Abstract
Despite blood transfusions are administered to restore adequate tissue oxygenation, transfusion guidelines consider only hemoglobin as trigger value, which gives little information about the balance between oxygen delivery and consumption. Central venous oxygen saturation is an alternative, however its changes reflect systemic metabolism and fail to detect regional hypoxia. A complementary parameter to ScvO2 may be central venous-to-arterial carbon dioxide difference (CO2-gap). Our aim was to investigate the change of alternative transfusion trigger values in experimental isovolemic anemia. After splenectomy, anesthetized Vietnamese mini pigs (n = 13, weight range: 18–30 kg) underwent controlled bleeding in five stages (T1–T5). During each stage approximately 10% of the estimated starting total blood volume was removed and immediately replaced with an equal volume of colloid. Hemodynamic measurements and blood gas analysis were then performed. Each stage of bleeding resulted in a significant fall in hemoglobin, the O2-extraction increased significantly from T3 and ScvO2 showed a similar pattern and dropped below the physiological threshold of 70% at T4. By T4 CO2-gap increased significantly and well correlated with VO2/DO2 and ScvO2. To our knowledge, this is the first study to show that anemia caused altered oxygen extraction may have an effect on CO2-gap.
Introduction
Transfusion of red blood cells is an everyday practice in critical care with the primary aim of restoring adequate tissue oxygenation. Transfusion guidelines consider certain levels of hemoglobin as transfusion trigger [1], [2], which on its own gives little information if any about the balance between oxygen delivery (DO2) and consumption (VO2). Hence, there is a clear need for additional physiologic transfusion trigger values. One of the potentially useful physiological parameters is the central venous oxygen saturation (ScvO2), which has been shown to be a potential physiologic transfusion trigger in hemodynamically stable but anemic patients [3]. Its normal value is around 70–75% and it is the product of the VO2 and DO2 relationship. Low ScvO2 usually indicates inadequate DO2, but higher than physiological values may be difficult to interpret as these can indicate reduced oxygen consumption, but may also mean inappropriate oxygen uptake [4], [5]. Under these circumstances additional parameters are needed.
Central venous-to-arterial carbon dioxide difference (CO2-gap) may be one of the potential alternatives to complement ScvO2. Under physiological circumstances its value is less than 6 mmHg [6], [7]. Transport of carbon dioxide in blood ensues in three forms: dissolved in plasma, as bicarbonate ion and bound to hemoglobin. The CO2-gap may be higher during anaerobic respiration when lactic acid has to be buffered by bicarbonate or under aerobic respiration in poorly perfused tissues when flow stagnation results in an accumulation of CO2 [8], [9], [10]. From previous experiments it seems that increased CO2-gap during ischemia is related to decreased blood flow and impaired CO2 washout rather than to hypoxemia [10]. Whether anemia caused tissue hypoxemia is reflected in changes of the CO2-gap has not been investigated before.
Another additional parameter may be the central venous-to-arterial pCO2 difference divided by the difference of the arterio-venous oxygen content, P(v-a)CO2/C(a-v)O2, which is considered to give information about tissue oxygenation. It was found in a retrospective study that this ratio reflected the occurrence of anaerobic metabolism better than other oxygen-, or CO2-derived parameters [11].
Our aim was to investigate how CO2-gap and P(v-a)CO2/C(a-v)O2 change during experimental isovolemic anemia.
Materials and Methods
The study protocol was approved by the local ethics committee at the University of Szeged and the study was carried out in the research laboratory of the Institute of Surgical Research. The current experiment complements our previously published data on the relationship of ScvO2 and isovolemic anemia [12]. Vietnamese mini pigs (n = 13) weighing 24±3 kg were anaesthetized and mechanically ventilated in pressure control mode. Anesthesia was induced with an intramuscular injection of a mixture of ketamine (20 mg/kg) and xylazine (2 mg/kg) and maintained with a continuous infusion of propofol (6 mg/kg/h i.v.). The tidal volume was set at 13±2 ml/kg and the respiratory rate was adjusted to maintain the end-tidal carbon dioxide and the partial pressure of arterial carbon dioxide in the range of 35–45 mmHg and the arterial pH between 7.35 and 7.45. The adequacy of the depth of anesthesia was assessed by monitoring the jaw tone. After the initiation of anesthesia, the right carotid artery and jugular vein and the right femoral artery and vein were dissected and catheterized. The animals underwent suprapubic urinary catheter placement and laparotomy for splenectomy. Splenectomy in swine hemorrhage models are performed because of the distensibility of the spleen and the resultant variation in the amounts of sequestered blood [13]. The core temperature was maintained at 37±1°C through use of an external warming device.
For invasive hemodynamic monitoring, a transpulmonary thermodilution catheter (PiCCO, PULSION Medical Systems AG, Munich, Germany) was placed in the femoral artery and a pulmonary artery catheter (PV2057 VoLEF Catheter, PULSION Medical Systems AG) by pressure tracings via the femoral vein. The latter was also used to draw mixed venous blood gas samples. The femoral artery served as the site of arterial blood gas samples and the central venous line was used for central venous blood gas sampling and for the injection of cold saline boluses for thermodilution measurements. Central venous catheter was positioned by using guidewire attached intracavital ECG. During the experiment blood was drained from the catheter in the right carotid artery, which was also used to replace the blood loss with the same amount of colloid, in order to avoid a sudden increase in right ventricular preload.
At baseline (T0) hemodynamic and blood gas parameters were recorded, and heparin sulfate (200 IU/kg) was administered through the central venous line. Isovolemic anemia was achieved in five intervals (T1–T5). During each interval 10% of the estimated total blood volume was withdrawn over a 5- to 10-min period. Hemodynamic parameters were recorded and the amount of blood drained was immediately replaced by an equal volume of colloid (hydroxyethyl starch 130 kDa/0.4, 6%, Voluven, Fresenius, Germany). To achieve a steady state, the animals were allowed to rest for 10 min between intervals. At the end of each cycle, hemodynamic and blood gas parameters were measured. At the end of the experiment the animals were humanely euthanized.
Arterial, central venous, and mixed venous blood gas samples (Cobas b 221, Roche Ltd., Basel, Switzerland) were drawn and analyzed by cooximetry simultaneously at baseline and at the end of each cycle. From these parameters the oxygen delivery (DO2), oxygen consumption (VO2), oxygen extraction ratio (VO2/DO2) and the simplified oxygen extraction ratio (ERO2) were calculated according to standard formulae:
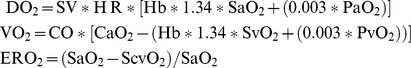 |
Central venous-to-arterial CO2-gap (cvCO2-gap), mixed venous-to-arterial CO2-gap (vCO2-gap), the P(cv-a)CO2/C(a-cv)O2 and P(v-a)CO2/C(a-v)O2 were also calculated from the arterial, central venous and mixed venous blood gas samples.
These were calculated according to standard formulae:
 |
Analysis
Data are reported as median±standard deviation unless indicated otherwise. For testing normal distribution the Kolmogorov-Smirnov test was used. Changes in all parameters throughout the experiment were tested by Friedman test and repeated measures analysis of variance (RM ANOVA), and the number of degrees of freedom was adjusted to Greenhouse-Geisser epsilon when needed. For pairwise comparisons Pearson's correlation was used. To evaluate the performance in detecting altered oxygen extraction of >30% (considered as the “physiological threshold”), receiver operating characteristics (ROC) curve analysis was performed. Post-hoc calculation showed a power of 86% with an effect of 25% increase in VO2/DO2, for a sample size of 13 and α = 0.05. For statistical analysis SPSS version 20.0 for Windows (SPSS, Chicago, IL, USA) was used and p<0.05 was considered statistically significant.
Results
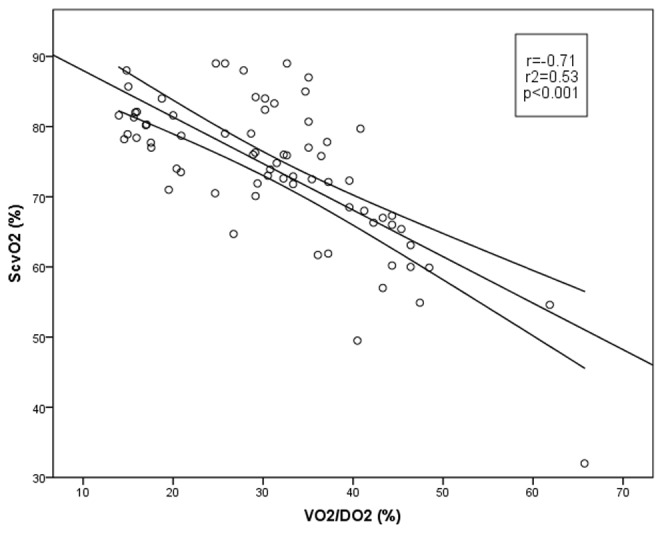
All 13 animals survived the study. The bleeding caused a gradual decrease in hemoglobin level after each phase and by the end of the experiment it had fallen by 61% of the baseline value. The hemodynamic parameters are summarized in Table 1. The SaO2 remained in the normal range throughout the experiment. DO2 fell significantly from T2, VO2 at T4, VO2/DO2 increased significantly from T3, and exceeded the physiologic threshold of 30% (Table 2). The change in ScvO2 displayed a similar pattern as VO2/DO2 and changed significantly and also fell below 70% only at T4. There was strong negative correlation between VO2/DO2 and ScvO2 (Fig. 1).
Table 1. Hemodynamic effects of isovolemic anemia. These data have been published earlier [12].
| T0 | T1 | T2 | T3 | T4 | T5 | |
| Hb (g/L) | 125(113–134) | 102(90–109)* # | 79(73–93)* # | 68(60–76)* # | 59(53–67)* # | 49(43–55)* # |
| HR (beats/min) | 125(91–135) | 119(100–138)* | 123(102–146)* | 129(110–159) * | 139(118–179) * | 147(131–177)* |
| MAP (mm Hg) | 91(79–105) | 89(79–101) | 83(75–98)* | 82(68–90)* | 72(59–85)* | 72(63–86)* |
| CVP (mm Hg) | 6(5–8) | 8(5–9) | 7(4–9) | 7(5–9) | 7(5–9) | 7(3–10) |
| CI (L/min/m2) | 2.6(2.3–2.8) | 3.3(2.7–3.6)* # | 3.6(2.9–3.8)* # | 3.6(3.3–4.1)* | 3.5(3.2–4.0)* | 3.9(3.6–4.1)* |
| GEDI (mL/m2) | 270 (243–284) | 271 (245–320) | 276 (248–298) | 274 (236–305) | 268 (227–302) | 261 (232–298) |
| ELWI (mL/kg) | 9 (9–10) | 10 (10–10) | 9 (9–10) | 10 (9–10) | 10 (9–10) | 10 (9–11) |
| dPmx (mm Hg/s) | 540(485–790) | 700(540–985)* | 800(570–1075)* | 810(540–1480)* | 880(560–1360)* | 975(562–1275)* |
Hb- Hemoglobin, HR- Heart rate, MAP- Mean arterial pressure, CVP- Central venous pressure, CI- Cardiac index, GEDI- Global end-diastolic volume index, ELWI- extravascular lung water index, dPmx- Index of left ventricular contractility. T0- Baseline measurement, T1-T5- Five intervals of bleeding.
*p<.05 compared with T0; #p<.05 compared with previous; GLM repeated measures ANOVA.
Table 2. Descriptives (Median±IQR).
| Time intervals | ||||||
| T0 | T1 | T2 | T3 | T4 | T5 | |
| cvCO2-gap (mmHg) | 5.0(2.6–8.5) | 6.0(3.1–7.0) | 5.0(3.5–5.5) | 5.4(4.4–7.0) | 8.0(4.3–8.5)* | 6.3(5.9–11.0)* |
| vCO2-gap (mmHg) | 5.5(4.0–9.0) | 6.5(4.5–7.8) | 6.5(5.1–7.0) | 5.5(3.7–6.0) | 5.4(5.0–8.0) | 6.2(5.5–8.0) |
| PcvCO2/C(a-cv)O2 | 2.01(1.42–2.23) | 2.27(1.76–3.34) | 2.67(1.71–2.85) | 2.59(1.50–4.47) | 3.30(2.89–3.74)* | 3.93(2.55–5.11)* |
| PvCO2/C(a-v)O2 | 1.57(0.77–1.99) | 1.69(0.91–2.00) | 1.71(1.36–1.99) | 1.61(0.96–2.17) | 2.14(1.58–2.23) | 2.30(1.93–3.56)* |
| ScvO2 (%)# | 76(69–83) | 73(72–82) | 77(75–83) | 77(68–81) | 68(61–76)* | 66(60–76)* |
| SvO2 (%)# | 68 (64–77) | 67 (64–77) | 68 (63–79) | 64 (58–76) | 62 (55–72)* | 58 (52–72)* |
| DO2 (ml/min/m2) # | 431 (362–474) | 438 (323–524) | 378 (302–412)* | 344 (252–376)* | 284 (236–333)* | 247 (216–292)* |
| VO2 (ml/min/m2) # | 119 (82–139) | 130 (77–151) | 93 (66–136) | 113 (67–141) | 98 (72–120)* | 105 (70–120)* |
| VO2/DO2 (%)# | 29(18–33) | 29(17–33) | 29(18–32) | 35(21–40)* | 37(26–43)* | 41(27–47)* |
| ERO2 (%)# | 19(13–26) | 19(14–24) | 20(14–22) | 21(16–28) | 30(22–37)* | 32(21–39)* |
| Lactate (mmol/L) # | 4.5 (3.2–5.3) | 4.2 (3.0–5.1) | 5.0 (3.2–6.0) | 4.1 (2.9–6.0) | 4.2 (2.9–6.5) | 4.0 (3.0–6.4) |
| vLactate (mmol/L) | 4.6(3.7–5.3) | 4.3(3.3–5.3) | 4.4(3.1–5.4) | 4.4(2.8–5.2) | 4.4(3.0–5.2) | 4.1(3.0–6.4) |
| cvLactate (mmol/L) | 4.5(3.5–5.5)§ | 3.9(3.4–5.4)§ | 4.2(3.3–6.3)§ | 4.1(3.1–5.6)§ | 3.9(2.9–5.7)§ | 3.9(3.0–6.4)§ |
| PaCO2 (mmHg) # | 39(35–44) | 38(35–45) | 37(34–45) | 39(34–46) | 37(34–42) | 38(35–41) |
| PaO2 (mmHg) # | 76(66–80) | 75(72–80) | 76(73–80) | 77(72–82) | 79(75–85) | 81(77–90) |
cvCO2-gap: central venous-to-arterial carbon dioxide difference; vCO2-gap: mixed venous-to-arterial carbon dioxide difference; P(cv-a)CO2/C(a-cv)O2: the central venous-to-arterial pCO2 difference divided by the difference of the arterio-venous oxygen content; P(v-a)CO2/C(a-v)O2: the mixed venous-to-arterial pCO2 difference divided by the difference of the arterio-venous oxygen content; ScvO2: central venous oxygen saturation; SvO2: mixed venous oxygen saturation; DO2: oxygen delivery; VO2: oxygen consumption; VO2/DO2: oxygen extraction ratio; ERO2: simplified oxygen extraction ratio; PaCO2: arterial partial pressure of carbon dioxide; PaO2: arterial partial pressure of oxygen * p<.05 as compared to baseline, § p<.05 significant difference between mixed venous and central venous blood with Friedman and Wilcoxon tests, # Data published earlier [12].
Figure 1. The association between VO2/DO2 and ScvO2. VO2/DO2: oxygen extraction ratio; ScvO2: central venous oxygen saturation.
The CO2-gap was calculated for both, central venous (cvCO2-gap) and mixed venous blood (vCO2-gap). By T4 cvCO2-gap increased significantly, however vCO2-gap did not change. The correlations of VO2/DO2 and ScvO2 were significant with cvCO2-gap, while there were only weak correlations with vCO2-gap (Fig. 2).
Figure 2. Correlation between oxygen balance parameters and CO2-gap.
cvCO2-gap and VO2/DO2 and ScvO2 (on the left); vCO2-gap and VO2/DO2 and ScvO2 (on the right). cvCO2-gap: central venous-to-arterial carbon dioxide difference; VO2/DO2: oxygen extraction ratio; ScvO2: central venous oxygen saturation; vCO2-gap: mixed venous-to-arterial carbon dioxide difference.
P(cv-a)CO2/C(a-cv)O2 increased by T4 and P(v-a)CO2/C(a-v)O2 by T5. The correlations of VO2/DO2 and ScvO2 were significant with P(cv-a)CO2/C(a-cv)O2, but it was found to be weak between P(v-a)CO2/C(a-v)O2 and VO2/DO2, and there was no significant correlation with ScvO2 (Fig. 3).
Figure 3. Correlation between tissue oxygenation and oxygen balance parameters.
P(cv-a)CO2/C(a-cv)O2 and VO2/DO2 and ScvO2 (on the left); P(v-a)CO2/C(a-v)O2 and VO2/DO2 and ScvO2 (on the right).P(cv-a)CO2/C(a-cv)O2: the central venous-to-arterial pCO2 difference divided by the difference of the arterio-venous oxygen content; VO2/DO2: oxygen extraction ratio; ScvO2: central venous oxygen saturation; P(v-a)CO2/C(a-v)O2: the mixed venous-to-arterial pCO2 difference divided by the difference of the arterio-venous oxygen content.
ROC analysis revealed the same tendency as the correlation. With 30% taken as the physiologic threshold for VO2/DO2, the area under the curve (AUC), its standard error and that of the 95% confidence interval were >0.5 only for cvCO2-gap, P(cv-a)CO2/C(a-cv)O2 ratio, ScvO2 (Table 3.)
Table 3. ROC analysis for determining VO2/DO2>30%.
| Test Result Variable(s) | Area | Std. Error | Sig. | 95% CI | |
| cvCO2-gap | ,769 | ,078 | ,007 | ,617 | ,921 |
| vCO2-gap | ,553 | ,097 | ,598 | ,363 | ,742 |
| P(cv-a)CO2/C(a-cv)O2 ratio | ,742 | ,070 | ,016 | ,604 | ,879 |
| P(v-a)CO2/C(a-v)O2 ratio | ,641 | ,096 | ,157 | ,453 | ,829 |
| ScvO2 | ,768 | ,056 | ,000 | ,657 | ,879 |
| SvO2 | ,986 | ,010 | ,000 | ,967 | 1,000 |
| Lactate | ,517 | ,078 | ,867 | ,363 | ,670 |
cvCO2-gap: central venous-to-arterial carbon dioxide difference;
vCO2-gap: mixed venous-to-arterial carbon dioxide difference;
P(cv-a)CO2/C(a-cv)O2: the central venous-to-arterial pCO2 difference divided by the difference of the arterio-venous oxygen content;
P(v-a)CO2/C(a-v)O2: the mixed venous-to-arterial pCO2 difference divided by the difference of the arterio-venous oxygen content;
ScvO2: central venous oxygen saturation; SvO2: mixed venous oxygen saturation.
Linear regression revealed a significant relationship between ScvO2 (r = 0.71, r2 = 0.50, p<.001) and VO2/DO2. This relationship became significantly stronger when cvCO2-gap was added to ScvO2 (r = 0.74, r2 = 0.54, p = .015). According to the Pratt's importance coefficient, ScvO2 was responsible for this increase in 63% and cvCO2-gap in 37%.
Discussion
Our results in this isovolemic anemia animal model show that besides ScvO2, both central venous-to-arterial CO2-gap and the P(cv-a)CO2/C(a-cv)O2 correlated well with changes in anemia caused increase in VO2/DO2. Furthermore, mixed venous blood driven indices, such as vCO2-gap and P(v-a)CO2/C(a-v)O2 failed to indicate changes in oxygen extraction. When oxygen extraction ratio started to increase (from T3) it was followed by a decrease of ScvO2 and an increase of cvCO2-gap and P(cv-a)CO2/C(a-cv)O2, and both performed well in the ROC analysis, with the cvCO2-gap's AUC being marginally better. In addition, in our experiment neither vCO2-gap nor P(v-a)CO2/C(a-v)O2 or lactate could detect the increase in VO2/DO2>30% as revealed by ROC analysis.
An interesting finding of our experiment is that despite isovolemia was maintained as indicated by the stable global end diastolic volume index values and there were in fact increasing cardiac output and stroke volume, we observed a rise in cvCO2-gap. This observation seemingly contradicts previously published results to some extent. The occurrence of increased CO2-gap has fundamentally been explained by the CO2 stagnation phenomenon [5]. This was based on the finding that there was inverse correlation between CO2-gap and cardiac index during non-septic and septic low flow states [5], [9], [10]. Moreover, it was also found that the amount of CO2 produced is negligible when anaerobic respiration is present and CO2-gap therefore cannot serve as a marker of tissue hypoxia [10]. The paramount study on this theory by Vallet et al. used an isolated hind limb model and reached hypoxia either by decreasing flow or decreasing arterial oxygen content [10]. They found that occurrence of an increased CO2-gap during ischemia was related to decreased blood flow and impaired carbon dioxide washout; moreover, dysoxia per se was not sufficient to increase CO2-gap. However, the latter could also be due to the Haldane's effect. As the carbon dioxide dissociation curve is influenced by the saturation of hemoglobin with oxygen, the lower the saturation of hemoglobin with oxygen, the higher the saturation of hemoglobin with carbon dioxide for a given carbon dioxide partial pressure is [14]. In our experiment arterial oxygen saturation and PaO2 remained in the normal range and did not change over time, hence the CO2 dissociation curve was not influenced by low saturation of hemoglobin with oxygen.
Nevertheless, anemia resulted in increased VO2/DO2 above the baseline and also above the physiological 30% after the 3rd bleeding event, which was followed by the significant decrease of SvO2 and ScvO2. (It is important to note that there is mathematical coupling between VO2 and SvO2, which is not the case considering ScvO2). The most interesting finding of the current study is the increase of cvCO2-gap during the last two stages of the experiment, without any change in the vCO2-gap. One of the possible reasons for this difference is that due to isovolemia cardiac output was maintained to avoid low flow in the systemic circulation, which is also reinforced by the unchanged lactate levels. Therefore when CO2 was measured in the mixed venous blood it was unchanged and within the normal range almost throughout. As central venous blood driven variables mostly reflect blood flow and metabolism of the brain [15], our hypothesis is that anemia reached such a degree by T4 that it caused tissue hypoxia and consecutive anaerobic respiration with CO2 production. However, due to the low hemoglobin levels the Haldane effect could not take effect, hence there was a significant increase in central venous pCO2. But these changes in the brain did not have significant effects on the systemic level, to be picked up in mixed venous blood. As anemia has greater influence on arterial oxygenation than hypoxemia [16], this might explain the observed increase in cvCO2-gap. This is further reinforced by the P(cv-a)CO2/C(a-cv)O2 results. Both the P(cv-a)CO2/C(a-cv)O2 and the P(v-a)CO2/C(a-v)O2 increased at T4 and T5, but there was a more pronounced change in central venous as compared to mixed venous blood, which is also reflected in the results of the ROC analysis. We also measured mixed venous and central venous lactate levels and found that central venous lactate was significantly lower than in the mixed venous blood, which might give further proof to this hypothesis [17], [18]. In a previous animal experiment by Hare et al, it was found that hemodilutional isovolemic anemia led to cerebral hypoxia, and they also reported a gradual increase in the jugular venous pCO2 with a CO2-gap of 2.9 to 7.8 mmHg (mean) 60 minutes after hemodilution in the traumatic brain injured animals [19]. Although this finding was not discussed in the article, as the authors mainly focused on oxygenation, but nevertheless this is in accord with our results and gives some support to our hypothesis.
There is increasing evidence that untreated anemia can be associated with a worse outcome and increased mortality, while transfusion may cause various infectious and non-infectious adverse effects [20], [21]. cvCO2-gap may be an additional quantitative parameter, beyond Hb and ScvO2, that would give information on anemia related altered oxygen extraction and hence the need for blood administration. cvCO2-gap is a choice of plausible alternatives as it can be easily obtained via the central venous and arterial catheters already in situ in most critically ill patients and no additional invasive device is needed; moreover we found that mixed venous blood driven indices failed to indicate changes in oxygen extraction.
There are several limitations of our study. As the experiment was not designed to measure the effects of isovolemic anemia specifically on the brain, our hypothesis cannot be supported by specific measurements, such as regional cerebral blood flow, cerebral tissue oxygen and carbon dioxide tension. Furthermore, splenectomy and the length of the preparation of the animals may have been too long, which resulted in increased levels of lactate from baseline to the end of the experiment. The steady-state periods may also have been relatively short, although, the same time intervals have been used previously [22]. Another concern might be the type of fluid replacement, as one cannot exclude the possibility that the use of different types of colloid or crystalloid solutions would affect these results.
Conclusions
To our knowledge, this is the first study to show that anemia caused altered oxygen extraction may have an effect on cvCO2-gap and P(cv-a)CO2/C(a-cv)O2 that cannot be detected from mixed venous blood. The clinical relevance of this finding has to be further tested in both experimental and clinical studies.
Acknowledgments
The authors would like to thank the assistants, medical students and staff at the Institute of Surgical Research for their help.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Relevant data have been deposited to Figshare with the DOIs: http://dx.doi.org/10.6084/m9.figshare.1110007, http://dx.doi.org/10.6084/m9.figshare.1110008.
Funding Statement
The authors have no support or funding to report.
References
- 1. Retter A, Wyncoll D, Pearse R, Carson D, McKechnie S, et al. (2013) British Committee for Standards in Haematology (2013) Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. Br J Haematol 160(4): 445–464. [DOI] [PubMed] [Google Scholar]
- 2. Blood Observational Study Investigators of ANZICS-Clinical Trials Group, Westbrook A, Pettilä V, Nichol A, Bailey MJ, Syres G, et al (2010) Transfusion practice and guidelines in Australian and New Zealand intensive care units. Intensive Care Med 36: 1138–46. [DOI] [PubMed] [Google Scholar]
- 3. Adamczyk S, Robin E, Barreau O, Fleyfel M, Tavernier B, et al. (2009) Contribution of central venous oxygen saturation in postoperative blood transfusion decision. Ann Fr Anesth Reanim 28: 522–30. [DOI] [PubMed] [Google Scholar]
- 4. Vallet B, Robin E, Lebuffe G (2010) Venous oxygen saturation as a physiologic transfusion trigger. Critical Care 14: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vallée F, Vallet B, Mathe O, Parraquette J, Mari A, et al. (2008) Central venous-to-arterial carbon dioxide difference: an additional target for goal-directed therapy in septic shock? Intensive Care Med 34: 2218–2225. [DOI] [PubMed] [Google Scholar]
- 6. Geers C, Gros G (2000) Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol Rev 80: 681–715. [DOI] [PubMed] [Google Scholar]
- 7.Guyton AC, Hall JE (2006) Transport of Oxygen and Carbon Dioxide in Blood and Tissue Fluids. In: Guyton AC, Hall JE, editors. Textbook of Medical Physiology. Eleventh Edition. Philadelphia, Elsevier Saunders, pp. 502–513.
- 8. Schlichtig R, Bowles SA (1994) Distinguishing between aerobic and anaerobic appearance of dissolved CO2 in intestine during low flow. J Appl Physiol 76: 2443–2451. [DOI] [PubMed] [Google Scholar]
- 9. Lamia B, Monnet X, Teboul JL (2006) Meaning of arterio-venous PCO2 difference in circulatory shock. Minerva Anestesiol 72: 597–604. [PubMed] [Google Scholar]
- 10. Vallet B, Teboul JL, Cain S, Curtis S (2000) Venoarterial CO2 difference during regional ischemic or hypoxic hypoxia. J Appl Physiol 89: 1317–1321. [DOI] [PubMed] [Google Scholar]
- 11. Mekontso-Dessap A, Castelain V, Anguel N, Bahloul M, Schauvliege F, et al. (2002) Combination of venoarterial PCO2 difference with arteriovenous O2 content difference to detect anaerobic metabolism in patients. Intensive Care Med 28: 272–277. [DOI] [PubMed] [Google Scholar]
- 12. Kocsi S, Demeter G, Fogas J, Érces D, Kaszaki J, et al. (2012) Central venous oxygen saturation is a good indicator of altered oxygen balance in isovolemic anemia. ACTA Anaesthesiol Scand 56: 291–297. [DOI] [PubMed] [Google Scholar]
- 13. Phillips CP, Vinecore K, Hagg DS, Sawai RS, Differding JA, et al. (2009) Resuscitation of haemorrhagic shock with normal saline vs. lactated Ringer's: effects on oxygenation, extravascular lung water and haemodynamics. Critical Care 13: R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.West JB (1990) Gas transport to the periphery. In: West JB. Baltimore MD, editor, Respiratory Physiology: The Essentials (4th ed.), Williams and Wilkins, pp. 69–85.
- 15. Maddirala S, Khan A (2010) Optimizing hemodynamic support in septic shock using central and mixed venous oxygen saturation. Crit Care Clin 26: 323–333. [DOI] [PubMed] [Google Scholar]
- 16.Marino PL (2014) Systemic Oxygenation. In: Marino PL, editor, The ICU Book (4th ed.) Wolters Kluwer Health/Lippincot Williams and Wilkins, pp. 171–192.
- 17. Jalloh I, Helmy A, Shannon RJ, Gallagher CN, Menon DK, et al. (2013) Lactate uptake by the injured human brain: evidence from an arteriovenous gradient and cerebral microdialysis study. J Neurotrauma 30(24): 2031–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gallagher CN, Carpenter KL, Grice P, Howe DJ, Mason A, et al. (2009) The human brain utilizes lactate via the tricarboxylic acid cycle: a 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain 132(Pt10): 2839–2849. [DOI] [PubMed] [Google Scholar]
- 19. Hare GM, Mazer CD, Hutchison JS, McLaren AT, Liu E, et al. (2007) Severe hemodilutional anemia increases cerebral tissue injury following acute neurotrauma. J Appl Physiol 103: 1021–9. [DOI] [PubMed] [Google Scholar]
- 20. Vincent JL, Piagnerelli M (2006) Transfusion in the intensive care unit. Crit Care Med 34: S96–S101. [DOI] [PubMed] [Google Scholar]
- 21.Galvin I, Ferguson ND (2011) Acute lung injury in the ICU: focus on prevention. In: Vincent JL, editor. Annual update in intensive care and emergency medicine. Berlin: Springer Science+Business Media LLC. pp. 117–28. [Google Scholar]
- 22. Meletti JFA, Módolo NSP (2003) Hemorrhagic Shock Hemodynamic and Metabolic Behavior: Experimental Study in Dogs. Revista Brasielira de Anestesiologia 53: 623–632. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Relevant data have been deposited to Figshare with the DOIs: http://dx.doi.org/10.6084/m9.figshare.1110007, http://dx.doi.org/10.6084/m9.figshare.1110008.




