Abstract
Research efforts around the world have been increasingly devoted to investigating changes in C3 and C4 species' abundance or distribution with global warming, as they provide important insight into carbon fluxes and linked biogeochemical cycles. However, changes in the early life stage (e.g. germination) of C3 and C4 species in response to global warming, particularly with respect to asymmetric warming, have received less attention. We investigated germination percentage and rate of C3 and C4 species under asymmetric (+3/+6°C at day/night) and symmetric warming (+5/+5°C at day/night), simulated by alternating temperatures. A thermal time model was used to calculate germination base temperature and thermal time constant. Two additional alternating temperature regimes were used to test temperature metrics effect. The germination percentage and rate increased continuously for C4 species, but increased and then decreased with temperature for C3 species under both symmetric and asymmetric warming. Compared to asymmetric warming, symmetric warming significantly overestimated the speed of germination percentage change with temperature for C4 species. Among the temperature metrics (minimum, maximum, diurnal temperature range and average temperature), maximum temperature was most correlated with germination of C4 species. Our results indicate that global warming may favour germination of C4 species, at least for the C4 species studied in this work. The divergent effects of asymmetric and symmetric warming on plant germination also deserve more attention in future studies.
Introduction
Climate change may strongly shift the distribution of C3 and C4 vegetation. C3 and C4 distribution helps to estimate global vegetation primary production and carbon uptake by the terrestrial biosphere [1]. Many previous examinations of the distribution of C3 and C4 grasses have attempted to predict changes due to the effect of rising atmospheric CO2 concentration, and have suggested that the direct and indirect effects are likely to push C3/C4 relative abundances in opposite directions [2], [3]. A recent study indicates that C4 species tend to spread toward more northern latitudes and higher altitudes in the Inner Mongolia grassland. This was mainly triggered by increasing temperature, which overwhelmed the positive effect of rising CO2 concentrations on C3 species [4]. Others have found that temperature is the primary driver of C4 grass species distribution [5] and the aboveground productivity ratios of C3 and C4 plants [6], [7]. Long-term data sets from the shortgrass steppe have indicated that increased spring minimum temperature was correlated with decreased net primary production by the dominant C4 grass (Bouteloua gracilis) and increased abundance and production by exotic and native C3 forbs [8].
Global warming has advanced the yearly first-flowering times of plants [9]. This may change reproductive output and seed maturation time, which affects seed germination [10]. Germination percentage and rate of C4 species increased with temperature until 35°C to 40°C, while germination of C3 species was favored by cooler conditions [11]. Different germination responses of C3 and C4 species may affect ecosystem structure and functioning via species' altered relative competitive ability and/or net primary productivity. However, there has been little research on the consequences of global warming for plant seed germination [12], let alone for the effects on germination of C3 and C4 species.
Global warming has been found to be asymmetric [13], i.e. there are greater increases in daily minimum than maximum temperatures, resulting in declining diurnal temperature ranges [14], [15]. This pattern has been empirically demonstrated in several regions [16], [17]. To date, however, most modelling efforts and experimental manipulations investigating plant or ecosystem responses to climate change have assumed that future warming will occur primarily during the day or uniformly over the diurnal cycle [18], [19]. Only a few researchers have studied the effect of nocturnal temperature elevation on ecosystem functions [20]–[22]. To our knowledge, there is no study that has investigated the effect of asymmetric warming on plant regeneration from seed.
Predicting germination timing changes of C3 and C4 species under long term asymmetric warming compared with symmetric warming helps to elucidate C3 and C4 vegetation distribution changes. A thermal time approach can be used to model these aspects, though no one has attempted this before, except in modelling dormancy change [23] and seed germination [24], as well as seedling emergence [25] in current climate.
Previous research on germination response to alternating temperature has mainly focused on how seasonal changes in temperature regulate seed dormancy and germination [26]–[28] or on the effect of temperature fluctuations on germination [29]–[31]. In the current study, we alternated temperature regimes to simulate asymmetric and symmetric global warming and analyzed changes in germination patterns of C4 and C3 species using a thermal time model. The main objectives of this study were: (1) to determine the comparative effects of global warming on seed germination of C3 and C4 species; and (2) to compare the different effects on germination resulting from asymmetric versus symmetric warming.
Materials and Methods
Plant materials and seed collection
Six wild species were selected in this study, of which Cynanchum chinense R. Br., Lappula myosotis V. Worf., and Saussurea amara DC. were C3 species, and Amaranthus retroflexus L., Portulaca oleracea L., and Echinochloa crusgalli (L.) Beauv. were C4 species. These species are widely distributed in northern China. Mature seeds of the six target species were collected during autumn 2006 from wild populations in the Songnen grassland of China (sites near 44o40' N, 123o44' E), and stored in cloth bags at 4°C. The seeds were permitted to be collected around the land of the Grassland Ecosystem Experimental Station of Northeast Normal University. No protected species were sampled.
Temperature regimes
To simulate global asymmetric and symmetric warming, two alternating temperature regimes were established (Table 1): a) The differential rate of warming between maximum and minimum temperatures (asymmetric warming, AW). Under this scenario, minimum/maximum temperatures were incrementally increased by +6°C/+3°C for each treatment from 5°C/20°C to 35°C/35°C. As a result, the mean temperature increased by 4.5°C and diurnal temperature range (DTR) decreased by 3°C for each set of treatments, with 6 treatments total in this regime; b) The same rate of warming between maximum and minimum temperatures (symmetric warming, SW). Under this scenario, both minimum and maximum temperatures increased by 5°C for each treatment from 5°C/15°C to 30°C/40°C. As a result, the mean temperatures incrementally increased by 5°C while the DTR remained constant at 10°C, with 6 treatments total in this regime. To test temperature metrics effects, two additional temperature regimes were established (Table 1): c) Minimum temperatures increased from 5°C to 35°C with 3°C increments, but the maximum temperature remained constant at 35°C across treatments (TmaxC). Effectively, the mean temperature increased by 1.5°C and the DTR decreased by 3°C across treatments, with 11 treatments total in this regime; d) minimum temperatures increased from 5°C to 20°C in 3°C increments, but maximum temperatures decreased from 35°C to 20°C in 3°C decrements (TaveC). Effectively, the mean temperature remained constant at 20°C, while the DTR decreased by 6°C across treatments, with 6 treatments total in this regime. To complete statistical analysis of thermal time model parameters, germination at 5–40°C constant temperatures with 5°C interval was also tested, with 8 treatments total in this regime. Together, 37 temperature regimes were established in this study. The DTR is defined as the difference between the diurnal maximum and minimum temperature. Alternating temperatures can be dissected into minimum temperature, maximum temperature and the calculated DTR and average temperature.
Table 1. The alternating temperature regimes used in the study.
| Maximum temperature constant (TmaxC) | Average temperature constant (TaveC) | Asymmetric warming (AW) | Symmetric warming (SW) |
| TmaxC1: 5/35 (20, 30)* | TaveC1: 5/35 (20, 30) | AW1: 5/20 (12.5, 15) | SW1: 5/15 (10, 10) |
| TmaxC2: 8/35 (21.5, 27) | TaveC2: 8/32 (20, 24) | AW2: 11/23 (17, 12) | SW2: 10/20 (15, 10) |
| TmaxC3: 11/35 (23, 24) | TaveC3: 11/29 (20, 18) | AW3: 17/26 (21.5, 9) | SW3: 15/25 (20, 10) |
| TmaxC4: 14/35 (24.5, 21) | TaveC4: 14/26 (20, 12) | AW4: 23/29 (26, 6) | SW4: 20/30 (25, 10) |
| TmaxC5: 17/35 (26, 18) | TaveC5: 17/23 (20, 6) | AW5: 29/32 (30.5, 3) | SW5: 25/35 (30, 10) |
| TmaxC6: 20/35 (27.5, 15) | TaveC6: 20/20 (20, 0) | AW6: 35/35 (35, 0) | SW6: 30/40 (35, 10) |
| TmaxC7: 23/35 (29, 12) | |||
| TmaxC8: 26/35 (30.5, 9) | |||
| TmaxC9: 29/35 (32, 6) | |||
| TmaxC10: 32/35 (33.5, 3) | |||
| TmaxC11: 35/35 (35, 0) |
*The mean temperature and diurnal temperature range of each temperature regime were presented in parenthesis with comma between them.
Germination test
Germination studies were conducted in growth chambers in August 2007. Each chamber corresponded to each temperature treatment. A 12-h photoperiod (Sylvania cool white fluorescent lamps, 25 µmol m−2 s−1, 400–700 nm) with 12-h dark period was maintained throughout the experiment. The temperatures cycled with half-hour linear transition periods between minimum and maximum temperatures (8:00–8:30 and 20:00–20:30, respectively).
Fifty seeds from each species were placed on two layers of filter paper in a grid (100 mm diameter) of plastic trays, and replicated 4 times within each temperature treatment. The filter paper was kept moistened with distilled water. Seeds were considered to have germinated when the radicle emerged. Germination status was recorded twice a day during the first 5 days, then once a day during the second 5 days. Germination was measured for 10 days because preliminary tests indicated that most of the germination events occurred within this period. The final germination percentage (GP) and germination rate (GR, the reciprocal of germination time) of each species were calculated with respect to the cumulative germination curves of each temperature treatment.
Thermal time model theory
Garcia-Huidobro et al. [32] presented a model with two equations relating thermal time (degree days above a base temperature) to germination rate (the reciprocal of the time taken for a given fraction of seed to germinate) at a constant temperature. The two equations of the thermal time model (TT model) are:
For any given subpopulation g, germination rate GR can be described by two straight lines. The slopes of the two lines are θ 1(g) and θ 2(g) (thermal time constant at suboptimal and supra-optimal temperatures) with the intersection of the two lines defined as T o (optimum temperature, at which maximum germination rate occurs). The two points where germination percentages equal zero are defined as Tb(g) and Tc(g) (the minimum and maximum temperature, below or above which no germination occurs). The parameters are useful for field predictions and can be used to compare germination in different species, climates, and locations.
If alternating temperatures were all above or all below the optimum temperature, then the formulas were no different to those for constant temperature. However, if the temperature fluctuated from below the optimum to above, then the equation changed [33]. In such cases, the predicted germination rate for any subpopulation g is:
where t 1 is the time below T o, with mean temperature of T 1, and t 2 is the time above T o, with mean temperature T 2.
Data analysis
Data were analysed using SPSS (version 13.0, SPSS Inc., Chicago, Illinois, USA). Germination percentage and germination rate of C3 or C4 species were the average of the three species with the same photosynthetic type. The effects of different alternating temperature treatments in each regime and the plant photosynthesis type (C3 and C4) on germination percentage and germination rate were examined using two-way ANOVA. Germination times for 1 percent seed (1% germination) were calculated from the cumulative germination curves in AW and SW temperature regimes for the six species and converted to rates in this study, which represents the initiative germination time of each batch of seeds. This standard (1% germination) was used instead of 50% germination because seed germination of certain species was less than 50% for some temperature treatments (see Figure S1). Thermal time model (TT model) parameters were analyzed using repeated probit regression as described previously [33]. The average daily temperatures were used for determining alternating temperatures. The model parameter (T
b and θ
1) differences between C3 and C4 species, or between AW and SW temperature regimes within one photosynthetic type, were tested by Paired-Samples T-tests (2-tailed). The relationships between germination percentages/rates and the average temperatures in AW and SW regimes were fitted by either linear 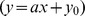 or quadratic regressions
or quadratic regressions 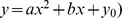 using SigmaPlot (version 10.0, Systat Software Inc., Richmond, California, USA), with 95% confidence of the fitted lines given. The differences in the slope (a in the equation) of the regression between AW and SW regimes for germination of C4 species were tested using the SMATR package in R software [34]. Pearson correlation analysis was carried out to test the correlation between GP/GR and temperature metrics (minimum, maximum, DTR, average) from the four alternating temperature regimes, totally 29 treatments.
using SigmaPlot (version 10.0, Systat Software Inc., Richmond, California, USA), with 95% confidence of the fitted lines given. The differences in the slope (a in the equation) of the regression between AW and SW regimes for germination of C4 species were tested using the SMATR package in R software [34]. Pearson correlation analysis was carried out to test the correlation between GP/GR and temperature metrics (minimum, maximum, DTR, average) from the four alternating temperature regimes, totally 29 treatments.
Results
Germination responses to different temperature regimes
The interaction of plant photosynthetic type and temperature treatment had significant effects on germination percentage (GP) and germination rate (GR), except in the case for GR under TmaxC and TaveC temperature regimes (Table 2).
Table 2. The two-way ANOVA analysis of the effects of plant photosynthetic type (PPT) and temperature treatments (T) in four temperature regimes on germination percentage and germination rate.
| PPT (df = 1) | T (df = 10† or 5) | PPT * T (df = 10† or 5) | |
| GP | |||
| TmaxC | 32.24*** | 1.24ns | 2.38* |
| TaveC | 0.13ns | 0.30ns | 3.23* |
| SW | 0.43ns | 0.88ns | 3.18* |
| AW | 0.05ns | 0.19ns | 3.40* |
| GR | |||
| TmaxC | 557.85*** | 5.37** | 1.02ns |
| TaveC | <0.01ns | 0.07ns | 1.56ns |
| SW | 1.05ns | 1.11ns | 14.16*** |
| AW | 0.84ns | 1.18ns | 4.97** |
ns, P>0.05;
*, P<0.05;
**, P<0.01;
***, P<0.001
The degree of freedom for TmaxC temperature regime is 10 and df of other temperature regimes is 5.
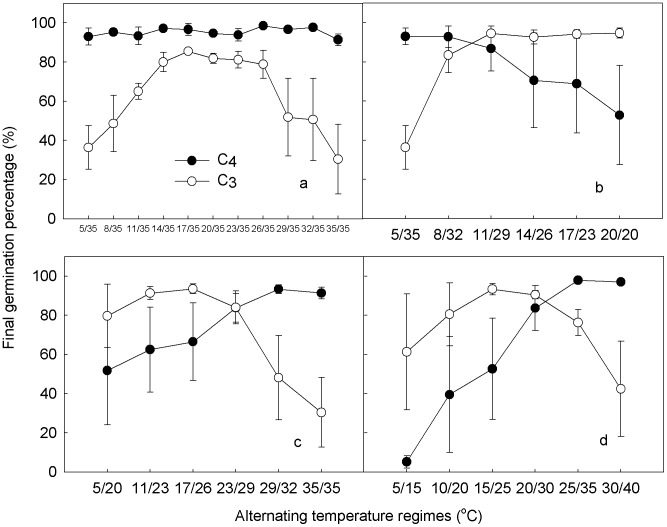
GP of C4 species were all above 90% in the TmaxC temperature regime, while GP of C3 species increased linearly from 5/35°C to 17/35°C and remained around 80%–85% from 17/35°C to 26/35°C, then decreased sharply as the minimum temperature increased thereafter (Figure 1a). In the TaveC temperature regime, GP of C4 species decreased as the minimum temperature increased, while GP of C3 species increased, reached its highest value at 11/29°C, and remained subsequently constant (Figure 1b). In the AW and SW temperature regimes, GP of C4 species increased, whereas GP of C3 species increased and then decreased with the increasing temperatures (Figure 1c, d).
Figure 1. Germination percentages of C4 (closed circles, average of E. crusgalli, P. oleracea and A. retroflexus) and C3 species (open circles, average of L. myosotis, S. amara and C. chinense) under different alternating temperature regimes (a, TmaxC; b, TaveC; c, AW; d, SW).
Mean ± S.E.
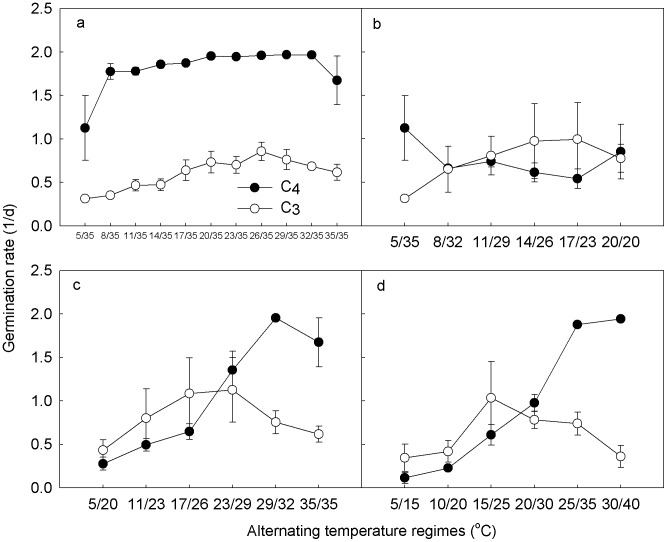
In the TmaxC temperature regime, GR of C4 species at 5/35°C was significantly lower than those at the other altered temperatures (P<0.05, Figure 2a). As the minimum temperature increased, GR of C3 species increased gradually and reached peak at 26/35°C, and then decreased. In the TaveC temperature regime, GR of C4 species tended to decrease with the increasing minimum temperature, while GR of C3 species tended to increase until 17/23°C (Figure 2b). However, there were no significant differences in all values except GR at 5/35°C between C4 and C3 species (P<0.05). In the AW and SW temperature regimes, GR of C4 species increased until 30°C of average temperature (29/32°C for AW or 25/35°C for SW), while GR of C3 species increased and then decreased with the increasing temperatures (Figure 2c, d). GR of the three C3 and C4 species responded consistently to the temperature regimes, with only magnitude difference (Figure S2).
Figure 2. Germination rates of C4 and C3 species under different alternating temperature regimes.
See Fig. 1 for symbols.
Comparison of TT model parameters and germination under asymmetric and symmetric warming
For all species in the AW and SW alternating temperature regimes (except C3 species L. myosotis in the SW temperature regime), the linear regressions of the TT model explained more than 87% variation (Table 3). For the three C4 species and the average, the estimated Tb of 1% germination in the AW temperature regime was higher than those in the SW temperature regime (P = 0.059). However, the estimated θ 1 in the AW regime were lower than those in the SW regime (P = 0.057). Therefore, the germination rates were difficult to compare between the two temperature regimes according to the TT model equation (Figure 3c). For C. chinense and the average of three C3 species, Tb and θ 1 of 1% germination in the AW temperature regime were both lower than that in the SW temperature regime (P = 0.891 for Tb, P = 0.081 for θ 1), such that germination rates of the C3 species in the AW temperature regime were higher than values in the SW temperature regime (Figure 3d). Compared with C3 species, Tb of C4 species were significantly higher in both the AW and SW temperature regimes (P<0.05). If C3 species L. myosotis is excluded, θ 1 of C4 species was also significantly lower than C3 species in both temperature regimes (P<0.05).
Table 3. Thermal time model parameter estimates (Tb, minimum temperature; θ 1, thermal time constant) for C4 and C3 species under the symmetric warming (SW) and asymmetric warming (AW) alternating temperature regimes.
| Tb (°C) | θ 1 (°C•d) | R2 | P | ||
| C4 species | |||||
| P. oleracea | SW | 9.6 | 12.4 | 0.94 | 0.0013 |
| AW | 10.4 | 11.3 | 0.94 | 0.0015 | |
| E. crusgalli | SW | 10.4 | 12.3 | 0.95 | 0.0011 |
| AW | 10.7 | 10.0 | 0.93 | 0.0079 | |
| A. retroflexus | SW | 12.6 | 11.7 | 0.92 | 0.0028 |
| AW | 13.3 | 10.5 | 0.87 | 0.0206 | |
| C4 species average | SW | 10.9 | 12.1 | ||
| AW | 11.5 | 10.6 | |||
| C3 species | |||||
| L. myosotis | SW | 6.3 | 8.2 | 0.76 | 0.3275 |
| AW | 7.2 | 7.3 | 0.97 | 0.1123 | |
| S. amara | SW | 1.7 | 34.6 | 0.93 | 0.0349 |
| AW | 1.8 | 31.8 | 0.95 | 0.1372 | |
| C. chinense | SW | 5.6 | 28.6 | 0.90 | 0.0133 |
| AW | 4.3 | 25.5 | 0.94 | 0.0057 | |
| C3 species average | SW | 4.5 | 23.8 | ||
| AW | 4.4 | 21.5 |
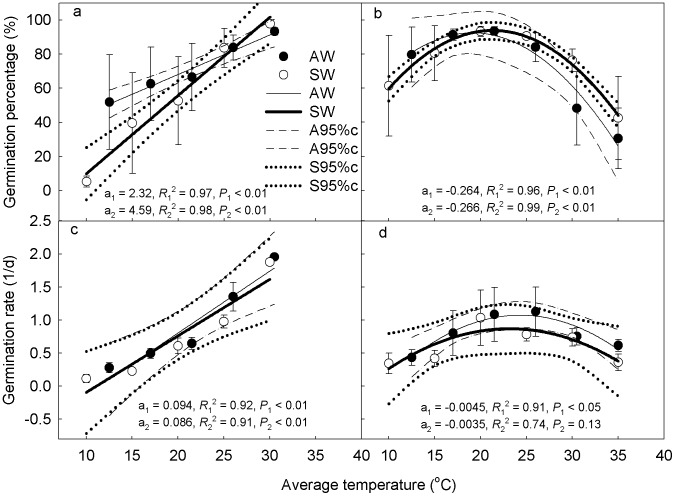
Figure 3. Germination percentage (a, b) and germination rate (c, d) changes of C4 (a, c) and C3 (b, d) species with increasing average temperature in the AW and SW temperature regimes.
The relationship between C4 species and the average temperature is fitted by linear equation; the relationship between C3 species and the average temperature is fitted by polynomial equation. Dash-dot lines represent 95% confidence of AW temperature regime and dotted lines represent 95% confidence of SW temperature regime. a1, R 1 2, P 1 represent the parameter, coefficient of determination and probability for the fitting in the AW regime and a2, R 2 2, P 2 represent the parameter, coefficient of determination and probability for the fitting in the SW regime.
For relationship between germination percentage and the average temperature, the parameter a in the AW regime was smaller than that in the SW regime, regardless of photosynthetic type (Figure 3a, b) and the difference was significant for C4 species (P<0.01). This suggests that changes were more intense under symmetric warming (SW) than asymmetric warming (AW), especially for C4 species. For relationship between germination rate and the average temperature, the parameter a in the AW regime was larger than that in SW regime for both C4 and C3 species. However, the parameter a between the two temperature regimes was not significantly different for C4 species (P = 0.74, Figure 3c) and the linear regression between germination rate and average temperature for C3 species in the SW regime was not significant (P = 0.13, Figure 3d).
Correlation between GP/GR and temperature metrics
For C4 species average, GP/GR were positively correlated with minimum, maximum and average temperature (P<0.05, Table 4), with the highest correlation coefficients between GP/GR and maximum temperature (r = 0.939 or r = 0.876). GRs of C4 species were negatively correlated with DTR, though it was not significant (P = 0.798). All of the three C4 species responded similarly. For C3 species however, GPs were negatively correlated with maximum and average temperatures (P<0.05), and GRs were negatively correlated with DTR (P<0.01). Furthermore, the results of the three C3 species were inconsistent.
Table 4. Pearson correlation analysis of germination percentage (GP) and germination rate (GR) with temperature metrics (TM, minimum, maximum, average, diurnal temperature range (DTR)) from the four alternating temperature regimes for C4 and C3 species (r, correlation coefficient; P, probability for the correlation).
| Species | TM | GP | GR | ||
| r | P | r | P | ||
| C4 species | |||||
| P. oleracea | Minimum | 0.388 | 0.037 | 0.590 | 0.001 |
| Maximum | 0.870 | <0.001 | 0.863 | <0.001 | |
| DTR | 0.221 | 0.250 | −0.005 | 0.981 | |
| Average | 0.678 | <0.001 | 0.815 | <0.001 | |
| E. crusgalli | Minimum | 0.200 | 0.298 | 0.624 | <0.001 |
| Maximum | 0.434 | 0.019 | 0.822 | <0.001 | |
| DTR | 0.103 | 0.594 | −0.072 | 0.712 | |
| Average | 0.343 | 0.069 | 0.819 | <0.001 | |
| A. retroflexus | Minimum | 0.462 | 0.012 | 0.636 | <0.001 |
| Maximum | 0.958 | <0.001 | 0.846 | <0.001 | |
| DTR | 0.206 | 0.285 | −0.067 | 0.728 | |
| Average | 0.771 | <0.001 | 0.839 | <0.001 | |
| C4 species average | Minimum | 0.439 | 0.017 | 0.640 | <0.001 |
| Maximum | 0.939 | <0.001 | 0.876 | <0.001 | |
| DTR | 0.216 | 0.260 | −0.050 | 0.798 | |
| Average | 0.746 | <0.001 | 0.855 | <0.001 | |
| C3 species | |||||
| L. myosotis | Minimum | −0.416 | 0.025 | −0.002 | 0.992 |
| Maximum | −0.679 | <0.001 | −0.440 | 0.017 | |
| DTR | −0.049 | 0.801 | −0.324 | 0.087 | |
| Average | −0.608 | <0.001 | −0.208 | 0.278 | |
| S. amara | Minimum | −0.576 | 0.001 | 0.438 | 0.017 |
| Maximum | −0.594 | 0.001 | −0.071 | 0.713 | |
| DTR | 0.189 | 0.326 | −0.531 | 0.003 | |
| Average | −0.679 | <0.001 | 0.270 | 0.157 | |
| C. chinense | Minimum | 0.539 | 0.003 | 0.802 | <0.001 |
| Maximum | 0.312 | 0.100 | 0.385 | 0.039 | |
| DTR | −0.358 | 0.057 | −0.590 | 0.001 | |
| Average | 0.521 | 0.004 | 0.737 | <0.001 | |
| C3 species average | Minimum | −0.299 | 0.115 | 0.333 | 0.077 |
| Maximum | −0.514 | 0.004 | −0.197 | 0.307 | |
| DTR | −0.055 | 0.777 | −0.509 | 0.005 | |
| Average | −0.449 | 0.015 | 0.138 | 0.474 | |
Discussion
Germination percentage and germination rate of C4 species increased linearly under the AW regime (Figure 1c and 2c), while those of C3 species increased and then decreased with the increasing temperature. This suggests that long term global asymmetric warming may favor seed germination of C4 species, at least for the tested species, which is consistent with the model prediction by Thorpe et al. [35] and inference from Sage and Kubien [36]. Spring events are changing more than autumn events as they are more sensitive to climate and are also undergoing the greatest alterations of climate relative to other seasons [37]. Germination and emergence are mainly spring events in most temperate regions [38], which are important research topics related to global warming. Though we just studied three C3 and three C4 species and more research is needed on this topic, our results somehow reflected the trends of C3 and C4 species germination and emergence shift under global warming.
In our study, symmetric and asymmetric warming had different impacts on germination of C4 species. The symmetric warming treatments significantly overestimated the speed of germination percentage change with temperature (P<0.05), compared to the asymmetric warming (Figure 3a). Another evidence is that the average base temperature is higher and the average thermal time constant is lower in the AW alternating temperature regime compared to the SW regime (Table 3), which is near significant (P = 0.059 for base temperature, P = 0.057 for thermal time constant).
Ecosystem warming studies have been performed for more than 20 years using a variety of methods including heat-resistance cables, infrared (IR) lamps, field chambers (e.g. OTC), and night-time warming. Historic air temperature data and most models suggest that much of the global warming increase will occur during the night-time hours. Therefore, artificial night-time warming is ideally suited for replicating a potentially relevant form of climate change [39]. Field night warming has been achieved by heating with IR at night-time [21], by reflective curtains covering the vegetation at night [40], or by light-weight aluminum fabric shelters (mounted on rollers similar to a window shade) that are drawn across the warming plots at night [41]. In this study, we first used alternating temperature regimes controlled by different growth chambers to simulate consecutive global asymmetric (+3/+6°C at day/night continuously) and symmetric (+5/+5°C at day/night continuously) warming (Table 2). This method can be used to predict the trend of shifts in seed germination and seedling growth under global warming.
Phillips et al. [42] suggest that it is significant to understand the influence of both day and night-time warming on the carbon balance of plants and concluded that changes in daily mean temperatures, rather than changes in minimum or maximum temperature, are sufficient for predicting ecosystem carbon fluxes in a Mediterranean grassland system. Another report indicates that a changing daily temperature regime may be important in determining plant responses to warming temperatures and should be considered in predictions of plant and ecosystem responses to future climate change [43]. The correlation between germination and the temperature metrics (minimum, maximum, diurnal temperature range (DTR) and average temperature) from the four temperature regimes for C4 and C3 species were analyzed in this study (Table 4). For C4 species, GP/GR was most correlated with the maximum temperature, while the results of the three C3 species were inconsistent. Von Fischer et al. [7] suggest that daily maximum temperature better predicts percent of C4 species than daily average or minimum temperature. Hattersley [44] also found summer (January) temperature in Australia had highest correlation with percent of C4 species. DTR is an important index of climate change [45]. In our study, average and minimum temperature was significantly correlated with germination of C4 species, but not C3 species. However, DTR was not correlated with germination, except with the germination rates of the C3 species S. amara and C. chinense.
Conclusions
This work indicates that global symmetric and asymmetric warming favors seed germination of C4 species, rather than C3 species, according to the results of the tested species in this study. Compared to asymmetric warming, symmetric warming overestimated the speed of germination percentage change with temperature for C4 species. Asymmetric and symmetric warming had no significant effects on germination of C3 species. Among the temperature metrics (minimum, maximum, diurnal temperature range and average of the alternating temperature), maximum temperature (day-time temperature) was found to be most correlated with germination for C4 species. Germination of C3 species responded inconsistently to the temperature metrics. Using alternating temperature regimes controlled by different growth chambers to simulate consecutive global asymmetric and symmetric warming is a good method, which can be used to predict the trend of shifts in plant early growth under global warming.
Supporting Information
Final germination percentages of six species under different alternating temperature regimes (TmaxC, a, b; TaveC, c, d; AW, e, f; SW, g, h). C4 species: a, c, e, g; C3 species: b, d, f, h.
(TIF)
Germination rates of six species under different alternating temperature regimes. See S1 for symbols.
(TIF)
Acknowledgments
We thank Dr Ping Wang, Wisdom Japhet, and Louis Irving for the comments on the previous version of the manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the paper and in the Supporting Information files.
Funding Statement
This research has been supported by the National Natural Science Foundation of China (41330640). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Auerswald K, Wittmer MHOM, Männel TT, Bai YF, Schäufele R, et al. (2009) Large regional-scale variation in C3/C4 distribution pattern of Inner Mongolia steppe is revealed by grazer wool carbon isotope composition. Biogeosciences 6: 795–805. [Google Scholar]
- 2. Collatz GJ, Berry JA, Clark JS (1998) Effects of climate and atmospheric CO2 partial pressure on the global distribution of C4 grasses: present, past, and future. Oecologia 114: 441–454. [DOI] [PubMed] [Google Scholar]
- 3. Winslow JC, Hunt ER, Piper SC (2003) The influence of seasonal water availability on global C3 versus C4 grassland biomass and its implications for climate change research. Ecol Model 163: 153–173. [Google Scholar]
- 4. Wittmer MHOM, Auerswald K, Bai YF, Schaufele R, Schnyder H (2010) Changes in the abundance of C3/C4 species of Inner Mongolia grassland: evidence from isotopic composition of soil and vegetation. Glob Change Biol 16: 605–616. [Google Scholar]
- 5. Bremond L, Boom A, Favier C (2012) Neotropical C3/C4 grass distributions-present, past and future. Glob Change Biol 18: 2324–2334. [Google Scholar]
- 6. Paruelo JM, Lauenroth WK (1996) Relative abundance of plant functional types in grasslands and shrublands of North America. Ecol Appl 6: 1212–1224. [Google Scholar]
- 7. Von Fischer JC, Tieszen LL, Schimel DS (2008) Climate controls on C3 vs. C4 productivity in North American grasslands from carbon isotope composition of soil organic matter. Glob Change Biol 14: 1141–1155. [Google Scholar]
- 8. Alward RD, Detling JK, Milchunas DG (1999) Grassland vegetation changes and nocturnal global warming. Science 283: 229–231. [DOI] [PubMed] [Google Scholar]
- 9. Abu-Asab MS, Peterson PM, Shetler SG, Orli SS (2001) Earlier plant flowering in spring as a response to global warming in the Washington, DC, area, Biodivers Conserv. 10: 597–612. [Google Scholar]
- 10. De Frenne P, Brunet J, Shevtsova A, Kolb A, Graae BJ, et al. (2011) Temperature effects on forest herbs assessed by warming and transplant experiments along a latitudinal gradient Glob Change Biol. 17: 3240–3253. [Google Scholar]
- 11.Zhang H (2008) Research on seed germination ecology. Ph. D thesis.
- 12. Hovenden MJ, Wills KE, Chaplin RE, Schoor JKV, Williams AL, et al. (2008) Warming and elevated CO2 affect the relationship between seed mass, germinability and seedling growth in Austrodanthonia caespitosa, a dominant Australian grass. Glob Change Biol 14: 1633–1641. [Google Scholar]
- 13. Xia J, Chen J, Piao S, Ciais P, Luo Y, et al. (2014) Terrestrial carbon cycle affected by non-uniform climate warming. Nat Geosci 7: 173–180. [Google Scholar]
- 14. Easterling DR, Horton B, Jones PD, Peterson TC, Karl TR, et al. (1997) Maximum and minimum temperature trends for the glob. Science 277: 364–367. [Google Scholar]
- 15. Vose RS, Easterling DR, Gleason B (2005) Maximum and minimum temperature trends for the globe: an update through 2004. Geophys Res Let 32: 5. [Google Scholar]
- 16. Karl TR, Jones PD, Knight RW, Kukla G, Plummer N, et al. (1993) A new perspective on recent global warming: asymmetric trends of daily maximum and minimum temperature. B Am Meteorol Soc 74: 1007–1023. [Google Scholar]
- 17. Liu B, Xu M, Henderson M, Qi Y, Li YQ (2004) Taking China's Temperature: daily range, warming trends, and regional variations, 1955–2000. J Climate 17: 4453–4462. [Google Scholar]
- 18. Zavaleta ES, Thomas BD, Chiariello NR, Asner GP, Shaw MR, et al. (2003) Plants reverse warming effect on ecosystem water balance. P Natl Acad Sci USA 100: 9892–9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luo YQ (2007) Terrestrial carbon-cycle feedback to climate warming. Annu Rev Ecol Evol S 38: 683–712. [Google Scholar]
- 20. Lobell DB, Ortiz-Monasterio JI (2007) Impacts of day versus night temperatures on spring wheat yields: a comparison of empirical and CERES model predictions in three locations. Agron J 99: 469–477. [Google Scholar]
- 21. Wan SQ, Xia JY, Liu WX, Niu SL (2009) Photosynthetic overcompensation under nocturnal warming enhances grassland carbon sequestration. Ecology 90: 2700–2710. [DOI] [PubMed] [Google Scholar]
- 22. Bai WM, Xia JY, Wan SQ, Zhang WH, Li LH (2012) Day and night warming have different effect on root lifespan. Biogeosciences 9: 375–384. [Google Scholar]
- 23. Wang WQ, Song SQ, Li SH, Gan YY, Wu JH, et al. (2009) Quantitative description of the effect of stratification on dormancy release of grape seeds in response to various temperatures and water contents. J Exp Bot 60 (2): 3397–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trudgill DL, Squire GR, Thompson K (2000) A thermal time basis for comparing the germination requirements of some British herbaceous plants. New Phytol 145: 107–114. [Google Scholar]
- 25. Qiu J, Bai Y, Coulman B, Romo JT (2006) Using thermal time models to predict seedling emergence of orchardgrass (Dactylis glomerata L.) under alternating temperature regimes. Seed Sci Res 16: 261–271. [Google Scholar]
- 26. Khan MA, Ungar IA (1996) Influence of salinity and temperature on the germination of Haloxylon recurvum . Ann Bot 78: 547–551. [Google Scholar]
- 27. Gul B, Webber DJ (1999) Effect of salinity, light and temperature on germination in Allenrolfea occidentalis . Can J Bot 77: 240–246. [Google Scholar]
- 28. Khan MA, Gul B, Weber DJ (2000) Germination responses of Salicornia rubra to temperature and salinity. J Arid Environ 45: 207–214. [Google Scholar]
- 29. Ellis RH, Barrett S (1994) Alternating temperatures and rate of seed germination in Lentil. Ann Bot 74: 519–524. [Google Scholar]
- 30. Ekstam B, Johannesson R, Milberg P (1999) The effect of light and number of diurnal temperature fluctuations on germination of Phragmites australis . Seed Sci Res 9: 165–170. [Google Scholar]
- 31. Markus B (2004) The role of temperature in the regulation of dormancy and germination of two related summer-annual mudflat species. Aquat Bot 79: 15–32. [Google Scholar]
- 32. Garcia-Huidobro J, Monteith JL, Squire GR (1982a) Time, temperature and germination of pearl millet (Pennisetum typhoides S. & H.) 1. Constant temperature. J Exp Bot 33: 287–295. [Google Scholar]
- 33. Garcia-Huidobro J, Monteith JL, Squire GR (1982b) Time, temperature and germination of pearl millet (Pennisetum typhoides S. & H.) 2. Alternating temperature. J Exp Bot 33: 297–302. [Google Scholar]
- 34.Falster DS, Warton DI, Wright IJ (2006) SMATR: standardised major axis tests and routines, ver 2.0. http://www.bio.mq.edu.au/ecology/SMATR/.
- 35. Thorpe J, Wolfe SA, Houston B (2008) Potential impacts of climate change on grazing capacity of native grasslands in the Canadian prairies. Can J Soil Sci 88: 595–609. [Google Scholar]
- 36. Sage RF, Kubien DS (2003) Que vadis C4? An ecophysiological perspective on global change and the future of C4 plants. Photosynth Res 77: 209–225. [DOI] [PubMed] [Google Scholar]
- 37. Gordo O, Sanz JJ (2010) Impact of climate change on plant phenology in Mediterranean ecosystems. Glob Change Biol 16: 1082–1106. [Google Scholar]
- 38. Zhang H, Zhou D, Wang P, Wang T, Jin Y (2007) Germination responses of four wild species to diurnal increase or decrease in temperature. Seed Sci Technol 35: 291–302. [Google Scholar]
- 39. Aronson EL, McNulty SG (2009) Appropriate experimental ecosystem warming methods by ecosystem, objective and practicality. Agr Forest Meteorol 149: 1791–1799. [Google Scholar]
- 40. Beier C, Emmett B, Gundersen P, Tietema A, Penuelas J, et al. (2004) Novel approaches to study climate change effects on terrestrial ecosystems in the field: Drought and passive nighttime warming. Ecosystems 7: 583–597. [Google Scholar]
- 41. Collins SL, Fargione JE, Crenshaw CL, Nonaka E, Elliott JR, et al. (2010) Rapid plant community responses during the summer monsoon to nighttime warming in a northern Chihuahuan Desert grassland. J Arid Environ 74: 611–617. [Google Scholar]
- 42. Phillips CL, Gregg JW, Wilson JK (2011) Reduced diurnal temperature range does not change warming impacts on ecosystem carbon balance of Mediterranean grassland mesocosms. Glob Change Biol 17: 3263–3273. [Google Scholar]
- 43. He JS, Wolfe-Bellin KS, Bazzaz FA (2005) Leaf-level physiology, biomass, and reproduction of Phytolacca americana under conditions of elevated CO2 and altered temperature regimes. Int J Plant Sci 166: 615–622. [Google Scholar]
- 44. Hattersley PW (1983) The distribution of C3 and C4 grasses in Australia in relation to climate. Oecologia 57: 113–128. [DOI] [PubMed] [Google Scholar]
- 45. Sun DL, Pinker R (2014) Factors contributing to the spatial variability of Satellite estimates of diurnal temperature range in the United States. IEEE Geosci Remote S 11: 1524–1528. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Final germination percentages of six species under different alternating temperature regimes (TmaxC, a, b; TaveC, c, d; AW, e, f; SW, g, h). C4 species: a, c, e, g; C3 species: b, d, f, h.
(TIF)
Germination rates of six species under different alternating temperature regimes. See S1 for symbols.
(TIF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the paper and in the Supporting Information files.