Abstract
Venous thromboembolism (VTE) mostly presenting as deep venous thrombosis (DVT) and pulmonary embolism (PE) affects up to 600,000 individuals in United States each year. Clinical symptoms of VTE are nonspecific and sometimes misleading. Additionally, side effects of available treatment plans for DVT are significant. Therefore, medical imaging plays a crucial role in proper diagnosis and avoidance from over/under diagnosis, which exposes the patient to risk. In addition to conventional structural imaging modalities, such as ultrasonography and computed tomography, molecular imaging with different tracers have been studied for diagnosis of DVT. In this review we will discuss currently available and newly evolving targets and tracers for detection of DVT using molecular imaging methods.
Keywords: FDG-PET/CT, venous thromboembolism, deep vein thrombosis, SPECT, molecular imaging
Introduction
Venous thromboembolism (VTE), mostly presenting as deep vein thrombosis (DVT) and pulmonary embolism (PE), affects approximately 300,000 to 600,000 individuals and 60,000 to 100,000 die of VTE each year in the United States [1-4] more than prostate and breast cancer combined [5]. VTE has a relatively high mortality rate of 6% for DVT cases and 12% of PE cases within the first month of diagnosis [6,7]. One-third VTE cases are manifested as PE and 2/3 present with DVT alone [4]. Eighty to 90% of pulmonary embolism cases are caused by DVT or a thrombus formed in the pelvis [8]. US healthcare system carries a huge burden for treatment of VTE and its complications, which is estimated to be $1.5 billion/year [9]. It is very important to correctly diagnose VTE before instituting an intervention, however, currently available diagnostic methods have pitfalls and is sometimes misleading [10].
The established modalities and current gold standards for evaluation of VTE may be inapplicable in some situations. Ultrasonography (US) has replaced contrast venography for the diagnosis of DVT because of availability, performance, elimination of radiation and contrast agents [11]. However, US is dependent on user experience and also could be compromised by mechanical obstacles. US contrast medium is highly allergenic and not suitable for cardiac patients. It is also not applicable for body cavity and non-occlusive thrombi [12,13]. In patients with involvement of the vasculature below the knee or in the pelvic veins, in asymptomatic patients, and in patients with duplicate veins, US might show false negative results [14-16]. Venography and US can only reflect changes in venous anatomy, which is caused by filling defects and cannot show the metabolic activity of the clot. Since morphologic changes may remain present for years after an episode of DVT, patients with a prior history of DVT represent a challenge to diagnosis because of difficulty in differentiating new clots from residual ones [15,17]. Up to 11% of CT venograms are insufficient for diagnosis of DVT [10,18] and are not recommended for the initial assessment of DVT due to invasiveness, technical difficulties and potential complications (e.g., hematoma, allergic reaction to contrast media) [19]. Patients with implanted electronic devices and intractable claustrophobia or renal failure cannot undergo magnetic resonance imaging (MRI) with contrast media [13].
With the emergence of nuclear medicine methods, new perspectives were opened early on for diagnosis of DVT [20]. Initial trials for diagnosis of DVT using radiolabeled antibodies targeting fibrin, activated platelets, plasminogen, plasmin, factor XIII were not promising due to their long blood circulation time and radioactivity accumulation in the lungs and problems with timing of availability of the epitope which antibody was designed to bind, causing low clot to blood ratios [21-23]. Later on, studies focusing on specific synthetic peptides targeting fibrin and platelet receptors have shown more promising results [15,22,24-43], which will be discussed in this review (Figure 1). These new tracers might be able to aid the currently used modalities for detection of DVT.
Figure 1.

A schematic view depicting elements of the venous thrombus and binding sites for different radiotracers. 1. FDG taken up by metabolically active inflammatory cells and platelets. 2. Radiolabeled platelets indicating sites of aggregated platelets. 3. GP IIb/IIIa cyclic RGD peptides (Apcitide, DMP 444, Bitistatin) targeting GP IIb/IIIa receptors on activated platelets. 4. TP850 pentapeptide targeting fibrin α chain. 5. 59D8, T2G1, GC4, 64C5 targeting fibrin β chain. 6. Cyclic fibrin binding peptide EP-2104R. 7. DI-80B3 targeting D-domain of the fibrin. 8. Fibronectin-binding domain targeting lysine residue in fibrin. 9. Recombinant tissue plasminogen activatior (rt-PA) binding to C-terminal lysine residue of fibrin.
Here, we will discuss currently available and newly evolving targets and tracers for detection of DVT using molecular imaging methods and evaluate potential of 2-deoxy-2-[18F]fluoro-D-glucose-positron emission tomography/CT (FDG-PET/CT) as an accurate diagnostic tool for assessment of DVT (Table 1). We will also briefly discuss the role of FDG-PET/CT in detection of tumor thrombosis and septic thromboembolism.
Table 1.
List of targets and tracers studied for detection of venous thrombosis
| Binding site | Tracer | Modality | Study, Publication year | Study population (number) | Reference |
|---|---|---|---|---|---|
| Platelet | 111In labeled platelet | Planar Scintigraphy | Thakur et al., 1976; Knight et al., 1978, … | Animal/Human (multiple studies) | [98,99] |
| Platelet, GP IIb/IIIa receptor | Cyclic RGD Peptide (99mTc-Apcitide (P280)) | Planar Scintigraphy | Dunzinger et al., 2008; Taillefer et al., 2000 | Human (n=19); Human (n=280, Phase III) | [33,39] |
| Platelet, GP IIb/IIIa receptor | Cyclic RGD Peptide (99mTc-DMP 444, 99mTc-P4 DMP 444) | Planar Scintigraphy | Fang et al., 2011; Klem et al., 2000 | Animal; Human (n=11) | [22,41] |
| Platelet, GP IIb/IIIa receptor | Cyclic RGD Peptide (99mTc-Bitistatin) | Planar Scintigraphy | Knight et al., 2000; Knight et al., 2007 | Animal; Human (n=4, Phase I) | [34,119] |
| Platelet, Thrombospondin receptor | 99mTc-TP 1201, 99mTc-TP 1301 | Planar Scintigraphy | Pallela et al., 1999 | Animal | [151] |
| Fibrin, Alpha chain | 99mTc-TP850 | Planar Scintigraphy | Aruva et al., 2006; Thakur et al., 2000 | Animal | [36,75] |
| Fibrin, Beta Chain | 111In-59D8, 111In-T2G1, 111In-GC4, 111In-64C5 | Planar Scintigraphy | Morris et al., 1997; Alavi et al., 1990; Jung et al., 1989; Lusiani et al., 1989; DeFaucal et al., 1991; Stratton et al., 1994, … | Animal/Human (multiple studies) | [64,65,67,69,74,79,94] |
| Fibrin | Cyclic fibrin binding peptide (EP-2104R) | MRI | Vymazal et al., 2009; Spuentrup et al., 2008 | Human (n=14, n=10) | [32,85] |
| Fibrin | EP-2104R | NIRF | Hara et al., 2012 | Animal | [68] |
| Fibrin | 111In-EP-2104R (FibPep) | SPECT | Starmans et al., 2013 | Animal | [25,26] |
| Fibrin | 64Cu-DOTA-FBP EP-2104R | PET/CT | Cieskienski et al., 2013 | Animal | [27] |
| Fibrin | EP-2104R based dual PET/MR probe | PET/MR | Uppal et al., 2011 | Animal | [87] |
| D-Domain | Anti D-dimer 99mTc-DI-80B3 Fab’ fragment (ThromboView®) | Planar Scintigraphy | Douketis et al., 2012 | Human (n=82, Phase II) | [24] |
| D-Domain | 99mTc-DI-DD3B6/22-80B3 | SPECT | Macfarlane et al., 2009 | Human (Phase I, n=21) | [31] |
| D-Domain | 111In-anti D-dimer | Planar Scintigraphy | Morris et al., 2004 | Animal | [72] |
| Region 102-10 | 89Zr-102-10 (anti-Bβ and anti-γ mAb) | PET/CT | Hisada et al., 2013 | Animal | [82] |
| Factor XIII | CLIO-FXIII | NIRF/MRI | McCarthy et al., 2009, Tung et al., 2003 | Animal | [38,97] |
| Fibronectin | 111In-FBD | Planar Scintigraphy | Rosenthall et al., 1995 | Human (n=62) | [95] |
| Plasmin | 131I-Plasminogen, 123I-Plasminogen | Planar Scintigraphy | Harwig et al., 1976 | Animal | [128] |
| Plasmin | 99mTc-Plasmin | Planar Scintigraphy | Dahlborn et al., 1984 | Animal | [121] |
| Tissue plasminogen activator | 99mTc-rt-PA | Planar Scintigraphy | Brighton et al., 2007 | Human (n=74) | [35] |
| Tissue plasminogen activator | 123I-t-PA | Planar Scintigraphy | De Bruyn et al., 1995 | Animal | [152] |
| Active inflammatory cells | 18F-FDG | PET/CT | Rondina et al., 2012 | Human (n=36) | [12] |
| Active inflammatory cells | 18F-FDG | PET/CT | Hess et al., 2014 | Human (n=15) | [137] |
Pathophysiology of thrombosis
Since we will focus on agents involved in molecular mechanisms of thrombosis, the complex cascade of blood coagulation will be reviewed briefly. Hemostasis of blood is a complex mechanism for maintaining blood fluidity and conversion to insoluble gel in sites of vascular injury. Platelets and coagulation proteins are two major forces interacting with each other. In arterial thrombosis, loss of endothelial layer exposes platelets to subendothelial ligands and activates them causing cascade of procoagulant molecules such as factor V, Von Willebrand factor (VWF) and fibrinogen to be released and promotes the flip-flop reaction exposing phosphatidylserine on outer membrane leaflet of platelets, providing surface for generation of thrombin and fibrin deposition [44]. Arterial thrombi are platelet rich and composed of a core of platelets over the vessel injury site and a mesh of fibrin covering platelets [45]. Mechanisms behind venous thrombosis are less clear. Vessel wall injury is not considered the prominent initiating event in venous thrombosis [44,46]. Two distinct regions are present in venous thrombi, red thrombi consisting of fibrin and trapped red blood cells and white thrombi composed of aggregated platelets [44]. Fibrin rich region attaches the thrombi to the vessel wall and platelet rich regions are attached to the fibrin rich region distally [44,47,48]. According to Virchow’s triad, which described the pathogenesis of VTE many years ago [49], stasis, endothelial changes and increase in blood thrombogenicity contribute to VTE. Additional factors are also described which include inflammation and abnormalities of fibrinolytic mechanisms [50]. Many VTE patients fulfill most or all of Virchow’s triad [45,51,52].
Targeting fibrinogen, fibrin and their derivatives
Fibrinogen is a soluble glycoprotein circulating in blood consisting of two outer D domains connected by a coiled segment to its central E domain. Fibrinogen contains two sets of three polypeptide chains: alpha, beta and gamma. Thrombin mediates cleavage of fibrinopeptides A and B from alpha and beta chains of fibrinogen, producing fibrin monomers, which polymerize and form fibrin fibers and subsequent insoluble fibrin network, making the scaffold for thrombus [53,54]. Fibrin and its related proteins have been a main target for investigations related to VTE in the literature which will be discussed in the following paragraphs.
Traditional anti-fibrin antibodies
Fibrinogen labeled with 125I was popular in the early 1990s but abandoned because of low sensitivity, low specificity and the need for a long delay from injection to imaging [21,55-57]. Polyclonal antibodies against fibrin were labeled with 131I, 111In and used for detection of venous thrombi. However, the problem of long half-life and time consuming imaging was still present [47,58]. Later on, production of monoclonal antibodies, increased the specificity of fibrin detection and techniques of antibody fragmentation helped faster clearance from blood. These factors altogether enabled applicability of radiolabels with shorter half-life like 99mTc [47,59-63].
Fibrin beta chain antibodies
Fibrin has been a target for various studies focusing on imaging of thrombus and different types of antibodies have been introduced to the literature [20,22,25,27,32,36,57,60,61,63-76]. Antifibrin antibodies 59D8 [77] and T2G1 [59,78] bind to a 7 amino acid sequence on beta chain of human fibrin with a binding site specific for cross-linked fibrin undergoing active thrombosis [47]. Preliminary results from human studies of 111In labeled 59D8 showed a combined sensitivity and specificity of 87% up to 4 hours post injection compared to venography in total of 159 patients [58,64,65,67,69,79]. However, as mentioned above, the problem of blood clearance and half-life of tracer still persisted. The preliminary results from a study on 99mTc labeled T2G1 in DVT patients showed an overall sensitivity of 80% and 94% for proximal DVT [58,65]. It has been hypothesized that heparin might interfere with antibody binding to fibrin [58,65]. It has been shown that GC4, which is a monoclonal antibody against fragment D of fibrin, has tendency to old thrombi which has greater uptake during heparinization compared to T2G1 [73]. Another example of fibrin specific molecules is fragment E, a plasmin degradation product from cross-linked fibrin [80]. 111In labeled monoclonal antibody 64C5 specific for the beta-chain was used for canine (n = 6) pulmonary emboli [81].
The published human studies for detection of thrombi using fibrin antibodies [65-67,69] have shown acceptable sensitivity and specificity. For example, Alavi et al. [64,65] in 1990 used 111In labeled antifibrin antibody in patients suspected of VTE with a sensitivity of 97% in 33 patients versus venography. Jung et al. [69] showed sensitivity and specificity of 84% and 81%, respectively. However, the patient group had a high pretest probability for DVT. The study of De faucal et al. had a sensitivity of 85% and specificity of 100% in 10 patients [67].
Bautovich [66] used another anti-fibrin antibody named DD3B6/22 and noted a sensitivity of 100% in 20 patients. However, none of these studies could optimally bind thrombi during anti-coagulation [72].
Hisada et al. [82] have recently discovered an uncovered region (named 102-10) in fibrin clot with specificity for insoluble fibrin against fibrinogen, soluble fibrin and D-dimer. They investigated its applicability in clot detection in microhemorrhages of tumoral masses in mice by designing monoclonal antibodies (mAb) against elements of this region and radiolabeling them with 89Zr (89Zr labeled 102-10) and subsequent immunohistochemical assessment. They concluded that their newly developed antibodies against insoluble fibrin mAbs are feasible for detection of high-grade and aggressive tumors.
Cyclic fibrin-binding peptides
Cyclic fibrin binding peptide EP-2104R, composed of a fibrin-binding motif and four Gd-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) moieties, has been studied for MRI visualization of fibrin deposition in PE, atherosclerosis, coronary and carotid artery thrombosis [32,83-86]. EP-2104R has successfully passed phase II clinical trials [32,83-86]. A similar structure introduced by Starmans et al. [26], 111In labeled fibrin-binding peptide named FibPep, containing cyclic fibrin binding motif has been studied in vivo using mice and in vitro. They showed enhanced binding compared to control tracer to fibrin and blood clots in vitro with 100 fold higher affinity. In vivo studies showed clear visualization of the thrombi and rapid blood clearance making it feasible for sensitive detection of thrombi using SPECT. Further studies are needed to investigate the potentials of this compound in thrombosis, atherosclerosis and cancer research. Starmans et al. in a newer study [25], introduced EPep, 111In-labeled fibrin binding peptide which incorporated EP-2104R’s fibrin binding site and compared it with FibPep. Both peptides were approximately similar in metabolic stability and affinity.
Uppal et al. [87] used a dual modality PET/MR probe EP-2104R on rat arterial thrombus model. They observed thrombus enhancement in all animals at both MR and PET after injection of probe.
Ciesienski et al. [27] designed three new simplified fibrin-targeted PET probes based on EP-2104R and two other similar sequences previously shown to bind to fibrin with high affinity and conjugated them to 64Cu-DOTA [84,88]. They used PET imaging in rat models of arterial thrombosis. Two of the probes showed enhanced metabolic stability in vivo with four-fold thrombus to background ratio and accurate detection of arterial thrombus and imaging efficacy in hybrid MR-PET imaging. However, these probes have a long residual activity in blood even after 120 minutes, therefore future work is needed to modify these probes.
Ay et al. [89] have recently introduced a fibrin-binding peptide 7 (FBP7) for detection of thrombosis with promising results in animal models of arterial thrombosis. Although their model was designed for occlusive and non-occlusive arterial thrombi in the carotid arteries, this 64Cu labeled compound might be useful in venous thrombosis as well.
Hara et al. [68] investigated the avidity and specificity of a newly synthesized near infrared fluorescence (NIRF) thrombus imaging agent based on already known EP-2104R, namely FTP11-Cy7 in acute and sub-acute murine DVT in vivo using high-resolution intra-vital fluorescence microscopy and noninvasive integrated fluorescence molecular tomography-CT. They found FTP11-Cy7 binds specifically and avidly to thrombus with high target to background ratios enabling non-invasive and sensitive detection of acute and sub-acute murine DVT.
Fibrin alpha chain: TP850
Laudano and Doolittle introduced a tripeptide corresponding to alpha chain of fibrin with inhibitory properties of fibrin polymerization [90]. Kawasaki et al. [91] prepared additional analogs with more potent activity against thrombin clots. Thakur et al. [75] have investigated 99mTc-TP 850, a fibrin alpha chain N-terminal pentapeptide similar to mentioned peptide that binds to C terminal of gamma chain of fibrin for detection of chronic and acute DVT or PE in animal models of DVT and PE. The resulting images showed stability in vivo and in vitro, the tracer cleared rapidly from blood and was able to delineate experimental DVT and PE.
Aruva et al. [36] imaged thromboembolism with TP 850 in swine models of DVT and PE. They measured modest affinity for 99mTc-TP 850 with rapid blood clearance and high DVT and PE uptake.
D-dimer (thromboview)
The D-domains of fibrin connect covalently with unique antigenic sites which antibodies are made for binding to these structures [31,76,92,93]. Murine monoclonal antibodies (DI-DD-3B6/22) were the first class of these antibodies [31,66]. The next generation with modified structure and optimized efficacy by replacing murine specific sequences not involved in antigen detection with human equivalent was DI-DD3B6/22-80B3 Fab’ fragments [71,72]. DI-DD3B6/22-80B3 has unique properties such as anatomical localization of clot, differentiating new and old lesions in patients with suspected recurrence, elimination of intravenous contrast dye. Furthermore, it has some advantages over US by having the ability to identify non-occlusive proximal DVT or distal DVT and obese or edematous patients in whom US may be difficult to interpret [24] (See Figure 2).
Figure 2.

99mTc-DI-80B3 (ThromboView®) images of right leg proximal deep vein thrombosis. Reprinted from [24]. Copyright (2012), with permission from Elsevier.
Morris et al. [71] used 99mTc labeled deimmunized antifibrin Fab’ fragments and SPECT to detect DVT and PE in 5 dogs with induced DVT and subsequent embolization and were able to visualize all PEs and DVTs with mass of 0.4 g or greater. PEs (0.48 ± 0.09 g) were intensely radiolabeled, yielding clot/blood radioactivity ratios of 22.8 ± 5.6. DVTs (0.45 ± 0.31 g) also had high clot/blood ratios (11.7 ± 2.6). Imaging was done at 4 hours after injection. However, the study was not able to detect sub-segmental emboli in dogs.
Morris et al. [94] also compared imaging of DVT during anticoagulation by 111In labeled antibodies against D-Dimer, fibrin beta chain in dogs by gamma scan. D-dimer was 100% sensitive but anti-beta was only 60% sensitive. The clot/blood ratio was 24.5 ± 2.8 in the anti-D-dimer group, but only 7.8 ± 2.0 in the anti-beta group.
In phase I of a trial to image DVT using radiolabeled anti D-Dimer fab’ fragment, Macfarlane et al. [31] have evaluated 99mTc-DI-80B3 imaging of 26 patients with acute DVT for safety. Douketis et al. [24] did the multicenter phase II prospective cohort trial on 82 patients to investigate diagnostic accuracy and safety of 99mTc-DI-80B3 in DVT patients. They found no serious adverse effect, 84% sensitivity, and 97% specificity for proximal DVT and lower accuracy for distal DVT. They could not comment on recurrent DVT due to lack of adequate number of patients.
Fibronectin
Fibronectin is one of the most abundantly found proteins in plasma and is involved in cross-linking of fibrin dimers with multifunctional adhesive properties [15]. The 99mTc-FBD contains part of fibronectin which is responsible for directing it to blood clots.
Initial pilot study [95] was done using 111In-FBD after 18 to 24 hours of injection of radiotracer which showed preliminary encouraging results, further pilot studies by Thaillefer with 99mTc-FBD showed sensitivity of 80% by considering equivocal as normal and better sensitivity in proximal DVT (87% to 97%) than distal DVT ( 56% to 78%) [15,95].
Factor XIII
Clotting factor XIII is an endogenous protein with ability to crosslink polymerized fibrin molecules together and adding stability to the forming thrombus. Since factor XIII crosslinks to fibrin, it is a good target for detecting active thrombosis.
Preliminary studies on factor XIII showed relative similarity in ability to achieve an acceptable thrombus-to-blood ratio [47]. However, further studies were halted presumably due to its low blood clearance and large molecular weight. In 2003 a novel, thrombosis-specific diagnostic probe based on the factor XIII transglutaminase activity and previously identified peptide substrates with near-infrared fluorescence probe and gadolinium (Gd) chelating MR probe became available [38]. McCarthy et al. [96,97] have also synthesized efficient multimodal nanoagents targeting activated factor XIII.
Targeting platelet and its receptors
Radiolabeled platelet
Since the late 1970s, 111In labeled platelets have provided physiologic data of platelet for therapeutic decisions and assessment of atherosclerosis, angioplasty, vascular grafts and venous thrombosis and it was once the only FDA approved method of functional imaging of thrombus formation [98,99]. However, this method was time-consuming requiring up to 24 hours of imaging delay [74,100]. Signal-to-noise ratio was also low, and there was a lack of documentation of underlying pathological condition [101]. Therefore, methods that are more rapid were needed.
Cyclic Arg-Gly-Asp (RGD) peptides: GPIIB/IIIA platelet receptor antibody
DVT can be detected by targeting membrane glycoproteins IIb/IIIa (GPIIb/IIIa), which are expressed on platelets to form platelet bridges, found predominantly in thrombi. It is estimated that activated platelets present more than 80,000 GP IIb/IIIa receptors on their membrane [29,102].
Short synthetic peptides containing analogues of Arg-Gly-Asp (RGD) have been extensively tested for their ability to image thrombosis and tumors [30,103-107]. The problem of long circulation time of radiolabeled antibodies can be alleviated using small peptides that are cleared quickly from the blood circulation [22,33,36,39-43,108-116]. These peptides showed rapid clearance from blood, but their tissue-to-background contrast was not satisfactory [110,117]. Zhou et al. [30] have extensively reviewed cyclic RGD peptides and their applications. Here are examples of these peptides, which have been studied for imaging thrombosis.
Apcitide (AcuTec)
Apcitide is a synthetic RGD-mimicking polypeptide, which binds to glycoprotein IIb/IIIa receptors on activated platelets. Apcitide was approved for clinical use in 1998 and has since been studied for detection of acute DVT and PE [30,43,118].
Dunzinger et al. [33] have studied patients with DVT and PE using 99mTc-apcitide to detect thrombosis. The results of 19 patients were compared to ultrasonography and/or phlebography. The results showed acute clot formation in 14 out of 16 patients with DVT up to 17 days after onset of clinical symptoms. Only one out of six patients with imaging proven segmental or sub-segmental PE was detected by 99mTc-apcitide. Apcitide had a sensitivity of 87% and specificity of 100% for acute DVT, however the sensitivity for PE was poor.
Thaillefer et al. [39] in their Phase III multicenter trial for detection of thromboembolic disease compared 99mTc-apcitide scintigraphy with contrast venography in 280 patients within 10 days of onset of signs and symptoms of acute DVT or 10 days of surgery associated with high risk of DVT. Blinded reading of 99mTc-apcitide scintigraphy and contrast venography had a sensitivity of 73.4%, specificity of 67.5%, and agreement of 69.1%. The study included patients with history of previous DVT, which might confound the venography results. In a subset of patients without previous DVT episodes, sensitivity, specificity and agreement were 90.6%, 83.9% and 87.3% respectively. Thus apcitide could be used as a sensitive imaging for acute DVT. Same lead author in another study compared 99mTc-apcitide at multiple time points with contrast venography. When images at multiple time points were analyzed together, sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio rose to 85.4%, 88.3%, 7.38, and 0.154, respectively [37,113].
Bates et al. [108] have used radiolabeled peptide apcitide on 38 patients with newly diagnosed DVT, and 40 patients with previous DVT, post thrombotic symptoms, and ultrasonography abnormalities. Sensitivity of 99mTc-apcitide was 92% for two expert readers and specificity was 82% and 90%, respectively. However, they reported lower accuracy and inter-reader agreement for inexperienced physicians.
DMP 444
Another peptide targeting activated platelets as a GP IIb/IIIa receptor antagonist is 99mTc-DMP444 [42]. It actively incorporates into arterial [111,116] and venous thrombi in DVT and PE. It has also been used in imaging infective endocarditis [101,109,115].
Kelm et al. [41] reported detection of DVT with 99mTc labeled DMP 444 in 10 patients with clinical suspicion of DVT confirmed with US and D-Dimer. All patients underwent planar imaging, and no significant adverse effect was noted. Eight patients demonstrated areas of increased activity with correlation to ultrasonographic findings. This preliminary study showed safety and potential value of DMP 444 in future studies.
Anti GP IIIa
Ji et al. [29] have investigated human and mouse/human chimeric versions of monoclonal antibody against platelet GPIIIa, SZ21, and chSZ21, respectively in canine PE and DVT models. Their results showed focal uptake on planar images as early as after 30 minutes and clearer uptakes after 3 hours. Lesion-to-background ratio was 12.8 for PE/lung and 7.2 for DVT/blood and 117 for DVT/muscle.
Bitistatin
Bitistatin is an 83 amino acid polypeptide isolated from viper venom, which binds avidly to GPIIb/IIIa receptor of the platelets. Knight et al. labeled bitistatin with 99mTc and assessed the ability of bitistatin for detection of PE and DVT. In their preclinical model, the uptake in thrombus and embolus was higher than other thrombus targeting tracers 0.5 to 4 h after injection [119]. Subsequently, recombinant version of bitistatin replaced the native one [34] and in phase I clinical trial showed safety and was able to bind to circulating platelets. Future studies are needed to elucidate the role of bitistatin in clinic.
As a summary, various peptides and antibodies are being developed and examined in animal and human studies targeting receptors on the activated platelets. However, most of these agents are in either pre-clinical setting or phase I clinical trials. Therefore, more time is needed to be able to make an accurate comparison among these agents.
Targeting plasmin and its derivatives
Fibrinolytic system associated with hemostasis consists of two types of proteolytic enzymes: plasminogen activator and plasmin. Plasminogen activator has two types: tissue-type plasminogen activator (TPA) and urokinase-type plasminogen activator; both synthesized in vascular endothelial cells and released to bloodstream. When fibrin clots form, circulating plasminogen and TPA bind to fibrin. Then, TPA activates fibrin-bound plasminogen to plasmin, which in turn degrades in situ fibrin [120]. This close relationship with thrombus formation and degradation makes plasmin and TPA a good target for imaging thrombus.
Plasmin
Several studies have evaluated DVT by gamma camera detection of 99mTc-Plasmin [121-127] and compared with phlebography. Although their results showed relatively favorable sensitivity, the specificity, predictive values and accuracy were not satisfactory for DVT diagnosis, specifically after hip replacement surgery [124].
Plasminogen
Harwig et al. [128] investigated radio iodinated plasminogen for localization of canine preformed thrombi. Two days after thrombus formation, radiolabeled plasminogen was injected and thrombus-to-blood activity ratio of 7.8 ± 2.4 was obtained. Even 6 days old thrombi were visible in 80% of cases. However there was variability in thrombus weight and thrombus blood ratio in 1-day-old thrombi, which might have been caused by plasminogen release accompanying thrombus retraction.
Tissue-type plasminogen activator (TPA)
Brighton et al. [35] examined relative uptake of 99mTc-rt-PA in acute DVT over first 30 days after diagnosis. Plasminogen activation site of rt-PA undergoes inactivation but fibrin binding is retained. As the thrombus ages fewer fibrin sites will be available for 99mTc-rt-PA binding. They studied 74 patients with acute symptomatic DVT who underwent ultrasound and 99mTc-rt-PA imaging and found 72% uptake on day 7 and 0% uptake after 30 days. They concluded 99mTc-rt-PA could distinguish new from old thrombus.
Targeting inflammatory cells involved in VTE
The role of 18F-FDG-PET/CT in detection and assessment of DVT
18F-FDG is an analog of glucose, which is the major source of energy and therefore taken up by various cells in the body, including tumoral cells and the ones involved in inflammation and coagulation such as macrophages, endothelial cells and lymphocytes. It is suggested the process of VTE is closely related to inflammation and expression of cell adhesion molecules and leukocyte adhesion and thrombosis and subsequent uptake of FDG in thrombosed areas [12,129]. Incidental findings of thrombosis detected by FDG-PET/CT in various malignant and non-malignant diseases including hepatic lymphoma, colon cancer, pancreatic neuroendocrine tumor, alcoholic cirrhosis, renal cell carcinoma, have been reported in the literature [130-136], mainly considered as tumor thrombi.
Ability of FDG-PET/CT for recurrent and new VTE differentiation, detection of malignancy secondary to VTE, VTE and tumor thrombosis discrimination has been reviewed previously [10]. However applicability of FDG-PET/CT as an all in one imaging method and use in new patient categories and non proximal lower extremity areas (like calf, pelvic area, upper extremity, sagittal sinus thrombosis and PE) is still unclear and further studies are needed to clarify that.
Rondina et al. [12] prospectively studied 12 patients with confirmed proximal thrombosis of the lower limb using FDG-PET/CT and detected thrombosis with sensitivity of 87.5-100% and specificity of 87.5-100% varying with two thresholds for maximum standard uptake value (SUVmax) (1.64 and 1.49). There was a negative correlation between maximum metabolic activity in thrombosed veins (SUVmax) and time from DVT symptom onset suggesting a steady decrease over time, a prerequisite for differentiation between old and new thrombi, and for response evaluation.
In another study, Hess et al. [137] prospectively examined 15 patients with suspected DVT and/or PE with subsequent FDG-PET/CT less than 24 hours after the diagnosis had been confirmed or ruled out based on local guidelines. Images were interpreted visually, i.e. DVT/PE was considered present with focal or linear increased FDG uptake within the veins/pulmonary vasculature, and absent with no pathologic FDG uptake. They found FDG-PET/CT able to correctly diagnose or rule out acute DVT in all patients (Figures 3, 4), whereas PE cases were less favorable with only 2 out of 6 PE patients being positive on FDG-PET/CT.
Figure 3.

New deep vein thrombosis in right leg. FDG MIP image (A), axial PET (B), and axial PET/CT (C) show high metabolic activity (red arrows) in a dilated vein consistent with a fresh new clot.
Figure 4.

Suspected recurrence of deep vein thrombosis in right leg. Axial CT (A) shows a dilated vein with no metabolic activity in combined axial PET/CT (B, red arrows) consistent with inactive old changes; no new clot.
Based on results of abovementioned studies and these preliminary results of volumetric FDG-PET/CT parameters, FDG-PET/CT biomarkers appear to be promising for detection of deep vein thrombosis and other VTE subcategories. However, confounding factors contributing to false positive results, such as co-existing inflammation or infection should be considered while interpreting FDG-PET/CT for diagnosis of DVT [138,139].
Gynecologic thrombotic disorders such as VTE in pregnancy pose a major diagnostic challenge since no test diagnostic test has shown adequate accuracy with acceptable margin of safety [140,141]. DVT in pregnancy usually occurs in proximal veins and therefore, currently accepted first test in patients with clinical suspicion of DVT is compression ultrasonography but based on represented data, the test results appear suboptimal [140]. One of the advantages of molecular imaging based technique is its ability to visualize clots in the venous system in the anatomical regions such as pelvis, which is not feasible by other modalities. Since pelvis is considered a source of clot formation and PE, molecular imaging and specifically FDG-PET might be a useful modality for detection of clots in this region.
Tumor thrombosis and FDG-PET/CT
In most patient studies reported in the literature about VTE and FDG-PET/CT, the thrombus was found incidentally or retrospectively in patients investigated because of underlying malignancy, and thus, presented data on the differential diagnosis between “benign” (bland) and “malignant” (tumor) thrombi.
Davidson et al. [142] retrospectively evaluated 11 patients with suspected tumor thrombosis. FDG-PET/CT scans were considered positive if they demonstrated focal or linear uptake in the involved vessel. Eight occult tumor thromboses were found by FDG-PET/CT, in patients with gastrointestinal, renal, head and neck malignancies or lymphoma. Three thrombotic lesions were PET negative and these were due to VTE or leiomyomatosis. Thus in this study, FDG-PET/CT could distinguish benign from malignant thrombosis in all patients.
Lee et al. [143] have retrospectively evaluated FDG-PET/CT for the detection of tumor thrombosis. They reviewed 24 sites of thromboembolism in 15 patients with contrast enhanced CT scan, metabolic activity was measured by drawing regions of interest (ROI) and recording SUVmax of the site of thrombosis. ROC analysis was done to determine cutoff point of SUVmax for detection of tumor thrombosis. There was a significant difference between tumor and bland thombus SUVmax (P<0.005) and a cutoff of 2.25 with sensitivity of 78% and specificity of 100% was determined for SUVmax to differentiate tumor thrombus and bland thrombus.
Sharma et al. [144] retrospectively reviewed FDG-PET/CT of patients with known malignancy and FDG avid thrombosis. SUVmax was measured for blood pool, tumor, and thrombus. Average SUVmax in benign thrombosis groups was 3.2 and 6 in tumor thrombosis group with a significant difference (P=0.013). ROC analysis showed a cut-off SUVmax of 3.63 (71.4% Sensitivity and 90% Specificity) for differentiation of benign and malignant thromboembolism.
Rondina et al. [145] evaluated FDG-PET/CT as a comprehensive screening strategy for occult malignancy. In their pilot study they prospectively investigated 40 patients with unprovoked VTE by use of FDG-PET/CT for detection of occult malignancy. Patients underwent whole body FDG-PET/CT and followed for two years. In 62.5% of patients FDG-PET/CT identified abnormal findings requiring additional evaluations. In this pilot study, occult malignancy was present in only one patient, and FDG-PET/CT was able to rule out malignancy in the remainder.
Callejas et al. [146] in their retrospective survival study of 1331 cancer patients who underwent FDG-PET/CT, detected VTE in 19 patients. Incidental VTE detected by PET-CT was associated with poor survival independently with a hazard ratio of 2.03.
Ravina et al. [147] in another study from our center retrospectively reviewed all FDG-PET/CT scans of oncology cases during a 5 year period and found 21 patients with tumor thrombosis. Average SUVmax of the primary tumors was 10.3 and average SUVmax of thrombi was 7.85. The results from this study were in line with other studies regarding usefulness of FDG-PET/CT in tumor thrombosis, and the authors suggested that SUVmax and patterns of FDG uptake can be helpful for differentiating bland thrombus from tumor thrombus in oncological patients (Figure 5).
Figure 5.

The FDG-PET image above demonstrates relatively intense uptake of FDG in superior vena cava of an 8-year-old patient with neuroendocrine malignancy, which was interpreted to represent active thrombotic lesion in this location. Metastatic uptake in the cervical lymph nodes on the left side is also visible.
In another unpublished study, we investigated FDG-PET/CT evidence of VTE among cancer patients with clinical VTE diagnosis. In 10 multiple myeloma patients sequential FDG-PET/CT scans before and after VTE diagnosis were reviewed. In 9 out of 10 cases, VTE associated FDG uptake was found at the time of the clinical VTE diagnosis as well as in all FDG-PET/CT performed prior to the clinical VTE diagnosis. Thus, FDG-PET/CT appears to be sensitive for early detection of VTE processes, even in pre-symptomatic patients.
Septic thrombophlebitis and FDG-PET/CT
Miceli et al. [148] evaluated the role of FDG-PET/CT in diagnosis and management of septic deep thrombophlebitis. They prospectively observed patients with cancer and suspected septic thrombophlebitis and retrospectively reviewed patients with cancer and DVT using FDG-PET/CT. They found a strong uptake in septic thrombophlebitis patients while none of 27 DVT cases had increased FDG uptake. FDG PET was also able to detect central vein septic thrombophlebitis in five patients and caused change in management, while other modalities such as duplex scan and venography were less helpful.
Bleeker-Rovers et al. [149] reported a case of septic thrombophlebitis in portal vein in a 73-year-old man with fever of unknown origin diagnosed by FDG PET.
We have also previously reported the incidental detection of septic thrombophlebitis (both DVT and PE) in a patient with bacteraemia in which the diagnosis of VTE was not considered until the FDG-PET/CT scan was done [150] (Figure 6).
Figure 6.
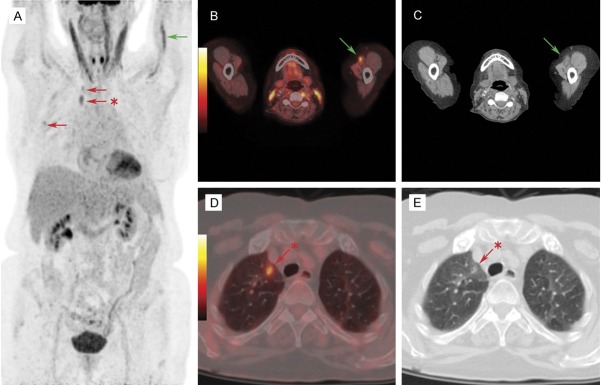
A: A 77-year-old female patient presented with Staphylococcus aureus bacteremia presumed to originate from a peripherally inserted central catheter. The catheter had been removed and the patient underwent FDG PET/CT to establish the extent of disease. Maximum intensity projection image showed unexpected FDG accumulation in the left brachial vein proximal to the removed catheter (green arrow) and in the right lung (red arrows). B, C: Transaxial image of FDG-avid left brachial vein (green arrow). Corresponding CT image revealed stranding in surrounding fat suggestive of inflammation. Subsequent ultrasound confirmed deep venous thrombosis (DVT) of the left brachial vein. D, E: FDG PET showed intense FDG uptake peripherally in the parenchyma of the right lung with barely visible findings on CT (red arrows). Adapted with permission from Lippincott Williams and Wilkins/Wolters Kluwer Health: Clinical Nuclear Medicine [150], copyright (2013).
These studies show FDG-PET/CT is able to diagnose septic thrombophlebitis, especially in hard to reach areas and in patients with unsuspected VTE/septic thrombophlebitis.
Summary and prospective on future imaging techniques for suspected vte
In this review, we have described in great detail imaging techniques that have been employed for detecting clots in the venous system as an important health care issue worldwide. Recent efforts in establishing and optimizing molecular imaging probes that target specific ingredients in the blood clots, opened new horizons for detection of thrombus and overcoming shortcomings of the existing techniques. Examples of these targets are GP IIb/IIIa cyclic RGD peptides and cyclic fibrin binding peptides. Apcitide is the only FDA approved agent among these tracers, which is in phase III multicenter study for DVT. Further studies using other modalities such as PET are suggested to evaluate the role of Apcitide in detection of DVT. Another recently introduced agent investigated in multimodality MRI/PET/SPECT/NIRF studies is EP-2104R. However, only the MR modality has proceeded to human feasibility studies with positive results. Anti D-dimer DI-80B3 Fab’ fragment (Thromboview) is another newly introduced agent which has shown favorable sensitivity and specificity for detection of suspected DVT and acute PE.
FDG-PET/CT has been able to detect thrombosis, mainly in the venous system anywhere in the body in patients with suspected DVT. Preliminary data suggest an acceptable sensitivity in the early, even in pre-symptomatic patients. It can also differentiate acute from chronic thrombi that are no longer active and eliminate the need for unnecessary treatment. Furthermore, accurate quantification with FDG-PET/CT allows monitoring response to treatment. Although molecular imaging is not likely to replace established methods of detection of DVT as a first line modality in the near future it is useful in certain clinical settings. FDG-PET/CT might be a good candidate for aiding diagnosis of DVT because of the limited value of conventional structural imaging modalities. For example, patients with renal disease with contraindication for contrast-enhanced studies might benefit from this modality.
We believe current imaging techniques including radiologic modalities as well as non-PET based approaches that have been described in the literature suffer from certain deficiencies. This is particularly true when we wish to distinguish acute from chronic disease. Therefore, we believe FGD-PET might play a role in detecting thrombosis in the venous system in some clinical settings. Other tracers might be applicable in the future.
At this juncture, FDG-PET imaging appears very promising for suspected VTE and therefore promises a potential role in managing this common disorder. We hope that soon a multicentric trial, sponsored by the National Institute of Health (NIH) or American College of Radiology Imaging Network (ACRIN) will determine the definitive role of FDG-PET imaging in this setting and this eventually will lead to routine use of FDG-PET as an effective method in this common and potentially fatal disease.
Disclosure of conflict of interest
The authors have declared that no competing interest exists.
References
- 1.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 2.Spencer FA, Goldberg RJ, Lessard D, Reed G, Emery C, Gore JM, Pacifico L, Weitz JI. Factors associated with adverse outcomes in outpatients presenting with pulmonary embolism: the Worcester Venous Thromboembolism Study. Circ Cardiovasc Qual Outcomes. 2010;3:390–394. doi: 10.1161/CIRCOUTCOMES.110.937441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White RH, Zhou H, Murin S, Harvey D. Effect of ethnicity and gender on the incidence of venous thromboembolism in a diverse population in California in 1996. Thromb Haemost. 2005;93:298–305. doi: 10.1160/TH04-08-0506. [DOI] [PubMed] [Google Scholar]
- 4.Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38:S495–501. doi: 10.1016/j.amepre.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 6.Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29:943–953. doi: 10.1592/phco.29.8.943. [DOI] [PubMed] [Google Scholar]
- 7.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107:I4–8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 8.Nitta D, Mitani H, Ishimura R, Moriya M, Fujimoto Y, Ishiwata S, Yamaguchi T, Ohno M. Deep vein thrombosis risk stratification. Int Heart J. 2013;54:166–170. doi: 10.1536/ihj.54.166. [DOI] [PubMed] [Google Scholar]
- 9.Spyropoulos AC, Hurley JS, Ciesla GN, de Lissovoy G. Management of acute proximal deep vein thrombosis: pharmacoeconomic evaluation of outpatient treatment with enoxaparin vs inpatient treatment with unfractionated heparin. Chest. 2002;122:108–114. doi: 10.1378/chest.122.1.108. [DOI] [PubMed] [Google Scholar]
- 10.Hess S, Madsen PH, Basu S, Hoilund-Carlsen PF, Alavi A. Potential role of FDG PET/CT imaging for assessing venous thromboembolic disorders. Clin Nucl Med. 2012;37:1170–1172. doi: 10.1097/RLU.0b013e318279bf73. [DOI] [PubMed] [Google Scholar]
- 11.Wheeler HB, Anderson FA Jr. Diagnostic methods for deep vein thrombosis. Haemostasis. 1995;25:6–26. doi: 10.1159/000217140. [DOI] [PubMed] [Google Scholar]
- 12.Rondina MT, Lam UT, Pendleton RC, Kraiss LW, Wanner N, Zimmerman GA, Hoffman JM, Hanrahan C, Boucher K, Christian PE, Butterfield RI, Morton KA. (18)F-FDG PET in the evaluation of acuity of deep vein thrombosis. Clin Nucl Med. 2012;37:1139–1145. doi: 10.1097/RLU.0b013e3182638934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drescher R, Freesmeyer M. PET angiography: Application of early dynamic PET/CT to the evaluation of arteries. AJR Am J Roentgenol. 2013;201:908–911. doi: 10.2214/AJR.12.10438. [DOI] [PubMed] [Google Scholar]
- 14.Screaton NJ, Gillard JH, Berman LH, Kemp PM. Duplicated superficial femoral veins: a source of error in the sonographic investigation of deep vein thrombosis. Radiology. 1998;206:397–401. doi: 10.1148/radiology.206.2.9457192. [DOI] [PubMed] [Google Scholar]
- 15.Taillefer R. Radiolabeled peptides in the detection of deep venous thrombosis. Semin Nucl Med. 2001;31:102–123. doi: 10.1053/snuc.2001.21268. [DOI] [PubMed] [Google Scholar]
- 16.Cronan JJ. Venous thromboembolic disease: the role of US. Radiology. 1993;186:619–630. doi: 10.1148/radiology.186.3.8430164. [DOI] [PubMed] [Google Scholar]
- 17.Baglin T. What happens after venous thromboembolism? J Thromb Haemost. 2009;7(Suppl 1):287–290. doi: 10.1111/j.1538-7836.2009.03409.x. [DOI] [PubMed] [Google Scholar]
- 18.Nazaroglu H, Ozmen CA, Akay HO, Kilinc I, Bilici A. 64-MDCT pulmonary angiography and CT venography in the diagnosis of thromboembolic disease. AJR Am J Roentgenol. 2009;192:654–661. doi: 10.2214/AJR.07.3939. [DOI] [PubMed] [Google Scholar]
- 19.Wilbur J, Shian B. Diagnosis of deep venous thrombosis and pulmonary embolism. Am Fam Physician. 2012;86:913–919. [PubMed] [Google Scholar]
- 20.Hobbs JT, Davies JW. Detection of venous thrombosis with 131I-labelled fibrinogen in the rabbit. Lancet. 1960;2:134–135. doi: 10.1016/s0140-6736(60)91272-1. [DOI] [PubMed] [Google Scholar]
- 21.Perrier A. Labeling the thrombus: the future of nuclear medicine for venous thromboembolism? Am J Respir Crit Care Med. 2004;169:977–978. doi: 10.1164/rccm.2403001. [DOI] [PubMed] [Google Scholar]
- 22.Fang W, He J, Kim YS, Zhou Y, Liu S. Evaluation of 99mTc-labeled cyclic RGD peptide with a PEG4 linker for thrombosis imaging: comparison with DMP444. Bioconjug Chem. 2011;22:1715–1722. doi: 10.1021/bc2003742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerqueira MD, Stratton JR, Vracko R, Schaible TF, Ritchie JL. Noninvasive arterial thrombus imaging with 99mTc monoclonal antifibrin antibody. Circulation. 1992;85:298–304. doi: 10.1161/01.cir.85.1.298. [DOI] [PubMed] [Google Scholar]
- 24.Douketis JD, Ginsberg JS, Haley S, Julian J, Dwyer M, Levine M, Eisenberg PR, Smart R, Tsui W, White RH, Morris TA, Kaatz S, Comp PC, Crowther MA, Kearon C, Kassis J, Bates SM, Schulman S, Desjardins L, Taillefer R, Begelman SM, Gerometta M. Accuracy and safety of (99m)Tc-labeled anti-D-dimer (DI-80B3) Fab’ fragments (ThromboView(R)) in the diagnosis of deep vein thrombosis: a phase II study. Thromb Res. 2012;130:381–389. doi: 10.1016/j.thromres.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Starmans LW, van Duijnhoven SM, Rossin R, Berben M, Aime S, Daemen MJ, Nicolay K, Grull H. Evaluation of In-Labeled EPep and FibPep as Tracers for Fibrin SPECT Imaging. Mol Pharm. 2013;10:4309–21. doi: 10.1021/mp400406x. [DOI] [PubMed] [Google Scholar]
- 26.Starmans LW, van Duijnhoven SM, Rossin R, Aime S, Daemen MJ, Nicolay K, Grull H. SPECT imaging of fibrin using fibrin-binding peptides. Contrast Media Mol Imaging. 2013;8:229–237. doi: 10.1002/cmmi.1521. [DOI] [PubMed] [Google Scholar]
- 27.Ciesienski KL, Yang Y, Ay I, Chonde DB, Loving GS, Rietz TA, Catana C, Caravan P. Fibrin-targeted PET probes for the detection of thrombi. Mol Pharm. 2013;10:1100–1110. doi: 10.1021/mp300610s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calvete JJ. The continuing saga of snake venom disintegrins. Toxicon. 2013;62:40–49. doi: 10.1016/j.toxicon.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Ji S, Fang W, Dong N, He Z, Ruan C. Detection of thromboembolism with (99)mTc-labeled F(ab)(2) fragment of anti-glycoprotein IIIa chimeric monoclonal antibody in beagle canines. Thromb Res. 2012;130:703–708. doi: 10.1016/j.thromres.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Chakraborty S, Liu S. Radiolabeled Cyclic RGD Peptides as Radiotracers for Imaging Tumors and Thrombosis by SPECT. Theranostics. 2011;1:58–82. doi: 10.7150/thno/v01p0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macfarlane D, Socrates A, Eisenberg P, Larcos G, Roach P, Gerometta M, Smart R, Tsui W, Scott AM. Imaging of deep venous thrombosis in patients using a radiolabelled anti-D-dimer Fab’ fragment (99mTc-DI-DD3B6/22-80B3): results of a phase I trial. Eur J Nucl Med Mol Imaging. 2009;36:250–259. doi: 10.1007/s00259-008-0934-7. [DOI] [PubMed] [Google Scholar]
- 32.Spuentrup E, Botnar RM, Wiethoff AJ, Ibrahim T, Kelle S, Katoh M, Ozgun M, Nagel E, Vymazal J, Graham PB, Gunther RW, Maintz D. MR imaging of thrombi using EP-2104R, a fibrin-specific contrast agent: initial results in patients. Eur Radiol. 2008;18:1995–2005. doi: 10.1007/s00330-008-0965-2. [DOI] [PubMed] [Google Scholar]
- 33.Dunzinger A, Hafner F, Schaffler G, Piswanger-Soelkner JC, Brodmann M, Lipp RW. 99mTc-apcitide scintigraphy in patients with clinically suspected deep venous thrombosis and pulmonary embolism. Eur J Nucl Med Mol Imaging. 2008;35:2082–2087. doi: 10.1007/s00259-008-0863-5. [DOI] [PubMed] [Google Scholar]
- 34.Knight LC, Romano JE, Bright LT, Agelan A, Kantor S, Maurer AH. Platelet binding and biodistribution of [99mTc] rBitistatin in animal species and humans. Nucl Med Biol. 2007;34:855–863. doi: 10.1016/j.nucmedbio.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brighton T, Janssen J, Butler SP. Aging of acute deep vein thrombosis measured by radiolabeled 99mTc-rt-PA. J Nucl Med. 2007;48:873–878. doi: 10.2967/jnumed.106.039396. [DOI] [PubMed] [Google Scholar]
- 36.Aruva MR, Daviau J, Sharma SS, Thakur ML. Imaging thromboembolism with fibrin-avid 99mTc-peptide: evaluation in swine. J Nucl Med. 2006;47:155–162. [PMC free article] [PubMed] [Google Scholar]
- 37.Davison JM, Bridwell R, Montilla JL, Jackson E, Moores LK. A novel diagnostic method for acute pulmonary embolism: technetium-99m apcitide scintigraphy. Ann Intern Med. 2004;140:936–939. doi: 10.7326/0003-4819-140-11-200406010-00028. [DOI] [PubMed] [Google Scholar]
- 38.Tung CH, Ho NH, Zeng Q, Tang Y, Jaffer FA, Reed GL, Weissleder R. Novel factor XIII probes for blood coagulation imaging. Chembiochem. 2003;4:897–899. doi: 10.1002/cbic.200300602. [DOI] [PubMed] [Google Scholar]
- 39.Taillefer R, Edell S, Innes G, Lister-James J. Acute thromboscintigraphy with (99m)Tc-apcitide: results of the phase 3 multicenter clinical trial comparing 99mTc-apcitide scintigraphy with contrast venography for imaging acute DVT. Multicenter Trial Investigators. J Nucl Med. 2000;41:1214–1223. [PubMed] [Google Scholar]
- 40.Mitchel J, Waters D, Lai T, White M, Alberghini T, Salloum A, Knibbs D, Li D, Heller GV. Identification of coronary thrombus with a IIb/IIIa platelet inhibitor radiopharmaceutical, technetium-99m DMP-444: A canine model. Circulation. 2000;101:1643–1646. doi: 10.1161/01.cir.101.14.1643. [DOI] [PubMed] [Google Scholar]
- 41.Klem JA, Schaffer JV, Crane PD, Barrett JA, Henry GA, Canestri L, Ezekowitz MD. Detection of deep venous thrombosis by DMP 444, a platelet IIb/IIIa antagonist: a preliminary report. J Nucl Cardiol. 2000;7:359–364. doi: 10.1067/mnc.2000.106967. [DOI] [PubMed] [Google Scholar]
- 42.Mousa SA, Bozarth JM, Edwards S, Carroll T, Barrett J. Novel technetium-99m-labeled platelet GPIIb/IIIa receptor antagonists as potential imaging agents for venous and arterial thrombosis. Coron Artery Dis. 1998;9:131–141. [PubMed] [Google Scholar]
- 43.Muto P, Lastoria S, Varrella P, Vergara E, Salvatore M, Morgano G, Lister-James J, Bernardy JD, Dean RT, Wencker D, et al. Detecting deep venous thrombosis with technetium-99m-labeled synthetic peptide P280. J Nucl Med. 1995;36:1384–1391. [PubMed] [Google Scholar]
- 44.Lopez JA, Chen J. Pathophysiology of venous thrombosis. Thromb Res. 2009;123(Suppl 4):S30–34. doi: 10.1016/S0049-3848(09)70140-9. [DOI] [PubMed] [Google Scholar]
- 45.Lowe GD. Virchow’s triad revisited: abnormal flow. Pathophysiol Haemost Thromb. 2003;33:455–457. doi: 10.1159/000083845. [DOI] [PubMed] [Google Scholar]
- 46.Sevitt S. The structure and growth of valve-pocket thrombi in femoral veins. J Clin Pathol. 1974;27:517–528. doi: 10.1136/jcp.27.7.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cerqueira MD. Current status of radionuclide tracer imaging of thrombi and atheroma. Semin Nucl Med. 1999;29:339–351. doi: 10.1016/s0001-2998(99)80021-x. [DOI] [PubMed] [Google Scholar]
- 48.Lopez JA, Kearon C, Lee AY. Deep venous thrombosis. Hematology Am Soc Hematol Educ Program. 2004:439–456. doi: 10.1182/asheducation-2004.1.439. [DOI] [PubMed] [Google Scholar]
- 49.Bagot CN, Arya R. Virchow and his triad: a question of attribution. Br J Haematol. 2008;143:180–190. doi: 10.1111/j.1365-2141.2008.07323.x. [DOI] [PubMed] [Google Scholar]
- 50.Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol. 2008;28:387–391. doi: 10.1161/ATVBAHA.108.162289. [DOI] [PubMed] [Google Scholar]
- 51.Chung I, Lip GY. Virchow’s triad revisited: blood constituents. Pathophysiol Haemost Thromb. 2003;33:449–454. doi: 10.1159/000083844. [DOI] [PubMed] [Google Scholar]
- 52.Blann AD. How a damaged blood vessel wall contibutes to thrombosis and hypertenasion. Pathophysiol Haemost Thromb. 2003;33:445–448. doi: 10.1159/000083843. [DOI] [PubMed] [Google Scholar]
- 53.Wolberg AS. Determinants of fibrin formation, structure, and function. Curr Opin Hematol. 2012;19:349–356. doi: 10.1097/MOH.0b013e32835673c2. [DOI] [PubMed] [Google Scholar]
- 54.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 55.Kakkar VV, Nicolaides AN, Renney JT, Friend JR, Clarke MB. 125-I-labelled fibrinogen test adapted for routine screening for deep-vein thrombosis. Lancet. 1970;1:540–542. doi: 10.1016/s0140-6736(70)90769-5. [DOI] [PubMed] [Google Scholar]
- 56.Browse NL. The 125I fibrinogen uptake test. Arch Surg. 1972;104:160–163. doi: 10.1001/archsurg.1972.04180020040007. [DOI] [PubMed] [Google Scholar]
- 57.Lensing AW, Hirsh J. 125I-fibrinogen leg scanning: reassessment of its role for the diagnosis of venous thrombosis in post-operative patients. Thromb Haemost. 1993;69:2–7. [PubMed] [Google Scholar]
- 58.Schaible TF, Alavi A. Antifibrin scintigraphy in the diagnostic evaluation of acute deep venous thrombosis. Semin Nucl Med. 1991;21:313–324. doi: 10.1016/s0001-2998(05)80134-5. [DOI] [PubMed] [Google Scholar]
- 59.Rosebrough SF, Kudryk B, Grossman ZD, McAfee JG, Subramanian G, Ritter-Hrncirik CA, Witanowski LS, Tillapaugh-Fay G. Radioimmunoimaging of venous thrombi using iodine-131 monoclonal antibody. Radiology. 1985;156:515–517. doi: 10.1148/radiology.156.2.4011916. [DOI] [PubMed] [Google Scholar]
- 60.Rosebrough SF, Grossman ZD, McAfee JG, Kudryk BJ, Subramanian G, Ritter-Hrncirik CA, Witanowski LS, Tillapaugh-Fay G, Urrutia E, Zapf-Longo C. Thrombus imaging with indium-111 and iodine-131-labeled fibrin-specific monoclonal antibody and its F(ab’)2 and Fab fragments. J Nucl Med. 1988;29:1212–1222. [PubMed] [Google Scholar]
- 61.Rosebrough SF, Grossman ZD, McAfee JG, Kudryk BJ, Subramanian G, Ritter-Hrncirik CA, Witanowski LS, Tillapaugh-Fay G, Urrutia E. Aged venous thrombi: radioimmunoimaging with fibrin-specific monoclonal antibody. Radiology. 1987;162:575–577. doi: 10.1148/radiology.162.2.3797675. [DOI] [PubMed] [Google Scholar]
- 62.Knight LC, Maurer AH, Ammar IA, Epps LA, Dean RT, Pak KY, Berger HJ. Tc-99m antifibrin Fab’ fragments for imaging venous thrombi: evaluation in a canine model. Radiology. 1989;173:163–169. doi: 10.1148/radiology.173.1.2781002. [DOI] [PubMed] [Google Scholar]
- 63.Knight LC, Maurer AH, Ammar IA, Shealy DJ, Mattis JA. Evaluation of indium-111-labeled anti-fibrin antibody for imaging vascular thrombi. J Nucl Med. 1988;29:494–502. [PubMed] [Google Scholar]
- 64.Alavi A, Gupta N, Palevsky HI, Kelley MA, Jatlow AD, Byar AA, Berger HJ. Detection of thrombophlebitis with 111In-labeled anti-fibrin antibody: preliminary results. Cancer Res. 1990;50:958s–961s. [PubMed] [Google Scholar]
- 65.Alavi A, Palevsky HI, Gupta N, Meranze S, Kelley MA, Jatlow AD, Schaible TF, Brown J, Berger HJ. Radiolabeled antifibrin antibody in the detection of venous thrombosis: preliminary results. Radiology. 1990;175:79–85. doi: 10.1148/radiology.175.1.2315506. [DOI] [PubMed] [Google Scholar]
- 66.Bautovich G, Angelides S, Lee FT, Greenough R, Bundesen P, Murray P, Schmidt P, Waugh R, Harris J, Cameron K, et al. Detection of deep venous thrombi and pulmonary embolus with technetium-99m-DD-3B6/22 anti-fibrin monoclonal antibody Fab’ fragment. J Nucl Med. 1994;35:195–202. [PubMed] [Google Scholar]
- 67.De Faucal P, Peltier P, Planchon B, Dupas B, Touze MD, Baron D, Scaible T, Berger HJ, Chatal JF. Evaluation of indium-111-labeled antifibrin monoclonal antibody for the diagnosis of venous thrombotic disease. J Nucl Med. 1991;32:785–791. [PubMed] [Google Scholar]
- 68.Hara T, Bhayana B, Thompson B, Kessinger CW, Khatri A, McCarthy JR, Weissleder R, Lin CP, Tearney GJ, Jaffer FA. Molecular imaging of fibrin deposition in deep vein thrombosis using fibrin-targeted near-infrared fluorescence. JACC Cardiovasc Imaging. 2012;5:607–615. doi: 10.1016/j.jcmg.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jung M, Kletter K, Dudczak R, Koppensteiner R, Minar E, Kahls P, Stumpflen A, Pokieser P, Ehringer H. Deep vein thrombosis: scintigraphic diagnosis with In-111-labeled monoclonal antifibrin antibodies. Radiology. 1989;173:469–475. doi: 10.1148/radiology.173.2.2678259. [DOI] [PubMed] [Google Scholar]
- 70.Morris TA, Gerometta M, Yusen RD, White RH, Douketis JD, Kaatz S, Smart RC, Macfarlane D, Ginsberg JS. Detection of pulmonary emboli with 99mTc-labeled anti-D-dimer (DI-80B3)Fab’ fragments (ThromboView) Am J Respir Crit Care Med. 2011;184:708–714. doi: 10.1164/rccm.201104-0624OC. [DOI] [PubMed] [Google Scholar]
- 71.Morris TA, Marsh JJ, Chiles PG, Konopka RG, Pedersen CA, Schmidt PF, Gerometta M. Single photon emission computed tomography of pulmonary emboli and venous thrombi using anti-D-dimer. Am J Respir Crit Care Med. 2004;169:987–993. doi: 10.1164/rccm.200306-735OC. [DOI] [PubMed] [Google Scholar]
- 72.Morris TA, Marsh JJ, Konopka R, Pedersen CA, Chiles PG. Improved imaging of deep venous thrombi during anticoagulation using radiolabelled anti-D-dimer antibodies. Nucl Med Commun. 2004;25:917–922. doi: 10.1097/00006231-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 73.Rosebrough SF, McAfee JG, Grossman ZD, Kudryk BJ, Ritter-Hrncirik CA, Witanowski LS, Maley BL, Bertrand EA, Gagne GM. Thrombus imaging: a comparison of radiolabeled GC4 and T2G1s fibrin-specific monoclonal antibodies. J Nucl Med. 1990;31:1048–1054. [PubMed] [Google Scholar]
- 74.Stratton JR, Cerqueira MD, Dewhurst TA, Kohler TR. Imaging arterial thrombosis: comparison of technetium-99m-labeled monoclonal antifibrin antibodies and indium-111-platelets. J Nucl Med. 1994;35:1731–1737. [PubMed] [Google Scholar]
- 75.Thakur ML, Pallela VR, Consigny PM, Rao PS, Vessileva-Belnikolovska D, Shi R. Imaging vascular thrombosis with 99mTc-labeled fibrin alpha-chain peptide. J Nucl Med. 2000;41:161–168. [PubMed] [Google Scholar]
- 76.Walker KZ, Milner LJ, Bautovich GJ, Borham P, Wood AK, Rylatt DB, Martin P, Bundesen PG, Boniface GR. Detection of experimental thrombi in rabbits with an 131I-labelled fibrin-specific monoclonal antibody. Eur J Nucl Med. 1990;16:787–794. doi: 10.1007/BF00833012. [DOI] [PubMed] [Google Scholar]
- 77.Hui KY, Haber E, Matsueda GR. Monoclonal antibodies to a synthetic fibrin-like peptide bind to human fibrin but not fibrinogen. Science. 1983;222:1129–1132. doi: 10.1126/science.6648524. [DOI] [PubMed] [Google Scholar]
- 78.Kudryk B, Rohoza A, Ahadi M, Chin J, Wiebe ME. Specificity of a monoclonal antibody for the NH2-terminal region of fibrin. Mol Immunol. 1984;21:89–94. doi: 10.1016/0161-5890(84)90093-2. [DOI] [PubMed] [Google Scholar]
- 79.Lusiani L, Zanco P, Visona A, Breggion G, Pagnan A, Ferlin G. Immunoscintigraphic detection of venous thrombosis of the lower extremities by means of human antifibrin monoclonal antibodies labeled with 111In. Angiology. 1989;40:671–677. doi: 10.1177/000331978904000710. [DOI] [PubMed] [Google Scholar]
- 80.Knight LC, Abrams MJ, Schwartz DA, Hauser MM, Kollman M, Gaul FE, Rauh DA, Maurer AH. Preparation and preliminary evaluation of technetium-99m-labeled fragment E1 for thrombus imaging. J Nucl Med. 1992;33:710–715. [PubMed] [Google Scholar]
- 81.Kanke M, Matsueda GR, Strauss HW, Yasuda T, Liau CS, Khaw BA. Localization and visualization of pulmonary emboli with radiolabeled fibrin-specific monoclonal antibody. J Nucl Med. 1991;32:1254–1260. [PubMed] [Google Scholar]
- 82.Hisada Y, Yasunaga M, Hanaoka S, Saijou S, Sugino T, Tsuji A, Saga T, Tsumoto K, Manabe S, Kuroda J, Kuratsu J, Matsumura Y. Discovery of an uncovered region in fibrin clots and its clinical significance. Sci Rep. 2013;3:2604. doi: 10.1038/srep02604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sirol M, Fuster V, Badimon JJ, Fallon JT, Moreno PR, Toussaint JF, Fayad ZA. Chronic thrombus detection with in vivo magnetic resonance imaging and a fibrin-targeted contrast agent. Circulation. 2005;112:1594–1600. doi: 10.1161/CIRCULATIONAHA.104.522110. [DOI] [PubMed] [Google Scholar]
- 84.Botnar RM, Perez AS, Witte S, Wiethoff AJ, Laredo J, Hamilton J, Quist W, Parsons EC Jr, Vaidya A, Kolodziej A, Barrett JA, Graham PB, Weisskoff RM, Manning WJ, Johnstone MT. In vivo molecular imaging of acute and subacute thrombosis using a fibrin-binding magnetic resonance imaging contrast agent. Circulation. 2004;109:2023–2029. doi: 10.1161/01.CIR.0000127034.50006.C0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vymazal J, Spuentrup E, Cardenas-Molina G, Wiethoff AJ, Hartmann MG, Caravan P, Parsons EC Jr. Thrombus imaging with fibrin-specific gadolinium-based MR contrast agent EP-2104R: results of a phase II clinical study of feasibility. Invest Radiol. 2009;44:697–704. doi: 10.1097/RLI.0b013e3181b092a7. [DOI] [PubMed] [Google Scholar]
- 86.Spuentrup E, Buecker A, Katoh M, Wiethoff AJ, Parsons EC Jr, Botnar RM, Weisskoff RM, Graham PB, Manning WJ, Gunther RW. Molecular magnetic resonance imaging of coronary thrombosis and pulmonary emboli with a novel fibrin-targeted contrast agent. Circulation. 2005;111:1377–1382. doi: 10.1161/01.CIR.0000158478.29668.9B. [DOI] [PubMed] [Google Scholar]
- 87.Uppal R, Catana C, Ay I, Benner T, Sorensen AG, Caravan P. Bimodal thrombus imaging: simultaneous PET/MR imaging with a fibrin-targeted dual PET/MR probe--feasibility study in rat model. Radiology. 2011;258:812–820. doi: 10.1148/radiol.10100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Overoye-Chan K, Koerner S, Looby RJ, Kolodziej AF, Zech SG, Deng Q, Chasse JM, McMurry TJ, Caravan P. EP-2104R: a fibrin-specific gadolinium-Based MRI contrast agent for detection of thrombus. J Am Chem Soc. 2008;130:6025–6039. doi: 10.1021/ja800834y. [DOI] [PubMed] [Google Scholar]
- 89.Ay I, Blasi F, Rietz TA, Rotile NJ, Kura S, Brownell AL, Day H, Oliveira BL, Looby RJ, Caravan P. In Vivo Molecular Imaging of Thrombosis and Thrombolysis Using a Fibrin-binding Positron Emission Tomography Probe. Circ Cardiovasc Imaging. 2014;7:697–705. doi: 10.1161/CIRCIMAGING.113.001806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Laudano AP, Doolittle RF. Synthetic peptide derivatives that bind to fibrinogen and prevent the polymerization of fibrin monomers. Proc Natl Acad Sci U S A. 1978;75:3085–3089. doi: 10.1073/pnas.75.7.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kawasaki K, Miyano M, Hirase K, Iwamoto M. Amino acids and peptides. XVIII. Synthetic peptides related to N-terminal portion of fibrin alpha-chain and their inhibitory effect on fibrinogen/thrombin clotting. Chem Pharm Bull (Tokyo) 1993;41:975–977. doi: 10.1248/cpb.41.975. [DOI] [PubMed] [Google Scholar]
- 92.Rylatt DB, Blake AS, Cottis LE, Massingham DA, Fletcher WA, Masci PP, Whitaker AN, Elms M, Bunce I, Webber AJ, et al. An immunoassay for human D dimer using monoclonal antibodies. Thromb Res. 1983;31:767–778. doi: 10.1016/0049-3848(83)90108-1. [DOI] [PubMed] [Google Scholar]
- 93.Walker KZ, Boniface GR, Phippard AF, Harewood W, Bautovich GJ, Bundesen PG. Preclinical evaluation of 99m technetium-labeled DD-3B6/22 Fab’ for thrombus detection. Thromb Res. 1991;64:691–701. doi: 10.1016/0049-3848(91)90069-9. [DOI] [PubMed] [Google Scholar]
- 94.Morris TA, Marsh JJ, Konopka R, Pedersen CA, Chiles PG, Fagnani R, Hagan M, Moser KM. Antibodies against the fibrin beta-chain amino-terminus detect active canine venous thrombi. Circulation. 1997;96:3173–3179. doi: 10.1161/01.cir.96.9.3173. [DOI] [PubMed] [Google Scholar]
- 95.Rosenthall L, Leclerc J. A new thrombus imaging agent. Human recombinant fibrin binding domain labeled with In-111. Clin Nucl Med. 1995;20:398–402. doi: 10.1097/00003072-199505000-00004. [DOI] [PubMed] [Google Scholar]
- 96.McCarthy JR. Nanomedicine and Cardiovascular Disease. Curr Cardiovasc Imaging Rep. 2010;3:42–49. doi: 10.1007/s12410-009-9002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McCarthy JR, Patel P, Botnaru I, Haghayeghi P, Weissleder R, Jaffer FA. Multimodal nanoagents for the detection of intravascular thrombi. Bioconjug Chem. 2009;20:1251–1255. doi: 10.1021/bc9001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thakur ML, Welch MJ, Joist JH, Coleman RE. Indium-LLL labeled platelets: studies on preparation and evaluation of in vitro and in vivo functions. Thromb Res. 1976;9:345–357. doi: 10.1016/0049-3848(76)90135-3. [DOI] [PubMed] [Google Scholar]
- 99.Knight LC, Primeau JL, Siegel BA, Welch MJ. Comparison of In-111-labeled platelets and iodinated fibrinogen for the detection of deep vein thrombosis. J Nucl Med. 1978;19:891–894. [PubMed] [Google Scholar]
- 100.Seabold JE, Rosebrough SF. Will a radiolabeled antibody replace indium-111-platelets to detect active thrombus? J Nucl Med. 1994;35:1738–1740. [PubMed] [Google Scholar]
- 101.Glaudemans AW, Slart RH, Bozzao A, Bonanno E, Arca M, Dierckx RA, Signore A. Molecular imaging in atherosclerosis. Eur J Nucl Med Mol Imaging. 2010;37:2381–2397. doi: 10.1007/s00259-010-1406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wagner CL, Mascelli MA, Neblock DS, Weisman HF, Coller BS, Jordan RE. Analysis of GPIIb/IIIa receptor number by quantification of 7E3 binding to human platelets. Blood. 1996;88:907–914. [PubMed] [Google Scholar]
- 103.Shi J, Zhou Y, Chakraborty S, Kim YS, Jia B, Wang F, Liu S. Evaluation of In-Labeled Cyclic RGD Peptides: Effects of Peptide and Linker Multiplicity on Their Tumor Uptake, Excretion Kinetics and Metabolic Stability. Theranostics. 2011;1:322–340. doi: 10.7150/thno/v01p0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lang L, Li W, Jia HM, Fang DC, Zhang S, Sun X, Zhu L, Ma Y, Shen B, Kiesewetter DO, Niu G, Chen X. New Methods for Labeling RGD Peptides with Bromine-76. Theranostics. 2011;1:341–353. doi: 10.7150/thno/v01p0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fani M, Maecke HR, Okarvi SM. Radiolabeled peptides: valuable tools for the detection and treatment of cancer. Theranostics. 2012;2:481–501. doi: 10.7150/thno.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y, Yang Y, Cai W. Multimodality Imaging of Integrin alpha(v)beta(3) Expression. Theranostics. 2011;1:135–148. doi: 10.7150/thno/v01p0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou Y, Shao G, Liu S. Monitoring Breast Tumor Lung Metastasis by U-SPECT-II/CT with an Integrin alpha(v)beta(3)-Targeted Radiotracer(99m)Tc-3P-RGD(2) Theranostics. 2012;2:577–588. doi: 10.7150/thno.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bates SM, Lister-James J, Julian JA, Taillefer R, Moyer BR, Ginsberg JS. Imaging characteristics of a novel technetium Tc 99m-labeled platelet glycoprotein IIb/IIIa receptor antagonist in patients With acute deep vein thrombosis or a history of deep vein thrombosis. Arch Intern Med. 2003;163:452–456. doi: 10.1001/archinte.163.4.452. [DOI] [PubMed] [Google Scholar]
- 109.Brouwers FM, Oyen WJ, Boerman OC, Barrett JA, Verheugt FW, Corstens FH, Van der Meer JW. Evaluation of Tc-99m-labeled glycoprotein IIb/IIIa receptor antagonist DMP444 SPECT in patients with infective endocarditis. Clin Nucl Med. 2003;28:480–484. doi: 10.1097/01.RLU.0000067508.82824.75. [DOI] [PubMed] [Google Scholar]
- 110.Lister-James J, Knight LC, Maurer AH, Bush LR, Moyer BR, Dean RT. Thrombus imaging with a technetium-99m-labeled activated platelet receptor-binding peptide. J Nucl Med. 1996;37:775–781. [PubMed] [Google Scholar]
- 111.Sakuma T, Sari I, Goodman CN, Lindner JR, Klibanov AL, Kaul S. Simultaneous integrin alphavbeta3 and glycoprotein IIb/IIIa inhibition causes reduction in infarct size in a model of acute coronary thrombosis and primary angioplasty. Cardiovasc Res. 2005;66:552–561. doi: 10.1016/j.cardiores.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 112.Sakuma T, Sklenar J, Leong-Poi H, Goodman NC, Glover DK, Kaul S. Molecular imaging identifies regions with microthromboemboli during primary angioplasty in acute coronary thrombosis. J Nucl Med. 2004;45:1194–1200. [PubMed] [Google Scholar]
- 113.Taillefer R, Therasse E, Turpin S, Lambert R, Robillard P, Soulez G. Comparison of early and delayed scintigraphy with 99mTc-apcitide and correlation with contrast-enhanced venography in detection of acute deep vein thrombosis. J Nucl Med. 1999;40:2029–2035. [PubMed] [Google Scholar]
- 114.Carretta RF, Streek PV, Weiland FL. Optimizing images of acute deep-vein thrombosis using technetium-99m-apcitide. J Nucl Med Technol. 1999;27:271–275. [PubMed] [Google Scholar]
- 115.Oyen WJ, Boerman OC, Brouwers FM, Barrett JA, Verheugt FW, Ruiter DJ, Corstens FH, van der Meer JW. Scintigraphic detection of acute experimental endocarditis with the technetium-99m labelled glycoprotein IIb/IIIa receptor antagonist DMP444. Eur J Nucl Med. 2000;27:392–399. doi: 10.1007/s002590050521. [DOI] [PubMed] [Google Scholar]
- 116.Scharn DM, Oyen WJ, Klemm PL, Wijnen MH, vanderVliet JA. Assessment of prosthetic vascular graft thrombogenicity using the technetium-99m labeled glycoprotein IIb/IIIa receptor antagonist DMP444 in a dog model. Cardiovasc Surg. 2002;10:566–569. doi: 10.1016/s0967-2109(02)00077-7. [DOI] [PubMed] [Google Scholar]
- 117.Knight LC, Radcliffe R, Maurer AH, Rodwell JD, Alvarez VL. Thrombus imaging with technetium-99m synthetic peptides based upon the binding domain of a monoclonal antibody to activated platelets. J Nucl Med. 1994;35:282–288. [PubMed] [Google Scholar]
- 118.Bernarducci MP. Radiolabeled peptides: overcoming the challenges of post-surgical patient management of venous thromboembolism. Surg Technol Int. 2004;12:50–67. [PubMed] [Google Scholar]
- 119.Knight LC, Baidoo KE, Romano JE, Gabriel JL, Maurer AH. Imaging pulmonary emboli and deep venous thrombi with 99mTc-bitistatin, a platelet-binding polypeptide from viper venom. J Nucl Med. 2000;41:1056–1064. [PubMed] [Google Scholar]
- 120.Aoki N. Hemostasis associated with abnormalities of fibrinolysis. Blood Rev. 1989;3:11–17. doi: 10.1016/0268-960x(89)90021-0. [DOI] [PubMed] [Google Scholar]
- 121.Dahlborn M, Ahlborg G, Soderborg B, Virgin J, Wilczek B. Gamma camera detection of 99mTC-plasmin in the diagnosis of deep-vein thrombosis. Eur J Nucl Med. 1984;9:499–501. doi: 10.1007/BF00263253. [DOI] [PubMed] [Google Scholar]
- 122.Aronen HJ, Korppi-Tommola T, Suoranta HT, Taavitsainen MJ. 99mTc-plasmin test in deep vein thrombosis of the leg. Eur J Nucl Med. 1985;10:10–12. doi: 10.1007/BF00261755. [DOI] [PubMed] [Google Scholar]
- 123.Christensen SW, Wille-Jorgensen P, Kjaer L, Stadeager C, Widding A, Vestergaard A, Bjerg-Nielsen A. Contact thermography, 99mTc-plasmin scintimetry and 99mTc-plasmin scintigraphy as screening methods for deep venous thrombosis following major hip surgery. Thromb Haemost. 1987;58:831–833. [PubMed] [Google Scholar]
- 124.Dahl OE, Leistad E, Nyhus S, Havig O. 99mTc-plasmin uptake test is unreliable for diagnosing asymptomatic deep vein thrombosis after hip replacement surgery. Thromb Res. 1991;62:781–784. doi: 10.1016/0049-3848(91)90382-7. [DOI] [PubMed] [Google Scholar]
- 125.Lagerstedt C, Olsson CG, Fagher B, Oqvist B. 99mTc plasmin in 394 consecutive patients with suspected deep venous thrombosis. Eur J Nucl Med. 1989;15:771–775. doi: 10.1007/BF00255495. [DOI] [PubMed] [Google Scholar]
- 126.Luckers AE, Luth WJ, Teule GJ, Huijgens PC, Heidendal GA. Diagnosis of deep venous thrombosis with 99mTc plasmin using the gamma camera. Eur J Nucl Med. 1984;9:282–283. doi: 10.1007/BF00803251. [DOI] [PubMed] [Google Scholar]
- 127.Husted SE, Nielsen HK, Krusell L, Fasting H, Nielsen BO, Pedersen JB, Dalgaard E, Hansen HH. Deep vein thrombosis detection by 99mTc-plasmin test and phlebography. Br J Surg. 1984;71:65–66. doi: 10.1002/bjs.1800710121. [DOI] [PubMed] [Google Scholar]
- 128.Harwig SL, Harwig JF, Sherman LA, Coleman RE, Welch MJ. Radioiodinated plasminogen: an imaging agent for pre-existing thrombi. J Nucl Med. 1977;18:42–45. [PubMed] [Google Scholar]
- 129.Saha P, Humphries J, Modarai B, Mattock K, Waltham M, Evans CE, Ahmad A, Patel AS, Premaratne S, Lyons OT, Smith A. Leukocytes and the natural history of deep vein thrombosis: current concepts and future directions. Arterioscler Thromb Vasc Biol. 2011;31:506–512. doi: 10.1161/ATVBAHA.110.213405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aras M, Dede F, Dane F, Aktas B, Turoglu HT. FDG PET/CT appearance of portal vein tumor thrombus in the gastric primitive neuroectodermal tumor: uncommon primary tumor site with rare finding. Clin Nucl Med. 2013;38:47–49. doi: 10.1097/RLU.0b013e3182708530. [DOI] [PubMed] [Google Scholar]
- 131.Kaida H, Ishibashi M, Kurata S, Uchida M, Hayabuchi N. Tumor thrombus in the inferior vena cava from colon cancer detected by 18F-FDG-PET. Ann Nucl Med. 2007;21:185–188. doi: 10.1007/s12149-007-0003-5. [DOI] [PubMed] [Google Scholar]
- 132.Lim TC, Tan EH, Zaheer S. Use of Ga-68 DOTATATE PET/CT to confirm portal vein tumor thrombosis in a patient with pancreatic neuroendocrine tumor. Clin Nucl Med. 2011;36:498–499. doi: 10.1097/RLU.0b013e318217398b. [DOI] [PubMed] [Google Scholar]
- 133.Natsuizaka M, Kudo M, Suzuki M, Takano M, Tsuyuguchi M, Kawamura N, Noguchi S, Wada A, Nakata M, Ogasawara M, Kiyama Y, Asaka M, Kasai M. Diffuse large B-cell lymphoma with massive portal vein tumor thrombosis in a patient with alcoholic cirrhosis: a case report and literature review. Intern Med. 2009;48:805–808. doi: 10.2169/internalmedicine.48.1648. [DOI] [PubMed] [Google Scholar]
- 134.Probst S, Seltzer A, Chachoua A, Friedman K. Azygos venous tumor thrombus from renal cell carcinoma detected by F-18 FDG PET/CT. Clin Nucl Med. 2010;35:832–833. doi: 10.1097/RLU.0b013e3181ef0bae. [DOI] [PubMed] [Google Scholar]
- 135.Strobel K, Steinert HC, Bhure U, Koma AY, Gassmann N, Stockli SJ. Tumour thrombus in the superior vena cava from anaplastic carcinoma of the thyroid: FDG-PET/CT imaging findings. Eur J Nucl Med Mol Imaging. 2007;34:813. doi: 10.1007/s00259-006-0349-2. [DOI] [PubMed] [Google Scholar]
- 136.Tripathi M, Sharma R, Jaimini A, Singh N, Saw SK, Mishra AK, Mondal A. Metastatic follicular carcinoma of the thyroid with tumor thrombus in the superior vena cava and right brachiocephalic and internal jugular veins: FDG-PET/CT findings. Clin Nucl Med. 2008;33:426–428. doi: 10.1097/RLU.0b013e318170d508. [DOI] [PubMed] [Google Scholar]
- 137.Hess S, Madsen PH, Iversen ED, Frifelt JJ, Hoilund-Carlsen PF, Alavi A. Efficacy of FDG PET/CT Imaging for Venous Thromboembolic Disorders: Preliminary Results From a Prospective, Observational Pilot Study. Clin Nucl Med. 2014 doi: 10.1097/RLU.0000000000000453. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 138.Barrington SF, O’Doherty MJ. Limitations of PET for imaging lymphoma. Eur J Nucl Med Mol Imaging. 2003;30(Suppl 1):S117–127. doi: 10.1007/s00259-003-1169-2. [DOI] [PubMed] [Google Scholar]
- 139.Rosenbaum SJ, Lind T, Antoch G, Bockisch A. False-positive FDG PET uptake--the role of PET/CT. Eur Radiol. 2006;16:1054–1065. doi: 10.1007/s00330-005-0088-y. [DOI] [PubMed] [Google Scholar]
- 140.Middeldorp S. Thrombosis in women: what are the knowledge gaps in 2013? J Thromb Haemost. 2013;11(Suppl 1):180–191. doi: 10.1111/jth.12266. [DOI] [PubMed] [Google Scholar]
- 141.Chan WS, Rey E, Kent NE, Chan WS, Kent NE, Rey E, Corbett T, David M, Douglas MJ, Gibson PS, Magee L, Rodger M, Smith RE. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36:527–553. doi: 10.1016/s1701-2163(15)30569-7. [DOI] [PubMed] [Google Scholar]
- 142.Davidson T, Goitein O, Avigdor A, Zwas ST, Goshen E. 18F- FDG-PET/CT for the diagnosis of tumor thrombosis. Isr Med Assoc J. 2009;11:69–73. [PubMed] [Google Scholar]
- 143.Lee EY, Khong PL. The value of 18F-FDG PET/contrast-enhanced CT in detection of tumor thrombus. Clin Nucl Med. 2013;38:e60–65. doi: 10.1097/RLU.0b013e318266d53e. [DOI] [PubMed] [Google Scholar]
- 144.Sharma P, Kumar R, Jeph S, Karunanithi S, Naswa N, Gupta A, Malhotra A. 18F-FDG PET-CT in the diagnosis of tumor thrombus: can it be differentiated from benign thrombus? Nucl Med Commun. 2011;32:782–788. doi: 10.1097/MNM.0b013e32834774c8. [DOI] [PubMed] [Google Scholar]
- 145.Rondina MT, Wanner N, Pendleton RC, Kraiss LW, Vinik R, Zimmerman GA, Heilbrun M, Hoffman JM, Morton KA. A pilot study utilizing whole body 18 F-FDG-PET/CT as a comprehensive screening strategy for occult malignancy in patients with unprovoked venous thromboembolism. Thromb Res. 2012;129:22–27. doi: 10.1016/j.thromres.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Callejas MF, Errázuriz JI, Castillo F, Otárola C, Riquelme C, Ortega C, Huete Á, Bächler P. Incidental Venous Thromboembolism detected by PET-CT in Patients with Cancer: Prevalence and Impact on Survival rate. Thromb Res. 2014;133:750–5. doi: 10.1016/j.thromres.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 147.Ravina M, Hess S, Chauhan MS, Jacob MJ, Alavi A. Tumor Thrombus: Ancillary Findings on FDG PET/CT in an Oncologic Population. Clin Nucl Med. 2014 doi: 10.1097/RLU.0000000000000451. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 148.Miceli M, Atoui R, Walker R, Mahfouz T, Mirza N, Diaz J, Tricot G, Barlogie B, Anaissie E. Diagnosis of deep septic thrombophlebitis in cancer patients by fluorine-18 fluorodeoxyglucose positron emission tomography scanning: a preliminary report. J Clin Oncol. 2004;22:1949–1956. doi: 10.1200/JCO.2004.10.160. [DOI] [PubMed] [Google Scholar]
- 149.Bleeker-Rovers CP, Jager G, Tack CJ, Van Der Meer JW, Oyen WJ. F-18-fluorodeoxyglucose positron emission tomography leading to a diagnosis of septic thrombophlebitis of the portal vein: description of a case history and review of the literature. J Intern Med. 2004;255:419–423. doi: 10.1046/j.1365-2796.2003.01259.x. [DOI] [PubMed] [Google Scholar]
- 150.Nielsen AL, Thomassen A, Hess S, Alavi A, Hoilund-Carlsen PF. Deep venous thrombosis and pulmonary embolism detected by FDG PET/CT in a patient with bacteremia. Clin Nucl Med. 2013;38:276–277. doi: 10.1097/RLU.0b013e3182817aaf. [DOI] [PubMed] [Google Scholar]
- 151.Pallela VR, Thakur ML, Consigny PM, Rao PS, Vasileva-Belnikolavska D, Shi R. Imaging thromboembolism with Tc-99m-labeled thrombospondin receptor analogs TP-1201 and TP-1300. Thromb Res. 1999;93:191–202. doi: 10.1016/s0049-3848(98)00180-7. [DOI] [PubMed] [Google Scholar]
- 152.De Bruyn VH, Bergmann R, Keyt BA, Bennett WF, Sobel BE. Scintigraphic visualization of pulmonary thrombi with 123I-YPACK-TNK-tPA. Coron Artery Dis. 1995;6:715–721. [PubMed] [Google Scholar]


