Abstract
Purpose
To investigate the correlated factors for lymph node metastasis and prognosis for patients with T2 gastric cancer.
Methods
A total of 442 patients with T2 gastric cancer who underwent gastrectomy from January 1996 to December 2009 were evaluated. The clinicopathological parameters were analyzed for lymph node metastasis and prognosis, including gender, age, tumor size, tumor location, histological type, depth of invasion, vascular tumor emboli, nervous invasion, resection type, and pathological stage.
Results
The rate of lymph node metastasis was 45.9%. Univariate analysis showed that depth of invasion, tumor size, and vascular tumor emboli were associated with lymph node metastasis. Logistic regression demonstrated that depth of invasion, tumor size, and vascular tumor emboli were independently predictive factors for lymph node metastasis. The 5-year survival rate was 64.0%. Multivariate analysis showed that tumor size, tumor location, resection type, and pathological stage were independent prognostic factors. Based on tumor size, there were significant differences of 5-year survival between small size tumor (<6 cm) and large size tumor (≥6 cm) according to stage IIA (P = 0.006). Based on tumor location, there were significant differences of 5-year survival among different tumor location according to stage IB. Based on resection type, there were significant differences of overall 5-year survival between curative surgery and palliative surgery according to stage IIB (P = 0.015) and IIIA (P = 0.001).
Conclusion
Depth of invasion, tumor size, and vascular tumor emboli were independently predictive factors for lymph node metastasis. Tumor size, tumor location, resection type, and pathological stage were independent prognostic factors.
Introduction
Although the incidence of gastric cancer has been dramatically declining for past several decades, it remains the fourth most common cancer and the second most frequent cause of cancer-related death worldwide [1], [2]. The identification of prognostic factors is essential for predicting patients' survival and determining optimal therapeutic strategies. A variety of prognostic factors including pathological parameters and biological markers have been reported [3]–[6]. Among the prognostic factors now available for gastric cancer, the most precise and important prognostic factor still was the UICC TNM stage. Some studies have indicated that the depth of invasion and the status of lymph node metastasis were the most important prognostic factors in gastric cancer [7], [8].
The presence or absence of lymph node metastasis was closely related with depth of invasion [9]. The rate of lymph node metastasis was relatively low in T1 stage gastric cancer, the rate of lymph node metastasis was relatively high in T3 or more stage gastric cancer. However, there is a large variance in patients with T2 stage gastric cancer. Additionally, there was a significant modification in T2 stage between the sixth and seventh UICC/AJCC TNM classification. In the former, the T2 stage indicated that tumor extended into the muscularis propria (MP) or subserosa (SS) layer. In the latter, the tumor invading into subserosa (SS) was classified into T3 stage. There were relatively limited studies reporting the clinicopathological characteristics and prognosis of patients with T2 stage gastric cancer in the seventh UICC stage system. Therefore, we evaluated the correlated factors for lymph node metastasis and prognosis in patients with T2 gastric cancer.
Materials and Methods
Patients
From January 1996 to December 2009, 4550 patients with histologically confirmed primary gastric adenocarcinoma underwent gastrectomy at the Department of gastric cancer and soft sarcoma, Fudan University Shanghai Cancer Center. Inclusion criteria for this study were as follows: (1) tumor invading into but not penetrating muscularis propria; (2) complete pathological data. There were 477 patients presented with invasion of the muscularis propria (T2). Of 477 patients, 35 were excluded from the final analysis: 13 received preoperative chemotherapy; five due to gastric stump carcinoma; 17 had distant metastasis. In total, 442 patients were included in the current study. Data were retrieved from their operative and pathological reports, and follow-up data were obtained by phone outpatient and clinical database. The written informed consent had been obtained from all the patients, and this study was approved by the Ethical Committee of Shanghai Cancer Center of Fudan University. The study was retrospective. Patient records were anonymized and de-identified prior to analysis.
Preoperative evaluation and treatment
Imaging studies were routinely performed using upper gastrointestinal barium-meal, endoscopic examination, and computed tomographic scan in order to evaluate the preoperative stage. Staging was performed according to the American Joint Committee on Cancer (AJCC) TNM Staging Classification for Carcinoma of the Stomach (Seventh Edition, 2010). Gastrectomy was performed in accordance with the Japanese Classification of Gastric Carcinoma.
Follow-up
A follow-up of all patients was carried out according to our standard protocol (every three months for at least 2 years, every six months for the next 3 years, and after 5 years every 12 months for life). The check-up items included physical examination, tumor-marker examination, ultrasound, chest radiography, computed tomographic scan, and endoscopic examination. The median follow-up time was 70 months for all patients.
Statistical analysis
The patients' features and clinicopathological characteristics were analyzed using the two-tailed Student's t-test for continuous variable and χ2 test for categorical variable. Logistic regression was used to estimate related factors for lymph node metastasis. Five-year survival rate was calculated by Kaplan-Meier method, and the differences between survival curves were examined with long-rank test. Factors that were deemed of potential importance on univariate analysis were included in the multivariate analysis. The independent prognostic factors were examined by the multivariate survival analysis using Cox proportional hazards model. The accepted level of significance was P<0.05. Statistical analyses and graphics were performed using the SPSS 13.0 statistical package (SPSS, Inc., Chicago, IL).
Results
Clinicopathological characteristics
There were 302 males and 140 females (2.2∶1) with a mean age of 57 years. There were 263 (59.5%) patients with infiltration in shallow muscular (T2a), and 179 (40.5%) patients with infiltration in deep muscular invasion (T2b). According to histological type, well-differentiated tumors were observed in 13 (2.9%) patients, moderately-differentiated in 129 (29.2%) patients, poorly-differentiated tumors in 281 (63.6%) patients, and undifferentiated tumors remaining 19 (4.3%). Of these patients, 101 (22.9%) had tumors located in the upper third (70 patients with Siewert II, 31 patients with Siewert III), 65 (14.7%) had tumors in the middle third, 253 (57.2%) had tumors in the lower third of the stomach, and 23 (5.2%) had tumors occupied two-thirds or more of stomach. Total number of lymph nodes dissected was 7395 (mean 16.7, median 16.0). Lymph node metastasis was observed in 203 patients, the metastasis rate was 45.9%. There were 28 patients receiving palliative resection. The reason for palliative resection was that the standard D2 lymph node dissection was not enough for these patients with T2N3M0. There were 216 patients received adjuvant chemotherapy. The demographic data were shown in Table 1.
Table 1. Clinicopathologic characteristics of patients.
| Clinicopathologic features | Number (%) |
| Gender | |
| Female | 140 (31.7) |
| Male | 302 (68.3) |
| Age (years) | |
| ≤50 | 113 (25.6) |
| >50 | 329 (74.4) |
| Tumor size (cm) | |
| <6 | 390 (88.2) |
| ≥6 | 52 (11.8) |
| Tumor location | |
| Upper third | 101 (22.9) |
| Middle third | 65 (14.7) |
| Lower third | 253 (57.2) |
| ≥Two thirds | 23 (5.2) |
| Histological type | |
| Well-differentiated | 13 (2.9) |
| Moderately-differentiated | 129 (29.2) |
| Poorly-differentiated | 281 (63.6) |
| Undifferentiated | 19 (4.3) |
| Depth of invasion in muscularis propria | |
| Shallow muscular layer | 263 (59.5) |
| Deep muscular layer | 179 (40.5) |
| Vascular tumor emboli | |
| Yes | 105 (23.8) |
| No | 337 (76.2) |
| Nervous invasion | |
| Yes | 67 (15.2) |
| No | 375 (84.8) |
| Lymph node metastasis | |
| Yes | 203 (45.9) |
| No | 239 (54.1) |
| Pathological stage | |
| IB (T2N0M0) | 239 (54.1) |
| IIA (T2N1M0) | 91 (20.6) |
| IIB (T2N2M0) | 65 (14.7) |
| IIIA (T2N3M0) | 47 (10.6) |
Risk factors for lymph node metastasis
Of the 442 patients with invasion in muscularis propria, 203 patients (45.9%) had lymph node metastasis. Univariate analysis showed that depth of invasion, tumor size, vascular tumor emboli were associated with lymph node metastasis, whereas age, gender, histological type, tumor location, and nervous invasion were not (Table 2). Logistic regression was employed to identify the independent risk factors of lymph node metastasis. It was demonstrated that depth of invasion, tumor size, and vascular tumor emboli were independently predictive factors for lymph node metastasis (Table 3).
Table 2. Univariate analysis of predictors of lymph node metastasis.
| Variable | No metastasis | Metastasis | P |
| (n = 239) | (n = 203) | ||
| Gender | 0.335 | ||
| Male | 168 | 134 | |
| Female | 71 | 69 | |
| Age (years) | 0.526 | ||
| ≤50 | 64 | 49 | |
| >50 | 175 | 154 | |
| Tumor size (cm) | 0.007 | ||
| <6 | 220 | 170 | |
| ≥6 | 19 | 33 | |
| Tumor location | 0.662 | ||
| Upper third | 56 | 45 | |
| Middle third | 39 | 26 | |
| Lower third | 133 | 120 | |
| Two or more thirds | 11 | 12 | |
| Histological type | 0.147 | ||
| Well differentiated | 10 | 3 | |
| Moderate differentiated | 72 | 57 | |
| Poor differentiated | 144 | 137 | |
| Undifferentiated | 13 | 6 | |
| Depth of invasion in muscularis propria | 0.022 | ||
| Shallow layer | 154 | 109 | |
| Deep layer | 85 | 94 | |
| Vascular tumor emboli | 0.000 | ||
| Yes | 29 | 76 | |
| No | 210 | 127 | |
| Nervous invasion | 0.097 | ||
| Yes | 30 | 37 | |
| No | 209 | 166 |
Table 3. Multivariate analysis of predictors of lymph node metastasis.
| Variable | Hazard ratio | 95% confidence interval | P value |
| Age | 1.040 | 0.660–1.641 | 0.865 |
| Tumor size | 1.968 | 1.043–3.713 | 0.037 |
| Vascular tumor emboli | 4.056 | 2.493–6.599 | 0.000 |
| Depth of invasion | 1.517 | 1.013–2.271 | 0.043 |
Univariate analysis
The over-all 5-year survival rate was 64.0% for all 442 patients. The significant prognostic factors included: tumor size, tumor location, depth of invasion, lymph node status, resection type, and pathological stage (Table 4). The 5-year survival was higher in patients with small size tumor (<6 cm) compared with those patients with large size tumor (≥6 cm) (P = 0.001). The 5-year survival was longer in patients with lower third location tumor (P = 0.032), in patients with shallow muscular invasion (P = 0.017), in patients without lymph node metastais (P = 0.000), in patients with curative gastrectomy (P = 0.000). The survival of patients was better in early stage than that of late stage (P = 0.000). The sex, age, histological type, vascular tumor emboli and nervous invasion did not show any relationship with survival.
Table 4. Univariate analysis of all patients by Kaplan-Meier method.
| Variable | n | 5-Year survival rate (%) | P value |
| Gender | 0.936 | ||
| Female | 140 | 63 | |
| Male | 302 | 64 | |
| Age (y) | 0.386 | ||
| ≤50 | 113 | 61 | |
| >50 | 329 | 64 | |
| Tumor size (cm) | 0.001 | ||
| <6 | 390 | 66 | |
| ≥6 | 52 | 47 | |
| Tumor location | 0.032 | ||
| Upper third | 101 | 63 | |
| Middle third | 65 | 57 | |
| Lower third | 253 | 67 | |
| ≥Two thirds | 23 | 50 | |
| Histological type | 0.134 | ||
| Well-differentiated | 84 | ||
| Moderately-differentiated | 71 | ||
| Poorly-differentiated | 59 | ||
| Undifferentiated | 73 | ||
| Depth of invasion | 0.017 | ||
| Shallow muscularis layer | 263 | 66 | |
| Deep muscularis layer | 179 | 60 | |
| Vascular tumor emboli | 0.066 | ||
| Yes | 105 | 55 | |
| No | 337 | 66 | |
| Nervous invasion | 0.857 | ||
| Yes | 67 | 61 | |
| No | 375 | 64 | |
| Lymph node metastasis | 0.000 | ||
| Yes | 203 | 48 | |
| No | 239 | 77 | |
| Curability | 0.000 | ||
| Curative | 414 | 67 | |
| Palliative | 28 | 20 | |
| Pathological stage | 0.000 | ||
| IB (T2N0M0) | 239 | 77 | |
| IIA (T2N1M0) | 91 | 59 | |
| IIB (T2N2M0) | 65 | 42 | |
| IIIA (T2N3M0) | 47 | 35 |
Multivariate analysis
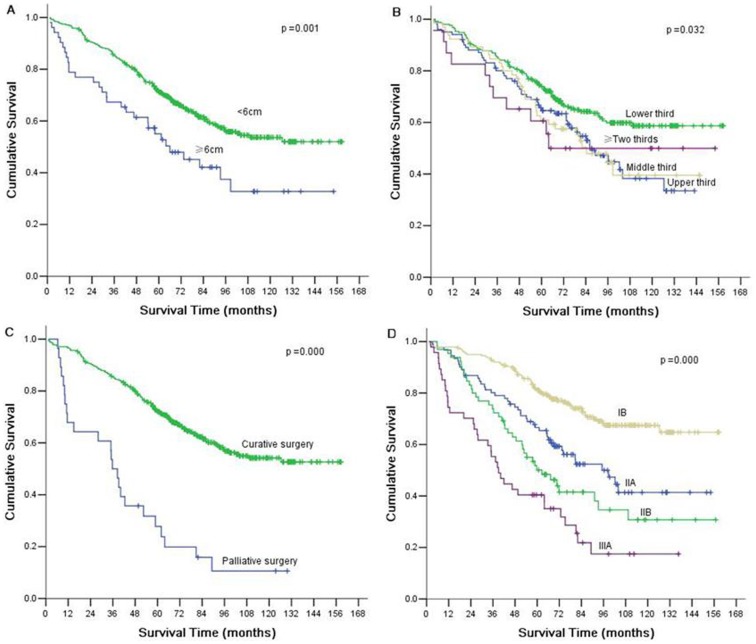
Multivariate survival analysis was performed to determine the independent prognostic factors for T2 gastric cancer. Multivariate analysis using Cox proportional hazards model showed that tumor size, tumor location, resection type, and pathological stage were independent prognostic factors (Table 5) (Figure 1).
Table 5. Multivariate analysis of patients by Cox model.
| Variable | χ2 | P vale | RR | 95% CI |
| Sex | 0.0.173 | 0.678 | 0.936 | 0.684–1.280 |
| Age | 0.035 | 0.851 | 1.034 | 0.726–1.473 |
| Tumor size | 4.962 | 0.026 | 1.574 | 1.056–2.347 |
| Tumor location | 5.906 | 0.015 | 0.819 | 0.697–0.962 |
| Depth of invasion | 0.815 | 0.367 | 1.148 | 0.851–1.548 |
| Lymph node status | 1.886 | 0.170 | 1.443 | 0.855–2.435 |
| Curability | 18.278 | 0.000 | 2.719 | 1.719–4.301 |
| Pathological stage | 8.273 | 0.004 | 1.396 | 1.112–1.753 |
Figure 1. Kaplan-Meier curves for overall survival of T2 gastric cancer according to tumor size (A), tumor location (B), curability (C), and pathological stage (D).
Comparison of survival according to tumor size, tumor location, and resection type
According to AJCC/TNM Staging System, tumor stage in this study was divided into 4 stages: stage IB, stage IIA, stage IIB, and stage IIIA. Based on tumor size, each stage was divided into small size tumor (<6 cm) and large size tumor (≥6 cm). There were significant differences of over-all 5-year survival between two groups according to stage IIA (P = 0.006). Based on tumor location, each stage was divided into upper third, middle third, lower third and two or more third. There were significant differences of over-all 5-year survival among four groups according to stage IB (P = 0.040). Based on resection type, each stage was divided into curative surgery and palliative surgery. There were significant differences of over-all 5-year survival among two groups according to stage IIB (P = 0.015), IIIA (P = 0.001).
Discussion
Gastric cancer was one of the most common malignancies worldwide. Although the prognosis of patients with gastric cancer has improved, gastric was still the second leading cause of cancer related deaths as a result of late diagnosis. The identification of prognostic factors is very important for predicting gastric cancer patients' survival and determining therapeutic strategies. It is universally acknowledged that the most significant factors affecting prognosis of gastric cancer patients are the depth of invasion (T staging) and the status of lymph node metastasis (N staging) [10]–[12]. According to the NCCN guideline, the T staging was classified as four categories including T1, T2, T3, and T4. Patients with T1 had a relatively good prognosis; patients with T3 or T4 had poor prognosis. T2 was considered as an intermediate stage between early and advanced gastric cancer. Therefore, there was a large variance in the prognosis of patients with T2 gastric cancer. In this study, the clinicopathological characteristics and prognostic factors were investigated in patients with T2 stage gastric cancer.
The status of lymph node metastasis was considered to be very important prognostic predictor for gastric cancer; patients with lymph node metastasis had a worse survival than those without lymph node metastasis [13]–[15]. Therefore, identification of predictive factors for lymph node metastasis was crucial to establish the staging and prognosis. Although many studies have reported the association between clinicopathological variables and lymph node metastasis in gastric cancer, studies in T2 gastric cancer have been rarely reported so far especially in large-scale. Therefore, we conducted this study in 442 patients with T2 gastric cancer. In this study, we found that depth of invasion, tumor size, vascular tumor emboli were associated with lymph node metastasis. Logistic regression demonstrated that depth of invasion, tumor size, and vascular tumor emboli were independently predictive factors for lymph node metastasis. This was consistent with previous some studies. Yamashita et al. [16] reported that lymphatic involvement, venous involvement, serosal invasion and tumor size were associated with lymph node metastasis in gastric cancer. Lim et al. [17] reported that tumor size, depth of invasion, macroscopic type, and lymphovascular invasion were related to lymph node metastasis in early gastric cancer. Wu et al. [18] reported that poor differentiation, submucosal invasion and large tumor size were independent risk factors for lymph node metastasis in early gastric cancer. All these studies indicated that some pathological parameters such as tumor size, depth of invasion, and vascular tumor emboli could be good predictors for lymph node metastasis. Therefore, we should pay more attention to T2 gastric cancer patients with these adverse factors, and conduct more appropriate treatment plan in clinical practice.
Despite the fact that the most T2 stage patients with lymph node metastasis had a bad prognosis, some of them still had a long term survival. Therefore, the identification of prognostic factors in patients with T2 gastric cancer was the primary purpose of this study. Many factors have been reported to influence the survival of patients with gastric cancer, such as tumor size, depth of invasion, lymphovascular invasion, tumor location, serosal invasion [19]–[23]. However, previous studies did not take into account the effect of the depth of invasion on other clinicopathological parameters. It was difficult to identify the most crucial variables with regard to prognosis because many variables were interrelated. Therefore, precise identification of prognostic factors can be feasible only when the depth of invasion was specified. Recently, Bu et al. [24] showed that TNM stage and lymphatic vascular invasion were independent prognostic factors for T2 gastric cancer patients. In this study, we found that pathological stage, tumor size, tumor location, and resection type were independent prognostic factors. Therefore, we suggested that T2 gastric patients with lymph node metastasis, large size tumor, more than two thirds location, and palliative gastrectomy should receive adjuvant chemotherapy or radiochemotherapy.
To evaluate whether tumor size, tumor location, and resection type can provide additional prognostic information on the basis of TNM stage system, we compared cumulative survival curves according to these independent prognostic factors. The results showed that there were significant differences of over-all 5-year survival between different tumor size groups according to stage IIA; different location groups according to stage IB; different resection groups according to stage IIB and IIIA. These findings indicated that tumor size, tumor location, and resection type could provide additional prognostic information in patients with T2 gastric cancer.
In conclusion, depth of invasion, tumor size, and vascular tumor emboli were independently predictive factors for lymph node metastasis. Tumor size, tumor location, resection type, and pathological stage were independent prognostic factors. These findings were important to develop individualized treatment plans for patients with gastric cancer. T2 gastric cancer patients with large tumor size, upper location, palliative gastrectomy, and late stage should be considered for adjuvant chemotherapy.
Acknowledgments
We thank Ben Liotta for editing our manucript's English language style.
Funding Statement
These authors have no support or funding to report.
References
- 1.Shibata A, Parsonnet J (2006) Stomach cancer. In: Schottenfeld D, Fraumeni JF, eds. Cancer epidemiology and prevention, 3rd edn. New York: Oxford University Press 707–720.
- 2. Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 3. Tang H, Deng M, Tang Y, Xie X, Guo J, et al. (2013) miR-200b and miR-200c as Prognostic Factors and Mediators of Gastric Cancer Cell Progression.Clin Cancer Res. 19: 5602–12. [DOI] [PubMed] [Google Scholar]
- 4. Shou ZX, Jin X, Zhao ZS (2012) Upregulated expression of ADAM17 is a prognostic marker for patients with gastric cancer. Ann Surg 256: 1014–22. [DOI] [PubMed] [Google Scholar]
- 5. Baiocchi GL, Tiberio GA, Minicozzi AM, Morgagni P, Marrelli D, et al. (2010) A multicentric Western analysis of prognostic factors in advanced, node-negative gastric cancer patients. Ann Surg 252: 70–73. [DOI] [PubMed] [Google Scholar]
- 6. Liu X, Xu Y, Long Z, Zhu H, Wang Y (2009) Prognostic significance of tumor size in T3 gastric cancer. Ann Surg Oncol 16: 1875–82. [DOI] [PubMed] [Google Scholar]
- 7. Adachi Y, Mori M, Maehara Y, Sugimachi K (1994) Dukes's classification: a valid prognostic indicator for gastric cancer. Gut 35: 1368–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maruyama K (1987) The most important prognostic factors for gastric cancer patients: a study using univariate and multivariate analyses. Scand J Gastroenterol 22: 63–68. [Google Scholar]
- 9. Nitti D, Marchet A, Mocellin S, Rossi GM, Ambrosi A, et al. (2009) Prognostic value of subclassification of T2 tumors in patients with gastric cancer. Br J Surg 96: 398–404. [DOI] [PubMed] [Google Scholar]
- 10. Gammerer G, Formentini A, Karletshofer M, Henne-Bruns D, Kornmann M (2012) Evaluation of important prognostic clinical and pathological factors in gastric cancer. Anticancer Res 32: 1839–1842. [PubMed] [Google Scholar]
- 11. Maruyama K (1987) The most important prognostic factors for gastric cancer patients: a study using univariate and multivariate analyses. Scand J Gastroenterol 22: 63–68. [Google Scholar]
- 12. Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, et al. (2005) Gastric adenocarcinoma: review and considerations for future directions. Ann Surg 241: 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim JP, Lee JH, Kim SJ, Yu HJ, Yang HK (1998) Clinicopathologic characteristics and prognostic factors in 10783 patients with gastric cancer. Gastric cancer 1: 125–133. [DOI] [PubMed] [Google Scholar]
- 14. Siewert JR, Bottcher K, Stein HJ, Roder JD (1998) Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 228: 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim JP, Kim YW, Yang HK, Noh DY (1994) Significant prognostic factors by multivariate analysis of 3926 gastric cancer patients. World J Surg 18: 872–877. [DOI] [PubMed] [Google Scholar]
- 16. Yamashita H, Kitayama J, Kanno N, Yatomi Y, Nagawa H (2006) Hyperfibrinogenemia is associated with lymphatic as well as hematogenous metastasis and worse clinical outcome in T2 gastric cancer. BMC Cancer 6: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lim MS, Lee HW, Im H, Kim BS, Lee MY, et al. (2011) Predictable factors for lymph node metastasis in early gastric cancer-analysis of single institutional experience. J Gastrointest Surg 15: 1783–1788. [DOI] [PubMed] [Google Scholar]
- 18. Wu CY, Chen JT, Chen GH, Yeh HZ (2002) Lymph node metastasis in early gastric cancer: a clinicopathological analysis. Hepatogastroenterology 49: 1465–1468. [PubMed] [Google Scholar]
- 19. Lu J, Huang CM, Zheng CH, Li P, Xie JW, et al. (2013) Consideration of tumor size improves the accuracy of TNM predictions in patients with gastric cancer after curative gastrectomy. Surg Oncol 22: 167–171. [DOI] [PubMed] [Google Scholar]
- 20. Liu X, Xu Y, Long Z, Zhu H, Wang Y (2009) Prognostic significant of tumor size in T3 gastric cancer. Ann Surg Oncol 16: 1875–1882. [DOI] [PubMed] [Google Scholar]
- 21. Hsu JT, Lin CJ, Sung CM, Yeh HC, Chen TH, et al. (2013) Prognostic significance of the number of examined lymph nodes in node-negative gastric adenocarcinoma. Eur J Surg Oncol 39: 1287–1293. [DOI] [PubMed] [Google Scholar]
- 22. Kim DY, Seo KW, Joo JK, Park YK, Ryu SY, et al. (2006) Prognostic factors in patients with node-negative gastric carcinoma: a comparison with node-positive gastric carcinoma. World J Gastroenterol 12: 1182–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qiu MZ, Wang ZQ, Zhang DS, Zhou ZW, Li YH, et al. (2011) Prognostic analysis in node-negative gastric cancer patients in China. Tumor Biol 32: 489–492. [DOI] [PubMed] [Google Scholar]