Abstract
Malignant gliomas, especially glioblastoma multiforme, are the most widely distributed and deadliest brain tumors because of their resistance to surgical and medical treatment. Research of glioma-specific bioconjugates for diagnosis and therapy developed rapidly during the past several years. Many studies have demonstrated that chlorotoxin (CTX) and Buthus martensii Karsch chlorotoxin (BmK CT) specifically inhibited glioma cells growth and metastasis, and accelerated tumor apoptosis. The bioconjugates of CTX or BmK CT with other molecules have played an increasing role in diagnostic imaging and treatment of gliomas. To date, CTX-based bioconjugates have achieved great success in phase I/II clinical trials about safety profiles. Here, we will provide a review on the important role of ion channels in the underlying mechanisms of gliomas invasive growth and how CTX suppresses gliomas proliferation and migration. We will summarize the recent advances in the applications of CTX bioconjugates for gliomas diagnosis and treatment. In addition, we will review recent studies on BmK CT bioconjugates and compare their efficacies with CTX derivatives. Finally, we will address advantages and challenges in the use of CTX or BmK CT bioconjugates as specific agents for theranostic applications in gliomas.
Keywords: Chlorotoxin (CTX), Buthus martensii Karsch chlorotoxin (BmK CT), glioma, imaging, therapy
Introduction
Gliomas, including glioblastoma multiforme (GBM), astrocytoma, anaplastic astrocytoma, and oligodendroglioma, are the most lethal types of primary brain tumors. However, effective treatment of gilomas clinically remains a big challenge [1]. Though survival rates of patients can be improved by complete surgical resection of tumor, it is very difficult for neurosurgeon to accurately locate and distinguish neoplastic tissue from healthy nervous tissue [2]. In addition, gliomas are resistant to chemotherapy and radiation for their special nature of infiltrating proliferation pattern with rapid growth rate and highly invasive ability [3,4]. Therefore, it is urgently needed to find a more effective therapeutic approach against this malignant disease. In recent years, scorpion toxins such as chlorotoxin (CTX) and the chlorotoxin-like toxin derived from Buthus martensii Karsch (BmK CT) have been explored as candidates for glioma diagnosis and therapy.
CTX was purified from the giant yellow Israeli scorpion Leiurus quinquestriatus venom in 1993. It contains 36-amino-acid residues including four disulfide bonds (amino acid sequence: MCMPCFTTDH QMARKCDDCCGGK GRGKCYGPQ CLCR [5]. Previous studies have shown that a matrix metalloproteinase-2 (MMP-2) receptor-associated chloride channel and a glioma-specific chloride channel (GCC) are specifically expressed in the membranes of glioma cells but not in normal human cell membranes. CTX blocks the GCC and specifically binds to MMP-2 receptor. Upon binding of CTX, the MMP-2 complex and GCC are internalized into the cell membrane lipid rafts, leading to inhibition of glioma cell migration and invasion. Up to date, CTX has passed preclinical safety test and entered a phase I clinical trial.
BmK CT is a peptide with 35 amino acids and four disulfide bonds, sharing approximately 68% amino acid homology with CTX. After the purification from the Chinese scorpion Buthus martensii Karsch venom, BmK CT demonstrated an ability of inhibiting glioma cells invasion and migration. Previous research suggests BmK CT selectively interacts with MMP-2 receptor and blocks the GCC in a mechanism similar to that of CTX. Indeed, CTX and BmK CT have been widely studied on glioma cells. The peptides have been demonstrated their glioma specificities in a variety of formats, including radiolabeled, fluorescent, and nanoparticle-based derivatives.
While some available reviews focus on the application of CTX-based agents for either imaging [6] or therapy [7], we believe this review is unique, which summarizes the recent progress on the development of CTX and BmK CT bioconjugates for theranostic applications in gliomas.
The role of ion channels in gliomas
Recent molecular biology studies have found that malfunctions of ion channels on cell membranes are associated with gliomas [8,9]. For example, intracellular Ca2+ is a main regulator for cell motility owing to Ca2+-activated ion channels [10]. Turner et al. [11] demonstrated that Ca2+-activated K+ channel (KCa3.1) elevates glioma migration. The data revealed a notable decrease in tumor invasion into around brain in vivo by ablating KCa3.1 with inducible siRNA. In addition, chloride channel (ClC) protein family, which contains nine members [12], namely ClC-Ka, ClC-Kb and ClC-1 through ClC-7, was absent in normal brain tissue, but abundantly expressed in glioma cell lines. Among them, ClC-3, in particular, has been suggested to affect the invasion and migration of glioma cells by contributing to the efflux of chloride ions and the associated obligatory movement of water [13]. ClC-3 forms protein complexes with membrane type-I MMP, MMP-2, tissue inhibitor of metalloprotein-2, and αvβ3 integrin, co-localizing with Ca2+-activated K+ channel to lipid raft domain of invadipodia. Furthermore, compared with normal tissues, chloride intracellular channel 1 (CLIC1), a non-classical ion channel protein, has been shown to be overexpressed in gliomas [14]. The data suggested that CLIC1 involved in the mechanism of glioma development through the association with cell cycle and functional expression during oxidative stress conditions [15]. Setti et al. demonstrated that CLIC1 is essential for self-renewal and proliferation of GBM cancer stem cells [16].
The initial findings on the effects of CTX on chloride ion channels suggested that a glioma-specific chloride channel facilitates shape changes during glioma cell migration and invasion [17]. However, additional experiments showed that submicromolar concentrations of chlorotoxin cannot block volume-regulated, Ca2+-activated and cystic fibrosis transmembrane conductance regulator (CFTR) chloride channels, suggesting CTX cannot be classified as a general chloride channel toxin [18]. Although the molecular mechanism of CTX in glioma remains elusive, there is a general agreement that CTX binds specifically to the ClC-3/MMP-2 membrane complex [7], which may cause endocytosis of ClC-3/MMP-2 and a reduction of glioma invasiveness.
Progress of CTX-related basic science and applications
CTX and CTX-like peptides
CTX can be prepared through chemical synthesis and subsequent oxidative folding, but the final yield of this process is usually insufficient [19]. Therefore, Wang et al. provided an efficient approach for the preparation of active CTX and its analogs through the recombinant expression of designed CTX precursors in Escherichia coli (E. coli) and subsequent in vitro enzymatic and oxidative refolding [20]. The designed CTX precursors were expressed in E. coli, which reformed by a glutathione transferase (GST)-tag and a 6xHis-tag. Subsequent to the removal of the Nterminal tag, the sulfonated CTX can be refolded with ~80% yield under optimized conditions.
TM-601 was purified from the naturally occurring peptide CTX using solid-phase chemical synthesis method. TM-601 shares physical and biologic properties with natural CTX, and it has been approved by the US Food and Drug Administration for clinical studies. In 2010, Kesavan et al. identified annexin A2 as a new targeting molecule for TM-601 in human umbilical vein endothelial cell and multiple human tumor cell lines [21]. They demonstrated that TM-601 bound to the surface of Panc-1 cells depending on the level of annexin A2 expression. Annexin A2 plays an important role in angiogenesis by regulating plasminogen activation and binding to tissue plasminogen activator on vascular endothelial cells. Since TM-601 also binds MT1-MMP and annexin A2 is overexpressed intracellularly in all cells, the molecular mechanism of TM-601 specificity toward cancers remains unclear. Jacoby et al. found that TM-601 not only binds a wide range of tumor cell types but also can be internalized by proliferating human vascular endothelial cells [19]. The study demonstrated an anti-angiogenic effect of TM-601 using both the chicken chorioallantoic membrane assays and the mouse Matrigel plug assays. Wiranowska et al. discovered that TM-601 localizes near trans-Golgi in glioma, lung carcinoma, and normal vascular endothelial cells, while it is dispersed in the cytoplasm in astrocytes and normal human dermal fibroblasts (NHDF) [22]. The uptake of TM-601 by U373 glioma cells is rapid and dependent on time and concentration. Chlorpromazine, a kind of clathrin-dependent intracellular transport of coated pits, can induce intracellular accumulation of the drug and clathrin near the Golgi; however, suppressers like amiloride (non-selective macropinocytosis) and filipin (caveolae-dependent endocytosis) lack this function. On the contrary, amiloride remarkably affected TM-601 uptake in NHDF cells, suggesting that macropinocytosis is the decisive uptake route of TM-601 in these cells.
Recently, a novel CTX-like peptide, namely AaCtx, was purified from the venom of the scorpion Androctonus australis. It has a 70% similarity with CTX in terms of amino acid sequence. Invasion and migration of human glioma cells can be both inhibited by native and synthetic AaCtx; however, while the inhibition activity of AaCtx was lower than that of CTX [23]. Nevertheless, the advance of novel CTX homologous peptides may offer an approach to achieve multiple-point mutations in the structure of CTX, leading to valuable information regarding the molecular mechanism of CTX.
Putative mechanism of gliomas invasion and metastasis suppressed by CTX
CTX has been demonstrated its capability to selectively and specifically act on MMP-2, but not on MMP-9, -3, and -1, which are also overexpressed in glioma cells. CTX can decrease the cell membrane expression of MMP-2 by inhibiting the MMP-2 enzyme activity. Recent studies suggested that CTX prevents cell shrinkage, thereby reducing the invasion ability of glioma cells through compact extracellular space in normal brain [24]. By decreasing MMP-2 activity, CTX prevents proteolytic degradation of ECM and the subsequent release of glioma cells from the constraints of cellular interactions with ECM.
Recently, the expression of ClCs and MMP-2 was studied in two human glioma cell lines (STTG1 and U251MG). A ClC-3 inhibitor (CTX), a non-specific ClC inhibitor (5-nitro-2,3-phenylpropylamino benzoic acid (NPPB)), and ClC-3 siRNA knockdown were used to study the inhibition effects of ClC [25]. Glioma cell invasion was remarkably but not completely inhibited by ClC-3 and MMP-2 siRNA knockdown, and by CTX treatment. Addition of CTX to siRNA-treated glioma cells only slightly increased the suppression of invasion. In contrast, the non-specific ClC blocker NPPB completely blocked the cell invasion, indicating that ClCs are crucial in glioma cell migration and invasion. This study demonstrated that ClC-3 is the primary ClC associated with invasiveness; however, pharmacological blockade of ClC-3 alone is not sufficient to inhibit glioma cell invasion. Therefore, future therapy for gliomas may increase the likelihood of success by aiming at pharmacologic blockade targeting multiple ClCs, as well as diminishing Ca2+/calmodulin-dependent protein kinase II and ClC-3 activities.
Application of CTX bioconjugates for gliomas imaging and therapy
CTX exhibits several advantages as a ligand for diagnosis and treatment of tumors as compared with the widely used antibodies. Firstly, it is a small peptide with compact structure consisting of an α-helix and a simple three-stranded antiparallel β-sheet. Secondly, it can penetrate the blood-brain barrier (BBB) due to its compact structure [26]. Furthermore, CTX interacts with the MMP-2 receptor to inhibit glioma metastasis and invasion [5]. However, the size of CTX bioconjugates coupled with imaging moiety or therapeutic agent may be significantly different as compared to that of CTX. Therefore, the BBB penetration property of newly developed CTX bioconjugates needs to be carefully studied.
Up to date, the CTX bioconjugates have played increasingly critical roles in diagnostic imaging and treatment of gliomas [27]. CTX can be complexed with a variety of moieties, including radioactive iodine isotopes, fluorescent molecules, nanoparticles (NPs), chemotherapy drugs, germ plasm, liposomes, immunogenic molecules and nitric oxide. The bioconjugates containing more than two components of the above elements are listed in Tables 1 and 2.
Table 1.
Representative CTX bioconjugates in the applications of glioma diagnosis and treatment
| Type | Cell Line | Study | Reference |
|---|---|---|---|
| Imaging | |||
| Cy5.5-CTX | 9L | In vitro & In vivo | [30] |
| Cy5.5-CTX (Modified) | ND2:SmoA1 | In vivo | [31] |
| BLZ-100 | LN229 | In vivo | [32] |
| IRDye 800CW-CTX | ND2:SmoA1, HTB-186, U87MG, A549, 22Rv1 | In vivo | [33] |
| Gd-DTPA-CTX | HEK293, C6, Bel-7402 | In vitro & In vivo | [48] |
| Therapy | |||
| 131I/125I-CTX | D54MG | In vivo | [58] |
| CTX-Fcs | PANC-1 | In vitro | [56] |
| A172 | In vitro | [63] | |
| NO-CTX | T98G, U87MG, NHAs, HBMECs | In vitro | [66] |
| T98G, U87MG, NHAs, HBMECs | In vitro | [67] | |
| Imaging and Therapy | |||
| CTX-LS/DOX-LS/DiR-LS | C6, U87MG, U251MG | In vitro & In vivo | [40] |
| PAMAM-PEG-CTX/DNA | C6, HEK293 | In vitro & In vivo | [74] |
Notes: D54MG, U87MG, U251MG, T98G, and LN229 are human glioblastoma cell lines; NHAs, normal human astrocytes; HBMECs, human brain microvascular endothelial cells; 9L and C6 are rat glioma cell lines; PANC-1, human pancreatic carcinoma cell line; A172, human glioma cell line; HEK293T and HEK293 are human embryonic kidney cell lines; ND2:SmoA1 is a transgenic mouse model of glioma; HTB-186, medulloblastoma cell line; A549, lung carcinoma cell line; 22Rv1, prostate carcinoma cell line; Bel-7402, human hepatocellular carcinoma cell line.
Table 2.
Representative CTX-based nanoparticles in the applications of glioma diagnosis and treatment
| Type | Cell Line | Study | Reference |
|---|---|---|---|
| Imaging | |||
| PEG iron oxide-CTX | 9L | In vitro & In vivo | [36] |
| PEG iron oxide-CTX-Cy5.5 | ND2:SmoA1 | In vitro & In vivo | [37] |
| 9L | In vitro | [39] | |
| PEI-NaYF(4): Yb, Er/Ce-CTX | C6 | In vitro & In vivo | [47] |
| Therapy | |||
| Iron oxide-PEG-CTX | C6 | In vitro | [25] |
| Ag/Ali @PNPs-CTX | U87MG | In vitro & In vivo | [56] |
| PBdot-CTX | ND2:SmoA1 | In vivo | [44] |
| Gene Delivery and Therapy | |||
| PEI-PEG-AF-CTX/DNA | C6, DAOY, NIH3T3 | In vitro | [38] |
| SNALPs-CTX/asOs, SNALPs-CTX/siRNA | U87MG, GL261, HEK293T | In vitro & In vivo | [41] |
| Iron oxide-PEG-PEI-CTX/DNA | C6 | In vivo | [75] |
| Iron oxide-PEG-PEI-CTX/siRNA | C6 | In vitro | [77] |
| Iron oxide-PEIb-CTX/siRNA | C6 | In vitro | [78] |
| Iron oxide-PEG-MTX-CTX | rCM, 9L, D283 | In vitro & In vivo | [82] |
| Iron oxide-PEG-CTX, iron oxide-PEG-RGD | U87MG, MCF-7, 9L | In vitro & In vivo | [46] |
Notes: DAOY, medulloblastoma tumor cell line; NIH3T3, mouse fibroblast cell line; rCM, rat cardiomyocytes; GL261, mouse glioma cell line; D283, human medulloblastoma cell line; MCF-7, human breast adenocarcinoma cell line.
Diagnostic imaging with CTX bioconjugates
Radioiodinated CTX
CTX has been radiolabeled with 125I or 131I to afford the complexes, such as 125I-CTX or 131I-CTX [27]. The characteristic radiation released from these decayed radionuclides have been subsequently detected and recorded by using SPECT and a gamma camera to accurately qualify, quantify, and localize the brain tumor tissues and cells. At 24 h after the injection of 131l-CTX, brain tumor-to-muscle ratio of accumulated radioactivity can reach to 39.13 ± 4.6 [28]. Up to date, 131I-TM-601 has been the most extensively studied complex of CTX in humans. 131I-TM-601 was shown to be safe to administer in patients with gliomas, and it can be used as a SPECT imaging agent for evaluating primary tumor extent in phase I/II clinical trials [29]. Further modification of 131I-TM-601 with isotopes that have better imaging capabilities may provide an important imaging tool for determining the extent of glioma growth.
Fluorescent dye conjugated CTX
In order to provide real-time biological information to distinctly excise small foci of cancer cells and tumor margins, Veiseh et al. conjugated CTX to a fluorescent dye (Cy5.5) to produce the complex of Cy5.5-CTX [30]. The specific binding of Cy5.5-CTX with tumor cells can be detected with light microscopy. A novel mono-labeled peptide containing a single near infrared fluorescent (NIRF) molecule was reported by Akcan et al. [31]. Substitution of Lys15 and Lys23 with either alanine or arginine retained the functional efficacy of CTX, while Lys27 can be specifically labeled with Cy5.5 (Figure 1). In another example, tumor paint BLZ-100, an indocyanine green (ICG)-CTX conjugate, was developed for an optimized NIR imaging system. By using a normal charge-coupled device (CCD) camera, Butte et al. reported a small, sensitive, and inexpensive NIR imaging system [32]. Using this NIR imaging system, it is possible to visualize BLZ-100 down to 50 nM concentration at fluence of 20 mW/cm2. Although no quantitative data was determined, the uptake of BLZ-100 in mouse brains implanted with human glioma was clearly visualized, whereas the uptake of BLZ-100 in normal brain tissue can barely be observed.
Figure 1.
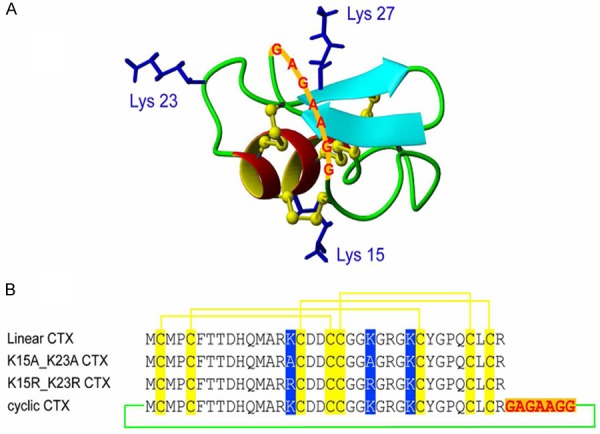
3D structure of chlorotoxin and sequences of the synthesized peptides: A: The structure of linear chlorotoxin has six backbone loops and the disulfide bonds are shown in yellow ball and stick format. B: The amino acid sequences of linear, Ala and Arg substituted and cyclic chlorotoxin. The disulfide connectivities between cysteine residues are shown by solid yellow lines, substituted residues are highlighted in blue boxes and the linker residues are shown in red. Reprinted with the permission of the Journal of Medicinal Chemistry, Akcan et al., 2011.
IRDye 800CW is a dye designed to limit interfering autofluorescence and exploit the enhanced permeability. It can ensure optimum signal view at deeper tissue with high signal-to-background ratios. In addition, operational imaging instruments, such as the Leica FL800 and Zeiss Pentero microscope, are compatible with IRDye 800CW. IRDye 800CW-CTX has been developed as a targeted imaging agent for brain tumors in ND2:SmoA1 model [33]. The targeting specificity of IRDye 800CW-CTX was evaluated using A549 (lung carcinoma), U87MG (glioblastoma), HTB-186 (medulloblastoma), and 22Rv1 (prostate carcinoma) cell lines. The results showed that IRDye 800CW-CTX binds to tumor cells specifically. Interestingly, blocking IRDye 800CW-CTX binding at room temperature was unsuccessful; however, blocking was observed at 4°C, a temperature at which internalization is slower. The fluorescence signal was reduced approximately 75% when A549 cells were pretreated with the MMP-2 inhibitor 1,10-phenanthroline at a dose (200 µM) that successfully inhibits glioma cell invasion. Individual tumor-bearing animals were injected with IRDye 800CW-CTX. Evan’s Blue perfusion was used to measure the integrity of the BBB. The extravasation of Evan’s Blue located only in fields of tumors but not anywhere else in the brain sections or whole brain, suggesting that the BBB permeability can be altered by the presence of the tumors.
CTX-conjugated nanoparticles
Novel tools for the diagnosis of gliomas have been developed based on CTX-containing nanoparticles [34,35]. For example, CTX-based superparamagetic iron oxide nanoparticles (NPs) have been reported [36]. The nanoparticles could bind to glioma cells specifically, and be used as a contrast agent for MRI to enhance the differentiation of the tumor from normal brain tissue. Later on, a dual modality optical/MR imaging nanoprobe was developed from the same group. Iron oxide NPs were used as the probe core, which was coated with biocompatible PEG-chitosan in the form of nanoparticle copolymer covalently conjugated with CTX and Cy5.5 [37]. The probe could label the tumor in genetically engineered mice, and be dually detected by optical imaging and MRI. The NPs were proved to be nontoxic, permeable through the BBB, and the tumor uptake of fluorescent NPs can be clearly visualized even at 120 h postinjection for optical imaging. Recently, the group reported a nonviral nanovector system P-PEG-AF-CTX comprising a fluorescent dye Alexa Fluor 647 (AF), polyethylenimine (PEI) polymer, PEG, and CTX [38]. The results demonstrated that the nanovector could be effectively loaded with genes for the specific labeling and genetic transfection of tumor cells. To date, CTX-functionalized iron oxide NPs have been extensively applied for imaging brain tumors by optical imaging and MRI [36,37,39]. The nanosystems are typically consisted of PEG, chitosan co-polymers, fluorescent dyes, targeting molecules, and an iron oxide core.
The CTX-modified liposomes have been recently developed for glioma imaging and therapy [40]. The optical imaging results showed that CTX-modified DiR-loaded liposomes can accumulate in the subcutaneous and intracranial glioma tumors (Figures 2 and 3). The U87MG cells bearing armpit tumor model revealed that CTX-modified doxorubicin (DOX)-loaded liposomes can inhibit tumor growth effectively. Overall, the results demonstrated that the CTX-modified liposome was a promising delivery system which could enhance the intracellular uptake of imaging agents or anticancer drugs. In another example, Costa et al. covalently combined CTX with liposomes which encapsulated small interfering RNAs (siRNAs) or antisense oligo-nucleotides (asOs) [41]. The data demonstrated that CTX coupled stable nucleic acid lipid particles (SNALPs) can be successfully applied for in vivo studies, and CTX-SNALPs exhibited excellent physicochemical properties, including electrical neutrality, low size, high protection against enzymatic degradation, and high encapsulation efficiency. Cellular association and internalization studies demonstrated that the CTX-SNALPs could stimulate particle internalization in glioma cells as compared to normal cells.
Figure 2.
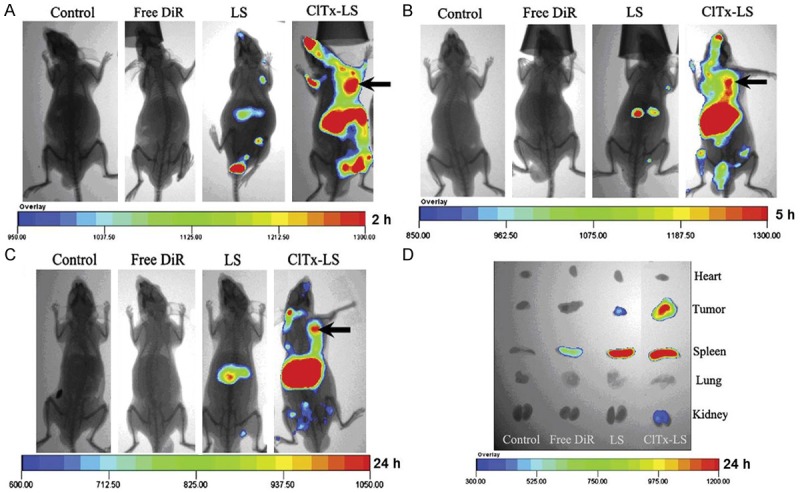
In vivo fluorescent images of U87MG tumor bearing mice of armpit model given physiological saline, free DiR, DiR-loaded LS, and DiR-loaded CTX-LS via tail vein, 2 h (A), 5 h (B) and 24 h (C) after administration, respectively, and fluorescent image of major organs and tumors ex vivo (D) 24 h post-injection. Reprinted with the permission of the Journal of Controlled Release, Xiang et al., 2011.
Figure 3.
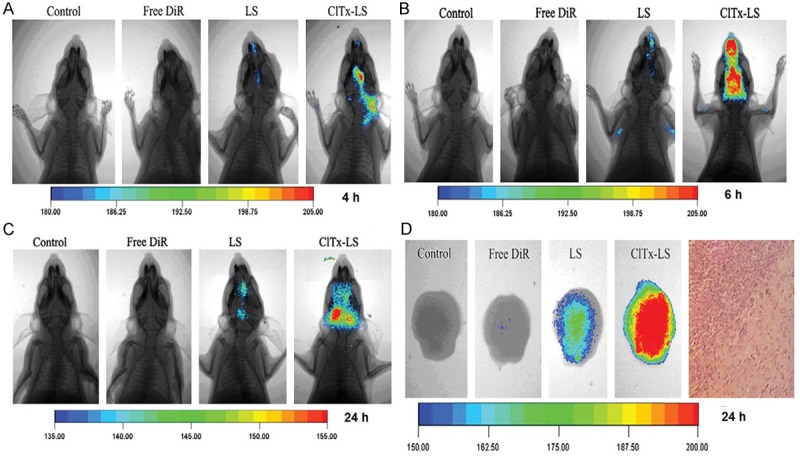
In vivo fluorescent images of U87MG tumor bearing mice of orthotopic model given physiological saline, free DiR, DiR-loaded LS, and DiR-loaded ClTx-LS via tail vein, 4 h (A), 6 h (B) and 24 h (C) after administration, respectively, and ex vivo fluorescent image of brains (D) after 24 h. Rightmost figure: H&E stained excised brains of the mice after in vivo imaging, exhibiting the infiltrative growth pattern of glioma cells. Reprinted with the permission of the Journal of Controlled Release, Xiang et al., 2011.
A new class of fluorescent probes, named semiconducting polymer dots (Pdots), has been applied in fluorescent-based tumor imaging [42,43]. As compared to quantum dots (Qdots), Pdot probes are small and extremely bright, making them attractive for serving as targeted imaging agents. Differing from Qdots, Pdots are made from highly biocompatible and non-toxic materials, rendering them an appealing candidate as a fluorescent imaging probe for clinical applications. Recently, polymer-blend dots (PBdots) were developed by using an efficient deep-red emitting polymer as the acceptor and a visible-light harvesting polymer as the donor. Wu et al. demonstrated the PBdot-CTX conjugate was able to permeate the BBB and specifically target tumor tissue in a transgenic mouse model (ND2:SmoA1) [44]. The probe with 15 nm in average size was resistant to photo-bleaching, 15 times brighter than Qdots, and stable in serum for over 72 h.
Integrin αvβ3 and MMP-2 play significant roles in neural tumor cell invasion and angiogenesis [45]. Fang et al. used biocompatible polymer-coated iron oxide to conjugate CTX or arginine-glycine-aspartic acid (RGD) on the surface to develop and assess two tumor-specific nanoprobes that target MMP-2 and αvβ3 integrin [46]. In this study, both nanoprobes presented long-term stability and excellent dispersion in cell culture media. NP-CTX diffused throughout the tumor, while NP-RGD showed high accumulation near the blood vessels. The results demonstrated that both NP-CTX and NP-RGD were target-specific to integrin MMP-2 and αvβ3. Compared to receptor-negative cell lines, both NP-CTX and NP-RGD exhibited enhanced cellular uptakes in receptor-positive cell lines. In vivo MRI results showed that nanoprobes provided contrast enhancement in the U87MG xenograft mouse model, and both NP-CTX and NP-RGD preferentially accumulated in U87MG tumors. In terms of R2, a contrast enhancement was observed for NP-RGD (11.939 ± 2.746 s-1) and NP-CTX (5.181 ± 1.567 s-1) in the tumors at 4 h post-injection, which is significant higher than that for NP-SIA (as control) (0.617 ± 1.447 s-1).
Rare-earth metals, exhibiting unique features for optical imaging, have recently been applied to tumor imaging. These nanoprobes are soluble in aqueous solutions, fluorescent and stable over a long period of time [34]. After functionalized with CTX, small polyethylenimine nanoprobes coated with hexagonal-phase NaYF4:Yb, Er/Ce were used to image C6 glioma xenografted tumors in vivo [47]. There was no perceptible indication of toxicity observed for the CTX:NPs. Significant upconversion fluorescence was observed in the xenograft tumors of the CTX:NPs-injected Balb-c nude mice, whereas no obvious fluorescence signal was observed in the tumors of the NPs-injected Balb-c nude mice, proving the specificity of the CTX:NPs for targeting xenograft gliomas.
Contrast agents are great helpful for clinical diagnosis of tumors using MRI, particularly for brain tumors at an early stage. However, the contrast agents in low molecular weight which are commonly used at present, such as gadoliniumion-diethylenetriaminepentaaceticn acid (Gd-DTPA), have several disadvantages, such as non-specificity, low contrast efficiency, and rapid renal clearance. A recent study showed that a macromolecular MRI contrast agent based on dendrigraft poly-L-lysines can be successfully synthesized by using CTX-modified conjugate as the main scaffold and Gd-DTPA as the payload [48]. Fluorescence microscopy results revealed that the modification of CTX could significantly boost the cellular ingestion in liver tumor cell and C6 glioma cell lines, but not in normal cell line. The MRI signal of mice treated with CTX-modified contrast was enhanced, which was remarkably higher than that of commercial control and unmodified conjugate. The signal improvement of CTX-modified contrast agent retained much longer in circulation than that of controls, which could be helpful for more accurate diagnosis of tumors. Taken together, CTX-modified dendrimer-based conjugate might be effective as a MRI contrast agent for accurate diagnosis of gliomas.
Targeted therapy with CTX bioconjugates
The success of targeted cancer therapy largely depends upon receptor-mediated ligand binding selectively to tumor cells. This method needs high selectivity or specificity of ligand targeting tumor cells or other tumor related cells, such as blood vessels. The key of success is that receptors over-express uniquely by tumor cells, but minimally by normal brain tissues. A lot of targeting molecules have been assessed containing epidermal growth factor receptor antibodies [49], CTX, transferring [50], F3 homingpeptide [51], insulin receptor [52], cationic albumin [53], and methotrexate [54]. The basic strategy is to identify a cellular toxin, modify the toxin to maximize antitumoral activity, and deliver the toxin directly to the tumor with a tumor-specific ligand acting as a carrier molecule. Among these identified targeting ligands so far, CTX has been recognized as an appropriate agent for its capability to specifically target a great number of cancers like brain tumors, breast and pancreatic cancers [55-57].
Radiolabeled CTX
125I- or 131I-labeled CTX was the first complex used in vivo targeting and biodistribution experiments, and the radiolabeled-CTX has been used in post-operative therapy [28]. Shen et al. evaluated radiation doses of 131I-CTX in human glioma xenografted model using athymic nude mices, and suggested that projected radiation doses in patients receiving 370 MBq of 131I-CTX [58]. In addition, Shirmardi et al. showed that 131I-CTX was stable in PBS solution and human serum [59]. Compared with the concentration of radioactivity in the liver, kidneys, stomach, and intestine, the blood clearance of 131I-CTX was moderate.
A phase I study of 131I-TM-601 has been finished to assess the safety, biodistribution, dosimetry and tolerability, in adult patients who suffer from recurrent high-grade glioma [60]. During the follow-up period, there were no grade III or IV toxicities associated with the therapeutic agent or the method of administration. A phase II study was then carried out to assess the safety and efficacy of multiple doses of radiolabeled TM-601. Improved survival and unnoticeable toxicity were found for patients receiving 6 versus 3 doses of 131I-TM-601 [61].
CTX-fused immunogenetic molecules
IgG antibodies play an important role in humoral immunity. They have been considered as proinflammatory mediators for a long time. The functions and specificities of antibodies are determined by the Fc (crystallizable) domain. Studies from Anthony et al. demonstrated that the sialylated IgG Fcs can affect in vivo activity of intravenous immunoglobulin [62]. Kasai et al. developed two forms of bioconjugate, which were human IgG-Fcs with/without a hinge region fused to CTX [63]. CTX combined to IgG-Fcs was operated as a monomer of 30 kDa without a hinge region (M-CTX-Fc) and a dimer of 60 kDa with a hinge region (D-CTX-Fc). The monomeric and dimeric CTXs inhibited human glioma A172 cell growth. Interestingly, the dimer showed a less inhibitory activity than the monomer, indicating M-CTX-Fc may be more suitable than D-CTX-Fc as a drug delivery system targeting to MMP-2. Recently, the same group has identified the inhibitory mechanism of M-CTX-Fc on MMP-2 in PANC-1 cells, the human cell line derived from pancreatic carcinoma [56]. The results suggested that the M-CTX-Fc fusion protein might be a promising agent for MMP-2 targeted treatment.
CTX-NO therapy
Nitric oxide (NO) is a small molecule which has been discovered to play multiple roles within the human body, including the maintenance of the tuned balance between tumor suppression and progression [64]. Due to the short half-life of NO, nitric oxide donors are utilized to release NO in order to prolong periods for therapeutic purposes. The study demonstrated that non-specific NO donors induced chemosensitivity in glioma cells by releasing NO [65]; however, the therapeutic efficacy requires high doses of NO to be delivered into tissues surrounding the tumor site. For instance, Safdar et al. reported that CTX can react with NO gas to provide a NO-releasing compound (CTX-NO), while retaining its capability to preferentially target glioma cells [66]. CTX-NO led to tumor cell death in a dose-dependent way, while normal cell viability was only affected at high NO concentrations. Later on, the same group studied a targeted NO donor as a pre-therapy to enhance the sensitivity of chemotherapy in glioma cells [67]. Their results demonstrated that CTX-NO can improve the therapeutic potential used in conjunction with carmustine (BCNU) or temozolomide (TMZ). CTX-NO was capable of reducing p53 expression in U87MG and T98G cells alone, or combined with chemotherapy. The NO released by CTX-NO led to unnoticeable alteration in the chemo-sensitivity of normal control cells. The pretreatment with CTX-NO remarkably decreased the expression of O6-methylguanine-DNA methyltransferase (MGMT) in glioma cells. MGMT is a 22 kDa protein repairing alkylation at the O6 position of guanine on DNA strands [68,69]. Compared with the study of NO alone, the combination of NO and TMZ had minimal effect on p53 expressions in T98G glioma cells. However, the combination of BCNU and NO induced noticeable decrease in p53 expressing levels. The data suggested that the activity of p53 may play a crucial role in determining cellular response and maintaining the integrity of the genome, either inducing apoptosis after being exposed to harmful stimuli such as chemotherapy and radiation, or activating DNA repair mechanisms [70]. Remarkable inhibition in cell invasion could be observed under the condition where chemotherapy was coupled with CTX-NO. The cell invasion was reduced by 54.3 ± 6.1% and 70.5 ± 8.6% using NO in conjunction with BCNU and the combination of NO and BCNU, respectively.
CTX combined with gene therapy
Gene therapy could enhance the dismal prognosis of patients suffering from glioma [71]. However, the degradation of nucleic acids by nucleases and the presence of the BBB restricting entry of therapeutic molecules into the brain, are main challenges for delivering nucleic acids in vivo [72,73]. Therefore, it is critical that oligonucleotides are appropriately transported by vehicles which are effective and dependable in conquering physiological and cellular obstacles, and exceedingly target-specific. Gene therapy of gliomas could be improved by using the approach of CTX-conjugated NPs.
Huang et al. designed a glioma-targeted gene delivery system [74]. A major vector was constructed by polyamidoamine (PAMAM), a nanoscopic high-branching dendrimer. Through biofunctional PEG, PAMAM was conjugated to CTX to produce PAMAM-PEG-CTX. The modification of CTX could prominently promote the cellular uptake of the DNA-loaded NPs and vectors in C6 cells. PAMAM-PEG-CTX/DNA NPs were more widely distributed in the brain than PAMAM-PEG/DNA NPs and PAMAM/DNA NPs in vivo. In addition, the gene expression of PAMAM-PEG-CTX/DNA NPs in glioma was broader and higher than that of PEG-modified and unmodified counterparts. The median survival time of CTX-modified group was remarkably longer than that in other groups. The results showed that PAMAM-PEG-CTX/DNA NPs can be used as a promising non-viral gene delivery system for gene therapy of glioma.
Kievit et al. attached CTX to an iron oxide nanoparticle core using a short PEG linker [75]. Green fluorescent protein (GFP) encoding DNA was then bound to the nanoparticle. A copolymer of chitosan and PEI were coated to the nanoparticles to yield NP-PEG-PEI-GFP-CTX. A control nanoparticle, NP-PEG-PEI-GFP, was also prepared. The C6 tumor bearing mice were intravenously injected with the DNA bound nanoparticles. The use of CTX targeted nanoparticles loaded with DNA specifically enhanced glioma cell uptake. CTX was found to enhance nanovector uptake by the tumor cells as proved by the increase of GFP expression, while the targeting ligand did not affect the accumulation and biodistribution of nanovector in the tumor site. The results showed that specific uptake of nanovectors into glioma cells could be improved by exposing a higher percentage of target cells to the delivered payload.
Although PEI nanoparticles have been recognized as effective gene delivery systems, the non-selective delivery and inherent toxicity of the material are the major problems for clinical translation. Veiseh et al. presented a non-viral nanovector P-PEG-AF-CTX comprising a PEI polymer, PEG, fluorescent dye AF, and CTX, which could be used to bind a series of tumor cells specifically for genetic treatment [38]. Since the nanovector can be specifically delivered to tumor cells, the toxicity of nanovector to healthy cells is minimized. The nanovector exhibited high levels of gene transfection efficiency and targeting specificity in both DAOY medulloblastoma and C6 glioma cells (Figure 4). Importantly, the nanovector with the CTX may serve as a broadly applicable gene carrier for a variety of cancer types.
Figure 4.
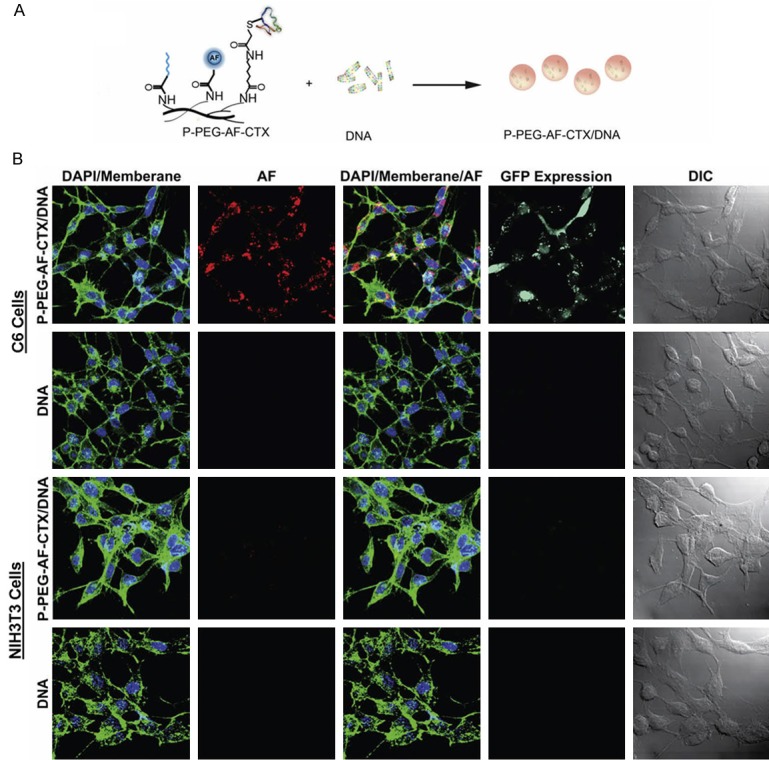
A: The polymeric construct complexed with DNA to generate the targeting nanovector (P-PEG-AF-CTX/DNA). B: Confocal fluorescence and differential interference contrast (DIC) images of C6 and NIH3T3 cells treated with 10 µg DNA mL-1 without a delivery vector (DNA) or with vectors complexed with PEGylated and CTX-enabled PEI (P-PEG-AF-CTX/DNA). Cellular membranes are shown in green, nuclei in blue, polymeric vectors in red, and GFP expression in turquoise. Scare bars correspond to 40 µm. Reprinted with the permission of the Journal of Biomaterials, Veiseh et al., 2009.
Ribonucleic acid interference (RNAi) is a rapidly developing technology that has been applied in gene therapy of cancer. The absence of site-targeting delivers that can effectively carry short interfering RNA (siRNA) to tumor cells is one of major challenges for translating this technology into the clinic [76]. In 2010, NP-siRNA-CTX, the first siRNA nanovector, was designed for glioma-targeted imaging and therapy [77]. The nanovector was constructed with PEI, PEG-grafted chitosan, and a superparamagnetic iron oxide nanoparticle core. The construct was also coated with CTX and siRNA. The data suggested that this CTX functionalized nanovector can deliver RNAi therapeutics to tumor cells. Later on, the same group developed a multifunctional nanosystem (NP-PEIb-siRNA-CTX) constructed with CTX, siRNA, and highly amine blocked PEI (PEIb). NP-PEIb-siRNA-CTX presented both gene silencing effects and significant cytotoxic at acidic conditions in C6 cells, but not at physiological conditions [78]. The CTX component of NP-PEIb-siRNA-CTX improved the nanovector uptake by cancer cells. The size (63 nm) of NP-PEIb-siRNA-CTX is suitable for this nanovector to be accumulated in tumor tissues through the enhanced permeability and retention effect. NP-PEIb-siRNA-CTX can not only maintain enough magnetism for MR imaging, but also offer an imaging tool to monitor the delivery of therapeutic payload in a real time [79].
CTX-functionalized nanoparticles
Nanoparticles have been emerged as contrast agents, drug delivery vehicles, and multifunctional devices for patient care. Development of multifunctional nanoparticles for targeting cancer cells has become a focus of research in the past few years [80]. Tumor-specific delivery, stability and biocompatibility are technological difficulties in the progress of developing ideal nanoparticle-based therapeutic agents [81]. At present, the main approaches are focused on delivering chemotherapeutic agents to induce apoptosis or DNA/siRNA by changing oncogene expression.
Veiseh et al. reported a nanoparticle system consisted of an iron oxide nanoparticle core, CTX and an amine-functionalized PEG silane [26]. They showed that the nanoparticle exhibited significantly increased cellular uptake and an invasion inhibition rate of 98% compared with unbound CTX (45%). Their studies demonstrated that the nanoparticle functionalized by CTX could reduce the activity of MMP-2 and increase internalization of lipid rafts that contain volume-regulating ion channels and membrane-expressed MMP-2 via receptor-mediated endocytosis, leading to the enhancement of invasion inhibition (Figure 5). Since upregulation of MMP-2 activity has also been observed in cancers of the skin, colon, breast, prostate, and lung, this nanovector system can be potentially applied for therapy of a broad spectrum of tumors.
Figure 5.
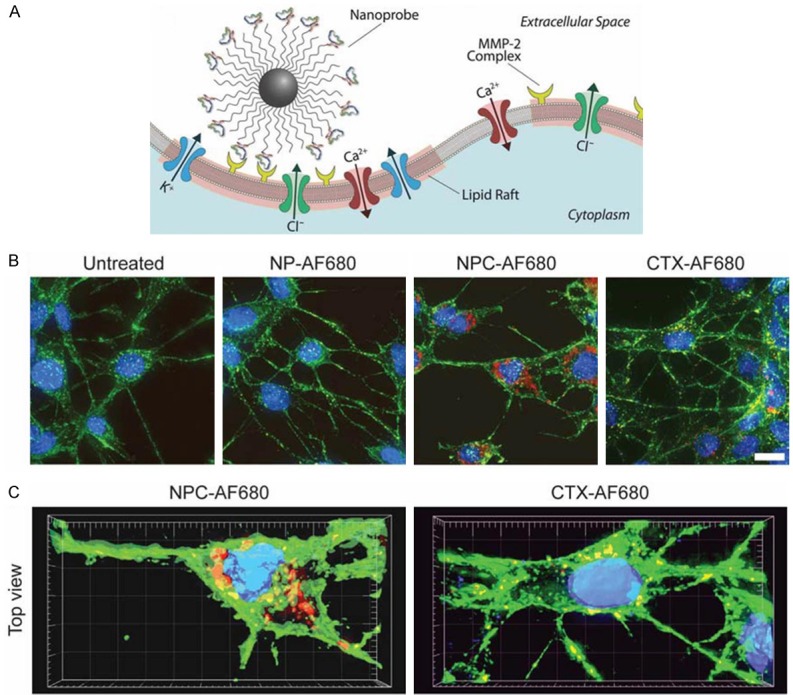
(A) NPC binding to lipid rafts of glioma cells containing MMP-2 and select ion channels. C6 cells incubated with AF680 fluorescently labeled NPC, NP or CTX and analyzed by (B) Z-stacked 2-D projections and (C) 3-D reconstructions (DAPI nuclear stain in blue, WGA 594 membrane stain in green, and AF680 in red). (scale bar: 10 μm). Reprinted with the permission of the Journal of Small, Veiseh et al., 2009.
A multifunctional nanoparticle system which comprises CTX, an iron oxide nanoparticl core, and methotrexate (MTX, a conventional chemotherapeutic agent) was presented by Sun et al [82]. The NP-MTX-CTX system can serve as a diagnostic and therapeutic tumor targeted nanovector. In this nanosystem, the functional ligands such as CTX and MTX were covalently attached to the iron oxide core through a PEG layer which is a biocompatible linking and coating molecule. The result indicated that the nanoparticle was able to carry MTX specifically to tumor cells, including glioma and medulloblastoma. Compared with NP-MTX, NP-MTX-CTX was more effective in inducing cytotoxicity in tumor cells, likely due to the increased uptake. At 24 h, the cell viability for NP-MTX-CTX reached a minimum at 25.6% and maintained this level for additional 24 h, while cells treated with NP-MTX began to recover with increasing proliferation reaching normal un-treated levels by 72 h. NP-MTX-CTX was also able to retain in the tumor tissue more than 14 days, and deliver combined chemotherapeutic molecules specifically to tumor cells.
Multifunctional nanovectors formed by polymeric nanoparticles (PNPs) were synthesized, which contain two cytotoxic elements - the silver nanoparticles and drug alisertib. PNPs are optimal nanocarriers for targeted drug delivery due to their small size and ability to entrap efficaciously drug molecules. The poly(lacticco-glycolic acid) (PLGA)-block-PEG-carboxylic acid (PLGA-b-PEG) copolymer is becoming one of the most promising system for drug loading and in vivo drug delivery applications. Locatelli et al. reported an PNPs, containing the drug alisertib (Ali), a selective aurora A kinase inhibitor, lipophilic silver (Ag)-loaded PNPs derived from the PLGA-b-PEG-COOH block copolymer and CTX, named Ag/Ali@PNPs-CTX [57]. The synergistic and individual property of these two cytotoxic molecules against GBM was evaluated both in vivo and in vitro. The result suggested that tumor reduction was achievable while using Ag/Ali @PNPs-CTX.
Application of BmK CT bioconjugates for gliomas imaging and therapy
CTX-like peptides derived from the venom of various scorpion species generally shares sequence homology with CTX. Buthus martensii Karsch chlorotoxin (BmK CT) which was purified from the venom of the Chinese scorpion, is the most important CTX-like peptide. The representative BmK CT bioconjugates are summarized in Table 3.
Table 3.
Representative BmK CT bioconjugates in the applications of glioma diagnosis and treatment
| Type | Cell Line | Study | Reference |
|---|---|---|---|
| Therapy | |||
| BmK CT | U251, BEL7404, CHOC400 | In vitro & In vivo | [89] |
| BmK CT | SHG-44 | In vitro & In vivo | [91] |
| 131I-BmK CT | C6 | In vitro | [92] |
| BmK CT | C6 | In vitro | [95] |
| Adenovirus-BmK CT | C6 | In vitro & In vivo | [96] |
| pEGFP-N1-BmK CT | C6 | In vitro | [97] |
| Imaging and Therapy | |||
| 131I-BmK CT, Cy5.5-BmK CT | C6 | In vivo | [90] |
| FND-BmK CT | C6 | In vitro | [98] |
Notes: BEL7404, hepatocellular carcinoma cell line; CHOC400, Chinese hamster ovary cell line; SHG-44, human glioma cell line.
Source and chemical structure of BmK CT
The Buthus martensi Karsch (BmK) is a kind of East-Asian scorpion extensively distributed in northwestern China, Korea, and Mongolia. A full-length cDNA sequence encoding BmK CT was purified from a cDNA library of the Chinese BmK venom glands. The encoding peptide of BmK CT has 59 amino acid residues in length which included a mature toxin of 35 residues with four disulfide bridges and a signal peptide of 24 residues. There are 68% similarities between BmK CT and CTX in sequence [83,84].
Purification of BmK CT
Through modifying BmK CT gene sequence based on the codon usage in Escherichia coli (E. Coli) and subcloning it into an expression vector pExSecI, recombinant BmK CT was successfully purified and expressed in E. Coli [85]. Plenty expression of a soluble and functional modality of BmK CT was thus obtained, which could be applied for the pharmaceutical function of this neurotoxin.
Putative mechanisms of BmK CT
The polypeptidy toxins purified from BmK venom can specifically interfere with a number of ion channels and change their functional features [86]. A study was conducted to determine the potential receptors of this CTX-like peptide in human glioma cell by using polycolonal antibodies to the purified protein raised in rats. Pull-down assay and overlay assay revealed that this toxin specifically binds to two proteins in the glioma cells with molecular weights of about 35 and 80 kDa. These proteins may be considered as candidate receptors or alternative cellular molecules interacting with BmK CT [87].
Electrostatic effects play an important role in the interaction between the small basic peptides of the scorpion venoms and the ion channels. The inhibition of BmK CT on MMP-2 activity has been studied using computational methods. Fu et al. proposed a model to elaborate the structural mechanism of this CTX-like peptide on glioma invation (Figure 6) [88]. In this study, the genes encoding BmK CTR14K15AA, BmK CTR17A, BmK CTK25A, and BmK CTR35A were amplified by PCR. The results from gelatin zymography assay showed that BmK CT and mutant forms could decrease glioma cells metastasis rate via MMP-2. The inhibitory effect of BmK CT, BmK CTK25A and BmK CTR35A was stronger than that of BmK CTR14K15AA and BmK CTR17A. The electrostatic surfaces of both catalytic domain of MMP-2 and BmK CT (mutants and wild type) were counted to determine the structure-function. The results demonstrated that the catalytic domains in BmK CTR17A-MMP-2 and BmK CTR14K15AA-MMP-2 complexes were less stable than those of BmK CTR35A-MMP-2, BmK CTK25A-MMP-2 and BmK CT-MMP-2 complexes. The molecular dynamics simulation showed that R14,17 and K15 residues may be three active residues of BmK CT interacting with the catalytic domain of MMP-2, which was proved by the in vitro experimental measurements [88].
Figure 6.
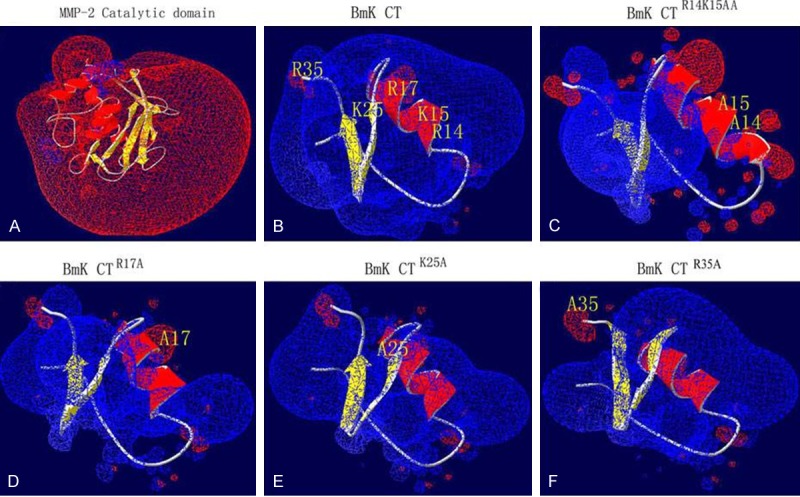
Three dimensional structures and electrostatic surfaces of MMP-2 catalytic domain (A), BmK CT (B) and mutants (C-F) Electrostatic surfaces are calculated by the program DeepView V.3.7. Different color codes are -1.8 kT/e (Red) and 1.8 kT/e (Blue) where k is the Blotzmann constant, T is the temperature in Kelvin, and e is the charge of the electron. Positively charged residues are shown in blue and negatively charged in red. The basic amino acid residues of BmK CT and site-directed mutagenesis are shown in yellow. Reprinted with the permission of the Journal of Biotechnology Letters, Fu et al., 2011.
Potential of BmK CT in glioma imaging and therapy
Wang et al. reported that BmK scorpion venom can induce U251-MG cell death at a dose of 10 mg/ml, but no effect on human hepatocellular carcinoma cells and Chinese hamster ovary cells was observed [89]. In a mouse U251-MG xenograft model, BmK venom showed remarkable inhibition of the tumor proliferation. Recent studies also revealed that both CTX and BmK CT could inhibit glioma cell proliferation, and Cy5.5 conjugated BmK CT is target-specific for gliomas in a rat xenograft model [90].
BmK CT has been characterized by in vivo and in vitro studies [91]. The cell proliferation assay revealed that BmK CT inhibits the glioma cell growth (SHG-44, human glioma cells) in a dose-dependent pattern with an IC50 value of 0.28 µM, while the IC50 value of BmK CT for normal astrocytes increased to 8 µM under the same conditions. The whole-cell patch-clamp recording showed that BmK CT could inhibit chloride current in SHG-44 in a voltage-dependent manner. The inhibition rates of BmK CT on ICl were determined to be 17.64 ± 3.06% and 55.86 ± 2.83% at the concentrations of 0.07 and 0.14 µM, respectively. Mice were also treated with rBmK CTa, a gene encoding chlorotoxin-like peptide from the scorpion, Buthus martensii Karsch. Histological analysis of rBmK CTa treated mice revealed that this toxin was distributed in brain, cardiac muscle, and leg muscle. These results suggested that rBmK CTa may have potential in the treatment of human gliomas.
In addition, BmK CT was labeled with 131I using the Bolton–Hunter method with the overall yield of 34.5% [92]. MTT assay demonstrated that both BmK CT and 131I-BmK CT could inhibit C6 growth. The ability of 131I-BmK CT to inhibit cell growth is superior to that of BmK CT. Whereas BmK CT could block the C6 glioma cell cycle in the G0/G1 stage, 131I-BmK CT is capable to block the cell cycle in the S stage at a radioactivity concentration of 50 µCi/mL. Hence, 131I-BmK CT may be useful as a glioma-targeted therapy agent better than BmK CT, while 131I-BmK CT may also be used for SPECT imaging.
BmK CT and lithium chloride (LiCl)
High-grade gliomas (HGGs) are rapidly progressive brain tumors with a low survival rate due to the devastating invasion and high recurrence rate. It is very difficult to completely remove the tumor tissue by surgical method [2]. Although specific pathophysiological mechanisms underlying resistance of HGGs to chemotherapy are still unclear, recent studies have revealed the importance of glycogen synthase kinase-3 (GSK-3) in inhibiting glioma cell progression and invasion. GSK-3 inhibition could result in glioma cell apoptosis by interfering with c-MYC activation and intracellular glucose metabolism [93]. Lithium as an inhibitor of GSK-3 has been applied in clinical therapy of bipolar disorder for several decades. A recent study showed that a high concentration of lithium is required to significantly inhibit the migration ability of most glioma cell lines [94]. However, lithium is often toxic at high concentrations, limiting its applicability as a therapeutic agent against gliomas.
Aiming to find out an approach to reduce the concentration of lithium at which the inhibitory effects remain significant, Fu et al. have studied whether the simultaneous administration of BmK CT and LiCl could be potentially beneficial for the treatment of HGGs [95]. In this study, the combination of BmK CT and LiCl could significantly inhibit the proliferation, invasion, and migration of C6 glioma cells, suggesting that BmK CT could reduce the “toxic” effects of lithium by preventing the metastatic spread of glioma cells to a certain extent. The results demonstrated that BmK CT could inhibit the lithium and the collagen type I-induced activation and overexpression of pro-MMP-2. Additionally, the combination treatment changed β-catenin localization patterns at the migration edge, disrupted cell-cell contacts, and caused the morphological alteration of C6 glioma cells.
BmK CT and gene therapy
Gene therapy has been served as a new strategy for the treatment of gliomas. Adenovirus-mediated delivery of the conditional cytotoxic gene was proved to be an adjuvant gene therapeutic method of gliomas. A recombinant adenoviral system was developed through a double-recombination product between a shuttle vector pShuttleIRES-hrGFP-2 carrying the BmK CT gene and a co-transformed adenoviral backbone plasmid vector, pAdEasy-1 [96]. This delivery system specifically targeted BmK CT to rat C6 glioma cells. The delivered BmK CT interacted with the MMP-2 and/or pro-MMP-2 in the glioma cells, which avoided immunologic rejection and degradation of BmK CT protein. The BmK CT mediated by adenovirus showed a high activity in preventing glioma cells from growing and invading, thereby proposing that this recombinant adenovirus is an effective approach for the treatment of glioblastoma. A type of green fluorescent protein encoded by pEGFP-N1 has been applied for brighter fluorescence and higher expression in mammalian cells. Recently, Fu et al. constructed a recombinant plasmid pEGFP-N1-BmK CT (Figure 7A) [97]. The results showed that pEGFP-N1 mediated BmK CT expression exhibited a high activity in inhibiting cell migration via MMP-2 (Figure 7B).
Figure 7.
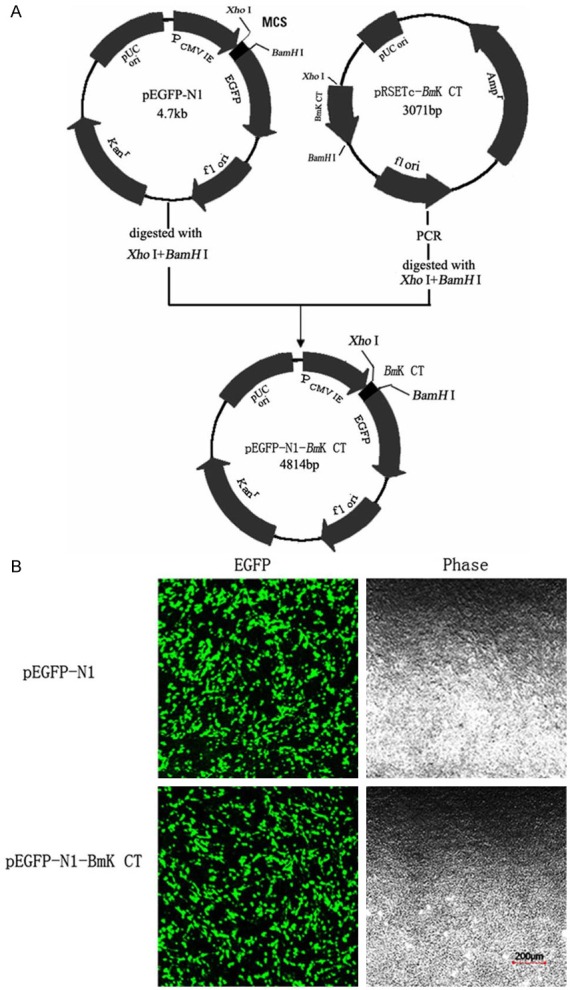
A: Construction of expression plasmid pEGFPN1-BmK CT. The gene BmK CT was fused with EGFP via theXhoI/BamH I linker. B: Photograph of the transfected cells sample obtained with a laser scanning confocal microscope, C6 cells were transfected with pEGFP-N1 and pEGFP-N1-BmKCT (scale bar: 200 µm). Reprinted with the permission of the Journal of Cytotechnology, Fu et al., 2013.
BmK CT-based nanoparticles
As optical imaging probes, fluorescent nanodiamonds (FND) have gained considerable attention. BmK CT-conjugated with FND was proposed as a new class of glioma-specific nanoparticles [98]. The confocal fluorescence assay confirmed receptor-mediated uptake of FND-BmK CT bioconjugates into rat C6 glioma cells by direct tumor visualization. The in vitro wound healing assay showed that FND-BmK CT had high inhibition rate during the migration of rat C6 glioma cells. Therefore, glioma-specific multifunctional nanoparticles (FND-BmK CT) might be useful for the development of more effective therapeutic agents for clinical treatment of gliomas.
Conclusions and perspectives
CTX and CTX-like peptide (BmK CT) represent novel and exciting platforms for glioma imaging and therapy due to the major advantages as follows: 1) small and condensed structure; 2) feasibility of artificial synthesis and the readily modified chemical structure with a tyrosine residue conjugating iodine or other molecules covalently; 3) rapid diffusion into tumor parenchymas and ability to penetrate the BBB; 4) slow elimination through the metabolism with a longer imaging time due to intracellular binding with glioma cells; 5) derivation from an invertebrate, being not rejected by human tissue, the absence of intimate toxicity without binding to normal tissue and cells; 6) antitumor activity in inhibiting tumor invasion and metastasis; and 7) antiangiogenic effects.
Currently, investigators have conjugated CTX and BmK CT with radioactive iodine isotopes, fluorescent molecules, gene (DNA and RNA) and nanoparticles, and subsequently localized these bioconjugants with the imaging tools, offering a unique and specific approach with theranostic potential for gliomas. Although the recent studies of CTX-based bioconjugates are promising, the development of an effective approach to treat gliomas remains a challenge due to high malignance of gliomas. Additionally, the in vivo toxicity of various bioconjugates should be monitored in a long term. With recent developments in nanotechnology, we can envision that CTX- or BmK CT-conjugated nanomedicine may have a great potential for diagnosis and treatment of gliomas.
Acknowledgements
This work was supported by the USC Department of Radiology, National Natural Science Foundation of China (No. 81171368, 812111366, and 81301245), and Japan Society for the Promotion of Science Fellowship (No. S-11109).
Disclosure of conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- CTX
chlorotoxin
- BmK CT
Buthus martensii Karsch Chlorotoxin
- GBM
glioblastoma multiforme
- MMP-2
matrix metalloproteinase-2
- ECM
extracellular matrix
- E. Coli
Escherichia coli.
- GCC
glioma-specific chloride channel
- ClC
chloride ion channels
- MT1-MMP
membrane type-1 MMP
- CLIC1
chloride intracellular channel 1
- CFTR
cystic fibrosis transmembrane conductance regulator
- GST
glutathione transferase
- AaCtx
Androctonus australis chlorotoxin
- BBB
blood-brain barrier
- NPs
nanoparticles
- NPPB
5-nitro-2-(3-phenylpropylamino)benzoic acid
- PEG
polyethylene glycol
- AF
Alexa Fluor 647
- PEI
polyethylenimine
- LS
liposomes
- DOX
doxorubicin
- NO
nitric oxide
- asOs
antisense oligonucleotides
- SNALPs
stable nucleic acid lipid particles
- PNPs
polymeric nanoparticles
- PLGA-b-PEG
poly(lacticco-glycolic acid) (PLGA)-block-PEG-carboxylic acid
- Ali
alisertib
- BCNU
carmustine
- MGMT
O6-Methylguanine-DNA Methyltransferase
- TMZ
temozolomide
- PAMAM
polyamidoamine
- GFP
green fluorescent protein
- siRNA
short interfering RNA
- RNAi
ribonucleic acid interference
- MTX
methotrexate
- Pdots
polymer dots
- Qdots
quantum dots
- PBdots
polymer-blend dots
- RGD
arginine-glycine-aspartic acid
- MRS
magnetic resonance spectroscopy
- MRI
magnetic resonance imaging
- SPECT
single photon emission computer tomography
- PET
positron emission computed tomography
- NIRF
near-infrared fluorescence
- Gd-DTPA
Gadoliniumion- diethylenetriaminepentaacetic acid
- ICG
indocyanine green
- CCD
charge-coupled device
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NHDF
normal human dermal fibroblasts
- HGGs
high-grade gliomas
- GSK-3
glycogen synthase kinase-3
- FND
fluorescent nanodiamonds
- rBmK CTa
a gene encoding chlorotoxin-like peptide from the scorpion, Buthus martensii Karsch
References
- 1.Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ Jr, Mehta MP. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hervey-Jumper SL, Berger MS. Role of surgical resection in low- and high-grade gliomas. Curr Treat Options Neurol. 2014;16:284. doi: 10.1007/s11940-014-0284-7. [DOI] [PubMed] [Google Scholar]
- 3.Alnaami IM, Al-Nuaimi SK, Senthilselvan A, Murtha AD, Walling S, Mehta V, Gourishankar S. Effectiveness of adjuvant temozolomide treatment in patients with glioblastoma. Neurosciences (Riyadh) 2013;18:349–355. [PubMed] [Google Scholar]
- 4.Mukherjee D, Manuel Sarmiento J, Nosova K, Boakye M, Lad SP, Black KL, Nuno M, Patil CG. Effectiveness of radiotherapy for elderly patients with anaplastic gliomas. J Clin Neurosci. 2014;21:773–778. doi: 10.1016/j.jocn.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Mamelak AN, Jacoby DB. Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601) Expert Opin Drug Deliv. 2007;4:175–186. doi: 10.1517/17425247.4.2.175. [DOI] [PubMed] [Google Scholar]
- 6.Stroud MR, Hansen SJ, Olson JM. In vivo bio-imaging using chlorotoxin-based conjugates. Curr Pharm Des. 2011;17:4362–4371. doi: 10.2174/138161211798999375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mamelak A. Targeted antitumor therapy with the scorpion venom chlorotoxin. Drugs Fut. 2011;36:615. [Google Scholar]
- 8.Murnyak B, Csonka T, Hegyi K, Mehes G, Klekner A, Hortobagyi T. Occurrence and molecular pathology of high grade gliomas. Ideggyogy Sz. 2013;66:312–321. [PubMed] [Google Scholar]
- 9.Murnyak B, Csonka T, Klekner A, Hortobagyi T. Occurrence and molecular pathology of low grade gliomas. Ideggyogy Sz. 2013;66:305–311. [PubMed] [Google Scholar]
- 10.Wei C, Wang X, Zheng M, Cheng H. Calcium gradients underlying cell migration. Curr Opin Cell Biol. 2012;24:254–261. doi: 10.1016/j.ceb.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Turner KL, Honasoge A, Robert SM, McFerrin MM, Sontheimer H. A proinvasive role for the Ca(2+)-activated K(+) channel KCa3.1 in malignant glioma. Glia. 2014;62:971–981. doi: 10.1002/glia.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jentsch TJ. Chloride and the endosomal-lysosomal pathway: emerging roles of CLC chloride transporters. J Physiol. 2007;578:633–640. doi: 10.1113/jphysiol.2006.124719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner KL, Sontheimer H. Cl- and K+ channels and their role in primary brain tumour biology. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130095. doi: 10.1098/rstb.2013.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, He S, Tu Y, Ji P, Zong J, Zhang J, Feng F, Zhao J, Zhang Y, Gao G. Elevated expression of chloride intracellular channel 1 is correlated with poor prognosis in human gliomas. J Exp Clin Cancer Res. 2012;31:44. doi: 10.1186/1756-9966-31-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Averaimo S, Milton RH, Duchen MR, Mazzanti M. Chloride intracellular channel 1 (CLIC1): Sensor and effector during oxidative stress. FEBS Lett. 2010;584:2076–2084. doi: 10.1016/j.febslet.2010.02.073. [DOI] [PubMed] [Google Scholar]
- 16.Setti M, Savalli N, Osti D, Richichi C, Angelini M, Brescia P, Fornasari L, Carro MS, Mazzanti M, Pelicci G. Functional role of CLIC1 ion channel in glioblastoma-derived stem/progenitor cells. J Natl Cancer Inst. 2013;105:1644–1655. doi: 10.1093/jnci/djt278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ullrich N, Sontheimer H. Biophysical and pharmacological characterization of chloride currents in human astrocytoma cells. Am J Physiol. 1996;270:C1511–1521. doi: 10.1152/ajpcell.1996.270.5.C1511. [DOI] [PubMed] [Google Scholar]
- 18.Maertens C, Wei L, Tytgat J, Droogmans G, Nilius B. Chlorotoxin does not inhibit volume-regulated, calcium-activated and cyclic AMP-activated chloride channels. Br J Pharmacol. 2000;129:791–801. doi: 10.1038/sj.bjp.0703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacoby DB, Dyskin E, Yalcin M, Kesavan K, Dahlberg W, Ratliff J, Johnson EW, Mousa SA. Potent pleiotropic anti-angiogenic effects of TM601, a synthetic chlorotoxin peptide. Anticancer Res. 2010;30:39–46. [PubMed] [Google Scholar]
- 20.Wang XM, Luo X, Guo ZY. Recombinant expression and downstream processing of the disulfide-rich tumor-targeting peptide chlorotoxin. Exp Ther Med. 2013;6:1049–1053. doi: 10.3892/etm.2013.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kesavan K, Ratliff J, Johnson EW, Dahlberg W, Asara JM, Misra P, Frangioni JV, Jacoby DB. Annexin A2 is a molecular target for TM601, a peptide with tumor-targeting and anti-angiogenic effects. J Biol Chem. 2010;285:4366–4374. doi: 10.1074/jbc.M109.066092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiranowska M, Colina LO, Johnson JO. Clathrin-mediated entry and cellular localization of chlorotoxin in human glioma. Cancer Cell Int. 2011;11:27. doi: 10.1186/1475-2867-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rjeibi I, Mabrouk K, Mosrati H, Berenguer C, Mejdoub H, Villard C, Laffitte D, Bertin D, Ouafik L, Luis J, Elayeb M, Srairi-Abid N. Purification, synthesis and characterization of AaCtx, the first chlorotoxin-like peptide from Androctonus australis scorpion venom. Peptides. 2011;32:656–663. doi: 10.1016/j.peptides.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Deshane J, Garner CC, Sontheimer H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J Biol Chem. 2003;278:4135–4144. doi: 10.1074/jbc.M205662200. [DOI] [PubMed] [Google Scholar]
- 25.Lui VC, Lung SS, Pu JK, Hung KN, Leung GK. Invasion of human glioma cells is regulated by multiple chloride channels including ClC-3. Anticancer Res. 2010;30:4515–4524. [PubMed] [Google Scholar]
- 26.Veiseh O, Gunn JW, Kievit FM, Sun C, Fang C, Lee JS, Zhang M. Inhibition of tumor-cell invasion with chlorotoxin-bound superparamagnetic nanoparticles. Small. 2009;5:256–264. doi: 10.1002/smll.200800646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu XS, Jian XC, Yin B, He ZJ. Development of the research on the application of chlorotoxin in imaging diagnostics and targeted therapies for tumors. Chin J Cancer. 2010;29:626–630. doi: 10.5732/cjc.009.10359. [DOI] [PubMed] [Google Scholar]
- 28.Soroceanu L, Gillespie Y, Khazaeli MB, Sontheimer H. Use of chlorotoxin for targeting of primary brain tumors. Cancer Res. 1998;58:4871–4879. [PubMed] [Google Scholar]
- 29.Hockaday DC, Shen S, Fiveash J, Raubitschek A, Colcher D, Liu A, Alvarez V, Mamelak AN. Imaging glioma extent with 131I-TM-601. J Nucl Med. 2005;46:580–586. [PubMed] [Google Scholar]
- 30.Veiseh M, Gabikian P, Bahrami SB, Veiseh O, Zhang M, Hackman RC, Ravanpay AC, Stroud MR, Kusuma Y, Hansen SJ, Kwok D, Munoz NM, Sze RW, Grady WM, Greenberg NM, Ellenbogen RG, Olson JM. Tumor paint: a chlorotoxin:Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res. 2007;67:6882–6888. doi: 10.1158/0008-5472.CAN-06-3948. [DOI] [PubMed] [Google Scholar]
- 31.Akcan M, Stroud MR, Hansen SJ, Clark RJ, Daly NL, Craik DJ, Olson JM. Chemical re-engineering of chlorotoxin improves bioconjugation properties for tumor imaging and targeted therapy. J Med Chem. 2011;54:782–787. doi: 10.1021/jm101018r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butte PV, Mamelak A, Parrish-Novak J, Drazin D, Shweikeh F, Gangalum PR, Chesnokova A, Ljubimova JY, Black K. Near-infrared imaging of brain tumors using the Tumor Paint BLZ-100 to achieve near-complete resection of brain tumors. Neurosurg Focus. 2014;36:E1. doi: 10.3171/2013.11.FOCUS13497. [DOI] [PubMed] [Google Scholar]
- 33.Kovar JL, Curtis E, Othman SF, Simpson MA, Olive DM. Characterization of IRDye 800CW chlorotoxin as a targeting agent for brain tumors. Anal Biochem. 2013;440:212–219. doi: 10.1016/j.ab.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Xing Y, Zhao J, Conti PS, Chen K. Radiolabeled nanoparticles for multimodality tumor imaging. Theranostics. 2014;4:290–306. doi: 10.7150/thno.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li G, Xing Y, Wang J, Conti PS, Chen K. Near-infrared fluorescence imaging of CD13 receptor expression using a novel Cy5.5-labeled dimeric NGR peptide. Amino Acids. 2014;46:1547–1556. doi: 10.1007/s00726-014-1727-x. [DOI] [PubMed] [Google Scholar]
- 36.Sun C, Veiseh O, Gunn J, Fang C, Hansen S, Lee D, Sze R, Ellenbogen RG, Olson J, Zhang M. In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small. 2008;4:372–379. doi: 10.1002/smll.200700784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veiseh O, Sun C, Fang C, Bhattarai N, Gunn J, Kievit F, Du K, Pullar B, Lee D, Ellenbogen RG, Olson J, Zhang M. Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier. Cancer Res. 2009;69:6200–6207. doi: 10.1158/0008-5472.CAN-09-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veiseh O, Kievit FM, Gunn JW, Ratner BD, Zhang M. A ligand-mediated nanovector for targeted gene delivery and transfection in cancer cells. Biomaterials. 2009;30:649–657. doi: 10.1016/j.biomaterials.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veiseh O, Sun C, Gunn J, Kohler N, Gabikian P, Lee D, Bhattarai N, Ellenbogen R, Sze R, Hallahan A, Olson J, Zhang M. Optical and MRI multifunctional nanoprobe for targeting gliomas. Nano Lett. 2005;5:1003–1008. doi: 10.1021/nl0502569. [DOI] [PubMed] [Google Scholar]
- 40.Xiang Y, Liang L, Wang X, Wang J, Zhang X, Zhang Q. Chloride channel-mediated brain glioma targeting of chlorotoxin-modified doxorubicine-loaded liposomes. J Control Release. 2011;152:402–410. doi: 10.1016/j.jconrel.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Costa PM, Cardoso AL, Mendonca LS, Serani A, Custodia C, Conceicao M, Simoes S, Moreira JN, Pereira de Almeida L, Pedroso de Lima MC. Tumor-targeted Chlorotoxin-coupled Nanoparticles for Nucleic Acid Delivery to Glioblastoma Cells: A Promising System for Glioblastoma Treatment. Mol Ther Nucleic Acids. 2013;2:e100. doi: 10.1038/mtna.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu C, Schneider T, Zeigler M, Yu J, Schiro PG, Burnham DR, McNeill JD, Chiu DT. Bioconjugation of ultrabright semiconducting polymer dots for specific cellular targeting. J Am Chem Soc. 2010;132:15410–15417. doi: 10.1021/ja107196s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Pang L, Ma C, Tu Q, Zhang R, Saeed E, Mahmoud AE, Wang J. Small molecule-initiated light-activated semiconducting polymer dots: an integrated nanoplatform for targeted photodynamic therapy and imaging of cancer cells. Anal Chem. 2014;86:3092–3099. doi: 10.1021/ac404201s. [DOI] [PubMed] [Google Scholar]
- 44.Wu C, Hansen SJ, Hou Q, Yu J, Zeigler M, Jin Y, Burnham DR, McNeill JD, Olson JM, Chiu DT. Design of highly emissive polymer dot bioconjugates for in vivo tumor targeting. Angew Chem Int Ed Engl. 2011;50:3430–3434. doi: 10.1002/anie.201007461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dearling JL, Barnes JW, Panigrahy D, Zimmerman RE, Fahey F, Treves ST, Morrison MS, Kieran MW, Packard AB. Specific uptake of 99mTc-NC100692, an alphavbeta3-targeted imaging probe, in subcutaneous and orthotopic tumors. Nucl Med Biol. 2013;40:788–794. doi: 10.1016/j.nucmedbio.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang C, Veiseh O, Kievit F, Bhattarai N, Wang F, Stephen Z, Li C, Lee D, Ellenbogen RG, Zhang M. Functionalization of iron oxide magnetic nanoparticles with targeting ligands: their physicochemical properties and in vivo behavior. Nanomedicine (Lond) 2010;5:1357–1369. doi: 10.2217/nnm.10.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu XF, Sun Z, Li M, Xiang Y, Wang QQ, Tang F, Wu Y, Cao Z, Li W. Neurotoxin-conjugated upconversion nanoprobes for direct visualization of tumors under near-infrared irradiation. Biomaterials. 2010;31:8724–8731. doi: 10.1016/j.biomaterials.2010.07.099. [DOI] [PubMed] [Google Scholar]
- 48.Huang R, Han L, Li J, Liu S, Shao K, Kuang Y, Hu X, Wang X, Lei H, Jiang C. Chlorotoxin-modified macromolecular contrast agent for MRI tumor diagnosis. Biomaterials. 2011;32:5177–5186. doi: 10.1016/j.biomaterials.2011.03.075. [DOI] [PubMed] [Google Scholar]
- 49.Johns TG, Perera RM, Vernes SC, Vitali AA, Cao DX, Cavenee WK, Scott AM, Furnari FB. The efficacy of epidermal growth factor receptor-specific antibodies against glioma xenografts is influenced by receptor levels, activation status, and heterodimerization. Clin Cancer Res. 2007;13:1911–1925. doi: 10.1158/1078-0432.CCR-06-1453. [DOI] [PubMed] [Google Scholar]
- 50.Ying X, Wen H, Lu WL, Du J, Guo J, Tian W, Men Y, Zhang Y, Li RJ, Yang TY, Shang DW, Lou JN, Zhang LR, Zhang Q. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J Control Release. 2010;141:183–192. doi: 10.1016/j.jconrel.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 51.Kim CK, Ahmed AU, Auffinger B, Ulasov IV, Tobias AL, Moon KS, Lesniak MS. N-acetylcysteine amide augments the therapeutic effect of neural stem cell-based antiglioma oncolytic virotherapy. Mol Ther. 2013;21:2063–2073. doi: 10.1038/mt.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minchenko DO, Kharkova AP, Hubenia OV, Minchenko OH. Insulin receptor, IRS1, IRS2, INSIG1, INSIG2, RRAD, and BAIAP2 gene expressions in glioma U87 cells with ERN1 loss of function: effect of hypoxia and glutamine or glucose deprivation. Endocr Regul. 2013;47:15–26. doi: 10.4149/endo_2013_01_15. [DOI] [PubMed] [Google Scholar]
- 53.Lu W, Sun Q, Wan J, She Z, Jiang XG. Cationic albumin-conjugated pegylated nanoparticles allow gene delivery into brain tumors via intravenous administration. Cancer Res. 2006;66:11878–11887. doi: 10.1158/0008-5472.CAN-06-2354. [DOI] [PubMed] [Google Scholar]
- 54.Trapani A, Denora N, Iacobellis G, Sitterberg J, Bakowsky U, Kissel T. Methotrexate-loaded chitosan- and glycol chitosan-based nanoparticles: a promising strategy for the administration of the anticancer drug to brain tumors. AAPS PharmSciTech. 2011;12:1302–1311. doi: 10.1208/s12249-011-9695-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qin C, He B, Dai W, Zhang H, Wang X, Wang J, Zhang X, Wang G, Yin L, Zhang Q. Inhibition of Metastatic Tumor Growth and Metastasis via Targeting Metastatic Breast Cancer by Chlorotoxin-Modified Liposomes. Mol Pharm. 2014 doi: 10.1021/mp400691z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 56.El-Ghlban S, Kasai T, Shigehiro T, Yin HX, Sekhar S, Ida M, Sanchez A, Mizutani A, Kudoh T, Murakami H, Seno M. Chlorotoxin-Fc fusion inhibits release of MMP-2 from pancreatic cancer cells. Biomed Res Int. 2014;2014:152659. doi: 10.1155/2014/152659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Locatelli E, Naddaka M, Uboldi C, Loudos G, Fragogeorgi E, Molinari V, Pucci A, Tsotakos T, Psimadas D, Ponti J, Franchini MC. Targeted delivery of silver nanoparticles and alisertib: in vitro and in vivo synergistic effect against glioblastoma. Nanomedicine (Lond) 2014;9:839–849. doi: 10.2217/nnm.14.1. [DOI] [PubMed] [Google Scholar]
- 58.Shen S, Khazaeli MB, Gillespie GY, Alvarez VL. Radiation dosimetry of 131I-chlorotoxin for targeted radiotherapy in glioma-bearing mice. J Neurooncol. 2005;71:113–119. doi: 10.1007/s11060-004-0890-4. [DOI] [PubMed] [Google Scholar]
- 59.Shirmardi SP, Shamsaei M, Gandomkar M, Maragheh MG. Synthesis and biodistribution study of a chlorotoxin derivative peptide labeled with 131-iodine for tumor therapy. Iran J Radiat Res. 2011;8:243–248. [Google Scholar]
- 60.Mamelak AN, Rosenfeld S, Bucholz R, Raubitschek A, Nabors LB, Fiveash JB, Shen S, Khazaeli MB, Colcher D, Liu A, Osman M, Guthrie B, Schade-Bijur S, Hablitz DM, Alvarez VL, Gonda MA. Phase I single-dose study of intracavitary-administered iodine-131-TM-601 in adults with recurrent high-grade glioma. J Clin Oncol. 2006;24:3644–3650. doi: 10.1200/JCO.2005.05.4569. [DOI] [PubMed] [Google Scholar]
- 61.Fiveash J, Badie B, Manelak AN. Society for Neuro-Oncology Annual Meeting. New Orleans, LA: 2009. A randomized phase II study of intracavitary 131-I-TM-601 in the treatment of recurrent malignant glioma. [Google Scholar]
- 62.Anthony RM, Ravetch JV. A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs. J Clin Immunol. 2010;30(Suppl 1):S9–14. doi: 10.1007/s10875-010-9405-6. [DOI] [PubMed] [Google Scholar]
- 63.Kasai T, Nakamura K, Vaidyanath A, Chen L, Sekhar S, El-Ghlban S, Okada M, Mizutani A, Kudoh T, Murakami H, Seno M. Chlorotoxin Fused to IgG-Fc Inhibits Glioblastoma Cell Motility via Receptor-Mediated Endocytosis. J Drug Deliv. 2012;2012:975763. doi: 10.1155/2012/975763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Costa ET, Barnabe GF, Li M, Dias AA, Machado TR, Asprino PF, Cavalher FP, Ferreira EN, Del Mar Inda M, Nagai MH, Malnic B, Duarte ML, Leite KR, de Barros AC, Carraro DM, Chammas R, Armelin HA, Cavenee W, Furnari F, Camargo AA. Intratumoral heterogeneity of ADAM23 promotes tumor growth and metastasis through LGI4 and nitric oxide signals. Oncogene. 2014 doi: 10.1038/onc.2014.70. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 65.Jahani-Asl A, Bonni A. iNOS: a potential therapeutic target for malignant glioma. Curr Mol Med. 2013;13:1241–1249. doi: 10.2174/1566524011313080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Safdar S, Taite LJ. Targeted diazeniumdiolates: localized nitric oxide release from glioma-specific peptides and proteins. Int J Pharm. 2012;422:264–270. doi: 10.1016/j.ijpharm.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 67.Safdar S, Payne CA, Tu NH, Taite LJ. Targeted nitric oxide delivery preferentially induces glioma cell chemosensitivity via altered p53 and O(6)-methylguanine-DNA methyltransferase activity. Biotechnol Bioeng. 2013;110:1211–1220. doi: 10.1002/bit.24775. [DOI] [PubMed] [Google Scholar]
- 68.Hegi ME, Liu L, Herman JG, Stupp R, Wick W, Weller M, Mehta MP, Gilbert MR. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26:4189–4199. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 69.Hansen RJ, Nagasubramanian R, Delaney SM, Samson LD, Dolan ME. Role of O6-methylguanine-DNA methyltransferase in protecting from alkylating agent-induced toxicity and mutations in mice. Carcinogenesis. 2007;28:1111–1116. doi: 10.1093/carcin/bgl218. [DOI] [PubMed] [Google Scholar]
- 70.Bernhart E, Damm S, Heffeter P, Wintersperger A, Asslaber M, Frank S, Hammer A, Strohmaier H, Devaney T, Mrfka M, Eder H, Windpassinger C, Ireson CR, Mischel PS, Berger W, Sattler W. Silencing of protein kinase D2 induces glioma cell senescence via p53-dependent and -independent pathways. Neuro Oncol. 2014;16:933–945. doi: 10.1093/neuonc/not303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Catuogno S, Esposito CL, Quintavalle C, Condorelli G, de Franciscis V, Cerchia L. Nucleic acids in human glioma treatment: innovative approaches and recent results. J Signal Transduct. 2012;2012:735135. doi: 10.1155/2012/735135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tobias A, Ahmed A, Moon KS, Lesniak MS. The art of gene therapy for glioma: a review of the challenging road to the bedside. J Neurol Neurosurg Psychiatry. 2013;84:213–222. doi: 10.1136/jnnp-2012-302946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Westphal M, Yla-Herttuala S, Martin J, Warnke P, Menei P, Eckland D, Kinley J, Kay R, Ram Z. Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:823–833. doi: 10.1016/S1470-2045(13)70274-2. [DOI] [PubMed] [Google Scholar]
- 74.Huang R, Ke W, Han L, Li J, Liu S, Jiang C. Targeted delivery of chlorotoxin-modified DNA-loaded nanoparticles to glioma via intravenous administration. Biomaterials. 2011;32:2399–2406. doi: 10.1016/j.biomaterials.2010.11.079. [DOI] [PubMed] [Google Scholar]
- 75.Kievit FM, Veiseh O, Fang C, Bhattarai N, Lee D, Ellenbogen RG, Zhang M. Chlorotoxin labeled magnetic nanovectors for targeted gene delivery to glioma. ACS Nano. 2010;4:4587–4594. doi: 10.1021/nn1008512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oh YK, Park TG. siRNA delivery systems for cancer treatment. Adv Drug Deliv Rev. 2009;61:850–862. doi: 10.1016/j.addr.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 77.Veiseh O, Kievit FM, Fang C, Mu N, Jana S, Leung MC, Mok H, Ellenbogen RG, Park JO, Zhang M. Chlorotoxin bound magnetic nanovector tailored for cancer cell targeting, imaging, and siRNA delivery. Biomaterials. 2010;31:8032–8042. doi: 10.1016/j.biomaterials.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mok H, Veiseh O, Fang C, Kievit FM, Wang FY, Park JO, Zhang M. pH-Sensitive siRNA nanovector for targeted gene silencing and cytotoxic effect in cancer cells. Mol Pharm. 2010;7:1930–1939. doi: 10.1021/mp100221h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fang C, Zhang M. Multifunctional Magnetic Nanoparticles for Medical Imaging Applications. J Mater Chem. 2009;19:6258–6266. doi: 10.1039/b902182e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mooney R, Weng Y, Tirughana-Sambandan R, Valenzuela V, Aramburo S, Garcia E, Li Z, Gutova M, Annala AJ, Berlin JM, Aboody KS. Neural stem cells improve intracranial nanoparticle retention and tumor-selective distribution. Future Oncol. 2014;10:401–415. doi: 10.2217/fon.13.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bhujbal SV, de Vos P, Niclou SP. Drug and cell encapsulation: Alternative delivery options for the treatment of malignant brain tumors. Adv Drug Deliv Rev. 2014;67-68:142–153. doi: 10.1016/j.addr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 82.Sun C, Fang C, Stephen Z, Veiseh O, Hansen S, Lee D, Ellenbogen RG, Olson J, Zhang M. Tumor-targeted drug delivery and MRI contrast enhancement by chlorotoxin-conjugated iron oxide nanoparticles. Nanomedicine (Lond) 2008;3:495–505. doi: 10.2217/17435889.3.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zeng XC, Li WX, Zhu SY, Peng F, Zhu ZH, Wu KL, Yiang FH. Cloning and characterization of a cDNA sequence encoding the precursor of a chlorotoxin-like peptide from the Chinese scorpion Buthus martensii Karsch. Toxicon. 2000;38:1009–1014. doi: 10.1016/s0041-0101(99)00212-3. [DOI] [PubMed] [Google Scholar]
- 84.Yang R, Peng F, Liu H. Functional analysis of a gene encoding a chlorotoxin-like peptide derived from scorpion toxin. Chin J Biochem Mol Biol. 2005;21:19–23. [Google Scholar]
- 85.Fu YJ, Yin LT, Wang W, Chai BF, Liang AH. Synthesis, expression and purification of a type of chlorotoxin-like peptide from the scorpion, Buthus martensii Karsch, and its acute toxicity analysis. Biotechnol Lett. 2005;27:1597–1603. doi: 10.1007/s10529-005-2514-2. [DOI] [PubMed] [Google Scholar]
- 86.Quintero-Hernandez V, Jimenez-Vargas JM, Gurrola GB, Valdivia HH, Possani LD. Scorpion venom components that affect ion-channels function. Toxicon. 2013;76:328–342. doi: 10.1016/j.toxicon.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fu YJ, Yin LT, Liang AH. Polyclonal antibody against a recombinant chlorotoxin-like peptide from the Chinese scorpion and detection of its putative receptors in human glioma cells. Biotechnol Lett. 2006;28:1439–1443. doi: 10.1007/s10529-006-9108-5. [DOI] [PubMed] [Google Scholar]
- 88.Fu YJ, An N, Chan KG, Wu YB, Zheng SH, Liang AH. A model of BmK CT in inhibiting glioma cell migration via matrix metalloproteinase-2 from experimental and molecular dynamics simulation study. Biotechnol Lett. 2011;33:1309–1317. doi: 10.1007/s10529-011-0587-7. [DOI] [PubMed] [Google Scholar]
- 89.Wang WX, Ji YH. Scorpion venom induces glioma cell apoptosis in vivo and inhibits glioma tumor growth in vitro. J Neurooncol. 2005;73:1–7. doi: 10.1007/s11060-004-4205-6. [DOI] [PubMed] [Google Scholar]
- 90.Fan S, Sun Z, Jiang D, Dai C, Ma Y, Zhao Z, Liu H, Wu Y, Cao Z, Li W. BmKCT toxin inhibits glioma proliferation and tumor metastasis. Cancer Lett. 2010;291:158–166. doi: 10.1016/j.canlet.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 91.Fu YJ, Yin LT, Liang AH, Zhang CF, Wang W, Chai BF, Yang JY, Fan XJ. Therapeutic potential of chlorotoxin-like neurotoxin from the Chinese scorpion for human gliomas. Neurosci Lett. 2007;412:62–67. doi: 10.1016/j.neulet.2006.10.056. [DOI] [PubMed] [Google Scholar]
- 92.Zhao J, Qiao W, Zhang Y, Shao X. Preparation and in vitro evaluation of 131I-BmK CT as a glioma-targeted agent. Cancer Biother Radiopharm. 2010;25:353–359. doi: 10.1089/cbr.2009.0704. [DOI] [PubMed] [Google Scholar]
- 93.Zou Q, Hou Y, Shen F, Wang Y. Polarized regulation of glycogen synthase kinase-3beta is important for glioma cell invasion. PLoS One. 2013;8:e81814. doi: 10.1371/journal.pone.0081814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fu Y, Zheng Y, Chan KG, Liang A, Hu F. Lithium chloride decreases proliferation and migration of C6 glioma cells harboring isocitrate dehydrogenase 2 mutant via GSK-3beta. Mol Biol Rep. 2014;41:3907–3913. doi: 10.1007/s11033-014-3258-7. [DOI] [PubMed] [Google Scholar]
- 95.Fu Y, Zheng S, Huang R, An N, Zheng Y, Zhang Z, Liang A. A potential strategy for high-grade gliomas: combination treatment with lithium chloride and BmK CT. Biotechnol Lett. 2012;34:9–17. doi: 10.1007/s10529-011-0741-2. [DOI] [PubMed] [Google Scholar]
- 96.Du J, Fu Y, Wang J, Liang A. Adenovirus-mediated expression of BmK CT suppresses growth and invasion of rat C6 glioma cells. Biotechnol Lett. 2013;35:861–870. doi: 10.1007/s10529-013-1167-9. [DOI] [PubMed] [Google Scholar]
- 97.Fu Y, Jiao Y, An N, Liang A. pEGFP-N1-mediated BmK CT expression suppresses the migration of glioma. Cytotechnology. 2013;65:533–539. doi: 10.1007/s10616-012-9518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fu Y, An N, Zheng S, Liang A, Li Y. BmK CT-conjugated fluorescence nanodiamond as potential glioma-targeted imaging and drug. Diam Relat Mater. 2012;21:73–76. [Google Scholar]


