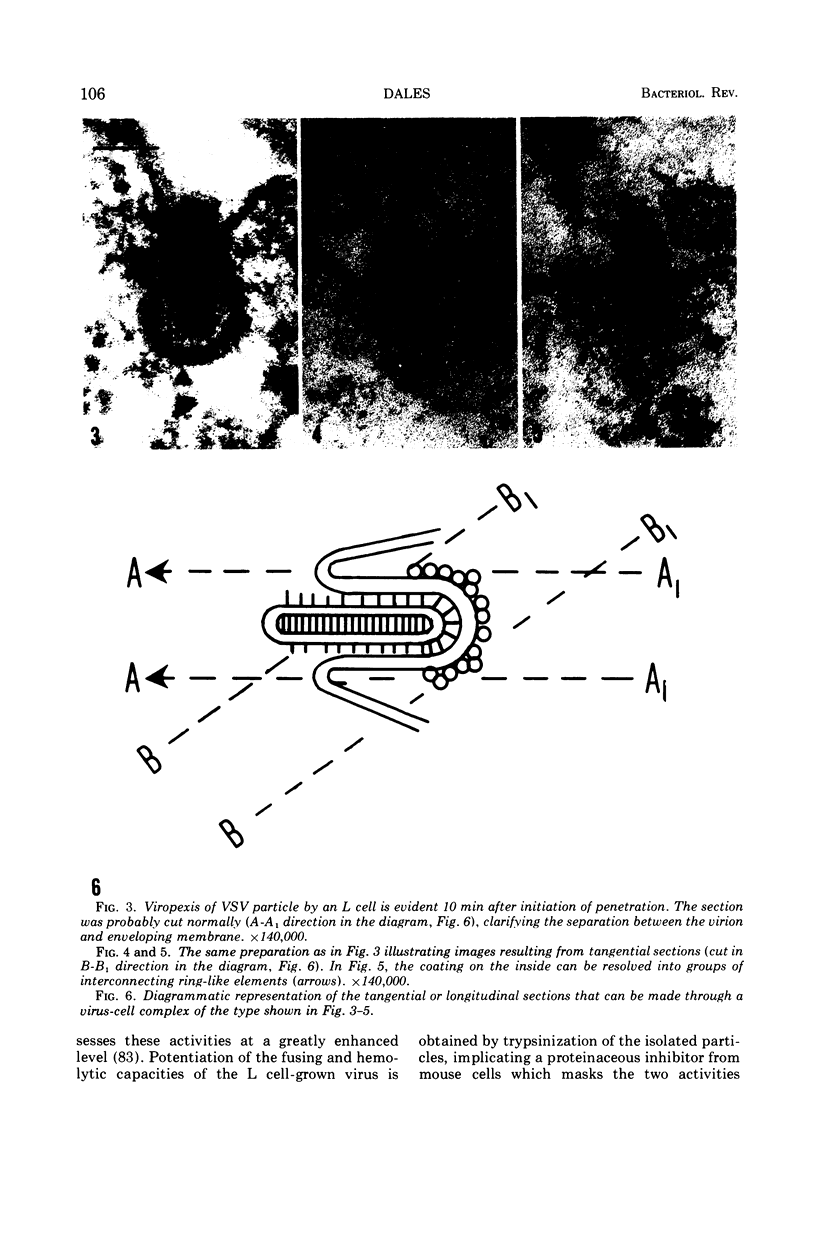
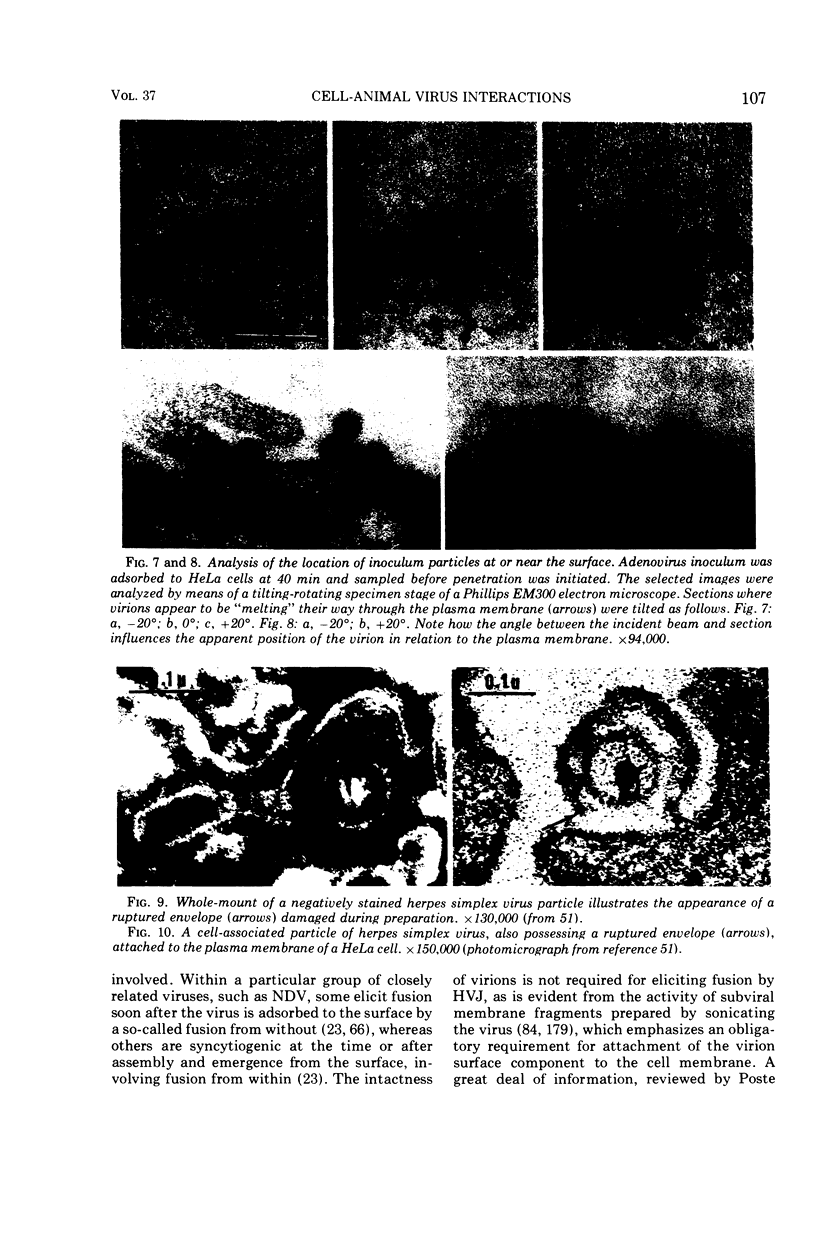
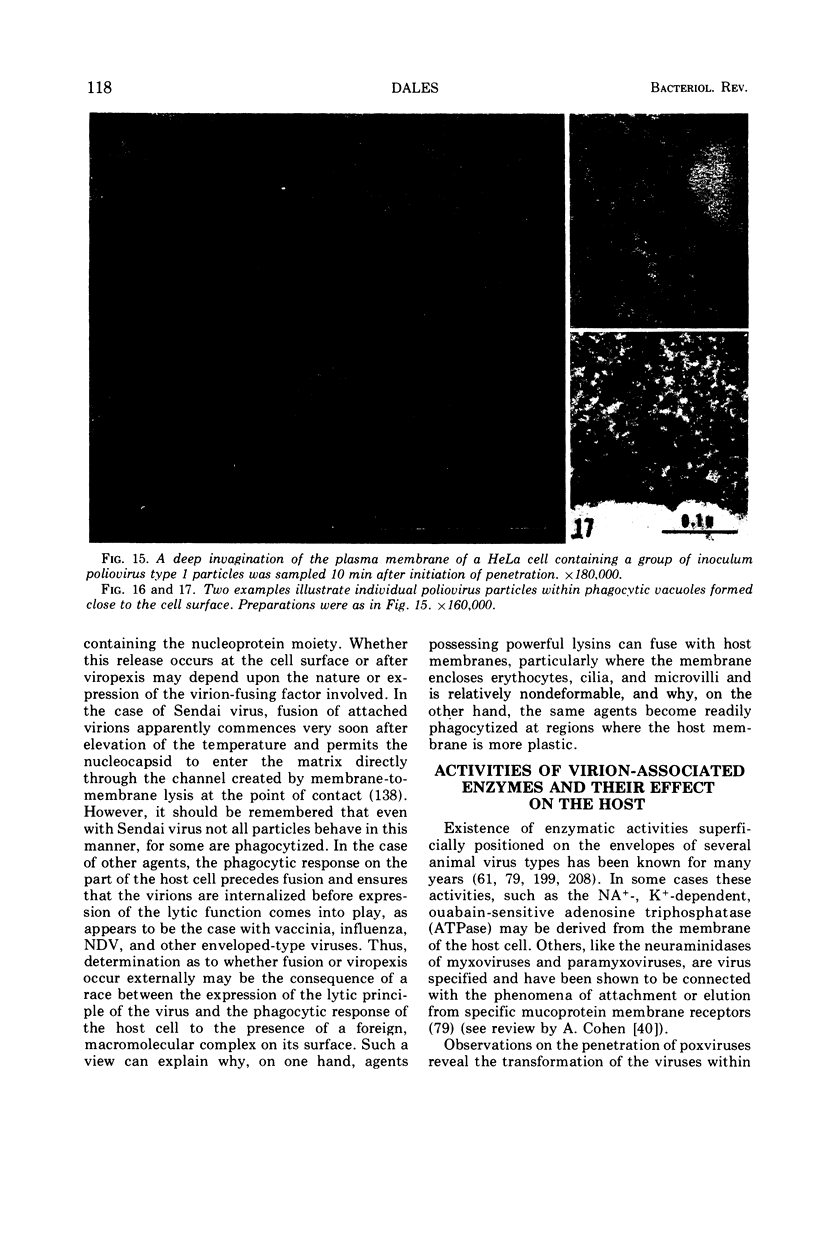
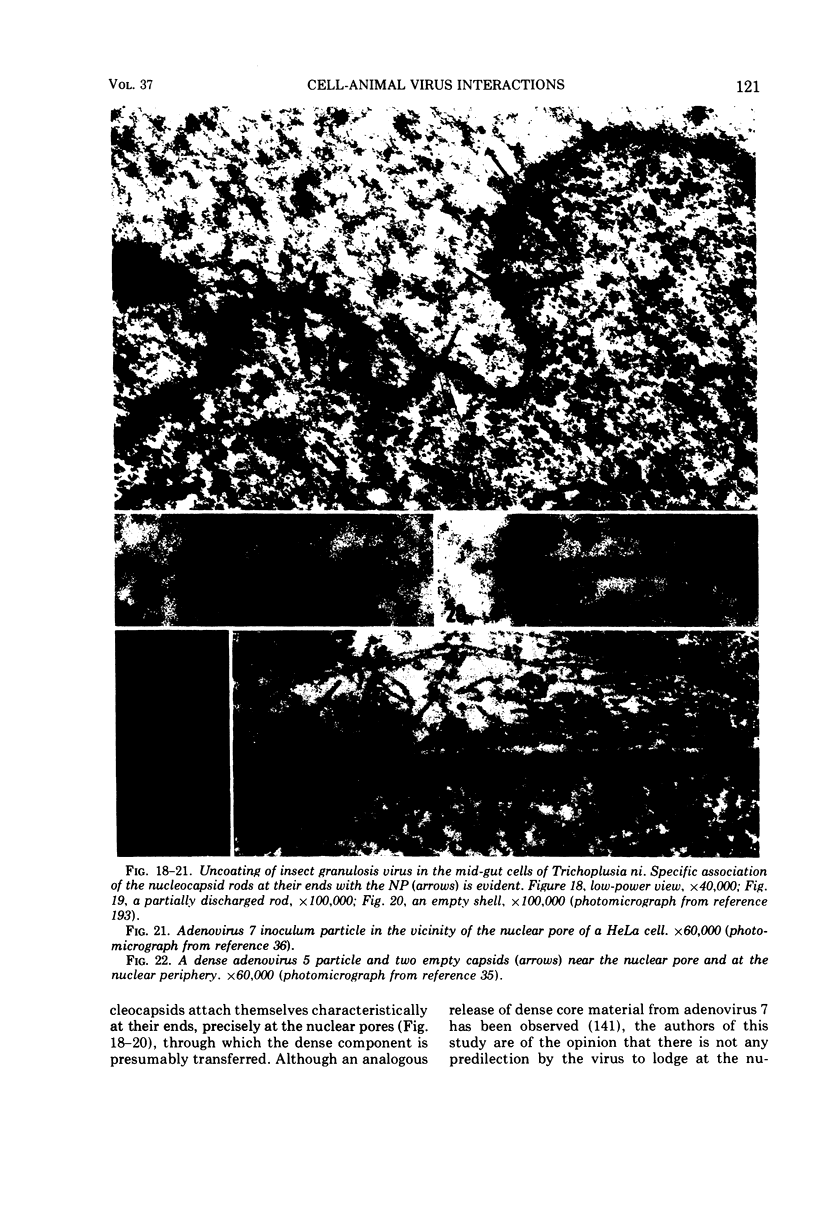
Full text
PDF




















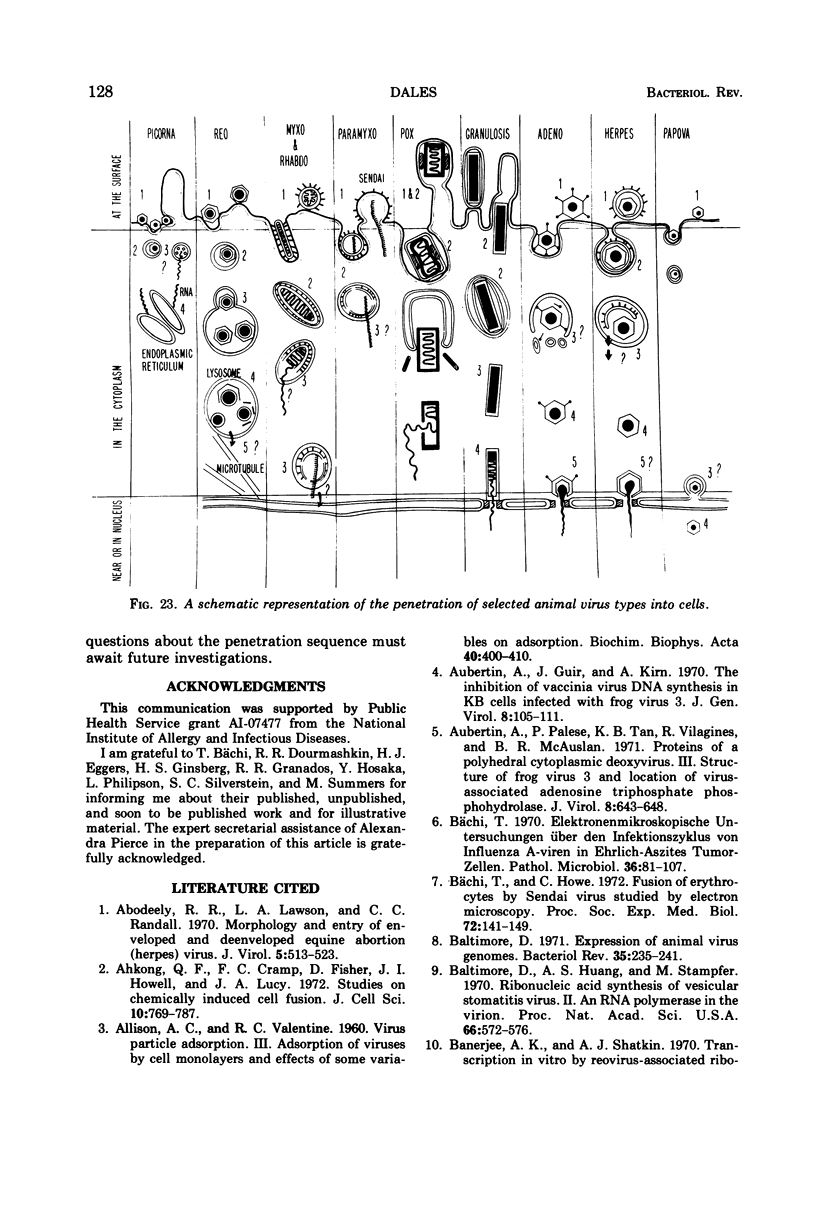







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., VALENTINE R. C. Virus particle adsorption, III. Adsorption of viruses by cell monolayers and effects of some variables on adsorption. Biochim Biophys Acta. 1960 Jun 3;40:400–410. doi: 10.1016/0006-3002(60)91380-9. [DOI] [PubMed] [Google Scholar]
- Abodeely R. A., Lawson L. A., Randall C. C. Morphology and entry of enveloped and deenveloped equine abortion (herpes) virus. J Virol. 1970 Apr;5(4):513–523. doi: 10.1128/jvi.5.4.513-523.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahkong Q. F., Cramp F. C., Fisher D., Howell J. I., Lucy J. A. Studies on chemically induced cell fusion. J Cell Sci. 1972 May;10(3):769–787. doi: 10.1242/jcs.10.3.769. [DOI] [PubMed] [Google Scholar]
- Aubertin A. M., Guir J., Kirn A. The inhibition of vaccinia virus DNA synthesis in KB cells infected with frog virus 3. J Gen Virol. 1970 Aug;8(2):105–111. doi: 10.1099/0022-1317-8-2-105. [DOI] [PubMed] [Google Scholar]
- Aubertin A., Palese P., Tan K. B., Vilagines R., McAuslan B. R. Proteins of a polyhedral cytoplasmic deoxyvirus. 3. Structure of frog virus 3 and location ov virus-associated adenosine triphosphate phosphohydrolase. J Virol. 1971 Nov;8(5):643–648. doi: 10.1128/jvi.8.5.643-648.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOAND A. V., Jr, KEMPF J. E., HANSON R. J. Phagocytosis of influenza virus. I. In vitro observations. J Immunol. 1957 Nov;79(5):416–421. [PubMed] [Google Scholar]
- BOURGAUX P. THE FATE OF POLYOMA VIRUS IN HAMSTER, MOUSE, AND HUMAN CELLS. Virology. 1964 May;23:46–55. doi: 10.1016/s0042-6822(64)80006-4. [DOI] [PubMed] [Google Scholar]
- Bachi T. Elektronenmikroskopische Untersuchungen über den Infektionszyklus von Influenza A-Viren in Ehrlich-Aszites-Tumor-Zellen. Pathol Microbiol (Basel) 1970;36(2):81–107. [PubMed] [Google Scholar]
- Baltimore D. Expression of animal virus genomes. Bacteriol Rev. 1971 Sep;35(3):235–241. doi: 10.1128/br.35.3.235-241.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. K., Shatkin A. J. Transcription in vitro by reovirus-associated ribonucleic acid-dependent polymerase. J Virol. 1970 Jul;6(1):1–11. doi: 10.1128/jvi.6.1.1-11.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbanti-Brodano G., Possati L., La Placa M. Inactivation of polykaryocytogenic and hemolytic activities of Sendai virus by phospholipase B (lysolecithinase). J Virol. 1971 Nov;8(5):796–800. doi: 10.1128/jvi.8.5.796-800.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbanti-Brodano G., Swetly P., Koprowski H. Early events in the infection of permissive cells with simian virus 40: adsorption, penetration, and uncoating. J Virol. 1970 Jul;6(1):78–86. doi: 10.1128/jvi.6.1.78-86.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialy H. S., Colby C. Inhibition of early vaccinia virus ribonucleic acid synthesis in interferon-treated chicken embryo fibroblasts. J Virol. 1972 Feb;9(2):286–289. doi: 10.1128/jvi.9.2.286-289.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Obijeski J. F., Simpson R. W. Transcription of the influenza ribonucleic acid genome by a virion polymerase. I. Optimal conditions for in vitro activity of the ribonucleic acid-dependent ribonucleic acid polymerase. J Virol. 1971 Jul;8(1):66–73. doi: 10.1128/jvi.8.1.66-73.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Obijeski J. F., Simpson R. W. Transcription of the influenza ribonucleic acid genome by a virion polymerase. II. Nature of the in vitro polymerase product. J Virol. 1971 Jul;8(1):74–80. doi: 10.1128/jvi.8.1.74-80.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaskovic P., Rhodes A. J., Doane F. W., Labzoffsky N. A. Infection of chick embryo tracheal organ cultures with influenza A2 (Hong Kong) virus. II. Electron microscopy. Arch Gesamte Virusforsch. 1972;38(2):250–266. doi: 10.1007/BF01249676. [DOI] [PubMed] [Google Scholar]
- Blough H. A., Lawson D. E. The lipids of paramyxoviruses: a comparative study of Sendai and Newcastle disease viruses. Virology. 1968 Oct;36(2):286–292. doi: 10.1016/0042-6822(68)90146-3. [DOI] [PubMed] [Google Scholar]
- Borsa J., Grover J., Chapman J. D. Presence of nucleoside triphosphate phosphohydrolase activity in purified virions of reovirus. J Virol. 1970 Sep;6(3):295–302. doi: 10.1128/jvi.6.3.295-302.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose H. R., Sagik B. P. The virus envelope in cell attachment. J Gen Virol. 1970 Nov;9(2):159–161. doi: 10.1099/0022-1317-9-2-159. [DOI] [PubMed] [Google Scholar]
- Bratt M. A., Clavell L. A. Hemolytic interaction of Newcastle disease virus and chicken erythrocytes. I. Quantitative comparison procedure. Appl Microbiol. 1972 Mar;23(3):454–460. doi: 10.1128/am.23.3.454-460.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinskaya A. G., Zhdanov V. M., Vorkunova G. K. Fate of Sendai virus ribonucleoprotein in virus-infected cells. J Virol. 1969 Aug;4(2):141–146. doi: 10.1128/jvi.4.2.141-146.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlingham B. T., Doerfler W. An endonuclease in cells infected with adenovirus and associated with adenovirions. Virology. 1972 Apr;48(1):1–13. doi: 10.1016/0042-6822(72)90108-0. [DOI] [PubMed] [Google Scholar]
- Burlingham B. T., Doerfler W., Pettersson U., Philipson L. Adenovirus endonuclease: association with the penton of adenovirus type 2. J Mol Biol. 1971 Aug 28;60(1):45–64. doi: 10.1016/0022-2836(71)90446-3. [DOI] [PubMed] [Google Scholar]
- Bächi T., Howe C. Fusion of erythrocytes by Sendai virus studied by electron microscopy. Proc Soc Exp Biol Med. 1972 Oct;141(1):141–149. doi: 10.3181/00379727-141-36733. [DOI] [PubMed] [Google Scholar]
- CAREY F. J., KUHN N. O., HARFORD C. G. EFFECTS OF ANTICELLULAR SERUM ON PHAGOCYTOSIS AND THE UPTAKE OF TRITIATED THYMIDINE AND URIDINE BY HELA CELLS. J Exp Med. 1965 Jun 1;121:991–1000. doi: 10.1084/jem.121.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. Membranous structures associated with translation and transcription of poliovirus RNA. Science. 1969 Nov 14;166(3907):885–886. doi: 10.1126/science.166.3907.885. [DOI] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Costanzo F., Mannini-Palenzona A., La Placa M. Impairment of host cell ribonucleic acid polymerase II after infection with frog virus 3. J Virol. 1972 Apr;9(4):698–700. doi: 10.1128/jvi.9.4.698-700.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Smale C. J., Brown F. Dissection of vesicular stomatitis virus into the infective ribonucleoprotein and immunizing components. J Gen Virol. 1970 Apr;7(1):19–32. doi: 10.1099/0022-1317-7-1-19. [DOI] [PubMed] [Google Scholar]
- Chan V. F., Black F. L. Uncoating of poliovirus by isolated plasma membranes. J Virol. 1970 Mar;5(3):309–312. doi: 10.1128/jvi.5.3.309-312.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. T., Zweerink H. J. Fate of parental reovirus in infected cell. Virology. 1971 Dec;46(3):544–555. doi: 10.1016/0042-6822(71)90058-4. [DOI] [PubMed] [Google Scholar]
- Chardonnet Y., Dales S. Early events in the interaction of adenoviruses with HeLa cells. 3. Relationship between an ATPase activity in nuclear envelopes and transfer of core material: a hypothesis. Virology. 1972 May;48(2):342–359. doi: 10.1016/0042-6822(72)90045-1. [DOI] [PubMed] [Google Scholar]
- Chardonnet Y., Dales S. Early events in the interaction of adenoviruses with HeLa cells. I. Penetration of type 5 and intracellular release of the DNA genome. Virology. 1970 Mar;40(3):462–477. doi: 10.1016/0042-6822(70)90189-3. [DOI] [PubMed] [Google Scholar]
- Chardonnet Y., Dales S. Early events in the interaction of adenoviruses with HeLa cells. II. Comparative observations on the penetration of types 1, 5, 7, and 12. Virology. 1970 Mar;40(3):478–485. doi: 10.1016/0042-6822(70)90190-x. [DOI] [PubMed] [Google Scholar]
- Chardonnet Y. Modications enzymatiques du cytoplasme de cellules (lignée établie T) infectées par l'adénovirus type 5. II. Enzymes des cellules infectées. Ann Inst Pasteur (Paris) 1969 Feb;116(2):159–180. [PubMed] [Google Scholar]
- Clavell L. A., Bratt M. A. Hemolytic interaction of Newcastle disease virus and chicken erythrocytes. II. Determining factors. Appl Microbiol. 1972 Mar;23(3):461–470. doi: 10.1128/am.23.3.461-470.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Holmes K. V., Dales S., Choppin P. W. An electron microscopic study of moderate and virulent virus-cell interactions of the parainfluenza virus SV5. Virology. 1966 Nov;30(3):411–426. doi: 10.1016/0042-6822(66)90119-x. [DOI] [PubMed] [Google Scholar]
- DALES S. An electron microscope study of the early association between two mammalian viruses and their hosts. J Cell Biol. 1962 May;13:303–322. doi: 10.1083/jcb.13.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S., GOMATOS P. J., HSU K. C. THE UPTAKE AND DEVELOPMENT OF REOVIRUS IN STRAIN L CELLS FOLLOWED WITH LABELED VIRAL RIBONUCLEIC ACID AND FERRITIN-ANTIBODY CONJUGATES. Virology. 1965 Feb;25:193–211. doi: 10.1016/0042-6822(65)90199-6. [DOI] [PubMed] [Google Scholar]
- DALES S., KAJIOKA R. THE CYCLE OF MULTIPLICATION OF VACCINIA VIRUS IN EARLE'S STRAIN L CELLS. I. UPTAKE AND PENETRATION. Virology. 1964 Nov;24:278–294. doi: 10.1016/0042-6822(64)90167-9. [DOI] [PubMed] [Google Scholar]
- DALES S., SIMINOVITCH L. The development of vaccinia virus in Earle's L strain cells as examined by electron microscopy. J Biophys Biochem Cytol. 1961 Aug;10:475–503. doi: 10.1083/jcb.10.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S. The uptake and development of vaccinia virus in strain L cells followed with labeled viral deoxyribonucleic acid. J Cell Biol. 1963 Jul;18:51–72. doi: 10.1083/jcb.18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVID-FERREIRA J. F., MANAKER R. A. AN ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF A MOUSE HEPATITIS VIRUS IN TISSUE CULTURE CELLS. J Cell Biol. 1965 Jan;24:57–78. doi: 10.1083/jcb.24.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R., Kates J. R. Intracellular structures containing vaccinia DNA: isolation and characterization. Virology. 1970 Oct;42(2):453–462. doi: 10.1016/0042-6822(70)90288-6. [DOI] [PubMed] [Google Scholar]
- Dahl R., Kates J. R. Synthesis of vaccinia virus "early" and "late" messenger RNA in vitro with nucleoprotein structures isolated from infected cells. Virology. 1970 Oct;42(2):463–472. doi: 10.1016/0042-6822(70)90289-8. [DOI] [PubMed] [Google Scholar]
- Dales S. Effects of streptovitacin A on the initial events in the replication of vaccinia and reovirus. Proc Natl Acad Sci U S A. 1965 Aug;54(2):462–468. doi: 10.1073/pnas.54.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Hanafusa H. Penetration and intracellular release of the genomes of avian RNA tumor viruses. Virology. 1972 Nov;50(2):440–458. doi: 10.1016/0042-6822(72)90396-0. [DOI] [PubMed] [Google Scholar]
- Dales S., Silverberg H. Viropexis of herpes simplex virus by HeLa cells. Virology. 1969 Mar;37(3):475–480. doi: 10.1016/0042-6822(69)90232-3. [DOI] [PubMed] [Google Scholar]
- Devauchelle G., Bergoin M., Vago C. Etude ultrastructurale du cycle de replicatin d'un entomopoxvirus dans les hémocytes de son hôte. J Ultrastruct Res. 1971 Nov;37(3):301–321. doi: 10.1016/s0022-5320(71)80126-0. [DOI] [PubMed] [Google Scholar]
- Dourmashkin R. R., Tyrrell D. A. Attachment of two myxoviruses to ciliated epithelial cells. J Gen Virol. 1970 Oct;9(1):77–88. doi: 10.1099/0022-1317-9-1-77. [DOI] [PubMed] [Google Scholar]
- Dunnebacke T. H., Levinthal J. D., Williams R. C. Entry and release of poliovirus as observed by electron microscopy of cultured cells. J Virol. 1969 Oct;4(4):505–513. doi: 10.1128/jvi.4.4.505-513.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLEM K. A., COLTER J. S. The interaction of infections ribonucleic acid with a mammalian cell line. I. Relationship between the osmotic pressure of the medium and the production of infectious centers. Virology. 1960 Jun;11:434–443. doi: 10.1016/0042-6822(60)90085-4. [DOI] [PubMed] [Google Scholar]
- EPSTEIN M. A., HOLT S. J. ELECTRON MICROSCOPE OBSERVATIONS ON THE SURFACE ADENOSINE TRIPHOSPHATASE-LIKE ENZYMES OF HELA CELLS INFECTED WITH HERPES VIRUS. J Cell Biol. 1963 Nov;19:337–347. doi: 10.1083/jcb.19.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton M. D., Scala A. R. Ammonium chloride and viral penetration. Arch Gesamte Virusforsch. 1967;20(4):411–420. doi: 10.1007/BF01275221. [DOI] [PubMed] [Google Scholar]
- FRASER K. B., CRAWFORD E. M. IMMUNOFLUORESCENT AND ELECTRON-MICROSCOPIC STUDIES OF POLYOMA VIRUS IN TRANSFORMATION REACTIONS WITH BHK21 CELLS. Exp Mol Pathol. 1965 Feb;76:51–65. doi: 10.1016/0014-4800(65)90023-7. [DOI] [PubMed] [Google Scholar]
- Fenner F., Sambrook J. F. Conditional lethal mutants of rabbitpox virus. II. Mutants (p) that fail to multiply in PK-2a cells. Virology. 1966 Apr;28(4):600–609. doi: 10.1016/0042-6822(66)90245-5. [DOI] [PubMed] [Google Scholar]
- Fletcher R. D., Hirschfield J. E., Forbes M. A common mode of antiviral action for ammonium ions and various amines. Nature. 1965 Aug 7;207(997):664–665. doi: 10.1038/207664a0. [DOI] [PubMed] [Google Scholar]
- Fong C. K., Bensch K. G., Hsiung G. D. Productive and abortive infections of simian and nonsimian cells with a simian adenovirus SV15. I. Microscopic observations. Virology. 1968 Jun;35(2):297–310. doi: 10.1016/0042-6822(68)90270-5. [DOI] [PubMed] [Google Scholar]
- Gallaher W. R., Bratt M. A. Temperature-dependent inhibition of fusion from without. J Virol. 1972 Jul;10(1):159–161. doi: 10.1128/jvi.10.1.159-161.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian G. A., Scott L. V. Quantitative estimation of phagocytosed herpes simplex virus in mouse peritoneal cells. Arch Gesamte Virusforsch. 1970;32(1):59–65. doi: 10.1007/BF01241520. [DOI] [PubMed] [Google Scholar]
- Gold P. H., Dales S. Localization of nucleotide phosphohydrolase activity within vaccinia. Proc Natl Acad Sci U S A. 1968 Jul;60(3):845–852. doi: 10.1073/pnas.60.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. A., Ratcliffe N. A., Basarab O., Smith H. Studies of the basis of localization of influenza virus in ferret organ cultures. Br J Exp Pathol. 1972 Feb;53(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Guggenheim M. A., Tyrrell S., Rabson A. S. Studies on Sendai virus cell fusion factor. Proc Soc Exp Biol Med. 1968 Dec;129(3):854–857. doi: 10.3181/00379727-129-33441. [DOI] [PubMed] [Google Scholar]
- Guir J., Braunwald J., Kirn A. Inhibition of host-specific DNA and RNA synthesis in KB cells following infection with frog virus 3. J Gen Virol. 1971 Sep;12(3):293–301. doi: 10.1099/0022-1317-12-3-293. [DOI] [PubMed] [Google Scholar]
- HANSON R. J., KEMPF J. E., BOAND A. V., Jr Phagocytosis of influenza virus. II. Its occurrence in normal and immune mice. J Immunol. 1957 Nov;79(5):422–427. [PubMed] [Google Scholar]
- HOLLAND J. J. Irreversible eclipse of poliovirus by HeLa cells. Virology. 1962 Feb;16:163–176. doi: 10.1016/0042-6822(62)90292-1. [DOI] [PubMed] [Google Scholar]
- HUANG A. S., WAGNER R. R. PENETRATION OF HERPES SIMPLEX VIRUS INTO HUMAN EPIDERMOID CELLS. Proc Soc Exp Biol Med. 1964 Aug-Sep;116:863–869. doi: 10.3181/00379727-116-29392. [DOI] [PubMed] [Google Scholar]
- Harter D. H., Choppin P. W. Cell-fusing activity of visna virus particles. Virology. 1967 Feb;31(2):279–288. doi: 10.1016/0042-6822(67)90172-9. [DOI] [PubMed] [Google Scholar]
- Hawkes R. A., Lafferty K. J. The enchancement of virus infectivity by antibody. Virology. 1967 Oct;33(2):250–261. doi: 10.1016/0042-6822(67)90144-4. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Schnaitman C. A. Entry of vesicular stomatitis virus into L cells. J Virol. 1971 Nov;8(5):786–795. doi: 10.1128/jvi.8.5.786-795.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Schnaitman C. A. Fusion of vesicular stomatitis virus with the cytoplasmic membrane of L cells. J Virol. 1969 Jun;3(6):619–622. doi: 10.1128/jvi.3.6.619-622.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg E., Becker Y. Adsorption, penetration and uncoating of herpes simplex virus. J Gen Virol. 1968 Mar;2(2):231–241. doi: 10.1099/0022-1317-2-2-231. [DOI] [PubMed] [Google Scholar]
- Hoffmann C. E., Neumayer E. M., Haff R. F., Goldsby R. A. Mode of Action of the Antiviral Activity of Amantadine in Tissue Culture. J Bacteriol. 1965 Sep;90(3):623–628. doi: 10.1128/jb.90.3.623-628.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M. Trypsin action on the growth of Sendai virus in tissue culture cells. II. Restoration of the hemolytic activity if L cell-borne Sendai virus by trypsin. J Virol. 1972 May;9(5):829–835. doi: 10.1128/jvi.9.5.829-835.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka Y. Biological activities of sonically treated Sendai virus. J Gen Virol. 1970 Jul;8(1):43–54. doi: 10.1099/0022-1317-8-1-43. [DOI] [PubMed] [Google Scholar]
- Hosaka Y., Koshi Y. Electron microscopic study of cell fusion by HVJ virions. Virology. 1968 Mar;34(3):419–434. doi: 10.1016/0042-6822(68)90062-7. [DOI] [PubMed] [Google Scholar]
- Hosaka Y., Shimizu Y. K. Artificial assembly of envelope particles of HVJ (Sendai virus). I. Assembly of hemolytic and fusion factors from envelopes solubilized by Nonidet P40. Virology. 1972 Sep;49(3):627–639. doi: 10.1016/0042-6822(72)90519-3. [DOI] [PubMed] [Google Scholar]
- Hosaka Y., Shimizu Y. K. Artificial assembly of envelope particles of HVJ (Sendai virus). II. Lipid components for formation of the active hemolysin. Virology. 1972 Sep;49(3):640–646. doi: 10.1016/0042-6822(72)90520-x. [DOI] [PubMed] [Google Scholar]
- Howatson A. F. Vesicular stomatitis and related viruses. Adv Virus Res. 1970;16:195–256. doi: 10.1016/s0065-3527(08)60024-x. [DOI] [PubMed] [Google Scholar]
- Howe C., Morgan C. Interactions between Sendai virus and human erythrocytes. J Virol. 1969 Jan;3(1):70–81. doi: 10.1128/jvi.3.1.70-81.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Bratt M. A. Ribonucleic acid polymerase in virions of Newcastle disease virus: comparison with the vesicular stomatitis virus polymerase. J Virol. 1971 Mar;7(3):389–394. doi: 10.1128/jvi.7.3.389-394.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus. 3. Multiple complementary messenger RNA molecules. Virology. 1970 Dec;42(4):946–957. doi: 10.1016/0042-6822(70)90343-0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Inhibition of cellular RNA synthesis by nonreplicating vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1579–1584. doi: 10.1073/pnas.54.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummeler K., Koprowski H., Wiktor T. J. Structure and development of rabies virus in tissue culture. J Virol. 1967 Feb;1(1):152–170. doi: 10.1128/jvi.1.1.152-170.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummeler K., Tomassini N., Sokol F. Morphological aspects of the uptake of simian virus 40 by permissive cells. J Virol. 1970 Jul;6(1):87–93. doi: 10.1128/jvi.6.1.87-93.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummeler K., Tomassini N., Zajac B. Early events in herpes simplex virus infection: a radioautographic study. J Virol. 1969 Jul;4(1):67–74. doi: 10.1128/jvi.4.1.67-74.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi Y., Matsumoto S., Dales S. Biogenesis of poxviruses: role of A-type inclusions and host cell membranes in virus dissemination. Virology. 1971 Dec;46(3):507–532. doi: 10.1016/0042-6822(71)90056-0. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., DARNELL J. E., Jr The adsorption and early fate of purified poliovirus in HeLa cells. Virology. 1961 Apr;13:439–447. doi: 10.1016/0042-6822(61)90275-6. [DOI] [PubMed] [Google Scholar]
- Jehn U., Lindahl T., Klein C. Fate of virus DNA in the abortive infection of human lymphoid cell lines by Epstein-Barr virus. J Gen Virol. 1972 Sep;16(3):409–412. doi: 10.1099/0022-1317-16-3-409. [DOI] [PubMed] [Google Scholar]
- Joklik W. K. The poxviruses. Bacteriol Rev. 1966 Mar;30(1):33–66. doi: 10.1128/br.30.1.33-66.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungwirth C., Launer J. Effect of poxvirus infection on host cell deoxyribonucleic acid synthesis. J Virol. 1968 May;2(5):401–408. doi: 10.1128/jvi.2.5.401-408.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAVERIN N. V. A NON-PROTEIN-SYNTHESIS-DEPENDENT STAGE OF LATENT PERIOD AND TIME OF PHOTOSENSITIVITY LOSS OF VIRUS IN ITS INTERACTION WITH THE CELL. Acta Virol. 1965 May;9:193–199. [PubMed] [Google Scholar]
- KOHN A. POLYKARYOCYTOSIS INDUCED BY NEWCASTLE DISEASE VIRUS IN MONOLAYERS OF ANIMAL CELLS. Virology. 1965 Jun;26:228–245. doi: 10.1016/0042-6822(65)90050-4. [DOI] [PubMed] [Google Scholar]
- Kang H. S., McAuslan B. R. Virus-associated nucleases: location and properties of deoxyribonucleases and ribonucleases in purified frog virus 3. J Virol. 1972 Aug;10(2):202–210. doi: 10.1128/jvi.10.2.202-210.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. C., Wilbert S. M., Black P. H. Endonuclease activity associated with purified simian virus 40 virions. J Virol. 1972 May;9(5):800–803. doi: 10.1128/jvi.9.5.800-803.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Poxvirus DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Jul;58(1):134–141. doi: 10.1073/pnas.58.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J., Beeson J. Ribonucleic acid synthesis in vaccinia virus. I. The mechanism of synthesis and release of RNA in vaccinia cores. J Mol Biol. 1970 May 28;50(1):1–18. doi: 10.1016/0022-2836(70)90100-2. [DOI] [PubMed] [Google Scholar]
- Kato N., Eggers H. J. Inhibition of uncoating of fowl plague virus by l-adamantanamine hydrochloride. Virology. 1969 Apr;37(4):632–641. doi: 10.1016/0042-6822(69)90281-5. [DOI] [PubMed] [Google Scholar]
- Kawanishi C. Y., Summers M. D., Stoltz D. B., Arnott H. J. Entry of an insect virus in vivo by fusion of viral envelope and microvillus membrane. J Invertebr Pathol. 1972 Jul;20(1):104–108. doi: 10.1016/0022-2011(72)90088-2. [DOI] [PubMed] [Google Scholar]
- Kidwell W. R., Saral R., Martin R. G., Ozer H. L. Characterization of an endonuclease associated with simian virus 40 virions. J Virol. 1972 Sep;10(3):410–416. doi: 10.1128/jvi.10.3.410-416.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Sharp D. G. Efficiency of the synergistic infection of L cells by groups of damaged vaccinia virus particles, MR. Virology. 1968 May;35(1):112–120. doi: 10.1016/0042-6822(68)90311-5. [DOI] [PubMed] [Google Scholar]
- Koch G., Bishop J. M. The effect of polycations on the interaction of viral RNA with mammalian cells: studies on the infectivity of single- and double-stranded poliovirus RNA. Virology. 1968 May;35(1):9–17. doi: 10.1016/0042-6822(68)90300-0. [DOI] [PubMed] [Google Scholar]
- Krizanová O., Kocisková D., Rathová V., Styk B. Interaction of plasma membranes with influenza virus. II. Release of viral nucleoprotein. Acta Virol. 1971 Sep;15(5):352–360. [PubMed] [Google Scholar]
- Lawrence W. C., Ginsberg H. S. Intracellular uncoating of type 5 adenovirus deoxyribonucleic acid. J Virol. 1967 Oct;1(5):851–867. doi: 10.1128/jvi.1.5.851-867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutenegger R. Early events of Sericesthis iridescent virus infection in hemocytes of Gallerio mellonella (L.). Virology. 1967 May;32(1):109–116. doi: 10.1016/0042-6822(67)90258-9. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Ginsberg H. S. Role of adenovirus structural proteins in the cessation of host-cell biosynthetic functions. J Virol. 1968 May;2(5):430–439. doi: 10.1128/jvi.2.5.430-439.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S., Magee W. E., Hamilton R. D., Miller O. V. Effect of interferon on early enzyme and viral DNA synthesis in vaccinia virus infections. Virology. 1967 May;32(1):33–40. doi: 10.1016/0042-6822(67)90249-8. [DOI] [PubMed] [Google Scholar]
- Lewandowski L. J., Kalmakoff J., Tanada Y. Characterization of a Ribonucleic Acid Polymerase Activity Associated with Purified Cytoplasmic Polyhedrosis Virus of the Silkworm Bombyx mori. J Virol. 1969 Dec;4(6):857–865. doi: 10.1128/jvi.4.6.857-865.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh P. C., Soergel M., Oie H. Growth characteristics of reovirus type 2: the intracellular fate of viral RNA. Arch Gesamte Virusforsch. 1967;22(3):398–408. doi: 10.1007/BF01242960. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. doi: 10.1128/jvi.4.4.323-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long W. F., Olusanya J. Adamantanamine and early events following influenza virus infection. Arch Gesamte Virusforsch. 1972;36(1):18–22. doi: 10.1007/BF01250291. [DOI] [PubMed] [Google Scholar]
- Lucy J. A. The fusion of biological membranes. Nature. 1970 Aug 22;227(5260):815–817. doi: 10.1038/227815a0. [DOI] [PubMed] [Google Scholar]
- MANDEL B. The use of sodium dodecyl sulfate in studies on the interaction of poliovirus and HeLa cells. Virology. 1962 Jun;17:288–294. doi: 10.1016/0042-6822(62)90119-8. [DOI] [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee W. E., Levine S., Miller O. V., Hamilton R. D. Inhibition by interferon of the uncoating of vaccinia virus. Virology. 1968 Aug;35(4):505–511. doi: 10.1016/0042-6822(68)90280-8. [DOI] [PubMed] [Google Scholar]
- Magee W. E., Miller O. V. Initiation of vaccinia virus infection in actinomycin D-pretreated cells. J Virol. 1968 Jul;2(7):678–685. doi: 10.1128/jvi.2.7.678-685.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak T. W., O'Callaghan D. J., Colter J. S. Studies of the early events of the replicative cycle of three variants of Mengo encephalomyelitis virus in mouse fibroblast cells. Virology. 1970 Dec;42(4):1087–1096. doi: 10.1016/0042-6822(70)90356-9. [DOI] [PubMed] [Google Scholar]
- Mandel B. The interaction of neutralized poliovirus with HeLa cells. I. Adsorption. Virology. 1967 Feb;31(2):238–247. doi: 10.1016/0042-6822(67)90167-5. [DOI] [PubMed] [Google Scholar]
- Mandel B. The relationship between penetration and uncoating of poliovirus in HeLa cells. Virology. 1967 Apr;31(4):702–712. doi: 10.1016/0042-6822(67)90198-5. [DOI] [PubMed] [Google Scholar]
- Mattern C. F., Takemoto K. K., Daniel W. A. Replication of polyoma virus in mouse embryo cells: electron microscopic observations. Virology. 1966 Oct;30(2):242–256. doi: 10.1016/0042-6822(66)90099-7. [DOI] [PubMed] [Google Scholar]
- Meiselman N., Kohn A., Danon D. Electron microscopic study of penetration of Newcastle disease virus into cells leading to formation of polykaryocytes. J Cell Sci. 1967 Mar;2(1):71–76. doi: 10.1242/jcs.2.1.71. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Gilden R. V. Electron microscopic studies of tumor viruses. I. Entry of murine leukemia virus into mouse embryo fibroblasts. J Virol. 1971 Mar;7(3):395–406. doi: 10.1128/jvi.7.3.395-406.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K., Morgan C. Structure and development of viruses as observed in the electron microscope. XI. Entry and uncoating of herpes simplex virus. J Virol. 1971 Dec;8(6):910–918. doi: 10.1128/jvi.8.6.910-918.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani S., Temin H. M. Enzymes and nucleotides in virions of Rous sarcoma virus. J Virol. 1971 Oct;8(4):409–416. doi: 10.1128/jvi.8.4.409-416.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Howe C. Structure and development of viruses as observed in the electron microscope. IX. Entry of parainfluenza I (Sendai) virus. J Virol. 1968 Oct;2(10):1122–1132. doi: 10.1128/jvi.2.10.1122-1132.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rose H. M., Mednis B. Electron microscopy of herpes simplex virus. I. Entry. J Virol. 1968 May;2(5):507–516. doi: 10.1128/jvi.2.5.507-516.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rose H. M. Structure and development of viruses as observed in the electron microscope. 8. Entry of influenza virus. J Virol. 1968 Sep;2(9):925–936. doi: 10.1128/jvi.2.9.925-936.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rosenkranz H. S., Mednis B. Structure and development of viruses as observed in the electron microscope. V. Entry and uncoating of adenovirus. J Virol. 1969 Nov;4(5):777–796. doi: 10.1128/jvi.4.5.777-796.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mss B., Filler R. Irreversible effects of cycloheximide during the early period of vaccinia virus replicaon. J Virol. 1970 Feb;5(2):99–108. doi: 10.1128/jvi.5.2.99-108.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Paoletti E., Grace J. T., Jr RNA polymerase activity in purified infectious vaccinia virus. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2280–2287. doi: 10.1073/pnas.58.6.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Paoletti E., Ospina J., Grace J. T., Jr Nucleotide phosphohydrolase in purified vaccinia virus. J Virol. 1968 Mar;2(3):167–172. doi: 10.1128/jvi.2.3.167-172.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson M. O., McAllister R. M. Infectivity of human adenovirus-1 DNA. Virology. 1972 Apr;48(1):14–21. doi: 10.1016/0042-6822(72)90109-2. [DOI] [PubMed] [Google Scholar]
- OKADA Y. Analysis of giant polynuclear cell formation caused by HVJ virus from Ehrlich's ascites tumor cells. I. Microscopic observation of giant polynuclear cell formation. Exp Cell Res. 1962 Feb;26:98–107. doi: 10.1016/0014-4827(62)90205-7. [DOI] [PubMed] [Google Scholar]
- OKADA Y. Analysis of giant polynuclear cell formation caused by HVJ virus from Ehrlich's ascites tumor cells. III. Relationship between cell condition and fusion reaction or cell degeneration reaction. Exp Cell Res. 1962 Feb;26:119–128. doi: 10.1016/0014-4827(62)90207-0. [DOI] [PubMed] [Google Scholar]
- OKADA Y., TADOKORO J. Analysis of giant polynuclear cell formation caused by HVJ virus from Ehrlich's ascites tumor cells. II. Quantitative analysis of giant polynuclear cell formation. Exp Cell Res. 1962 Feb;26:108–118. doi: 10.1016/0014-4827(62)90206-9. [DOI] [PubMed] [Google Scholar]
- OKADA Y., YAMADA K., TADOKORO J. EFFECT OF ANTISERUM ON THE CELL FUSION REACTION CAUSED BY HVJ. Virology. 1964 Mar;22:397–409. doi: 10.1016/0042-6822(64)90030-3. [DOI] [PubMed] [Google Scholar]
- Okada Y., Murayama F. Fusion of cells by HVJ: requirement of concentration of virus particles at the site of contact of two cells for fusion. Exp Cell Res. 1968 Sep;52(1):34–42. doi: 10.1016/0014-4827(68)90545-4. [DOI] [PubMed] [Google Scholar]
- Okada Y., Murayama F., Yamada K. Requirement of energy for the cell fusion reaction of Ehrlich ascites tumor cells by HVJ. Virology. 1966 Jan;28(1):115–130. doi: 10.1016/0042-6822(66)90312-6. [DOI] [PubMed] [Google Scholar]
- Ozer H. L., Takemoto K. K. Site of host restriction of simian virus 40 mutants in an established African green monkey kidney cell line. J Virol. 1969 Oct;4(4):408–415. doi: 10.1128/jvi.4.4.408-415.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILIPSON L., LIND M. ENTEROVIRUS ECLIPSE IN A CELL-FREE SYSTEM. Virology. 1964 Jul;23:322–332. doi: 10.1016/0042-6822(64)90254-5. [DOI] [PubMed] [Google Scholar]
- PHILIPSON L. THE EARLY INTERACTION OF ANIMAL VIRUSES AND CELLS. Prog Med Virol. 1963;5:43–78. [PubMed] [Google Scholar]
- Palese P., Koch G. Degradation of single- and double-stranded RNA by frog virus 3. Proc Natl Acad Sci U S A. 1972 Mar;69(3):698–701. doi: 10.1073/pnas.69.3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Moss B. Protein kinase and specific phosphate acceptor proteins associated with vaccinia virus cores. J Virol. 1972 Sep;10(3):417–424. doi: 10.1128/jvi.10.3.417-424.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L. Attachment and eclipse of adenovirus. J Virol. 1967 Oct;1(5):868–875. doi: 10.1128/jvi.1.5.868-875.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Lonberg-Holm K., Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968 Oct;2(10):1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Dales S., Bergoin M., Roberts D. W. Enzymes associated with an insect poxvirus. Virology. 1971 Jan;43(1):306–309. doi: 10.1016/0042-6822(71)90249-2. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Biogenesis of vaccinia: separation of early stages from maturation by means of hydroxyurea. Virology. 1971 Jan;43(1):144–151. doi: 10.1016/0042-6822(71)90232-7. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Regulation of the synthesis of nucleotide phosphohydrolase and neutral deoxyribonuclease: two activities present within purified vaccina virus. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1297–1303. doi: 10.1073/pnas.63.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Two deoxyribonuclease activities within purified vaccinia virus. Proc Natl Acad Sci U S A. 1969 Jul;63(3):820–827. doi: 10.1073/pnas.63.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R., Howell J. I., Lucy J. A. Lysolecithin and cell fusion. Nature. 1970 Aug 22;227(5260):810–814. doi: 10.1038/227810a0. [DOI] [PubMed] [Google Scholar]
- Poste G. Mechanisms of virus-induced cell fusion. Int Rev Cytol. 1972;33:157–252. doi: 10.1016/s0074-7696(08)61451-5. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Kates J., Kirkpatrick J. B. Replication of vaccinia virus DNA in enucleated L-cells. J Mol Biol. 1971 Aug 14;59(3):505–508. doi: 10.1016/0022-2836(71)90313-5. [DOI] [PubMed] [Google Scholar]
- Prose P. H., Friedman-Kien A. E., Vilcek J. Mollucum contagiosum virus in adult human skin cultures. An electron microscopic study. Am J Pathol. 1969 Jun;55(3):349–366. [PMC free article] [PubMed] [Google Scholar]
- REBEL G., FONTANGES R., COLOBERT L. [The lipid nature of substances responsible for the hemolytic activity of Myxo-virus paraninfluenzae I (Sendai virus)]. Ann Inst Pasteur (Paris) 1962 Feb;102:137–152. [PubMed] [Google Scholar]
- ROIZMAN B. Polykaryocytosis induced by viruses. Proc Natl Acad Sci U S A. 1962 Feb;48:228–234. doi: 10.1073/pnas.48.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBIN H., FRANKLIN R. M. On the mechanism of Newcastle disease virus neutralization by immune serum. Virology. 1957 Feb;3(1):84–95. doi: 10.1016/0042-6822(57)90025-9. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Lane W. T., Huebner R. J. Amantadine hydrochloride: inhibitory effect on murine sarcoma virus infection in cell cultures. Proc Soc Exp Biol Med. 1972 Apr;139(4):1258–1260. doi: 10.3181/00379727-139-36342. [DOI] [PubMed] [Google Scholar]
- Robinson H. J., Jr, Prose P. H., Friedman-Kien A. E., Neistein S., Vilcek J. The molluscum contagiosum virus in chick embryo cell cultures: an electron microscopic study. J Invest Dermatol. 1969 Jan;52(1):51–56. doi: 10.1038/jid.1969.7. [DOI] [PubMed] [Google Scholar]
- Robinson W. S. Ribonucleic acid polymerase activity in Sendai virions and nucleocapsid. J Virol. 1971 Jul;8(1):81–86. doi: 10.1128/jvi.8.1.81-86.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERSTEIN S. C., MARCUS P. I. EARLY STAGES OF NEWCASTLE DISEASE VIRUS-HELA CELL INTERACTION: AN ELECTRON MICROSCOPIC STUDY. Virology. 1964 Jul;23:370–380. doi: 10.1016/0042-6822(64)90259-4. [DOI] [PubMed] [Google Scholar]
- Salzman L. A. DNA polymerase activity associated with purified Kilham rat virus. Nat New Biol. 1971 Jun 9;231(23):174–176. doi: 10.1038/newbio231174a0. [DOI] [PubMed] [Google Scholar]
- Sambrook J. F., McClain M. E., Easterbrook K. B., McAuslan B. R. A mutant of its multiplication in three cell types. Virology. 1965 Aug;26(4):738–745. [PubMed] [Google Scholar]
- Schwartz J., Dales S. Biogenesis of poxviruses: identification of four enzyme activities within purified Yaba tumor virus. Virology. 1971 Sep;45(3):797–801. doi: 10.1016/0042-6822(71)90198-x. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Hosaka Y., Shimizu Y. K. Solubilization of envelopes of HVJ (Sendai virus) with alkali-emasol treatment and reassembly of envelope particles with removal of the detergent. J Virol. 1972 May;9(5):842–850. doi: 10.1128/jvi.9.5.842-850.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Astell C., Levin D. H., Schonberg M., Acs G. The mechanisms of reovirus uncoating and gene activation in vivo. Virology. 1972 Mar;47(3):797–806. doi: 10.1016/0042-6822(72)90571-5. [DOI] [PubMed] [Google Scholar]
- Silverstein S. Macrophages and viral immunity. Semin Hematol. 1970 Apr;7(2):185–214. [PubMed] [Google Scholar]
- Simon L. D., Anderson T. F. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. I. Attachment and penetration. Virology. 1967 Jun;32(2):279–297. doi: 10.1016/0042-6822(67)90277-2. [DOI] [PubMed] [Google Scholar]
- Simpson R. W., Hauser R. E., Dales S. Viropexis of vesicular stomatitis virus by L cells. Virology. 1969 Feb;37(2):285–290. doi: 10.1016/0042-6822(69)90209-8. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Smith W. R., McAuslan B. R. Biophysical properties of frog virus and its deoxyribonucleic acid: fate of radioactive virus in the early stage of infection. J Virol. 1969 Oct;4(4):339–347. doi: 10.1128/jvi.4.4.339-347.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spendlove R. S., McClain M. E., Lennette E. H. Enhancement of reovirus infectivity by extracellular removal or alteration of the virus capsid by proteolytic enzymes. J Gen Virol. 1970 Aug;8(2):83–94. doi: 10.1099/0022-1317-8-2-83. [DOI] [PubMed] [Google Scholar]
- Spring S. B., Roizman B. Herpes simplex virus products in productive and abortive infection. 3. Differentiation of infectious virus derived from nucleus and cytoplasm with respect to stability and size. J Virol. 1968 Oct;2(10):979–985. doi: 10.1128/jvi.2.10.979-985.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Cunningham C. H. Antibody-neutralized avian infectious bronchitis virus in chicken embryo kidney cells: entry and degradation. J Gen Virol. 1970 Sep;8(3):173–186. doi: 10.1099/0022-1317-8-3-173. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Protein kinase and phosphate acceptor proteins in Rauscher murine leukaemia virus. Nat New Biol. 1971 Sep 29;233(39):137–140. doi: 10.1038/newbio233137a0. [DOI] [PubMed] [Google Scholar]
- Summers M. D. Apparent in vivo pathway of granulosis virus invasion and infection. J Virol. 1969 Aug;4(2):188–190. doi: 10.1128/jvi.4.2.188-190.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. D. Electron microscopic observations on granulosis virus entry, uncoating and replication processes during infection of the midgut cells of Trichoplusia ni. J Ultrastruct Res. 1971 Jun;35(5):606–625. doi: 10.1016/s0022-5320(71)80014-x. [DOI] [PubMed] [Google Scholar]
- Takehara M., Schwerdt C. E. Infective subviral particles from cell cultures infected with myxoma and fibroma viruses. Virology. 1967 Jan;31(1):163–166. doi: 10.1016/0042-6822(67)90020-7. [DOI] [PubMed] [Google Scholar]
- Tan K. B., McAuslan B. R. Binding of deoxyribonucleic acid-dependent deoxyribonucleic acid polymerase to poxvirus. J Virol. 1972 Jan;9(1):70–74. doi: 10.1128/jvi.9.1.70-74.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanada Y., Leutenegger R. Multiplication of a granulosis virus in larval midgut cells of Trichoplusia ni and possible pathways of invasion into the hemocoel. J Ultrastruct Res. 1970 Mar;30(5):589–600. doi: 10.1016/s0022-5320(70)90053-5. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Mechanism of cell transformation by RNA tumor viruses. Annu Rev Microbiol. 1971;25:609–648. doi: 10.1146/annurev.mi.25.100171.003141. [DOI] [PubMed] [Google Scholar]
- Vilaginès R., McAuslan B. R. Interference with viral messenger RNA and DNA synthesis by superinfection with a heterologous deoxyvirus. Virology. 1970 Dec;42(4):1043–1053. doi: 10.1016/0042-6822(70)90352-1. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. DEAE-dextran: enhancement of cellular transformation induced by avian sarcoma viruses. Virology. 1967 Sep;33(1):175–177. doi: 10.1016/0042-6822(67)90109-2. [DOI] [PubMed] [Google Scholar]
- Wachsman J. T., Levin D. H., Acs G. Ribonucleoside triphosphate-dependent pyrophosphate exchange of Reovirus cores. J Virol. 1970 Oct;6(4):563–565. doi: 10.1128/jvi.6.4.563-565.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallbank A. M., Matter R. E., Klinikowski N. G. 1-Adamantanamine hydrochloride: inhibition of Rous and Esh Sarcoma viruses in cell culture. Science. 1966 Jun 24;152(3730):1760–1761. doi: 10.1126/science.152.3730.1760. [DOI] [PubMed] [Google Scholar]
- Warden D., Thorne H. V. Influence of diethylaminoethyl-dextran on uptake and degradation of polyoma virus deoxyribonucleic acid by mouse embryo cells. J Virol. 1969 Oct;4(4):380–387. doi: 10.1128/jvi.4.4.380-387.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Millward S., Graham A. F. Regulation of transcription of the Reovirus genome. J Mol Biol. 1968 Aug 28;36(1):107–123. doi: 10.1016/0022-2836(68)90223-4. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Trowbridge R. S., Kowalski J. B., O'Connell C. M., Peau C. J. Amantadine hydrochloride inhibition of early and late stages of lymphocytic choriomenigitis virus-cell interactions. Virology. 1971 Sep;45(3):679–686. [PubMed] [Google Scholar]
- Yaoi Y., Ogata M. The fate of vesicular stomatitis virus in cultured chick embryo cells. J Gen Virol. 1972 Sep;16(3):419–422. doi: 10.1099/0022-1317-16-3-419. [DOI] [PubMed] [Google Scholar]
- Yazgan S., Chapman A. L. Electron microscopic observations on the adenosine triphosphatase activity of a murine (Rauscher) and a canine leukemia virus. Virology. 1967 Jul;32(3):537–539. doi: 10.1016/0042-6822(67)90307-8. [DOI] [PubMed] [Google Scholar]
- Younghusband H. B., Lee P. E. Cytochemistry and autoradiography of Tipula iridescent virus in Galleria mellonella. Virology. 1970 Mar;40(3):757–760. doi: 10.1016/0042-6822(70)90223-0. [DOI] [PubMed] [Google Scholar]
- ZHDANOV V. M., BUKRINSKAYA A. G. Studies on the initial stage of virus-cell interaction. Acta Virol. 1962 Mar;6:105–113. [PubMed] [Google Scholar]
- Zee Y. C., Talens L. Entry of infectious bovine rhinotracheitis virus into cells. J Gen Virol. 1971 Apr;11(1):59–63. doi: 10.1099/0022-1317-11-1-59. [DOI] [PubMed] [Google Scholar]