Abstract
Overexpression of Interleukin-17 (IL-17) family has been shown in a variety of autoimmune diseases. IL-25 (IL-17E), as a member of this family of cytokines, induces the overexpression of IL-13 and impedes Th17/IL-17 responses. In the present study potential single nucleotide polymorphisms (SNP) of IL-25, its serum level in Multiple Sclerosis (MS) patients have been surveyed. Blood samples were obtained from 100 Relapsing-Remitting MS cases, and 100 healthy controls. Serum levels of IL-25 were measured by ELISA. IL-25 exons 1 and 2 were sequenced. IL-25 serum levels investigation showed significant association in cases compared to controls. Molecular analysis of IL-25exons 1 and 2 depicted significant differences in polymorphisms of exon 2 between two groups of study. However, no significant differences were found in polymorphisms for IL-25 exon. These results demonstrate that serum levels of IL-25 are reduced in MS patients compared to controls. This is the first study in Iran that shows polymorphisms in IL-25 among MS patients. Considering the role of IL-25 in suppression of the effects of IL-17A and active phase of Experimental Autoimmune Encephalomyelitis (EAE) in vivo, this cytokine seems to have therapeutic potentials for autoimmune diseases like MS.
Keywords: ELISA, gene polymorphism, IL-25, multiple sclerosis
Introduction
Multiple sclerosis (MS) is a chronic disease of central nervous system (CNS) that causes severe physical and cognitive disorders [1]. Among different clinical forms, Relapsing-Remitting type of MS is the most frequent and accounts for 85% to 90% of cases [2]. Since after trauma and rheumatic diseases is the third major cause of disability, MS has been the subject of extensive studies worldwide [3]. Although the main etiology of this disease is unknown, different factors such as genetic background, autoimmune mechanisms, environmental factors, and especially viruses have been demonstrated to play important roles in the occurrence of MS [4].
Researchers categorize MS into the group of inflammatory autoimmune diseases with the patterns of Th1 immune responses [5,6]. One of the hallmarks in pathology of MS is the presence of inflammatory areas in white matter of CNS and surrounding central coronary vessels. The main cells involved in this process are macrophages and T lymphocytes. B cells and antigen presenting cells are also found in these areas. Meanwhile, the role of Th17 cells has also been identified in the immunopathology of MS [7,8].
Th17 cells produce IL-17 to induce inflammatory mediators such as IL-1, IL-6, TNF-α, NOS-2, metalloprotease, and chemokines, consequent to activation of fibroblasts, endothelial cells, macrophages, and epithelial cells. IL-17, therefore, promotes inflammation and play pivotal roles in the pathogenesis of MS [9,10]. Hence, most of recent studies have been concentrated to find the source of increased Th17 cells in MS patients.
IL-17 family consists of six known members in mammals, and plays fundamental roles in the immunity against chronic infectious diseases [11]. Almost all members of this family of cytokines play roles in the induction of inflammation, except for one, which has anti-inflammatory function and shows the least homology to other members. This cytokine is known as IL-25 or IL-17E [11,12]. IL-25, as an anti-inflammatory cytokine, has been given intense attention in the field of autoimmune diseases during the last decades.
Several studies indicate that IL-25 diverges the immune system towards Th2 responses, and decreases the inflammation in autoimmune diseases that are caused by Th17 activity, through increased production of IL-5, IL-13 and IL-4[11,13-15]. It has been reported that treatment with IL-25 alleviates or suppresses the Th1 and Th17 induced inflammation in CNS, by directly inhibition of the IL-23, IL-1, and IL-6 production by active dendritic cells [13]. IL-25 also plays important roles in preventing the entrance of inflammatory cells to the CNS [16]. Studies have shown that IL-25 defective mice are highly sensitive to the EAE because of the decreased production of IL-13 [17]. These findings demonstrate that IL-25 has important roles in the maintenance of CNS, and prevention of MS and EAE. Since mutations and polymorphisms affect appropriate function or production of a protein, in this study we have investigated potential polymorphisms in IL-25 exons 1 and 2, and measured its serum levels in MS patient population in the East Azerbaijan, Iran.
Materials and methods
Subjects
In this study 100 cases (including 37 men, and 63 women with the mean age of 33.75 ± 7.67 years old) with relapsing-remitting MS were recruited from the individuals referred to the Neurology Department of Neurosciences Research Center, Imam Reza Hospital, Tabriz University of Medical Sciences, Tabriz, Iran in 2013. Patients were diagnosed by neurologist according to the diagnostic criteria of Poser et al [18]. Patients had not taken any drug pertaining to treatment of MS and they were in primary progressive grade of MS. Disability of patients was measured using the Expanded Disability Status Scale (EDSS). The mean EDSS score of patients was 1.3 ± 0.7. Also 100 age and sex matched healthy controls were selected from individuals without any autoimmune disease among themselves and their close relatives, and any history of chronic inflammatory diseases, migraine, and smoking. All procedures were performed by the Ethics Committee of Tabriz University of Medical Sciences, and all individuals enrolled in the study declared their informed consent in writing.
Sample size
In this study the sample size has been calculated through odds ratio formula. Considering the probability of increase in IL-25, p = 0.5, d = 0.11, and α = 0.05, the number of at least 28 samples were determined. However, to enhance the credibility of research we have considered 100 samples for each group of study.
ELISA test
In order to DNA extraction and preparation of serum, 10 cc peripheral blood was taken from individuals in EDTA (1 mg/ml) containing tubes. To measure the serum levels of IL-25 the USCN Co. commercial kit was used. In brief, 100 microliters (ml) from standard and samples were added to wells, and incubated for 2 hours (h) in 37°C. Wells were emptied, then 100 ml of reagent A were added to wells and incubated for 1 h in 37°C. After 3 steps of washing, 100 ml of reagent B were added and incubated for 30 min in 37°C. Afterwards, 5 times of washing were performed and then 90 ml of substrate were added. Then the wells were covered and incubated in 37°C. To stop the reactions, the stop solution was added, and then the reactions were read with the ELISA reader.
DNA extraction
PBMCs were isolated through Ficoll-Hypaque centrifugation from peripheral blood. In order to DNA extraction Cinnagen Inc. kit was used, and extraction was performed based on the protocol. Purity and amount of extracted DNA were determined using spectrophotometry.
Polymerase chain reaction (PCR)
Two primer pairs were designed for two regions of IL-25, consisting exons 1 and 2 using Primer3Plus (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi) and were blasted in NCBI website: http://www.ncbi.nlm.nih.gov/tools/primer-blast/. See Table 1 for the details of primers used in PCR. Primers were produced by the custom oligonucleotide synthesis service, Metabion (Martinsried, Germany).
Table 1.
Primers used in PCR, amplicon size, and Tm for each reaction
| Target | Sequence | Amplicon size (bp) | Tm (°C) |
|---|---|---|---|
| IL25-E1 F | 5’-TCACTCCCTAAAAAGACAGTGGA-3’ | 506 | 60 |
| IL25-E1 R | 5’-ACACACAGGGCAGGCATT-3’ | 59.8 | |
| IL25-E2 F | 5’-CTTTCCAAGGCCTGACAAGT-3’ | 705 | 61.7 |
| IL25-E2 R | 5’-CGAGATGTTCAGGCACCAC-3’ | 60.2 |
Tm; Melting Temperature.
PCR reactions were performed by Amplicon Inc. Master mix (Cat No: E245) in the final volume of 50 ml (25 ml Master mix: 13 ml sterile distilled water, 5 ml primer, and 7 ml sample). PCR reactions were performed in standard condition including: denaturation phase for 40 sec in 95°C, annealing phase for 40 sec in 64°C, elongation phases for 1 min in 72°C. These steps were repeated for 35 cycles, and the last cycle finished with an extension phase for 7 min in 72°C. To assess the quality of PCR products, gel electrophoresis was performed. Then, PCR products were sequenced (Macrogene, Korea) to reveal possible polymorphisms.
Statistical analysis
Data were shown in Mean ± SD. For comparison of the IL-25 level between case and control groups and sex ANOVA (Post-Hoc: Tukey, P < 0.05) test, and for the polymorphism analysis in cases and controls chai-square test were performed. Obtained data was analyzed by SPSS 20 software. P-values of less than 0.05 were considered significant.
Results
Serum level of IL-25
ELISA results for detection of IL-25 levels in MS patients and healthy controls showed that serum levels of IL-25 are significantly lower in MS patients compared to controls. However, serum level difference of IL-25 between women/men and healthy control was not significant. Figure 1 displays the results of IL-25 serum levels comparison between two groups of study.
Figure 1.
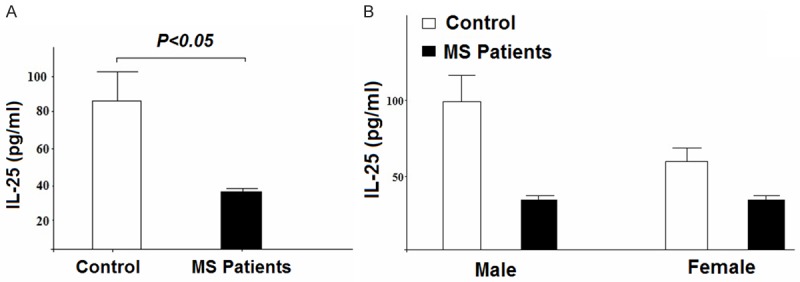
Investigating the serum levels of IL-25 in MS patients and control group. A: Statistical analysis showed a significant difference in serum levels of IL-25 between cases and controls (p < 0.05). B: Comparison in serum levels of IL-25 between men/women and controls showed no significant difference (p < 0.1).
IL-25 polymorphisms
Sequencing results for investigation of the polymorphisms of IL-25 exons 1 and 2 depicted that although sequence analysis unmasked a number of SNPs in IL-25 exon 1 of cases and controls, no significant differences in favor of association between SNPs and MS were proved. Table 2 shows the frequency of SNPs in exon 1 among MS patients and controls. Analysis of SNPs in exon 2, however, showed a significant association to MS compared to controls. Among detected SNPs of exon 2, 4076A>A, 3672T>TA, 3712G>GA, and 3463C>CA have shown to be more frequent. Among observed alterations 4076A>AG, 3672T>TA, and 3712G>GA were the most frequent in patients, and 3463C>CA was seen to be more frequent in healthy individuals compared to patients. In addition, some of these polymorphisms were observed to exist in a simultaneous pattern among patients, including 4076A>AG-3728G>GA and 3730G>GC-3728G>GA (Tables 3, 4). Analysis of these polymorphisms showed no significant association to the individual’s age. Table 3 illustrates the comparison of SNP frequencies in exon2 among MS patients and controls.
Table 2.
The frequency of SNPs in exon 1 in MS and control samples. SNPs with the frequency less than 2 are not reported
| Mutation situation | MS patients | Healthy control |
|---|---|---|
| Without mutation | 5 (5%) | 92 (92%) |
| 1118 G>GC | 5 (5%) | 2 (2%) |
MS; Multiple Sclerosis.
Table 3.
The frequency of SNPs in exon 2 in MS and control samples
| Mutation situation | MS patients | Healthy control | |
|---|---|---|---|
| Without mutation | 28 (28%) | 86 (86%) | |
| SNP | 3728G>GA | 31 (31%) | 11 (11%) |
| 4076A>AG c.1347 | 11 (11%) | - | |
| 3672T>TA c.943 | 9 (9%) | - | |
| 3712G>GA c.983 | 7 (7%) | - | |
| 3730G>GC c.1001 | 5 (5%) | - | |
| 3463C>CA | 9 (9%) | 3 (3%) | |
MS; Multiple Sclerosis.
Table 4.
The frequency of mutual SNPs in exon 2 in MS patients and controls
| SNP | MS patients | Healthy controls |
|---|---|---|
| 4076A>AG, 3728G>GA | 13 (13%) | 0 (0%) |
| 3712G>GA, 3728G>GA | 10 (10%) | 0 (0%) |
| 3730G>GC, 4076A>AG | 6 (6%) | 0 (0%) |
| 3730G>GC, 3728G>GA | 6 (6%) | 0 (0%) |
| 3463C>CA, 3728G>GA | 20 (20%) | 3 (3%) |
| 3463C>CA, 3730G>GC | 3 (3) | 0 (0%) |
MS; Multiple Sclerosis.
Discussion
The purpose of the current study was to investigate the polymorphisms in IL-25 exons 1 and 2, and serum levels of IL-25 in MS patients, in comparison to control group. MS is an inflammatory autoimmune disease that affects more than 2 million individuals around the world [19]. A variety of cells play roles in the induction of inflammation in MS. Among them Th17/Th1 and Treg/Th2 cells play roles in either induction or inhibition of the inflammation, through inflammatory and anti-inflammatory cytokines respectively [20]. Although studies have focused on IL-17A, it has been shown that IL-17F also has pre-inflammatory features like IL-17A, however, with reduced activity [21].
Considering the role of genetic factors in the activity and immune response against foreign antigens, these factors cannot be easily ignored. Genes that control the expression of major histocompatibility complex (MHC) and T cell receptor (TCR) in T cells are well known examples, which have indispensable roles in immune function. MHC genes family, in particular, has been linked to a variety of autoimmune diseases like MS [22]. In recent years, many genes have been under genetic investigation for possible association to MS; however, HLA-DRB1 was first identified to be associated with MS [23]. There are also extensive evidence of association between mutations in genes involved in the immune system and MS. Of those, IL-2Rα , CD58, CD226, EV15, and SH2B3 can be mentioned. Other evidence implies the association between mutations in IL-1, IL-10, IL-2, IL-2Rα, IL-2Rβ, and IFN-γ and MS. It has been indicated that IL-25 modulates the inflammatory respond in autoimmune diseases, through the control of Th17 inflammatory cytokines production. This function of IL-25 is mediated by IL13, IL-4, and IL-5 [23]. It has been identified that the treatment with IL-25 protects mice against EAE, showing the important role of IL-25 in the suppression of inflammation in EAE [23]. In the present study, as a first time, potential polymorphisms for IL-25 exons 1 and 2 among MS patient population in the East Azerbaijan of Iran have been investigated. Notably, based on the already conducted studies, there has not been a similar research investigating the polymorphisms for IL-25 in patients with MS. This gene was chosen because of its important role in controlling the pathogenesis of autoimmune diseases such as MS. To survey the coding region of the gene, sequencing has been used. There have not been many studies to investigate genetic alterations of IL-25 to date. The only research that has been seen was the study of IL-25 coding region sequencing in patients with IBD that reported C424C/A polymorphism in exon 2. However, this polymorphism did not demonstrate any significant differences in Crohn’s disease and ulcerative colitis, from the study of cases and controls (24). Despite finding polymorphisms in exon 1 of cases and controls in our study on Relapsing-Remitting MS patients, no significant differences showing the associations between these polymorphisms and the disease were seen. However, analysis of polymorphisms in exon 2 demonstrated significant association to disease, compared to controls. Since these polymorphisms are located in exons, their possible effects on the structure and function of protein should be investigated. Among the polymorphisms of exon 2, 4076A>AG, 3672T>TA, 3712G>GA, and 3463C>CA are the most frequent. Between these alterations 4076A>AG, 3672T>TA, and 3712G>GA are the most frequent in patients, and 3463C>CA in healthy controls. Furthermore, some of these polymorphisms, such as 4076A>AG-3728G>GA and 3730G>GC-3728G>GA are seen to occur together. Studying these polymorphisms showed no significant association related to individual’s age. Accordingly, the investigation of the role of these polymorphisms as a predisposing or preventive factor for MS disease may be useful in future studies with larger sample size. Current study demonstrated that serum levels of IL-25 are significantly lower in patients, compared to controls. Today, the importance of inflammation in CNS in the pathogenesis of MS is evidently verified through the beneficial clinical effects of Natalizumab in treatment of Relapsing MS. This drug prevents the entrance of circulating lymphocytes and monocytes into the CNS, and reduces the inflammation in CNS (25). Since the anti-inflammatory effects of IL-25 have been shown in a plenty of studies, it is expected that this cytokine could also be used to treat MS, or at least reduction of relapse. However, this issue requires more detailed studies in different population groups and different phases of the disease.
Acknowledgements
This study was supported by Immunology Department and Neuroscience Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Disclosure of conflict of interest
None.
References
- 1.McFarlin DE, McFarland HF. Multiple sclerosis (first of two parts) N Engl J Med. 1982;307:1183–1188. doi: 10.1056/NEJM198211043071905. [DOI] [PubMed] [Google Scholar]
- 2.Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- 3.Nagelkerken L. Role of Th1 and Th2 cells in autoimmune demyelinating disease. Braz J Med Biol Res. 1998;31:55–60. doi: 10.1590/s0100-879x1998000100007. [DOI] [PubMed] [Google Scholar]
- 4.Inglese M. Multiple sclerosis: new insights and trends. AJNR Am J Neuroradiol. 2006;27:954–957. [PMC free article] [PubMed] [Google Scholar]
- 5.Murray TJ. Diagnosis and treatment of multiple sclerosis. BMJ. 2006;332:525–527. doi: 10.1136/bmj.332.7540.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gold R, Luhder F. Interleukin-17--extended features of a key player in multiple sclerosis. Am J Pathol. 2008;172:8–10. doi: 10.2353/ajpath.2008.070862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pugliatti M, Sotgiu S, Rosati G. The worldwide prevalence of multiple sclerosis. Clin Neurol Neurosurg. 2002;104:182–191. doi: 10.1016/s0303-8467(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 8.Dean G. How many people in the world have multiple sclerosis? Neuroepidemiology. 1994;13:1–7. doi: 10.1159/000110351. [DOI] [PubMed] [Google Scholar]
- 9.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renoux C, Vukusic S, Mikaeloff Y, Edan G, Clanet M, Dubois B, Debouverie M, Brochet B, Lebrun-Frenay C, Pelletier J, Moreau T, Lubetzki C, Vermersch P, Roullet E, Magy L, Tardieu M, Suissa S, Confavreux C. Natural history of multiple sclerosis with childhood onset. N Engl J Med. 2007;356:2603–2613. doi: 10.1056/NEJMoa067597. [DOI] [PubMed] [Google Scholar]
- 12.Sedzik J, Toews AD, Blaurock AE, Morell P. Resistance to disruption of multilamellar fragments of central nervous system myelin. J Neurochem. 1984;43:1415–1420. doi: 10.1111/j.1471-4159.1984.tb05402.x. [DOI] [PubMed] [Google Scholar]
- 13.Hemmer B, Nessler S, Zhou D, Kieseier B, Hartung HP. Immunopathogenesis and immunotherapy of multiple sclerosis. Nat Clin Pract Neurol. 2006;2:201–211. doi: 10.1038/ncpneuro0154. [DOI] [PubMed] [Google Scholar]
- 14.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 15.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G, Coffman RL. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 16.Sonobe Y, Takeuchi H, Kataoka K, Li H, Jin S, Mimuro M, Hashizume Y, Sano Y, Kanda T, Mizuno T, Suzumura A. Interleukin-25 expressed by brain capillary endothelial cells maintains blood-brain barrier function in a protein kinase Cepsilon-dependent manner. J Biol Chem. 2009;284:31834–31842. doi: 10.1074/jbc.M109.025940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, Blumenschein WM, McClanahan T, Brombacher F, Hurst SD, Kastelein RA, Cua DJ. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204:161–170. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, Johnson KP, Sibley WA, Silberberg DH, Tourtellotte WW. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 19.Egg R, Reindl M, Deisenhammer F, Linington C, Berger T. Anti-MOG and anti-MBP antibody subclasses in multiple sclerosis. Mult Scler. 2001;7:285–289. doi: 10.1177/135245850100700503. [DOI] [PubMed] [Google Scholar]
- 20.Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, Grewal IS, de Sauvage FJ. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 21.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantegazza R, Cristaldini P, Bernasconi P, Baggi F, Pedotti R, Piccini I, Mascoli N, La Mantia L, Antozzi C, Simoncini O, Cornelio F, Milanese C. Anti-MOG autoantibodies in Italian multiple sclerosis patients: specificity, sensitivity and clinical association. Int Immunol. 2004;16:559–565. doi: 10.1093/intimm/dxh056. [DOI] [PubMed] [Google Scholar]
- 23.Henderson AP, Barnett MH, Parratt JD, Prineas JW. Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann Neurol. 2009;66:739–753. doi: 10.1002/ana.21800. [DOI] [PubMed] [Google Scholar]


