Child health in the United States improved dramatically over the twentieth century. Data from the National Center for Health Statistics indicate the infant mortality rate was 23 times greater in 1900 than in 2004. The mortality rate of one-to four-year-old children, although lower in absolute terms, had a larger proportionate decline: the value in 1900 was 66 times that in 2004.
The proximate cause of the mortality decline was a reduction in infectious disease. Between 1900 and 1998, the percentage of deaths of children age 1 to 19 due to infectious disease is estimated to have declined from 61.6 percent to 2 percent (Bernard Guyer et al. 2000). Major causes of child death included diarrhea, pneumonia and other respiratory infections, diphtheria, typhoid, measles, scarlet fever, whooping cough, and tuberculosis (Guyer et al. 2000). The mortality decline was accompanied by reductions in morbidity among surviving children. There were also declines in the prevalence of a host of illnesses, such as hookworm and trachoma, which were not deadly but which impaired children’s quality of life (C. Hoyt Bleakley 2007; Shannen K. Allen and Richard D. Semba 2002). Early life exposures to infectious disease may also have adverse effects on health and well-being into old age. If true, then the benefits of the twentieth century decline in infectious disease in the United States are still being realized.
We examine whether the disease environments experienced by American children in the first half of the twentieth century are associated with their cognitive abilities at older ages. We match region-level historical data on mortality from a variety of infectious diseases, as well as total infant mortality, with information on the cognitive function of older Americans followed by the Health and Retirement Study (HRS). We find evidence that the burden of disease in early life—measured using either mortality rates by cause or the overall infant mortality rate—is significantly associated with performance on cognitive tests in old age.
I. Background
An extensive literature documents how the disease environment in the prenatal period and early childhood influences adult health outcomes. Dora L. Costa (2000) discusses why childhood exposure to diseases such as measles and typhoid can affect cardiac and respiratory function later in life. Caleb E. Finch and Eileen M. Crimmins (2004) review evidence that infections can lead to chronic inflammation, which in turn influences morbidity and mortality in adulthood. Other research highlights the role of prenatal and early life nutrition on long-term health outcomes (see, for example, David J. P. Barker 1997).
Early disease environments may also influence cognitive outcomes. Some infections may affect brain development among children, presumably resulting in impairment throughout life. (See, for example, P. A. Holding and R. W. Snow (2001) on the effects of malaria on the developing brain.) Early life infections may also speed cognitive decline in old age, possibly through the effects of infection on later life cardiovascular health (Costa 2005) or the effects of inflammation on adult neurogenesis (C. T. Ekdahl et al. 2003).
An important issue has been to identify the periods in early life when disease exposures are particularly harmful. Douglas Almond (2006) conducted a careful study of the long-term effects of the 1918 influenza pandemic on the health, educational attainment, and economic status of those exposed early in life. He finds that prenatal exposure to influenza was particularly harmful, whereas exposure after birth was not. That influenza exposure influenced educational attainment suggests a possible effect on cognitive ability. It is unknown, however, whether other infectious diseases pose particular risks during the prenatal period, or whether the experiences of the very severe 1918 influenza generalize to other influenza exposures. In the research that follows, we pay particular attention to the issue of the timing of exposure to infectious disease.
II. Data
Our data on cognitive function at older ages come from the HRS. Since its inception in 1992, the HRS has been documenting the physical and mental health and life circumstances of a cohort of men and women in the United States over the age of 50. The measures of cognitive function that we use began to be collected in wave 3 of the study (1996), and for this reason we restrict our analysis to waves 3 to 7, which were collected in even years between 1996 and 2004. To reduce heterogeneity, we further restrict our analysis to non-Hispanic black and white men and women between the ages of 50 and 90 for whom no proxy respondent was used.
We use two markers of cognitive function. “Delayed word recall” records the number of words that the respondent remembers approximately five minutes after having heard a list of ten nouns read aloud. This measure has a sample mean of 4.60 and a standard deviation of 2.10. “Counting backward” is an indicator the respondent can count backward by one from 86 to 77. This assessment, which was conducted in waves 3 to 6, has a “pass rate” of 87.5 percent.
We examine the association between cognitive function and infant mortality, and mortality rates by-cause within census region of birth for typhoid, malaria, measles, influenza, and diarrhea. (Diarrhea deaths are for those under the age of two.) Mortality data from 1900 to 1936 are from Grant Miller’s data archive on the National Bureau of Economic Research Web site (available at http://www.nber.org/data/vital-statistics-deaths-historical). Data from 1937 to 1950 are from the Vital Statistics of the United States documents (downloaded from the Centers for Disease Control Web site). Mortality rates are expressed as the number of deaths per 100,000 population, and infant mortality rates are the number of infants deaths (under one year, exclusive of stillbirths) per 1,000 live births. We aggregated mortality rates to the level of the nine Census regions (New England, Mid-Atlantic, East-North Central, West-North Central, South Atlantic, East-South Central, West-South Central, Mountain, and Pacific).
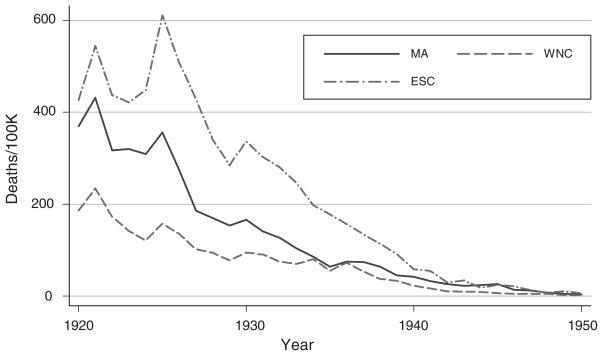
Infant mortality and by-cause mortality rates vary substantially across regions and over time. To illustrate, Figures 1 and 2 show typhoid and measles mortality in three regions (Mid-Atlantic, West-North Central, and East-South Central). Although all of the diseases we examine decline over this period, their initial levels and patterns of decline differ across regions. Typhoid shows relatively small year-to-year fluctuations, whereas measles mortality has the sawtooth pattern that is characteristic of this disease. (For a complete set of figures, see: http://www.princeton.edu/rpds/papers/pdfs/earlylifehealth_additions.pdf.)
Figure 1.
Typhoid Mortality in Three Regions
Figure 2.
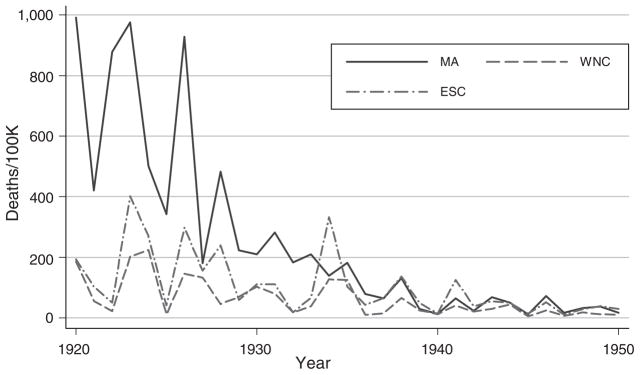
Measles Mortality in Three Regions
III. Results
We start by documenting differences in cognitive test scores across regions. Table 1 shows the results of regressions of our two cognitive outcomes on a set of indicators for the region of birth. All regressions include indicators for age at examination (in five-year categories), race, and sex. Columns 3 and 4 also include indicators for the current census region of residence. The mid-Atlantic region, which has the highest scores after adjusting for age, sex, and race, is the omitted category. The results indicate that southern regions have the lowest scores. There are substantial differences, however, even among nonsouthern regions.
Table 1.
Cognitive Function across Regions
| No controls for current region of residence
|
With controls for current region of residence
|
|||
|---|---|---|---|---|
| Delayed word recall | Counting backward | Delayed word recall | Counting backward | |
| New England | −0.167 (0.054) | −0.010 (0.007) | −0.206 (0.075) | −0.017 (0.010) |
| East-North Central | −0.128 (0.037) | −0.014 (0.005) | −0.091 (0.051) | −0.012 (0.007) |
| West-North Central | −0.156 (0.041) | −0.010 (0.007) | −0.131 (0.062) | −0.015 (0.008) |
| South Atlantic | −0.335 (0.040) | −0.057 (0.006) | −0.379 (0.048) | −0.041 (0.007) |
| East-South Central | −0.358 (0.047) | −0.048 (0.007) | −0.362 (0.062) | −0.030 (0.009) |
| West-South Central | −0.309 (0.046) | −0.054 (0.007) | −0.315 (0.068) | −0.041 (0.010) |
| Mountain | −0.000 (0.071) | −0.029 (0.011) | −0.169 (0.084) | −0.044 (0.012) |
| Pacific | −0.009 (0.058) | −0.015 (0.008) | −0.216 (0.072) | −0.028 (0.010) |
| F-test ( p-value): region of birth indicators jointly insignificant | 15.09 (0.000) | 18.77 (0.000) | 10.80 (0.000) | 6.01 (0.000) |
| Observations | 63,624 | 51,733 | 63,311 | 51,616 |
Table 2 presents results from OLS regressions on the association between delayed word recall and log of mortality rates in early life. Each column presents the results of three OLS regressions, all of which include indicators for age at examination (in five-year categories), race, sex, and census region of birth. In the top panel, delayed word recall is regressed on the log of the infant mortality rate in the cohort member’s year of birth (column 1), or the log of the mortality rate from different diseases (typhoid in column 2, malaria in column 3, and so on), all measured in the year of birth. The middle panel shows similar regressions, with the exception that measures of mortality in both the year of birth and the second year of life are included. The third panel shows regressions on mortality in the second year of life, and includes (in addition to the controls listed above) indicators for the current census region in which the individual resides. Standard errors, in parentheses, are clustered at the individual level.
Table 2.
Delayed Word Recall in the HRS and Disease Environment in Early Life
| Infant mortality rate | Deaths per 100,000 population from:
|
|||||
|---|---|---|---|---|---|---|
| Typhoid | Malaria | Measles | Influenza | Diarrhea | ||
| In birth year | −0.209 (0.073) | −0.056 (0.018) | −0.014 (0.017) | −0.003 (0.013) | 0.009 (0.018) | −0.057 (0.027) |
|
| ||||||
| In birth year | 0.108 (0.171) | 0.026 (0.042) | −0.014 (0.024) | −0.003 (0.013) | 0.019 (0.019) | 0.009 (0.046) |
| In second year of life | −0.371 (0.168) | −0.088 (0.038) | 0.000 (0.021) | −0.002 (0.013) | −0.042 (0.020) | −0.088 (0.045) |
|
| ||||||
| In second year of life | −0.277 (0.071) | −0.069 (0.016) | −0.009 (0.015) | −0.002 (0.013) | −0.040 (0.019) | −0.082 (0.027) |
|
| ||||||
| Observations | 59,412 | 62,029 | 61,583 | 62,029 | 62,029 | 59,686 |
The top panel shows a significant association between word recall at older ages and the log infant mortality rate, and the mortality rates for typhoid and diarrhea in the year of birth. However, the results in the second panel indicate that, in all cases, the inclusion of log mortality rates at age 2 erases the impact of the disease environment in the birth year. Instead, it is the disease environment at age 2 that is significantly correlated with cognitive function. This is true for overall infant mortality, typhoid, influenza, and diarrhea. These results are in contrast to those of Almond (2006), who finds largest effects for individuals in utero during the 1918 pandemic.
The final panel of Table 2 shows results that include log mortality rates at age 2 alone, with controls for current census region of residence (the presence of the latter has no significant effect on our results). We find that a halving of the infant mortality rate—which occurred between 1920 and 1940—is associated with an increase in delayed word recall test of 0.2 words, or nearly a tenth of a standard deviation.
We also estimated models that included all causes of mortality at once. Although the causes are jointly significantly different from zero, the point estimates for individual diseases are imprecisely estimated. Mortality rates within a census region are highly correlated, making it unwise, with these data, to claim that it was one disease (say, typhoid) rather than another (say, malaria) that is responsible for the significant association between measures of cognitive function and the disease environment. However, these results do support the idea that the disease environment in early childhood is associated with cognitive outcomes later in life.
In Table 3, we present a similar analysis for successfully counting backward from 86 to 77. Here, we have omitted the middle panel, and the second panel contains only the log mortality rates in the second year of life without indicators for the current region of residence. (However, there inclusion makes no difference to the results.) We find weaker associations between this measure of cognitive ability and the disease environment in early life. Typhoid and malaria are significantly associated with an inability to count backward, while measles, influenza, and diarrhea show no such effects. Indeed, influenza shows a positive association with successful counting. The inability to count backward is fairly uncommon—the test is (failed) less than 15 percent of the time—and failure may represent severe cognitive decline that the disease environment in early life cannot predict.
Table 3.
Counting Backward in the HRS and Disease Environment in Early Life
| Infant mortality rate | Deaths per 100,000 population from: | |||||
|---|---|---|---|---|---|---|
| Typhoid | Malaria | Measles | Influenza | Diarrhea | ||
| In birth year | −0.000 (0.013) | −0.004 (0.003) | 0.001 (0.003) | 0.002 (0.002) | 0.009 (0.003) | 0.009 (0.005) |
| In second year of life | −0.015 (0.013) | −0.005 (0.003) | −0.007 (0.002) | 0.003 (0.002) | 0.007 (0.003) | 0.005 (0.005) |
| Observations | 48,455 | 50,930 | 50,581 | 50,930 | 50,930 | 49,327 |
Changes in the disease environment within a census region may be correlated with other changes—such as changes in the quality of schools of health care facilities—that influenced cognitive development. It may be these other changes, rather than the disease environment, that lead to correlation between measures of cognitive ability at older ages and the disease burden in early life. That our results are robust to the inclusion (exclusion) of census region indicators suggests that these correlations are not simply picking up level differences in disease burden and level differences in cognitive function across space. However, they do not eliminate the possibility that we are picking up the impact of other changes taking place in census regions over time. Our results are not robust to adding census region–specific time trends—a result not terribly surprising, given that the level of variation in our data is at the census region–year level. In addition, selection effects with respect to who survives infancy, or early childhood, in a region experiencing a temporarily heavy disease burden, might be able to explain our results. All of these factors leave our results open to multiple interpretations.
We do not have good measures of the quality of educational or health care facilities in individuals’ places of birth. However, we do know individuals’ educational attainment, and can examine whether the associations between cognitive outcomes and early disease environment are altered when we control for education. In regressions of years of completed schooling that control for by-cause mortality, and region and year of birth, we find that educational attainment is significantly associated with only two measures of the early life disease environment: it is negatively associated with malaria mortality, but (unexpectedly) positively associated with diarrhea mortality. More important, adding years of education to our regressions for delayed word recall does little to alter our findings. Delayed word recall is still negatively and significantly associated with infant mortality, typhoid, and influenza. These findings suggest that to the extent an individual’s early life environment influences cognitive outcomes later in life, it does not do so through its effect on educational attainment.
These results, if confirmed in other work, have several implications. First, to the extent that the disease environments American children experience have both improved and converged across regions, we should expect regional differences in cognitive ability in old age to be smaller among the next cohorts of elderly individuals. This will be testable as later-born cohorts of individuals are brought into the HRS. (Currently, same-aged individuals in waves 3–7 of the HRS are observed only over a ten-year span of birth years, a period that is too short to examine this hypothesis.) Second, these results suggest that the benefits of reductions in childhood exposures to infectious disease in developing economies may extend far into the future.
Acknowledgments
We thank Douglas Almond for comments and acknowledge support from NIH grant P30 AG024361.
Contributor Information
Anne Case, 367 Wallace Hall, Princeton University, Princeton NJ 08544.
Christina Paxson, 316 Wallace Hall, Princeton University, Princeton NJ 08544.
References
- Allen Shannen K, Semba Richard D. The Trachoma Menace in the United States, 1897–1960. Survey of Opthalmology. 2002;47(5):500–09. doi: 10.1016/s0039-6257(02)00340-5. [DOI] [PubMed] [Google Scholar]
- Almond Douglas. Is the 1918 Influenza Pandemic Over? Long-term Effects of in Utero Influenza Exposure in the Post-1940 US Population. Journal of Political Economy. 2006;114(4):672–712. [Google Scholar]
- Barker David JP. Maternal Nutrition, Fetal Nutrition, and Disease in Later Life. Nutrition. 1997;13(9):807–13. doi: 10.1016/s0899-9007(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Bleakley C Hoyt. Disease and Development: Evidence from Hookworm Eradication in the American South. Quarterly Journal of Economics. 2007;122(1):73–117. doi: 10.1162/qjec.121.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Dora L. Understanding the Twentieth Century Decline in Chronic Conditions among Older Men. Demography. 2000;37(1):53–72. [PubMed] [Google Scholar]
- Costa Dora L. Causes of Improving Health and Longevity at Older Ages: A Review of the Explanations. Genus. 2005;LXI(1):21–38. [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is Detrimental for Neurogenesis in Adult Brain. Proceedings of the National Academy of Science. 2003;100:13632–37. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch Caleb E, Crimmins Eileen M. Inflammatory Exposure and Historical Changes in Human Life-Spans. Science. 2004;305(5691):1736–39. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- Guyer Bernard, Freedman Mary Anne, Strobino Donna M, Sondik Edward J. Annual Summary of Vital Statistics: Trends in the Health of Americans During the 20th Century. Pediatrics. 2000;106(6):1307–15. doi: 10.1542/peds.106.6.1307. [DOI] [PubMed] [Google Scholar]
- Holding PA, Snow RW. Impact of Plasmodium Falciparum Malaria on Performance and Learning: Review of the Evidence. American Journal of Tropical Medicine and Hygiene. 2001 Jan-Feb;64(supplement):68–75. doi: 10.4269/ajtmh.2001.64.68. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. Death Rates for Selected Causes by 10-Year Age Groups, Race, and Sex: Death Registration States, 1900–32, and United States, 1933–98. http://www.cdc.gov/nchs/datawh/statab/unpubd/mortabs/hist290.htm.