Abstract
The insect Rhodnius prolixus is responsible for the transmission of Trypanosoma cruzi, which is the etiological agent of Chagas disease in areas of Central and South America. Besides this, it can be infected by other trypanosomes such as Trypanosoma rangeli. The effects of these parasites on vectors are poorly understood and are often controversial so here we focussed on possible negative effects of these parasites on the reproductive performance of R. prolixus, specifically comparing infected and uninfected couples. While T. cruzi infection did not delay pre-oviposition time of infected couples at either temperature tested (25 and 30°C) it did, at 25°C, increase the e-value in the second reproductive cycle, as well as hatching rates. Meanwhile, at 30°C, T. cruzi infection decreased the e-value of insects during the first cycle and also the fertility of older insects. When couples were instead infected with T. rangeli, pre-oviposition time was delayed, while reductions in the e-value and hatching rate were observed in the second and third cycles. We conclude that both T. cruzi and T. rangeli can impair reproductive performance of R. prolixus, although for T. cruzi, this is dependent on rearing temperature and insect age. We discuss these reproductive costs in terms of potential consequences on triatomine behavior and survival.
Introduction
Chagas disease is a severe infection whose etiological agent is the protozoan Trypanosoma cruzi (Chagas, 1909). This parasite is transmitted to humans by insect vectors belonging to the subfamily Triatominae and the main tool to combat transmission of this disease is vector control, based on extensive use of residual insecticides [1], [2]. Rhodnius prolixus (Hemiptera: Reduviidae) Stål, 1859 is considered the primary vector of Chagas disease in Venezuela and Colombia but has also been responsible for disease transmission in much of Central America [3]–[5]. Beyond transmitting T. cruzi, this triatomine can also be infected by Trypanosoma rangeli Tejera, 1920, a protozoan that does not cause disease to humans. This parasite does, however, share surface antigens with T. cruzi [6], potentially leading to serological cross-reactivity and so misleading diagnosis of Chagas disease [7], [8].
These two parasites have distinct tropisms in the insect - T. cruzi multiplies exclusively in the digestive tract while T. rangeli initially infects insects through a similar process but is eventually able to cross the gut epithelium and invade the hemocoel and salivary glands [9]. To grow and multiply, parasites must obtain nutritional resources from their insect hosts. As a consequence, hosts may reorganize metabolic pathways and invest some of their energetic reserves in parasite elimination [10], [11], [ for review see 12]. Therefore, parasite infections have the potential to affect their insect hosts in various ways, directly or indirectly, with possible effects on fitness [13]–[16].
Conventionally, T. cruzi has been considered to be non-pathogenic to its insect hosts [17]–[20]: it normally does not increase mortality rates under optimal conditions (e.g. with unlimited food) [18], injure intestinal tissues [19] or even affect populations of symbionts [20]. There have, though, been some reports of alterations in developmental parameters [21], [22] and reproduction [23] of infected insects, depending on the parasite strain and the conditions to which insects were submitted, while some authors also suggest that the parasite may influence wing morphology [24] and dispersion patterns [25].
In contrast to T. cruzi, T. rangeli is known to be pathogenic to triatomines [9], [26]–[32]: generally, infected insects have difficulties feeding [33] and have increased mortality [32], [34] while those that survive have longer development times [15] and are damaged during ecdysis, leading to altered morphology [25], [35]. In addition, it has been shown that T. rangeli can reduce symbiont populations [26], [28] producing several negative effects on insect development [20].
Most studies to date have focused on effects of T. cruzi and T. rangeli on insect development, longevity and survival. In addition, there are still several controversies regarding the effect of these parasites on the fitness of triatomines. At least some of the differences in the results might be explained by the use of different methodologies and strains and evaluation of the infection of indistinct species of insects and for short periods. In the case of T. rangeli, to our knowledge, there are no studies to date showing the effects of the parasite on triatomine reproductive parameters.
We therefore infected R. prolixus with T. cruzi or T. rangeli and examined the effects of these parasites on reproductive aspects of adults. We evaluated pre-oviposion time (time to lay the first egg), the e-value (the insects' capacity to convert ingested blood into eggs) and egg viability. Since in the field triatomines can remain infected for long periods, the effect of infection on aspects of fitness was evaluated over several reproductive cycles. Our results indicate that these parasites affect, to differing degrees, most reproductive aspects we investigated.
Materials and Methods
Triatomines
All R. prolixus used in assays were obtained from a laboratory colony. This colony is derived from insects collected in Honduras around 1990. The colony was maintained by the Vector Behaviour and Pathogen Interaction Group in Centro de Pesquisas René Rachou, FIOCRUZ, MG, Brazil. Triatomines were reared, until infection, at 25±1°C, 60±10% RH and kept under a natural illumination cycle.
Ethics Statement
Before and after infection, in all instars, insects were fed on Swiss mice under Thiopental 2.5% anesthesia to minimize animal suffering. To infect the insects we used citrated rabbit blood obtained from Cecal (Centro de Criação de Animais de Laboratório) – Fundação Oswaldo Cruz (FIOCRUZ), RJ, Brazil. All experiments using live animals were performed in accordance to FIOCRUZ guidelines on animal experimentation and were approved by the Ethics Committee on Animal Experimentation (CEUA/FIOCRUZ) under the approved protocol number L-058/08. The protocol is from CONCEA/MCT (http://www.cobea.org.br/), which is associated with the American Association for Animal Science (AAAS), the Federation of European Laboratory Animal Science Associations (FELASA), the International Council for Animal Science (ICLAS) and the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
Parasites
Trypanosoma cruzi (CL strain) and T. rangeli (CHOACHI strain) epimastigotes were used to infect insects. These parasites were first isolated from naturally infected T. infestans [36] and R. prolixus [37], respectively. Most studies of the interaction between T. cruzi and R. prolixus use the DM28 strain (11 studies from a total of 13 found), which was originally isolated from the opossum Didelphis marsupialis [38]. The CL strain was chosen for the present study since it was isolated from a triatomine bug (rather than a vertebrate), albeit a distinct genus. The strains were cultured by two weekly passages in liver-infusion tryptose (LIT) medium supplemented with 15% fetal bovine serum, 100 mg/ml streptomycin and 100 units/ml penicillin [15].
Infection by trypanosomes
Infection by T. cruzi was induced in second instar nymphs (8±2 days old) to ensure a chronic infection in the adult phase. Inactivated (56°C, 30 min) citrated rabbit blood containing 5×106 parasites/ml was offered to triatomines at 37°C through an artificial feeder. Control group insects received parasite-free blood under the same conditions. The blood was stirred every 20 minutes to ensure homogeneity of parasite distribution during ad libitum bug feeding. Subsequently, insects that had fed to repletion were selected for the experiments. After this procedure, insects were split in two groups: one that was maintained in a chamber at 25°C during nymphal development and also after imaginal moult, hereafter called 25–25°C, and another that was maintained in a chamber at 30°C during nymphal development and transferred to 25°C after imaginal moult, hereafter called 30–25°C. Insects were maintained in 12∶12/L:D. The intention in rearing a group at 30°C was to induce a higher parasite load in these insects. Briefly, the previous experiments were performed in our laboratory and have proven that the growth rate of T. cruzi cultures is linearly and positively dependent on temperature over the range of 20–30°C [unpublished data]. Infection status was confirmed for all insects used in the experiments through microscopic examination of a drop of urine after blood feeding at the 3rd instar. Insects were fed 8–10 days after each molt in order to ensure continuity of infection [39], [40].
Similarly, infection by T. rangeli was achieved by feeding 3rd instar nymphs (8±2 days old) through an artificial feeder on inactivated (56°C, 30 min) citrated rabbit blood at 37°C, containing 1×105 parasites/ml. Nymphs belonging to the control group received parasite-free blood offered in identical conditions. To ensure that all insects contained parasites in hemolymph, seven days after moulting to 4th instar, nymphs were inoculated in the side of the thorax with 1 µl of PBS (0.15 M NaCl 0.01 M sodium phosphate, pH 7.4) containing 2.5×104 parasites/ml. A 50 µl syringe (Hamilton, needle 13×3.3; ½″) connected to a dispenser (model 705, Hamilton Company, USA) was used to inoculate the parasites. Nymphs belonging to the control group were inoculated with the same volume of PBS. Twenty-four hours post-inoculum, insects were allowed to feed ad libitum on anesthetized Swiss mice.
Hemolymph examination was performed under the microscope to confirm the infection of all experimental insects. Control insects had a drop of hemolymph sampled in the same manner to ensure equivalent manipulation of the insects studied. Both groups of insects were always maintained in a chamber at 25°C and 12∶12L/D and are hereafter called T. rangeli 25–25°C. No experiment was performed with insects infected with T. rangeli and exposed to 30°C due to the fact that previous experiments performed in our laboratory have shown that the culture growth rate of these parasites presents a peak at intermediate temperatures, i.e., 25°C, over the range 20–30°C.
For all experiments, both with T. cruzi and T. rangeli infected insects, we promoted early infections to generate a chronic pathological profile on the insects. We suggest that evaluating reproductive performance on adults infected as younger instars would allow showing effects accumulated throughout their lifespan. Infections by T. cruzi or T. rangeli were not conducted at the same instar since our main purpose was not to compare the effects of different parasites. As T. rangeli infected insects needed the injection of parasites into their hemocel after being orally infected, this had to be performed at a larger instar to avoid mortality due to this damaging procedure. In the light of existing knowledge on triatomine-trypanosome interactions, there is no evidence of parasite-induced virulence being dependant on the instar infected.
Bioassays
Fifteen-day-old virgin adults (previously individualized as 5th instar nymphs) were individually weighed before and immediately after feeding on anesthetized mice for 30 minutes or until the total distension of abdomen. Afterwards, insects were sorted into breeding pairs which were maintained for 21 days in separate plastic containers (5.5×8.0 cm). Inside each container a circular piece of filter paper and a strip of cardboard were added as substrate and climbing structure. The container was sealed with cloth. The feeding procedure was repeated with the same insects every 21 days for three times. Intervals between feeding were defined as reproductive cycles. The insects remained inside the containers until the end of the experiments or female death. Dead males were not replaced and females were kept alone until the end of the experiments. Only data generated from insects that fed in each cycle were considered for the experiment. After the first imaginal meal, bugs that did not engorge were excluded and the final number of experimental pairs established for each group (trying to keep figures as even as possible). It is relevant to mention that data obtained with a small proportion of pairs had to be excluded from the datasets of the 2nd and 3rd oviposition cycles (both for control and infected groups) as they had either not fed or died. The numbers of pairs for which data were obtained in the 2nd and 3rd cycles is given in figures.
Several experimental series were conducted to examine the effect of infection on the fecundity and fertility of R. prolixus.
Four treatments were used to study the effect of infection by T. cruzi:
uninfected pair (25–25°C) (n = 15 pairs);
infected pair (25–25°C) (n = 19 pairs);
uninfected pair (30–25°C) (n = 20 pairs);
infected pair (30–25°C) (n = 20 pairs).
Two treatments were developed to study the effect of infection by T. rangeli:
uninfected pair (25–25°C) (n = 20 pairs);
infected pair (25–25°C) (n = 20 pairs).
The eggs produced by each pair were collected daily and transferred to a plastic microplate (24 wells). The following parameters were recorded for each pair: a) pre-oviposition time (number of days spent from pairing to laying the first egg); b) e-value for each reproductive cycle [41], [42]; c) egg hatching rate for each reproductive cycle. The e-value is a variable that indicates the capacity of bugs to convert the ingested blood into egg production, taking in consideration the initial weight of the female in each cycle. This variable was calculated using the formula:
Statistical Analysis
All the statistical analyses were done in R software 3.0.2 [43]. Pre-oviposition time was analysed using a Wilcoxon rank-sum test. The number of days until first oviposition (pre-oviposition time) was compared between control and infected insects in every temperature and parasite species. Data on egg hatching rate and e-values (dependent variables) were analysed to compare the effects of: a) parasite infection (i.e. control vs infected insects) during three consecutive feeding cycles in every parasite treatment; b) T. cruzi infection (i.e. control vs infected insects) at two experimental temperatures (i.e. 25°C vs 30°C); and c) infection (i.e. control vs infected insects) of either parasite species (i.e. T. cruzi and T. rangeli) at the same temperature (i.e. 25°C). The variable individual was set as a random effect to account for repeated measures. Hatching rate data were analysed by a binomial generalized mixed-effects model (function glmer() in “lme4” package) [44] using “logit” as a link function. E-value data were analysed with a linear mixed-effects model (function lme() in “nlme” package) [45]. Full models with an interaction term were fitted initially and they were reduced to main effects models if the interaction was not significant. The goodness-of-fit of the models was visually inspected using the residual plots. Overdispersion was also checked for all models. Post-hoc interaction contrasts (function testInteractions() in “phia” package, De Rosario-Martinez [46] were performed to further explore the influence of each treatment in the data.
Results
Effect of trypanosome infection on R. prolixus reproduction
Pre-oviposition tim
Infection by T. cruzi did not affect the pre-oviposition time shown by females (Fig. 1A and 1B, Wilcoxon rank-sum test, W = 178.5, P = 0.33 and W = 152.5, P = 0.30, respectively at 25–25°C or 30–25°C). Infection of females by T. rangeli, however, induced a 2 day delay in their pre-oviposition time in comparison to uninfected females (Fig. 1C, Wilcoxon rank-sum test, W = 90.5, P<0.01).
Figure 1. Pre-oviposition time of R. prolixus females infected.
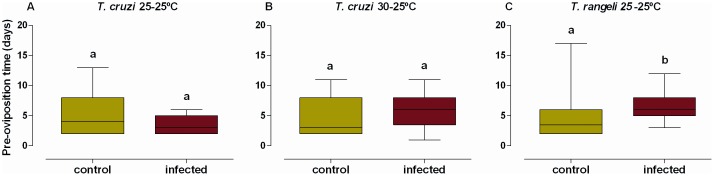
A) T. cruzi at 25–25°C; B) T. cruzi at 30–25°C and C) T. rangeli at 25–25°C. The median, quartiles and minimum and maximum numbers of days before the first oviposition are shown in each box plot. Data represent the mean ± s.e. of 15 control and 19 T. cruzi 25–25°C infected pairs (A), 20 control and 20 T. cruzi 30–25°C infected pairs (B) and 20 control and 20 T. rangeli 25–25°C infected pairs (C). Green indicates data from control insects, while red indicates data from infected insects. Distinct letters above columns indicate significant differences in pre-oviposition time (P<0.05).
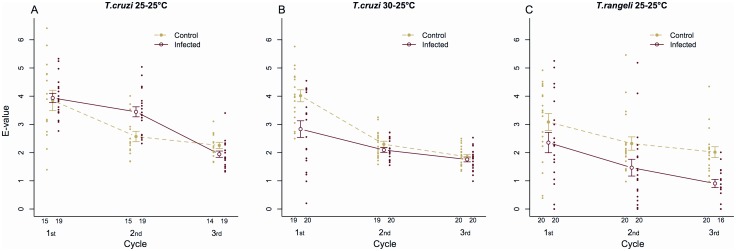
E-value and fertility of insects infected by T. cruzi 25–25°C
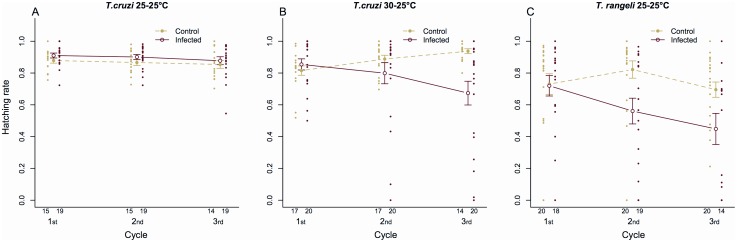
For T. cruzi 25–25°C a significant interaction between infection and cycle was observed in the e-value (Linear mixed-effects model, P = 0.01), indicating that the effect of infection depends on adult age. Post-hoc tests indicated a significant increase in the e-value of infected insects during the second cycle when compared to controls (Fig. 2A, control = 2.57±0.18, infected = 3.45±0.18, P = 0.004). Additionally, the hatching rate observed for infected insects was marginally increased (Fig. 3A, control = 0.87±0.01, infected = 0.90±0.01, binomial generalized mixed-effects model, P = 0.03).
Figure 2. E-value of R. prolixus pairs infected.
A) T. cruzi at 25–25°C; B) T. cruzi at 30–25°C and C) T. rangeli at 25–25°C. Data represent the mean e-value (± s.e.) of 15 control and 19 T. cruzi 25–25°C infected pairs (A), 20 control and 20 T. cruzi 30–25°C infected pairs (B) and 20 control and 20 T. rangeli 25–25°C infected pairs (C). Green indicates data from control insects, while red indicates data from infected insects. Cycles comprise periods of 21 days between meals and dots represent the e-value shown by each pair for a particular cycle.
Figure 3. Hatching rate of R. prolixus eggs from pairs infected.
A) T. cruzi at 25–25°C; B) T. cruzi at 30–25°C and C) T. rangeli at 25–25°C. Data represent the mean ± s.e. of 15 control and 19 T. cruzi 25–25°C infected pairs (A), 17 control and 20 T. cruzi 30–25°C infected pairs (B) and 20 control and 19 T. rangeli 25–25°C infected pairs (C). Green indicates data from control insects, while red indicates data from infected insects. Cycles comprise periods of 21 days between meals and dots represent the hatching rate shown by each pair for a particular cycle.
E-value and fertility of insects infected by T. cruzi 30–25°C
The analysis of the e-value of insects exposed to T. cruzi infection at 30–25°C revealed a strong interaction with the cycle, indicating that infection decreased the fertility of insects at particular phases of adult life (Linear mixed-effects model, P<0.001). Infected pairs produced eggs less efficiently during the first reproductive cycle in comparison to controls (Fig. 2B, control = 4.02±0.21, infected = 2.83±0.29, P<0.001). Nevertheless, no significant differences in e-values of control and infected insects were observed in the subsequent reproductive cycles (P = 0.65 for both cycles).
An interaction between infection and cycle was also seen in the egg hatching rates (Binomial generalized mixed-effects model, P<0.001), indicating that the decrease in hatching success of eggs laid by infected pairs also depended on adult age. Results from post-hoc tests showed that the hatching rate of these insects decreased significantly in the third reproductive cycle as compared to that of control group insects (Fig. 3B, control = 0.94±0.01, infected = 0.67±0.07, P<0.001)
E-value and fertility of insects infected by T. rangeli 25–25°C
The e-value of Trypanosoma rangeli-infected insects was affected both by infection (Linear mixed-effects model, P = 0.001) and cycle (P<0.001), but no significant interaction between the two treatments was observed. Figure 2C shows that T. rangeli-infected insects suffered a slight decrease in their e-value in all cycles compared to that shown by insects of the control group. Nevertheless, this difference was statistically significant during the second (control = 2.32±0.24, infected = 1.46±0.30; P = 0.04) and third (control = 2.02±0.19, infected = 0.91±0.14, P = 0.02) cycles. Moreover, infection status and reproductive cycle showed interactive effects on hatching rates (Binomial generalized mixed-effects model, P<0.001). In fact, parental infection by T. rangeli significantly decreased the hatching rate of eggs laid during the second (control = 0.82±0.05, infected = 0.56±0.08, P = 0.006) and third (control = 0.70±0.05, infected = 0.45±0.10, P = 0.03) cycles (Fig. 3C).
The effect of temperature on the outcome of T. cruzi infection
The e-value and fertility of insects infected by T. cruzi 25–25°C vs T. cruzi 30–25°C
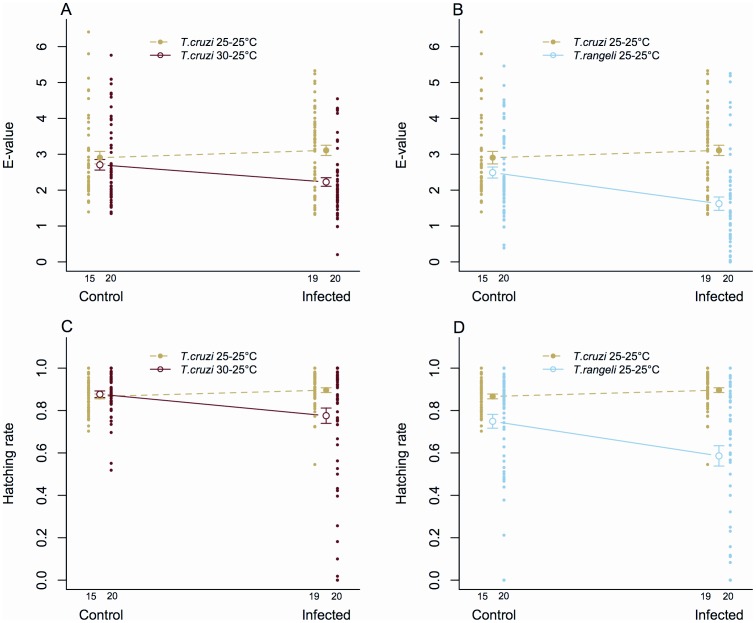
A statistically significant interaction between infection status and experimental temperature (Linear mixed-effects model, P = 0.007) indicates that T. cruzi affected the efficiency of egg production significantly depending on the temperature to which insects were exposed during nymphal phases. Although there were no significant differences in the e-value of control insects exposed to either temperature treatment (P = 0.84), infected insects exposed to 30–25°C experienced significant e-value reduction compared to those kept at 25–25°C (Fig. 4A, 25–25°C = 3.11±0.14, 30–25°C = 2.23±0.12, P<0.001).
Figure 4. Comparison of the effect of trypanosome infection on e-value and egg hatching rate of R. prolixus.
A) Variation of e-value between insects exposed to infection by T. cruzi 25–25°C and T. cruzi 30–25°C and their corresponding controls; B) variation of e-value between insects exposed to infection by T. cruzi 25–25°C and T. rangeli 25–25°C and their corresponding controls; C) variation of egg hatching rate between insects exposed to infection by T. cruzi 25–25°C and T. cruzi 30–25°C and their corresponding controls; D) variation of egg hatching rate between insects exposed to infection by T. cruzi 25–25°C and T. rangeli 25–25°C and their corresponding controls. Data represent the mean ± s.e. of 15 control and 19 T. cruzi 25–25°C infected pairs (A–D), 20 control and 20 T. cruzi 30–25°C infected pairs (A and C) and 20 control and 20 T. rangeli 25–25°C infected pairs (B and D). Green indicates data obtained from insects infected by T. cruzi 25–25°C and controls (A–D), red indicates data from insects infected by T. cruzi 30–25°C and controls (A and C) and blue indicates data from insects infected by T. rangeli 25–25°C and controls (B and D). Dots represent e-value and hatching rates shown by each pair.
Similar results were encountered in egg-hatching rates. A significant interaction found after comparing hatching rates as a function of insect infection by T. cruzi and temperature (Binomial generalized mixed-effects model, P = 0.02) indicates that eggs laid by infected pairs vary their hatching success depending on the temperatures to which they were exposed. The hatching of eggs laid by infected insects exposed to 30–25°C was significantly lower when compared to that of bugs at 25–25°C (Fig. 4C, 25–25°C = 0.90±0.01, 30–25°C = 0.78±0.04, P = 0.009). A similar tendency was not observed in non-infected bugs (P = 0.56).
The effect of different parasite infection
The e-value and fertility of insects infected by T. cruzi 25–25°C vs T. rangeli 25–25°C
The effects of infection on insects kept at the same temperature depended on parasite species, as reinforced by results obtained with insects infected by T. cruzi 25–25°C or T. rangeli 25–25°C (Linear mixed-effects model, P = 0.002). In fact, infection by T. rangeli strongly decreased the e-value of insects compared to that of bugs infected with T. cruzi (Fig. 4B, T . cruzi = 3.11±0.14, T. rangeli = 1.62±0.19, P<0.001).
The egg-hatching rates were clearly influenced by parasite species (Binomial generalized mixed-effects model, P = 0.02), as there was a hatching rate reduction on the eggs laid by T. rangeli infected pairs when compared to that of eggs laid T. cruzi infected insects (Fig. 4D, T . cruzi = 0.90±0.01, T. rangeli = 0.59±0.05, P<0.001).
Discussion
We initially aimed to evaluate reproductive performance effects of T. cruzi infection on R. prolixus adults. For this we chose to expose insects to temperatures they are known to prefer, i.e. 25°C [47]. We also evaluated whether parasite load would affect this outcome by comparing these results to those obtained with a group of insects held at 30°C to promote a higher parasitemia. Our results have shown for the first time that T. cruzi infection can impose costs on R. prolixus reproduction, which costs we would expect to see reflected in the insect's overall fitness. This seems to be the outcome of an interplay between parasite infection, insect age and environmental temperature. We have also shown that T. rangeli infection is costly for the reproductive performance of these bugs
Curiously, while T. rangeli is generally considered a pathogen of triatomines [27], [31], [34], T. cruzi has been mostly considered non-pathogenic to its insect vectors [17], [18], [20]. Our results seem to indicate that both trypanosomes can impose costs on their invertebrate hosts, depending in part on the environmental conditions governing their interaction. Several studies have shown negative effects of parasitism on vector hosts in a other insect models: Plasmodium spp. vs Anopheles and Aedes mosquitoes [12], [48], Dengue virus vs Aedes mosquitoes [49], [50] and Leishmania spp. vs sand flies [51], [52]. In order to measure the impact of parasite infections authors have evaluated parameters such as blood feeding capacity, duration of development, adult longevity, fecundity, fertility and mating performance. Moreover, effects of T. cruzi infection on triatomine fitness have been studied in Panstrongylus megistus (Burmeister, 1835) [23] and Triatoma brasiliensis Neiva, 1911 [22], both reared around 30°C. In the first study, infected P. megistus presented a significant decrease both in their fecundity and fertility [23]. Conversely, infected T. brasiliensis showed no significant effects on these parameters [22]. While the first insect species normally prefers temperatures lower than those at which infection effects on adult fitness were evaluated, T. brasiliensis prefer temperatures around 30°C and might be better adapted to deal with trypanosome infection at this temperature. Interestingly, the population growth rate of T. cruzi culture epimastigotes is affected by ambient temperature [unpublished data], being twice as fast at 30°C than at 27°C. Based on this, these parasites may achieve massive populations at higher temperatures if their access to nutrients is not restricted in the vector gut. In such a scenario, insects would probably lose a large amount of nutritional resources to the parasite population. Moreover, immunological responses to control parasite development would impose an additional energetic cost [10], [53]. Comparison of our results and those of previous reports seem to suggest that the environmental temperature at which insects were reared, as well as their adult age, have an impact in the outcome of triatomine-trypanosome interactions.
We have shown that infection by T. cruzi does not affect the time taken to initiate oviposition by R. prolixus pairs. This seems to be in agreement with a previous study [22] using T. brasiliensis. In our experiments, the temperature at which infected R. prolixus bugs were held did not have an impact on the pre-oviposition time shown as adults. Nevertheless, infection by T. rangeli significantly delayed the onset of oviposition by R. prolixus females, showing a cost in terms of reproductive capacity.
An unexpected result was the increase in the e-value of T. cruzi-infected insects held at 25°C. This effect was restricted to the second feeding cycle and for these insects, hatching rates were not affected by insect age, but were increased in the infected insects. At present, we can only speculate on the causes of this phenomenon but it might be an interesting topic to investigate further, especially given the possibility of environment-dependent costs of infection and even that costs become benefits under some situations. The phenomenon demonstrated here on T. cruzi-infected insects at 25°C has a profile similar to the hormesis reaction, characterized by a reversal of response between low and high doses of a stressor [see review 54].
The negative effects observed on T. cruzi-infected insects reared at 30°C may be the consequence of a higher parasite load affecting adult bug energetic balance. In fact, insects exposed to this treatment presented significant decreases in their e-value and the hatching rates of their eggs. The fact that the decrease in e-value was observed during the first reproductive cycle reinforces the idea of negative effects being triggered due to large parasite populations competing for nutrients with bugs. Insects were transferred to 25°C after imaginal molt to avoid inducing a reduction in their nutritional resources due to an increased metabolic rate at higher temperatures. In this way, the probably large parasite populations they bore might have impacted their nutritional resources, promoting the negative effects observed on e-value. In the next cycles, a combination of lower temperature (slowing parasite growth) and cyclic starvation (decreasing available resources) might have decreased parasite populations bringing them under a threshold where reproductive performance is not affected. However, even if T. cruzi-infected insects managed to maintain fecundity parameters similar to those of uninfected insects during the last two cycles studied, the quality of their eggs was significantly affected by infection as shown by their diminished hatching rates. Moreover, this reproductive parameter was affected by insect age, as the proportion of viable offspring decreased as pairs became older. This decreased reproductive performance of T. cruzi-infected insects exposed to higher temperatures might be a consequence of direct competition for energetic resources that could otherwise be used for reproduction [55], an outcome of committing extra resources to implementing immune responses [56] or a result of host manipulation by parasites that reduce host investments in fecundity and prolong parasite survival [55]. To our knowledge, this is the first time that the e-value was used to evaluate the impact of trypanosome infection on triatomines.
Infection by T. rangeli affected all reproductive parameters evaluated in the present study. As mentioned above, infected pairs showed delayed pre-oviposition times and both their e-values and hatching rates were significantly decreased. Moreover, these negative effects increased with insect age. The virulence of T. rangeli to triatomines has been broadly described and shown to vary according to parasite strain and experimental methodologies [57]. However, effects of T. rangeli on the reproductive performance of triatomines have not been shown to date. In this sense, our results show an additional negative effect that T. rangeli can cause to its insect vector. Differently from T. cruzi, that develops exclusively in the intestinal tract of bugs, parasites of this species can cross the intestinal epithelium and continue their development in the hemocoel and salivary glands. As the eggs of these insects need abundant energy reserves, females undergo an intense mobilization of lipids, proteins and carbohydrates during oogenesis [58], [59]. In R. prolixus females, lipids are transported from the fat body to the ovaries by a lipoprotein called lipophorin, to allow their incorporation by oocytes [60], [61]. As trypanosomes lack the complete synthesis pathways for some fundamental lipids they must obtain these from their hosts [62]–[65]. Trypanosoma rangeli epimastigotes incorporate lipids together with lipophorin from the host hemolymph [66], probably decreasing the amount of these protein carriers available to the host. In addition, infection by T. rangeli can result in large parasite populations found in both the intestine and hemolymph [9]. This could also decrease insect nutritional resources and impact fecundity and fertility. It is important to highlight that pairs were allowed to copulate ad libitum in all treatments, even though we did not control whether uninfected insects copulated more frequently than infected ones. It is known that a greater mating frequency improves the fecundity shown by triatomine pairs [67], [68] and the possibility of consequences of trypanosome infection on bug mating performance deserves to be analyzed in further studies.
Conclusions
The reproductive performance of R. prolixus, a key vector for Chagas disease transmission to humans, has been shown here to diminish due to trypanosome infection. This can reduce the insect's fitness and therefore exert selective pressures on their interaction with the parasites. It is still not clear whether infection induced a decrease in the reproductive performance of males, females or both, since we have not performed assays to check this possibility, i.e., tests with pairs of infected females mating with control males or infected males with control females. We suggest that much of the selective pressures may be manifested in behavioral effects – infected insects may select a thermal environment that ameliorates negative effects of the parasites, for example, and it is quite possible that mate choice is affected by infection status. It would also be interesting to investigate the fitness of the offspring of infected parents and other subtle, yet ecologically (and therefore epidemiologically) relevant, aspects of these vector-parasite interactions. As mentioned above, we have recently determined that trypanosome-triatomine interactions are drastically affected by environmental temperature, showing that parasite pathogenicity is modulated by this parameter (unpublished data). This might suggest that other known stressors such as starvation and low water vapor pressure may expose insects to conditions inducing significant pathogenicity by these parasites.
Acknowledgments
We would like to thank Dr. Thiago A. Belinato for helpful suggestions.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (AAG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (AAG and MGL), Instituto Nacional de Ciência e Tecnologia em Entomologia Molecular (AAG and MGL), Fundação Oswaldo Cruz/Centro de Pesquisas René Rachou (MRF, AAG and MGL) and Programa Estratégico de Apoio à Pesquisa em Saúde (AAG). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Schofield CJ (1994) Triatominae: Biology and Control. United Kingdom, West Sussex: Eurocommunica Publications. 76p.
- 2.WHO - World Health Organization (2014) Chagas disease (American trypanosomiasis). Geneva: Fact sheet 340 . Available: http://www.who.int/mediacentre/factsheets/fs340/en/. Accessed 2014 May 22. [Google Scholar]
- 3. Lent H, Wigodzinsky P (1979) Revision of the Triatominae (Hemiptera, Reduviidae), and theirs significance as vectores of Chagas's disease. Bull Am Mus Nat Hist 163: 127–520. [Google Scholar]
- 4. Schofield CJ, Galvão C (2009) Classification, evolution and species groups within the Triatominae. Acta Trop 110: 88–100. [DOI] [PubMed] [Google Scholar]
- 5. Hashimoto K, Schofield CJ (2012) Elimination of Rhodnius prolixus in Central America. Parasit Vectors 5: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Afchain D, Leray D, Fruit J, Capron A (1979) Antigenic make-up of Trypanosoma cruzi culture forms: Identification of a specific component. J Parasitol 65: 507–514. [PubMed] [Google Scholar]
- 7. O'Daly JA, Carrasco H, Fernandez V, Rodriguez MB (1994) Comparison of chagasic and non-chagasic myocardiopathies by ELISA and immunoblotting with antigens of Trypanosoma cruzi and Trypanosoma rangeli . Acta Trop 56: 265–287. [DOI] [PubMed] [Google Scholar]
- 8. Moraes MH, Guarneri AA, Girardi FP, Rodrigues JB, Eger I, et al. (2008) Different serological cross-reactivity of Trypanosoma rangeli forms in Trypanosoma cruzi-infected patients sera. Parasit Vectors 1: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D' Alessandro A (1976) The biology of Trypanosoma (Herpetosoma) rangeli. In: Lumsden WHM, Evans DA, editors.Biology of Kinetoplastida.London: Academic Press. pp. 327–403. [Google Scholar]
- 10. Cheon HM, Shin SW, Bian G, Park JH, Raikhel AS (2006) Regulation of lipid metabolism genes, lipid carrier protein lipophorin, and its receptor during immune challenge in the mosquito Aedes aegypti . J Biol Chem 281: 8426–8435. [DOI] [PubMed] [Google Scholar]
- 11. Botto-Mahan C, Ossa CG, Medel R (2008) Direct and indirect pathways of fitness-impact in a protozoan-infected kissing bug. Physiol Entomol 33: 25–30. [Google Scholar]
- 12. Ferguson HM, Read AF (2002) Why is the effect of malaria parasites on mosquito survival still unresolved? Trends Parasitol 18: 256–261. [DOI] [PubMed] [Google Scholar]
- 13. Elliot SL, Adler FR, Sabelis MW (2003) How virulent should a parasite be to its vector? Ecology 84: 2568–2574. [Google Scholar]
- 14. Lambrechts L, Scott TW (2009) Mode of transmission and the evolution of arbovirus virulence in mosquito vectors. Proc R Soc Lond B 276: 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferreira LL, Lorenzo MG, Elliot SL, Guarneri AA (2010) A standardizable protocol for infection of Rhodnius prolixus with Trypanosoma rangeli, which mimics natural infections and reveals physiological effects of infection upon the insect. J Invertebr Pathol 105: 91–97. [DOI] [PubMed] [Google Scholar]
- 16. Sylvestre G, Gandini M, Maciel-de-Freitas R (2013) Age-Dependent effects of oral infection with dengue virus on Aedes aegypti (Diptera: Culicidae) Feeding Behavior, Survival, Oviposition Success and Fecundity. PLoS One 8: 3e59933 doi:10.1371/journal.pone.0059933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zeledón R, Guardia VM, Zúñiga A, Swartzwelde JC (1970) Biology and ethology of Triatoma dimidiata (Latreille, 1811). II. Life span of adults and fecundity and fertility of females. J Med Entomol 7: 462–469. [DOI] [PubMed] [Google Scholar]
- 18. Schaub GA (1988) Developmental time and mortality of larvae of Triatoma infestans infected with Trypanosoma cruzi . Trans R Soc Med Hyg 82: 94–97. [PubMed] [Google Scholar]
- 19. Kollien AH, Schmidt J, Schaub GA (1998) Modes of association of Trypanosoma cruzi with the intestinal tract of the vector Triatoma infestans . Acta Trop 70: 127–141. [DOI] [PubMed] [Google Scholar]
- 20. Eichler S, Schaub GA (2002) Development of symbionts in Triatomine bugs and the effects of infections with trypanosomatids. Exp Parasitol 100: 17–27. [DOI] [PubMed] [Google Scholar]
- 21. Neves DP, Peres RB (1975) Aspectos da biologia de Rhodnius prolixus quando alimentado em animais sadios ou infectados com o Trypanosoma cruzi . Rev Bras Biol 35: 317–320. [Google Scholar]
- 22. Oliveira TG, Carvalho-Costa FA, Gomes TF, Sarquis O, Sposina R, et al. (2010) Developmental and reproductive patterns of Triatoma brasiliensis infected with Trypanosoma cruzi under laboratory conditions. Mem Inst Oswaldo Cruz 105: 1057–1060. [DOI] [PubMed] [Google Scholar]
- 23. Lima MM, Pereira JB, Santos JAA, Pinto ZT, Braga MV (1992) Development and reproduction of Panstrongylus megistus (Hemiptera: Reduviidae) infected with Trypanosoma cruzi, under laboratory conditions. Ann Entomol Soc Am 85: 458–461. [Google Scholar]
- 24. Nouvellet P, Dumonteil E, Gourbière S (2011) Effects of genetic factors and infection status on wing morphology of Triatoma dimidiata species complex in the Yucatan peninsula, Mexico. Infect Genet Evol 11(6): 1243–1249. [DOI] [PubMed] [Google Scholar]
- 25. Ramirez-Sierra MJ, Herrera-Aguilar M, Gourbière S, Dumonteil E (2010) Patterns of house infestation dynamics by non-domiciliated Triatoma dimidiata reveal a spatial gradient of infestation and potential insect manipulation by Trypanosoma cruzi . Trop Med Int Health 15: 77–86. [DOI] [PubMed] [Google Scholar]
- 26. Brecher G, Wigglesworth VB (1944) The transmission of Actinomyces rhodnii Erikson in Rhodnius prolixus Stal (Hemiptera) and its influence on the growth of the host. Parasitology 35: 220–224. [Google Scholar]
- 27. Grewal MS (1957) Pathogenicity of Trypanosoma rangeli Tejera, in the invertebrate host. Exp Parasitol 6: 123–130. [DOI] [PubMed] [Google Scholar]
- 28. Lake P, Friend WG (1967) A monoxenic relationship, Nocardia rhodnii Erikson in the gut of Rhodnius prolixus Stal (Hemiptera: Reduviidae). J Entomol Soc Ont 98: 53–57. [Google Scholar]
- 29. Tobie EJ (1970) Observations on the development of Trypanosoma rangeli in the hemocoel of Rhodnius prolixus . J Invertebr Pathol 15: 118–125. [DOI] [PubMed] [Google Scholar]
- 30. Eichler S, Schaub GA (1998) The effects of aposymbiosis and of an infection with Blastocrithidia triatomae (Trypanosomatidae) on the tracheal system of the reduviid bugs Rhodnius prolixus and Triatoma infestans . J Insect Physiol 44: 131–140. [DOI] [PubMed] [Google Scholar]
- 31. Garcia ES, Castro DP, Figueiredo MB, Azambuja P (2012) Parasite-mediated interactions within the insect vector: Trypanosoma rangeli strategies. Parasit Vectors 5: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tobie EJ (1965) Biological factors influencing transmission of Trypanosoma rangeli by Rhodnius prolixus . J Parasitol 51: 837–841. [PubMed] [Google Scholar]
- 33. Anez N, East JS (1984) Studies in Trypanosoma rangeli Tejera, 1920. II. Its effects on the feeding behavior in triatomine bugs. Acta Trop 41: 93–95. [PubMed] [Google Scholar]
- 34. Añez N (1984) Studies on Trypanosoma rangeli Tejera, 1920. VII. Its effect on the survival of infected triatomine bugs. Mem Inst Oswaldo Cruz 79: 249–255. [DOI] [PubMed] [Google Scholar]
- 35. Watkins R (1971) Histology of Rhodnius prolixus infected with Trypanosoma rangeli . J Invertebr Pathol 17: 59–66. [DOI] [PubMed] [Google Scholar]
- 36. Brener Z, Chiari E (1963) Variações morfológicas observadas em diferentes amostras de Trypanosoma cruzi. . Rev Inst Med Trop São Paulo 5: 220–224. [PubMed] [Google Scholar]
- 37. Schottelius J (1987) Neuraminidase fluorescent test for differentiation of Trypanosoma cruzi and Trypanosoma rangeli . Trop Med Parasitol 38: 323–327. [PubMed] [Google Scholar]
- 38. Zingales B, Andrade SG, Briones MRS, Campbell DA, Chiari E, et al. (2009) A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz 104: 1051–1054. [DOI] [PubMed] [Google Scholar]
- 39. Schaub GA, Böker CA (1986) Colonization of the rectum of Triatoma infestans by Trypanosoma cruzi: influence of starvation studied by scanning electron microscopy. Acta Trop 43: 349–354. [PubMed] [Google Scholar]
- 40. Kollien AH, Schaub GA (1998) The development of Trypanosoma cruzi (Trypanosomatidae) in the reduviid bug Triatoma infestans (Insecta): Influence of starvation. J Euk Microbiol 45: 59–63. [DOI] [PubMed] [Google Scholar]
- 41. Davey KG (1987) Inputs to the hormonal control of eggs development in Rhodnius prolixus. . Mem Inst Oswaldo Cruz 82: 103–108. [DOI] [PubMed] [Google Scholar]
- 42. Guarneri AA, Pereira MH, Diotaiuti L (2000) Influence of the Blood Meal Source on the Development of Triatoma infestans, Triatoma brasiliensis, Triatoma sordida, and Triatoma pseudomaculata (Heteroptera, Reduviidae). J Med Entomol 37: 373–379. [DOI] [PubMed] [Google Scholar]
- 43. R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0 Available: http://www.R-project.org/. [Google Scholar]
- 44. Bates D, Maechler M, Bolker B, Walker S (2013) lme4: Linear mixed-effects models using Eigen and S4. R package version 1.0–5 Available: http://CRAN.R-project.org/package=lme4. [Google Scholar]
- 45.Pinheiro J, Bates D, DebRoy S, Sarkar D, the R Development Core Team (2013) nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–113. Available: http://CRAN.R-project.org/package=nlme.
- 46. De Rosario-Martinez H (2013) phia: Post-Hoc Interaction Analysis. R package version 0.1–5; 2013 Available: http://CRAN.R-project.org/package=phia. [Google Scholar]
- 47. Schilman PE, Lazzari CR (2004) Temperature preference in Rhodnius prolixus, effects and possible consequences. Acta Trop 90: 115–122. [DOI] [PubMed] [Google Scholar]
- 48. Araujo RV, Maciel C, Hartfelder K, Capurro ML (2011) Effects of Plasmodium gallinaceum on hemolymph physiology of Aedes aegypti during parasite development. J Insect Physiol 57: 265–273. [DOI] [PubMed] [Google Scholar]
- 49. Grimstad PR, Ross QE, Craig GB Jr (1980) Aedes triseriatus (Diptera: Culicidae) infection. J Med Entomol 17: 1–7. [DOI] [PubMed] [Google Scholar]
- 50. Lima-Camara TN, Bruno RV, Luz PM, Castro MG, Lourenço-de-Oliveira R, et al. (2011) Dengue Infection Increases the Locomotor Activity of Aedes aegypti Females. PLoS One 6: 1–5 doi:10.1371/journal.pone.0060878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schlein Y, Jacobson RL, Messer G (1992) Leishmania infections damage the feeding mechanism of the sand fly vector and implement parasite transmission by bite. P Natl Acad Sci USA 89: 9944–9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rogers ME, Bates PA (2007) Leishmania manipulation of sand fly feeding behavior results in enhanced transmission. PLoS Pathog 3: 818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dettloff M, Wittwer D, Weise C, Wiesner A (2001) Lipophorin of lower density is formed during immune responses in the lepidopteran insect Galleria mellonella . Cell Tissue Res 306: 449–458. [DOI] [PubMed] [Google Scholar]
- 54. Guedes RNC, Cutler GC (2013) Insecticide-induced hormesis and arthropod pest management. Pest Management Science 70: 690–697. [DOI] [PubMed] [Google Scholar]
- 55. Hurd H (2001) Host fecundity reduction: a strategy for damage limitation? Trends Parasitol 17: 363–368. [DOI] [PubMed] [Google Scholar]
- 56. Hurd H (1990) Physiological and behavioural interactions between parasites and invertebrate hosts. Adv Parasitol 29: 271–318. [DOI] [PubMed] [Google Scholar]
- 57. Vallejo GA, Marinkelle CJ, Guhl F, de Sánchez N (1986) Laboratory maintenance of Trypanosoma (Herpetosoma) rangeli Tejera, 1920. Rev Biol Trop 34: 75–81. [PubMed] [Google Scholar]
- 58. Valle D (1995) Vitellogenesis in insects and other group – A review. Mem Inst Oswaldo Cruz 88: 1–26. [DOI] [PubMed] [Google Scholar]
- 59. Atella GC, Gondim KC, Machado EA, Medeiros MN, Silva-Neto MA, et al. (2005) Oogenesis and egg development in triatomines: a biochemical approach. An Acad Bras Cienc 77: 405–430. [DOI] [PubMed] [Google Scholar]
- 60. Gondim KC, Oliveira PL, Masuda H (1989) Lipophorin and oogenesis in Rhodnius prolixus: Transfer of phophoslipids. J Insect Physiol 35: 19–27. [Google Scholar]
- 61. Machado EA, Atella GC, Gondim KC, de Souza W, Masuda H (1996) Characterization and immunocytochemical localization of lipophorin binding sites in the oocytes of Rhodnius prolixus . Arch Insect Biochem Physiol 31: 185–196. [DOI] [PubMed] [Google Scholar]
- 62. Coppens I, Courtoy PJ (1995) Exogenous and endogenous sources of sterols in the culture-adapted procyclic trypomastigotes of Trypanosoma brucei . Mol Biochem Parasitol 73: 179–188. [DOI] [PubMed] [Google Scholar]
- 63. Coppens I, Levade T, Courtoy PJ (1995) Host plasma low density lipoprotein particles as an essential source of lipids for the bloodstream forms of Trypanosoma brucei . J Biol Chem 270: 5736–5741. [DOI] [PubMed] [Google Scholar]
- 64. Paul KS, Jiang D, Morita YS, Englund PT (2003) Fatty acid synthesis in African trypanosomes: a solution to the myristate mystery. Trends Parasitol 8: 381–387. [DOI] [PubMed] [Google Scholar]
- 65. Vial HJ, Eldin P, Tielens AG, Van Hellemond (2003) JJ (2003) Phospholipids in parasitic protozoa. Mol Biochem Parasitol 126: 143–154. [DOI] [PubMed] [Google Scholar]
- 66. Folly E, Cunha e Silva NL, Lopes AH, Silva-Neto MA, Atella GC (2003) Trypanosoma rangeli uptakes the main lipoprotein from the hemolymph of its invertebrate host. Biochem Biophys Res Commun 310: 555–561. [DOI] [PubMed] [Google Scholar]
- 67. Asin S, Crocco de Ayerbe LB (1992) Influence of mating on ovarian follicle development in Triatoma infestans (Klug, 1834). Mem Inst Oswaldo Cruz 87: 369–374. [DOI] [PubMed] [Google Scholar]
- 68. Daflon-Teixeira NF, Carvalho-Costa FA, Chiang RG, Lima MM (2009) Influence of blood meal and mating in reproduction patterns of Triatoma brasiliensis females (Hemiptera: Reduviidae) under laboratory conditions. Mem Inst Oswaldo Cruz 104: 1031–1034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.