growth stimulant. The aim of this study was to examine the relationship between urotensin II and its receptor’s messenger RNA expression in breast cancer.
Material/Methods
Fifty-nine women with breast cancer were included in this study. The median age was 48 years. The relationships between urotensin II and urotensin II receptor mRNA expressions, which were derived from fresh breast cancer tissues and adjacent normal breast tissues, and clinical and pathological parameters, were assessed.
Results
We found expressions of urotensin II mRNA and its receptor in 55 of 59 breast cancer tissues and in 55 of 59 normal breast tissues. We found a positive significant correlation between urotensin II and its receptor (p=0.001, r=0.632), and found a negative, but insignificant, correlation between urotensin II and age (p=0.038, r=−0.281). Urotensin II levels were higher in the premenopausal group compared to the postmenopausal group (p<0.05). The mean urotensin II receptor expression was higher in the premenopausal group (p<0.05) compared to the postmenopausal group, and its expression was also higher in the group without extra-nodal invasion compared to that of the group with extra-nodal invasion (p=0.001). Urotensin II levels were higher in the group without lymphatic invasion compared to the group with lymphatic invasion (p=0.048).
Conclusions
This study is the first in the English medical literature to determine the urotensin II and its receptor mRNA expressions in breast cancer tissues. Consequently, urotensin II seems be associated with menopausal status, and extra-nodal and lymphatic invasion.
MeSH Keywords: Breast Neoplasms - genetics, Urotensins - genetics, Urotensins - physiology
Background
It is known that some vasoactive polypeptides are produced and secreted by tumor cells, and act as a paracrine growth stimulant. For instance, somatostatin (SST) inhibits the secretion of growth factors and hormones, which stimulates the tumor cell growth. It inhibits the DNA synthesis and angiogenesis formed by growth factors, and increases vasoconstriction and coordinates the functions of immune cells [1,2]. SST has been shown to be present in many tumors, including breast cancer [3].
Urotensin II (U-II), another vasoactive substance with a sequential structure similar to SST, is a peptide hormone with a cyclic structure of 11 amino acids. U-II has been shown to be the endogenous ligand of an orphan G-protein-coupled receptor (GPR 14). Because of the high selectivity of GPR 14 for U-II, this receptor was later called the urotensin receptor (UTR) [4]. U-II expresses its effect in various tissues by binding to UTR. U-II is encoded by the U-II gene, which is located in 1p36.23. Since it has been identified, the mRNA of this molecule has been obtained from various tissues in the human body [5].
U-II is known to have many effects on angiogenesis [6] and mitogenesis [7]. In-vivo and in-vitro, U-II has also been determined to have a powerful angiogenic effect, comparable with that of the fibroblast growth factor (FGF)-2, which is a classical angiogenic cytokine [6]. This effect seems to be induced through UTR. A UTR antagonist called polasuran (ACT058362) has also been identified, which inhibits this process [8].
U-II has been shown to stimulate the proliferation of the human adrenocortical carcinoma (SW-13) and human renal cell carcinoma (VMRC-RCW) cell lines [9]. However, the effects of U-II and UTR have not been studied thoroughly in tumor cells. U-II serves as a growth-stimulating factor in tumor cells. It also has a mitogenic effect on various tumors, such as human adrenocortical carcinoma SW-13 [10], human renal cell carcinoma VMRC-RCW cell lines [9], and pheochromocytoma [11], and significantly stimulates tumor proliferation. SW-13 cells have been also demonstrated to secrete U-II [12].
Due to the above-mentioned effects, U-II may have a significant impact on the pathogenesis of breast cancer. An investigation of U-II can provide useful prognostic and predictive information about patients with breast cancer. In our study, we aimed to investigate the potential relationship of U-II and UTR messenger RNA (mRNA) expression in breast cancer patients with clinical and pathological parameters. This is the first study in the English literature to investigate the relationship between U-II and UTR and breast cancer.
Material and Methods
This study was approved by the Ethics Committee of Gaziantep University Faculty of Medicine, on June 30, 2011 (reference number 133). Patients who were newly diagnosed breast cancer patients in the Gaziantep University, Faculty of Medicine, Gaziantep Oncology Hospital, Department of Oncology were included in the study. None of them had received anticancer therapy before inclusion into the study. A signed informed consent form was obtained from each patient. U-II and UTR mRNA expression were examined in the samples of breast tumor tissue and healthy tissue from the patients. The samples were taken by biopsy during surgery. The normal tissue was the tissue surrounding the tumor.
Demographic characteristics of the patients such as age, sex, menopausal status, family history, smoking history, comorbidities, and tumor characteristics such as tumor size, nodal involvement, and stage of disease, were recorded. The stage of disease at diagnosis was determined by means of clinical examination, mammography, ultrasonography, computed tomography, and bone scintigraphy methods. Patients were divided into groups according to the presence of menopause, smoking history, tumor histology, tumor grade, extra-nodal invasion, nodal status, distant metastases, and the stage of the disease.
The amounts of U-II and UTR mRNA in cancer and normal tissues of the same patient were determined by real-Time PCR method and the relative expression method was used to compare them statistically. The starting amounts of tumor and normal tissues were about 30 mg for RNA isolation procedure. According to qRT-PCR results, the differences between Ct values of U-II and UTR genes and Ct values of the housekeeping gene were used as normalized expression values in statistical comparison of related gene expression between cancer and normal tissues of the same patients in a method based on partial quantity. ACTB, considered as a reference gene, was the housekeeping gene (expression levels were constant under certain conditions), was at baseline level in all tissues or cells and was expressed without any variation. These normalized values gave information about the expression level of the related gene with respect to the reference gene. These differences were calculated for tumor tissue and normal tissue in the same patient separately. These values were statistically compared – relative expression information was obtained from the ratio of normalized expression values in cancer and normal tissues. For further comparisons, the patients were divided into 3 groups for both U-II and UTR cases as follows: patients having the rate under 0.9, patients having the rate 0.9–1.1, and patients having the rate above 1.1.
Obtaining RNA from the Tissue: A QIAamp RNA Blood Mini Kit (Qiagen, Cat. No. 52304) was used. Synthesis of cDNA was done using the Reverse Transcription Quantitect Qiagen (cat no. 205311) kit.
Real-time PCR: Human UTS2 Sabiosciences Qiagen (Cat. No. PPH02516A-200) UTS2-sense, and UTS2-antisense primers and SYBR Green PCR Master Mix were used. Human UTS2 R region (Cat. No. PPH14061A-200) UTS2 R-sense and antisense primers were tested. As a housekeeping gene, ACTB (Ref. Seq Accession number: NM_001101.3) was used.
Each of the prepared mixes underwent PCR using the Qiagen Rotor-Gene Q real-time device for each patient. The samples were subjected to PCR conditions of 95°C for 10 min, 40 cycles of 95°C for 15 s, and 60°C for 60 s. Ct values were calculated for the same device.
Statistical analysis
For statistical analysis, SPSS for Windows version 11.5 package program was used. The compliance of the numeric variables to normal distribution was tested with the Kolmogorov-Smirnov test. While analyzing the correlation between 2 variables, Spearman’s correlation analysis was used if 1 of the variables was ordinal (ordered categorical), both variables had no normal distribution, the sample size was small, or there was no linear relationship between x and y. In other cases, the Pearson correlation analysis was used. For the comparison of 2 independent groups, the Mann-Whitney U test was used and for the comparison of more than 2 independent groups the Kruskal-Wallis test was used. Frequency, percentage, and mean ± standard deviation are given as descriptive statistics. A p value <0.05 was considered statistically significant.
Results
A total of 59 female patients with a diagnosis of breast cancer, who were admitted to Gaziantep University Sahinbey Research and Training Hospital, were included in the study. The demographic, clinical, and pathologic features are given in detail in Table 1. U-II and UTR expression was determined in the tumor and normal tissues of 55 (93.2%) for UTR and 53 (89.9%) for U-II patients (Table 2).
Table 1.
The demographic, clinical, and pathologic features of patients.
| Age (median) | 48 (24–78) |
| Premenapausal/Postmenapausal | 44 (24–53)/61 (43–78) |
| Sex: Woman | 59 (100%) |
| Menapausal status: Premenapausal/Postmenapausal | 35 (59.3%)/24 (40.7%) |
| Smoking: Yes/No | 8 (13.6%)/33 (55.9%) |
| Unknown | 18 (% 30.5) |
| Cancer history in family: Yes/No | 7 (11.9%)/29 (49.2%) |
| Unknown | 23 (39%) |
| Histopathology | |
| Invasive ductal carcinoma | 47 (79.7%) |
| Invasive lobular carcinoma | 4 (6.8%) |
| Mixt type | 3 (5.1%) |
| Invasive papillary | 1 (1.7%) |
| Medullary carcinoma | 1 (1.7%) |
| Others | 3 (5.1%) |
| Extranodal invasion: Yes/No | 12 (32.4%)/37 (67.6%) |
| Lymphatic invasion: Yes/No | 12 (20.3%)/47 (79.7%) |
| Vasculary invasion: Yes/No | 13 (22%)/46 (78%) |
| Perineural invasion: Yes/No | 3 (5.1%)/56 (94.9%) |
| Tumor grade | |
| Grade 1 | 5 (8.5%) |
| Grade 2 | 25 (42.4%) |
| Grade 3 | 23 (39%) |
| Unknown | 6 (10.2%) |
| ER status: Positive/ Negative | 36 (61%)/23 (39%) |
| PR status: Positive/ Negative | 32 (54.2%)/25 (42.4%) |
| Unknown | 2 (3.4%) |
| Her-2 status: Positive/ Negative | 17 (28.8%)/41 (69.5%) |
| Unknown | 1 (1.7%) |
| Median tumor size (cm) | 4 (2–10) |
| Distant metastasis: Yes/No | 4 (6.8%)/51 (86.4%) |
| Unknown | 4 (6.8%) |
| Axillary metastatic lymph node status | |
| N0=yok | 18 (30.5%) |
| N1=1–3 | 18 (28.8%) |
| N2=4–9 | 13 (22%) |
| N3 >10 | 6 (10.2%) |
| Unknown | 5 (8.5%) |
| Stage | |
| 1 | 4 (6.8%) |
| 2 | 26 (44.1%) |
| 3 | 20 (33.9%) |
| 4 | 4 (6.8%) |
| Unknown | 5 (8.5%) |
ER – estrogen receptor; PR – progesterone receptor.
Table 2.
Patient distribution for U2 and UTR.
| n | % | |
|---|---|---|
| U2 | ||
| <0.9 | 12 | 20.3 |
| 0.9–1.1 | 25 | 42.4 |
| >1.1 | 16 | 27.1 |
| Unknown | 6 | 10.2 |
| UTR | ||
| <0.9 | 7 | 11.9 |
| 0.9–1.1 | 25 | 42.4 |
| >1.1 | 23 | 39.0 |
| Unknown | 4 | 6.7 |
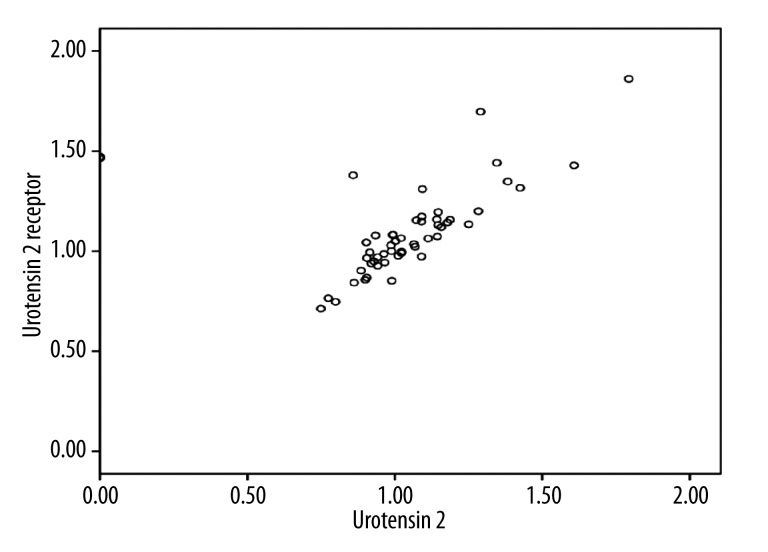
There was no statistically significant correlation between patient age and U-II (p=0.71, r=−0250), but a statistically weak negative correlation was found between patient age and UTR (p=0.038, r=−0281) (Table 3). U-II and UTR showed a strong positive correlation (p=0.001, r=0632) (Table 4, Figure 1).
Table 3.
Relationship between UTR and patient age.
| n | Median | Mean ±SD | r (Spearman’s) | p | |
|---|---|---|---|---|---|
| Age | 59 | 48 | 49.7797±12.57264 | −0.281 | 0.038 |
| UTR | 53 | 1.0650 | 1.1228±0.27928 |
Table 4.
Relationship between U2 and UTR.
| n | Median | Mean ±SD | r | p | |
|---|---|---|---|---|---|
| U2 | 53 | 1.0108 | 1.0235±0.28229 | 0.626 | 0.001 |
| UTR | 55 | 1.0650 | 1.1228±0.27928 |
Figure 1.
Positive correlation between U2 and UTR (showing the gene expression ratios of tumor tissue to normal tissue in the same patients).
The U-II levels were found to be higher in the premenopausal group compared to the postmenopausal group. This difference was statistically significant (p=0.04) (Table 5). The mean UTR levels were also higher in the premenopausal group compared to the postmenopausal group. The difference was statistically significant (p=0.021).
Table 5.
Relationship between the menapausal status and U2 and UTR.
| n | Median | Mean ±SD | p (Mann-Whitney U) | |
|---|---|---|---|---|
| U2 | ||||
| Postmenapausal | 22 | 0.9899 | 0.9848±0.15488 | 0.04 |
| Premenapausal | 31 | 1.0734 | 1.0510±0.34634 | |
| Total | 53 | 1.0108 | 1.0235±0.28279 | |
| UTR | ||||
| Postmenapausal | 22 | 0.9956 | 1.0174±0.17887 | 0.021 |
| Premenapausal | 31 | 1.0831 | 1.1932±0.31285 | |
| Total | 53 | 1.0650 | 1.1228±0.27928 | |
In patients with positive axillary lymph node involvement, there was no statistically significant difference between the groups with and without extra-nodal involvement in terms of U-II values (p=0174). In patients without extra-nodal invasion, UTR values were significantly higher than the group with extra-nodal involvement (p=0.001) (Table 6).
Table 6.
Relationship between extranodal invasion and U2 and UTR.
| Extranodal invasion | n | Median | Mean±SD | p (Mann-Whitney U) | |
|---|---|---|---|---|---|
| U2 | No | 26 | 1.0710 | 1.0126±0.35548 | 0.149 |
| Yes | 12 | 0.9537 | 1.0112±0.19800 | ||
| UTR | No | 26 | 1.1456 | 1.1817±0.24197 | 0.001 |
| Yes | 12 | 0.9680 | 0.9885±0.15426 |
In the group without lymphatic invasion, U-II levels were statistically significantly higher (p = 0.048), but this was a slight difference. There was no statistically significant difference between the 2 groups in terms of UTR (p=0714) (Table 7).
Table 7.
Relationship between lymphatic invasion and U2 and UTR.
| Lymphatic invasion | n | Median | Mean ±SD | p (Mann-Whitney U) | |
|---|---|---|---|---|---|
| U2 | Yes | 12 | 0.9122 | 0.8591±0.44934 | 0.048 |
| No | 41 | 1.0168 | 1.0716±0.19482 | ||
| UTR | Yes | 12 | 1.0545 | 1.1393±0.23542 | 0.714 |
| No | 43 | 1.0566 | 1.1182±0.29269 |
There was no statistically significant difference between the groups in terms of U-II and UTR, depending on the stage of the tumor, tumor grade, nodal involvement, molecular classification (luminal A, luminal B, or basal-like) ER, PR, c-erbB2 status, vascular invasion, and the presence of invasive ductal carcinoma (p>0.05).
Discussion
Researcher interest in Urotensin II is growing. Since the discovery of this SST-like peptide 43 years ago, a great amount of research has been conducted on its specific role in diseases and its mechanism of action. However, most studies examining UTR and U-II have focused on the cardiovascular system [13]. In this study, we aimed to investigate the potential relationship of U-II and UTR with the clinical and pathological parameters in breast cancer.
Although Urotensin II is known to stimulate cell growth and proliferation of the vascular smooth muscle cell [14,15], it has not been sufficiently determined whether the same effects take place in tumor cells. In recent years, in some studies, U-II has been shown to play an important role in some tumors. Takahashi et al. demonstrated that U-II and UTR are expressed in glioblastoma cell line T98G, neuroblastoma cell line IMR32, choriocarcinoma cell line BeWo, adrenocortical carcinoma cell line SW-13, colorectal carcinoma cell line DLD-1, and cervical carcinoma cell line HeLa. In the NB69 neuroblastoma cells, an expression of UTR was observed, but no U-II expression of could be determined [9]. U-II mRNA, U-II protein, and UTR expressions were clearly shown in the human lung adenocarcinoma A549 cells [16]. UTR and U-II have been immune-localized in adrenal tumors such as adrenal adenoma, adrenocortical carcinoma, and pheochromocytoma, as well as in non-neoplastic adrenal tissue [17]. In a study investigating the localization of U-II in normal kidney cells and renal carcinoma cells, U-II has been shown to have a moderate expression in cancer cells and vascular structures [18].
In our study, we have determined U-II and UTR mRNA expression in tumor and normal breast tissues in 89.9% and 93.2%, respectively, of patients with breast cancer. The 75% positivity of SSTR for breast tissue, which is another vasoactive polypeptide similar to U-II, has been previously shown in breast cancer [1]. However, the presence of U-II and UTR has not been shown in breast cancer and breast tissue. This study is the first to show U-II and UTR mRNA expression in the breast tissue and breast cancer tissue.
The mechanism of action and functions of U-II in cancer tissues are not thoroughly understood. The pathways of signal transduction that are formed following the activation of UTR by U-II are very complicated. RhoA/ROCK, ERK [7], EGFR transactivation, PKC, c-src tyrosine kinase, and p38 MAPK pathway are involved in this communication [19]. U-II has been shown to synergistically stimulate VEGF secretion in adventitial fibroblasts in angiogenesis, together with angiotensin II [20]. This constitutes one of the most important steps in tumor angiogenesis. Urotensin II is also involved in tumor cells as a growth-stimulating factor, and it has been reported to have a strong mitogenic effect in a variety of cell phenotypes [10–12]. However, U-II has been shown to protect heart muscle cells from doxorubicin-induced apoptosis by increasing their survival, partly through Akt and ERK [21]. Such an anti-apoptotic effect may also be present the cancer cells.
There are few studies indicating that the U-II plasma levels do not differ between women and men [22,23]. However, a study has shown it to be lower in women [24]. In our study, since all of our patients were women, we were unable to determine a possible difference between the sexes. We did not find a statistically significant relationship between patient age and U-II (p=0.71, r=−0250). There was a statistically weak correlation between age and UTR (p=0.038, r=−0281), but we believe this correlation does not have clinical meaning. The age and the sex of the patient may have an impact on the expression of U-II, but so far it has not been fully elucidated [25]. In our study, we found a strong positive correlation between UTR and U-II (r=0.632, p=0.001). U-II in tissues increased with increasing UTR.
In the premenopausal group, U-II and UTR levels were significantly higher compared to the postmenopausal group (p=0.04 and p<0.05, respectively). These significant differences between the 2 groups were not associated with age. There was no relationship between patient age and U-II (p=0.71, r= −0250), but a weak correlation was found between age and UTR (p=0.038, r=−0281). This finding was interpreted as a direct effect of menopause.
Axillary lymph node involvement is the most important prognostic factor in non-metastatic breast cancer [26]. There was no statistically significant difference between the groups in terms of U-II and UTR, depending on the nodal involvement (p=0935, p=0.171, respectively). Extra-nodal invasion is a negative prognostic factor and is known to be a marker of tumor invasion [26]. Federico et al. reported that U-II [4–11] induced a 20–40% increase in cell growth, and the blockade of the receptor with specific antagonists caused growth inhibition of 20–40% in colon adenocarcinoma. They determined that UTR mRNA expression was increased by 3-fold in adenomatous polyps and 8-fold in colon cancer, compared with normal colon. They also reported that inhibition of UTR induced an approximately 50% inhibition of both motility and invasion [27]. In our study, there was no statistically significant difference between the groups in terms of U-II, depending on the extra-nodal involvement (p=0.174). In the group with extra-nodal involvement, UTR levels were significantly lower compared to the patients without extra-nodal invasion (p<0.05).
Grieco et al. determined that decreased UTR was a negative prognostic marker of poor survival in high-grade and mid-grade patients with a Gleason score >7 [28]. The grade of the disease has a prognostic significance in invasive breast cancer. Grade III tumors have been shown to have an increased recurrence risk [26]. In our study, when the patients were grouped according to grade of the disease, there was no statistically significant difference in terms of U-II and UTR (p=0.381 and p=0550, respectively).
In breast cancer, both the hormone receptor and the HER-2 status have predictive and prognostic significance [26]. In our study, there were no statistically significant differences between ER-positive and ER-negative groups in terms of U-II and UTR (p=0.820 and p=0795, respectively). There were also no statistically significant differences between PR-positive and PR-negative groups in terms of U-II and UTR (p=0.690 and p=0.528, respectively). There were also no statistically significant differences between the HER-2-positive and HER-2-negative groups in terms of U-II and UTR (p=0.664 and p=0.220, respectively).
Somatostatin, which has sequential similarities to U-II, has inhibitory effects on invasion and migration, which are 2 important steps in carcinogenesis that occur through the inhibition of ERK 1/2 signals. Studies on this issue have generally indicated that SSTR expression in breast cancer is independent of age, menopausal status, and histological grade [3]. Retrospective studies have shown that breast cancer patients with SSTR expression have longer recurrence-free survival [29]. In our study, we determined the negative relationship between U-II, which is an SST-like vasoactive peptide, and UTR, which is its receptor, with the negative prognostic markers, such as extra-nodal and lymphatic invasion.
Conclusions
U-II and UTR mRNA is expressed in the breast tissue and breast cancer tissue. A highly significant positive correlation was found between U-II and UTR. U-II and UTR were found to be higher in premenopausal patients. UTR was lower in patients with extra-nodal invasion. U-II was significantly lower in patients with lymphatic invasion. These findings could be explained with good prognostic factors of U-II and its receptor UTR in breast cancer tumorigenesis. Due to lack of sufficient number of patients in each group according to the prognostic and the predictive properties, it is likely that some relationships could not be demonstrated. For this reason, in order to understand the relationship between breast cancer and U-II, cell culture studies and clinical trials with larger groups of patients are needed.
Footnotes
Conflict of interest
No conflicts of interest.
Source of support: Departmental sources
References
- 1.Watt HL, Kharmate G, Kumar U. Biology of SST in breast cancer. Mol Cell Endocrinol. 2008;286:251–61. doi: 10.1016/j.mce.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Ribatti D, Conconi MT, Nussdorfer GG. Nonclassic endogenous novel regulators of angiogenesis. Pharmacol Rev. 2007;59:185–205. doi: 10.1124/pr.59.2.3. [DOI] [PubMed] [Google Scholar]
- 3.Schulz S, Schulz S, Schmitt J, et al. Immunocytochemical detection of SST receptors sst1, sst2A, sst2B, and sst3 in paraffin-embedded breast cancer tissue using subtype-specific antibodies. Clin Cancer Res. 1998;4:2047–52. [PubMed] [Google Scholar]
- 4.Liu Q, Pong SS, Zeng Z, et al. Identification of urotensin II as the endogenous ligand for the orphan G-protein-coupled receptor GPR14. Biochem Biophys Res Commun. 1999;266:174–78. doi: 10.1006/bbrc.1999.1796. [DOI] [PubMed] [Google Scholar]
- 5.Sáez ME, Smani T, Ramírez-Lorca R, et al. Association analysis of urotensin II gene (UTS2) and flanking regions with biochemical parameters related to insulin resistance. PLoS One. 2011;6(4):e19327. doi: 10.1371/journal.pone.0019327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribatti D, Vacca A, Presta M. The discovery of angiogenic factors: a historical review. Gen Pharmacol. 2002;35:227–31. doi: 10.1016/s0306-3623(01)00112-4. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y, Matsumura K, Tsuchihashi T, et al. Role of ERK and Rho kinase pathways in central pressor action of urotensin II. J Hypertens. 2004;22:983–88. doi: 10.1097/00004872-200405000-00021. [DOI] [PubMed] [Google Scholar]
- 8.Clozel M, Binkert C, Birker-Robaczewska M, et al. Pharmacology of the urotensin-II receptor antagonist palosuran (ACT-058362; 1-[2-(4-benzyl-4-hydroxy-piperidin-1-yl)-ethyl]-3-(2-methyl-quinolin-4-yl)-urea sulfate salt): first demonstration of a pathophysiological role of the urotensin System. J Pharmacol Exp Ther. 2004;311:204–12. doi: 10.1124/jpet.104.068320. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi K, Totsune K, Kitamuro T, et al. Three vasoactive peptides, endothelin-1, adrenomedullin and urotensin-II, in human tumour cell lines of different origin: expression and effects on proliferation. Clin Sci (Lond) 2002;103(Suppl 48):35S–38S. doi: 10.1042/CS103S035S. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Totsune K, Murakami O, et al. Expression of urotensin II and its receptor in adrenal tumors and stimulation of proliferation of cultured tumor cells by urotensin II. Peptides. 2003;24:301–6. doi: 10.1016/s0196-9781(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 11.Zeng ZP, Liu GQ, Li HZ, et al. The effects of urotensin-II on proliferation of pheochromocytoma cells and mRNA expression of urotensin-II and its receptor in pheochromocytoma tissues. Ann NY Acad Sci. 2006;1073:284–89. doi: 10.1196/annals.1353.032. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi K, Totsune K, Murakami O, Shibahara S. Expression of urotensin II and urotensin II receptor mRNAs in various human tumor cell lines and secretion of urotensin II-like immunoreactivity by SW-13 adrenocortical carcinoma cells. Peptides. 2001;22:1175–79. doi: 10.1016/s0196-9781(01)00441-7. [DOI] [PubMed] [Google Scholar]
- 13.Ross B, McKendy K, Giaid A. Role of urotensin II in health and disease. Am J Physiol Regul Integr Comp Physiol. 2010;298:1156–72. doi: 10.1152/ajpregu.00706.2009. [DOI] [PubMed] [Google Scholar]
- 14.Mallamaci F, Cutrupi S, Pizzini P, et al. Urotensin II and biomarkers of endothelial activation and atherosclerosis in end-stage renal disease. Am J Hypertens. 2006;19:505–10. doi: 10.1016/j.amjhyper.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Sauzeau V, Mellionnec EL, Bertoglio J, et al. Human urotensin II-induced contraction and arterial smooth muscle cell proliferation are mediated by RhoA and Rho-kinase. Circ Res. 2001;88:1102–4. doi: 10.1161/hh1101.092034. [DOI] [PubMed] [Google Scholar]
- 16.Wu YQ, Song Z, Zhou CH, et al. Expression of urotensin II and its receptor in human lung adenocarcinoma A549 cells and the effect of urotensin II on lung adenocarcinoma growth in vitro and in vivo. Oncol Rep. 2010;24(5):1179–84. doi: 10.3892/or_00000970. [DOI] [PubMed] [Google Scholar]
- 17.Morimoto R, Satoh F, Murakami O, et al. Immunolocalization of urotensin II and its receptor in human adrenal tumors and attached non-neoplastic adrenal tissues. Peptides. 2008;29:873–80. doi: 10.1016/j.peptides.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Shenouda A, Douglas SA, Ohlstein EH, Giaid A. Localization of urotensin-II immunoreactivity in normal human kidneys and renal carcinoma. J Histochem Cytochem. 2002;50:885–89. doi: 10.1177/002215540205000702. [DOI] [PubMed] [Google Scholar]
- 19.Liu JC, Chen CH, Chen JJ, Cheng TH. Urotensin II induces rat cardiomyocyte hypertrophy via the transient oxidization of Src homology 2-containing tyrosine phosphatase and transactivation of epidermal growth factor receptor. Mol Pharmacol. 2009;76(6):1186–95. doi: 10.1124/mol.109.058297. [DOI] [PubMed] [Google Scholar]
- 20.Song N, Ding W, Chu S, et al. Urotensin II stimulates vascular endothelial growth factor secretion from adventitial fibroblasts in synergy with angiotensin II. Circ J. 2012;76:1267–73. doi: 10.1253/circj.cj-11-0870. [DOI] [PubMed] [Google Scholar]
- 21.Chen YL, Loh SH, Chen JJ, Tsai CS. Urotensin II prevents cardiomyocyte apoptosis induced by doxorubicin via Akt and ERK. Eur J Pharmacol. 2012;680:88–94. doi: 10.1016/j.ejphar.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 22.Bicak U, Karabiber H, Ozerol HI, et al. Possible pathogenic link between migraine and urotensin-II. J Child Neurol. 2008;23:1249–53. doi: 10.1177/0883073808318052. [DOI] [PubMed] [Google Scholar]
- 23.Ng LL, Loke I, O’Brien RJ, et al. Plasma urotensin in human systolic heart failure. Circulation. 2002;106:2877–80. doi: 10.1161/01.cir.0000044388.19119.02. [DOI] [PubMed] [Google Scholar]
- 24.Lim M, Honisett S, Sparkes CD, et al. Differential effect of urotensin II on vascular tone in normal subjects and patients with chronic heart failure. Circulation. 2004;109:1212–14. doi: 10.1161/01.CIR.0000121326.69153.98. [DOI] [PubMed] [Google Scholar]
- 25.Pearson D, Shively JE, Clark BR, et al. Urotensin II: a SST-like peptide in the caudal neurosecretory system of fishes. Proc Natl Acad Sci USA. 1980;77:5021–24. doi: 10.1073/pnas.77.8.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lønning PE. Breast cancer prognostication and prediction: are we making progress? Ann Oncol. 2007;18(Suppl 8):viii, 3–7. doi: 10.1093/annonc/mdm260. [DOI] [PubMed] [Google Scholar]
- 27.Federico A, Zappavigna S, Romano M, et al. Urotensin-II receptor is over-expressed in colon cancer cell lines and in colon carcinoma in humans. Eur J Clin Invest. 2014;44(3):285–94. doi: 10.1111/eci.12231. [DOI] [PubMed] [Google Scholar]
- 28.Grieco P, Franco R, Bozzuto G, et al. Urotensin II receptor predicts the clinical outcome of prostate cancer patients and is involved in the regulation of motility of prostate adenocarcinoma cells. J Cell Biochem. 2011;112(1):341–53. doi: 10.1002/jcb.22933. [DOI] [PubMed] [Google Scholar]
- 29.Foekens JA, Portengen H, van Putten WLJ, et al. Prognostic value of receptors for insulin-like growth factor 1, SST, and epidermal growth factor in human breast cancer. Cancer Res. 1989;49:7002–9. [PubMed] [Google Scholar]