Abstract
The Unfolded Protein Response is a homeostatic mechanism that permits eukaryotic cells to cope with Endoplasmic Reticulum (ER) stress caused by excessive accumulation of misfolded proteins in the ER lumen. The more conserved branch of the UPR relies on an ER transmembrane enzyme, Ire1, which, upon ER stress, promotes the unconventional splicing of a small intron from the mRNA encoding the transcription factor Xbp1. In mammals, two specific regions (the hydrophobic region 2 - HR2 - and the C-terminal translational pausing site) present in the Xbp1unspliced protein mediate the recruitment of the Xbp1 mRNA-ribosome-nascent chain complex to the ER membrane, so that Xbp1 mRNA can be spliced by Ire1. Here, we generated a Drosophila Xbp1 deletion mutant (Excision101) lacking both HR2 and C-terminal region, but not the Ire1 splicing site. We show that Ire1-dependent splicing of Xbp1 mRNA is reduced, but not abolished in Excision101. Our results suggest the existence of additional mechanisms for ER membrane targeting of Xbp1 mRNA that are independent of the C-terminal domain of Drosophila Xbp1unspliced.
Introduction
The endoplasmic reticulum (ER) is a membranous organelle where proteins targeted for secretion or for the plasma membrane are folded and processed. Physiological conditions that impose large amounts of proteins in the ER, as for example, the production of insulin by pancreatic β-cells, may represent a challenge to the ER folding capacity [1]. The Unfolded Protein Response (UPR) is a homeostatic mechanism that attempts to balance the load of incoming proteins into the ER to its folding capacity, to avoid the accumulation of toxic misfolded proteins, which otherwise would cause ER stress [2], [3].
In higher eukaryotes, the UPR has three signaling branches triggered by different ER transmembrane proteins: protein kinase (PKR)-like ER kinase (Perk), activating transcription factor 6 (Atf6) and Inositol-requiring enzyme 1 (Ire1). Ire1 is conserved across all eukaryotes, presenting a luminal domain that detects the accumulation of misfolded proteins in the ER lumen, and a cytoplasmic domain with kinase and RNase activities that trigger downstream signaling [4], [5], [6], [7], [8]. During ER stress, Ire1 is activated and catalyzes the unconventional splicing of an intron from X-box binding protein 1 (Xbp1) mRNA (in vertebrates, C. elegans and Drosophila) or from its functional yeast (S. cerevisiae) homolog Hac1 [9], [10], [11], [12], [13], [14], [15]. This Ire1 mediated unconventional splicing event causes a frameshift during the translation of Xbp1 mRNA that introduces a new C-terminus with a potent trans-activation domain, generating an effective transcription factor [11], [13]. Xbp1spliced enhances the expression of genes encoding ER chaperones, enzymes, and the ER protein degradation machinery [16], [17], [18]. Xbp1unspliced mRNA is translated into an unstable antagonist of Xbp1spliced and ATF6 signaling [19].
In addition to splicing of Xbp1 mRNA, Ire1 also cleaves a variety of mRNAs, mostly encoding proteins with signal peptide/transmembrane domains that would represent an additional challenge to the ER folding machinery under ER stress [20]. This mechanism was named RIDD (Regulated Ire1 Dependent Decay) and was also described in mammalian cells and in the fission yeast S. pombe (which lacks any Hac1/Xbp1 homologue) [21], [22], [23]. RIDD seems to be particularly important in cells undergoing very strong ER stress [24], [25].
The mechanism of targeting of a specific mRNA to RIDD seems to rely mostly on the existence of a signal peptide in its respective protein; the deletion of the signal peptide from known RIDD targets prevents their degradation and conversely, addition of a signal peptide to GFP is sufficient to promote the degradation of its mRNA by RIDD [26], [27]. One interesting exception is the mRNA encoding Smt3, a homologue of SUMO (small ubiquitin-like modifier), which is cleaved by RIDD although it does not have a signal peptide in its sequence [28].
Xbp1 also does not have a signal peptide in its sequence and the mechanism of recruitment of the Xbp1 mRNA to the ER membrane (and Ire1) is still unclear. Moreover, it seems that the mechanisms of recruitment to the ER membrane of Xbp1 mRNA in mammals and Hac1 mRNA in yeast are quite different. In yeast cells with no ER stress, the mRNA of unspliced Hac1 is found mostly in the cytoplasm, in association with stalled ribosomes. Upon ER stress, Hac1 mRNA is recruited to Ire1 clusters in the ER membrane, in a process that depends on a bipartite element (BE) present at the 3′ untranslated region of the Hac1 mRNA [29].
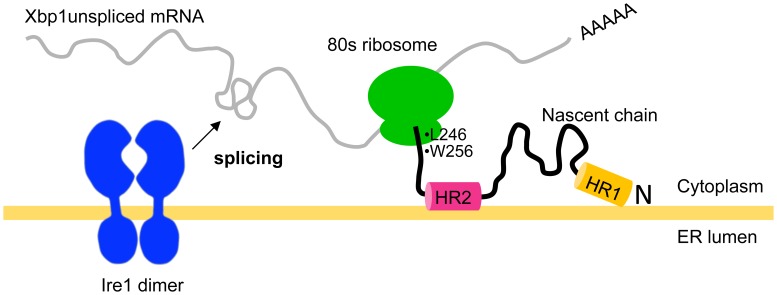
In contrast, ER membrane localization of mammalian Xbp1 is independent of the 3′ untranslated region of Xbp1 [30]. Instead, the mRNA of Xbp1unspliced is translated under normal conditions and originates a polypeptide that associates with the membrane of the ER through two hydrophobic regions (the N terminal hydrophobic region 1–HR1 and the C-terminal hydrophobic region 2–HR2) [30], [31] (Figure 1). The HR2 is a conserved region predicted to form a α-helix that has the propensity to interact with the lipid membrane [30]. Presumably, upon translation of Xbp1unspliced, the HR2 on the nascent polypeptide associates with the ER membrane and brings the Xbp1 mRNA-ribosome-nascent chain complex to the vicinity of Ire1, facilitating Ire1-mediated splicing. Xbp1spliced lacks HR2 due to the frameshift that occurs upon Ire1-mediated splicing event and localizes predominantly in the nucleus, where it is active as transcription factor.
Figure 1. Recruitment of Xbp1 mRNA to the ER membrane in mammalian cells.
XBP1unspliced originates translational pausing through its C-terminal region. The hydrophobic regions (HR1 and HR2) in XBP1unspliced target the XBP1 unspliced mRNA/ribosome/nascent chain complex to the ER membrane, giving the opportunity for Ire1 to splice Xbp1 mRNA. Adapted from [31].
In addition to HR1 and HR2, there is another motif in Xbp1unspliced that is also important for proper localization of Xbp1 mRNA at the ER membrane. The C-terminal region (CTR) of Xbp1unspliced is essential for translational pausing, just when HR2 is protruding from the ribosome exit tunnel [31] (Figure 1). Presumably, pausing of translation stabilizes the Xbp1 mRNA-ribosome-nascent chain complex in the vicinity of the ER membrane, giving the opportunity for activated Ire1 to cleave Xbp1 mRNA [31].
In Drosophila, a Xbp1-EGFP ER stress reporter lacking the HR2 and CTR of Xbp1unspliced was found to be activated under a variety of ER stress stimuli, including some specific physiological conditions during development, by the addition of ER stress-inducing drugs to tissues and cells or by using mutations that cause the accumulation of misfolded proteins in the ER [14], [32], [33], [34], [35], [36]. In these studies Xbp1-EGFP was expressed with the UAS/GAL4 system [37], but GFP is only observed upon the Ire1-dependent splicing of the Xbp1 intron present in the reporter. A modified “high gain” version of Xbp1-EGFP, where HR2, CTR and the 3′ UTR of Xbp1unspliced were included in the reporter, greatly increased the reporter sensitivity and GFP expression upon ER stress [38]. However, in all these cases, and due to the nature of the UAS/GAL4 system, Xbp1-EGFP was likely to be over-expressed in tissues or cells, which may overload the mechanisms regulating Xbp1 mRNA targeting to the ER membrane. Hence, the question whether or not the HR2 and the CTR of Xbp1unspliced are required for the targeting of Xbp1 mRNA to the ER membrane was not yet directly addressed in Drosophila. Here, we generated a deletion mutant (Excision101) of Drosophila Xbp1, lacking HR2 and CTR, but not the Ire1 splicing site. The transcription of Xbp1 mRNA in Excision101 is still at normal levels, since the upstream regulatory regions were not deleted in this mutant. We found that Ire1-dependent splicing of Xbp1 mRNA is reduced in Excision101, but not completely abolished. Our results suggest the existence of additional mechanisms for ER membrane targeting of Xbp1 mRNA that are independent of the C-terminal domain of Xbp1unspliced.
Methods
Drosophila genetics and molecular biology
Flies and crosses were raised with standard cornmeal food, at 25°C. Excision101 was generated by crossing the homozygous viable line carrying the transposon P{SUPor-P}CG9418KG05183 with Δ23 transposase. Males with mosaic orange eyes were selected and crossed with double balancer females. White eyed male progeny were tested for lethality complementation with P{lacW}xbp1 k13803.
Excision101 was balanced with CyO-GFP to collect homozygous mutant and heterozygote control larvae. Cages to collect larvae were set on apple juice plates and maintained at 25°C. For tunicamycin feeding experiments, 24 h. after egg laying larvae were exposed to yeast paste food with/without tunicamycin (5 µg/mL) for 6–8 hours and collected for RNA extraction.
Genomic DNA was isolated from larvae or flies using the High Pure PCR Template Preparation kit (Roche). Samples were snap frozen in liquid nitrogen and macerated with a motorized pestle in lysis buffer. The limits of Excision101 were determined by PCR and sequencing with the primers 5′AGT GAC GTT GCT TGG CTG AGT GAC and 5′GCA GCA CAA CACCAG ATG C. Total RNA was extracted with High Pure RNA Tissue kit (Roche) and used to synthesize cDNA with random hexamers (RevertAid First Strand kit - Thermo/Fermentas). Quantitative reverse transcription-PCR (RT-PCR) analysis was performed on the cDNA obtained using SSoFast EvaGreen Supermix and a Bio-Rad Cfx-96 detection system. All samples were analyzed in triplicates and from 3 independent RNA extractions. For each sample, the levels of mRNAs were normalized using rp49 as a loading control. PCR primers were: rp49 (Fwd: 5′AGA TCG TGA AGA AGC GCA CCA AGC, Rev: 5′GCA CCA GGA ACTT CTT GAA TCC GG), Xbp1 (Fwd1: 5′CAT CAA CGA GTC ACT GCT GGC CAA G, Rev3: 5′CGC TGA CGA CTG TGT GTC C), Pdi (Fwd: 5′TCA TCG AGA GTC CTG TCC AGG TTG, Rev: 5′AAC ACC TCC TTT CCC AGG AGC TG) and BiP (Fwd: 5′TGT CAC CGA TCT GGT TCT TCA GGC, Rev: 5′GTC CCA TGA CCA AGG ACA ACC ATC).
Analysis of Xbp1 mRNA splicing by PstI digestion and sequencing
Splicing of Xbp1 mRNA was accessed by restriction analysis with PstI of a fragment containing Ire1 splice site as described in [39]. Excision101/CyO heterozygous larvae were dissected in PBS and brains and eye imaginal discs were cultured in Schneider’s medium with or without 5 mM DTT for 5 hr. Total RNA was extracted with High Pure RNA Tissue kit (Roche) and used to synthesize cDNA with random hexamers (RevertAid First Strand kit - Thermo/Fermentas). Fragments flanking Ire1 splicing site were amplified using specific primers for the CyO chromosome (Fwd1 and Rev1) or Excision101 (Fwd1 and Rev2). PCR amplification fragments were digested with PstI overnight. Primers sequence: Fwd1: 5′CAT CAA CGA GTC ACT GCT GGC CAAG, Rev1: 5′GTC TGC TGT GAT ATC TGC GAG CAG AC, Rev2: 5′CTG GTT AAT GCA GCT CTG CGA AGC C3’. For DNA sequencing, the CyO chromosome or Excision101 specific PCR products were cloned in pJet (Thermo/Fermentas). Single colonies were grown for plasmid mini-preparation (NZYTech) and DNA sequencing was performed by Stab Vida using as sequencing primers Fwd1 and Rev1 or Rev2.
Results and Discussion
HR2 and CTR are conserved in Drosophila Xbp1unspliced
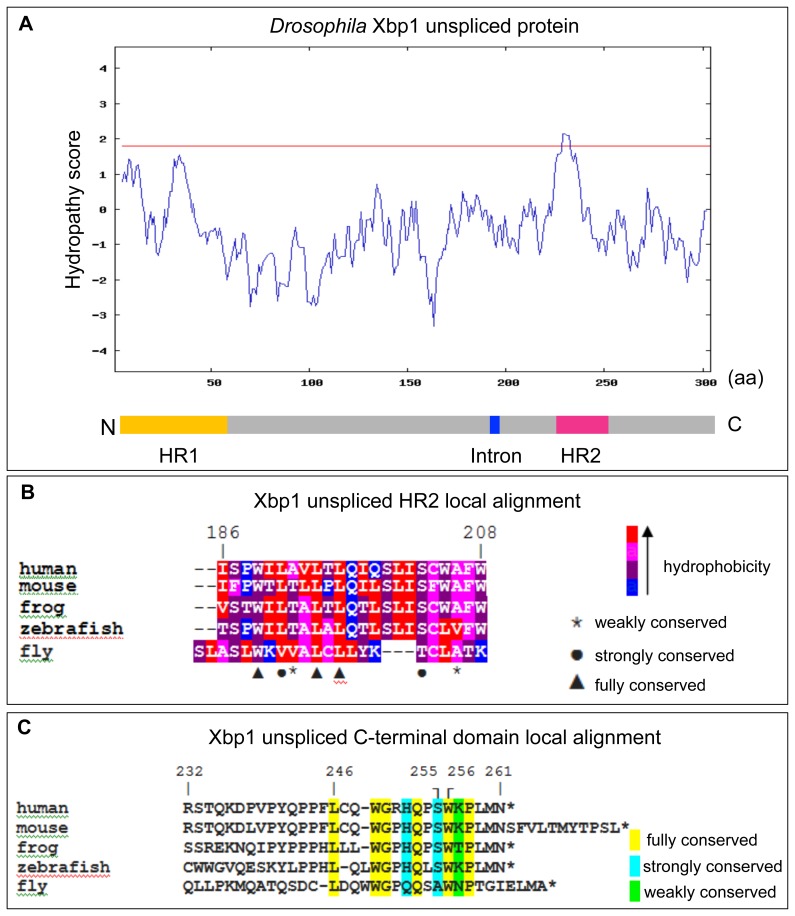
The mechanism of activation of Xbp1 by Ire1-mediated unconventional splicing of Xbp1 mRNA exists in Drosophila [14], [15], [32], as well as RIDD, which was first described in Drosophila S2 cells [20]. Moreover, the main UPR components, including Ire1, ATF6 and Perk, have homologs in Drosophila with high conservation scores [40]. To elucidate if the mechanism of Xbp1 mRNA transport to the ER surface in Drosophila is conserved with mammals, we started by performing Kyte and Doolittle hydrophobicity analysis, which indicated the existence of a highly hydrophobic region in the carboxyl end of the Drosophila Xbp1unspliced protein, corresponding to the HR2 (Figure 2A). Moreover, the Drosophila Xbp1unspliced protein has, within HR2, several amino acid residues that are fully or strongly conserved with human and other vertebrate species (Figure 2B).
Figure 2. Conservation of HR2 and C-terminal region of Xbp1unpsliced.
(A) Kyte and Doolittle hydrophobicity plot of Drosophila Xbp1unspliced, indicating the existence of two hydrophobic regions, HR1 and HR2. The horizontal red line indicates a score of 1.8. (B) Local amino acid sequence alignment of HR2 using several species. Amino acids that are fully, strongly or weakly conserved are indicated. The hydrophobicity of each amino acid is indicated by a color code. (C) Local amino acid sequence alignment of the C-terminal translational pausing region. Amino acids that are fully, strongly or weakly conserved are indicated.
In humans, the L246A and W256A mutations in the C-terminal domain of Xbp1unspliced abrogated translational pausing [31]. These Leucine and Tryptophan residues are conserved in humans, mice, zebrafish, Xenopus (frog) and Drosophila, further supporting the physiological role of these amino acid residues in translational pausing (Figure 2C). An S255-to-A255 mutation was previously reported to increase translational pausing in human Xbp1unspliced [31]. Interestingly, in Drosophila this position is occupied by an Alanine residue instead of the Serine, which is found in all other species (Figure 2C), which suggests that translational pausing should occur in Drosophila Xbp1unspliced with high efficiency.
Excision101 originates a truncated Xbp1 mRNA that is spliced by Ire1
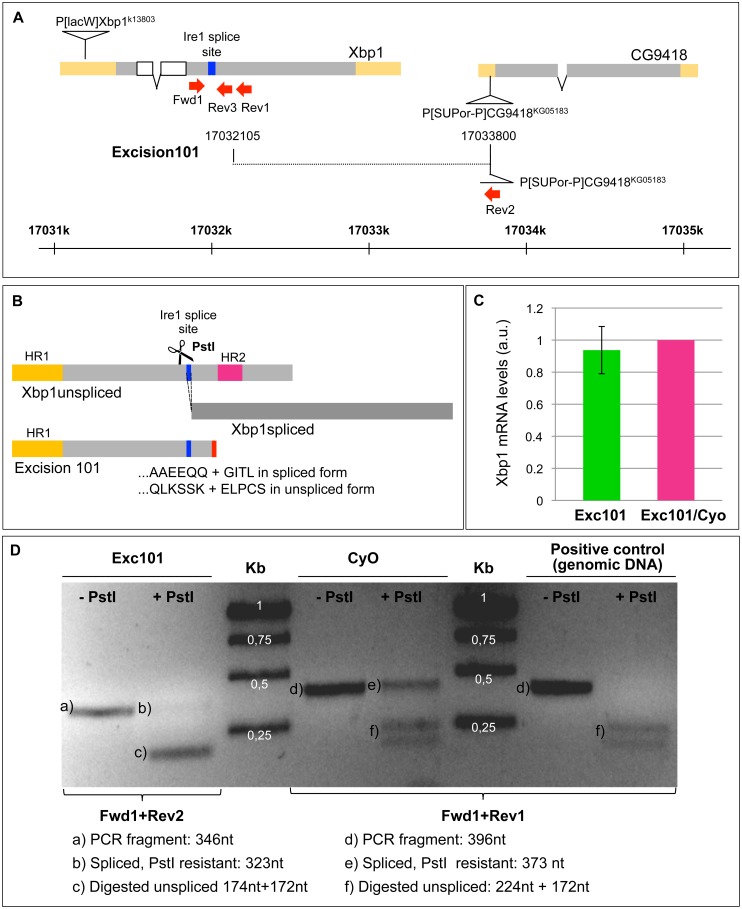
We generated a mutant allele of Xbp1, Excision101 (Exc101), by imprecise excision of P{SUPor-P}CG9418KG05183, a non-lethal transposable element inserted in the 5′UTR of CG9418, the gene immediately downstream of Xbp1. Excision101 is a deletion that originates Xbp1 proteins with truncated C-terminal regions (Figure 3A). In the Xbp1spliced open reading frame, the last 278 codons (at the 3′ region of the coding sequence) are deleted and 4 new codons (GITL) are present before the introduction of a nonsense STOP codon. In the Xbp1unspliced frame, the last 87 codons are deleted and 5 new codons (ELPCS) are followed by a nonsense STOP codon (Figure 3B). We confirmed by quantitative RT-PCR that the transcriptional regulation of Xbp1 mRNA is not affected in Excision101. Larvae homozygous for Excision101 present similar levels of Xbp1 mRNA to Excision101/CyO-GFP control larvae (Figure 3C).
Figure 3. Excision101 originates a truncated Xbp1 mRNA that is spliced by Ire1.
(A) Schematic representation of genomic region around Xbp1, with the localization of Excision101 deletion and the PCR primers Fwd1, Rev1, Rev2 and Rev3. In Excision 101, the breakpoint in P{SUPor-P}CG9418KG05183 is 5′GAA TTA CCT TGT AGT TGA TAT TTG AGA T (following the leading strand of Xbp1). (B) Schematic representation of the Xbp1unspliced and Xbp1spliced proteins in “wild-type” and in Excision101. PstI has a cleavage site in the intron spliced by Ire1. HR1: hydrophobic region 1. HR2: hydrophobic region 2. (C) Quantitative RT-PCR for total Xbp1 mRNA levels in larvae homozygous for Excision101or heterozygous Excision101/CyO-GFP, using the primers for Xbp1, Fwd1 and Rev3. Control Excision101/Cyo is set as 1, with the homozygous Excision101 Xbp1 mRNA levels indicated as a mean +/− standard deviation (a.u. - arbitrary units). (D) Agarose gel electrophoresis of RT-PCR products specific for Excision101 or CyO control chromosome after digestion with PstI restriction enzyme. PCR product specific for the Xbp1unspliced form is cleaved by PstI, while Xbp1spliced form is resistant to PstI digestion (because the PstI is in the intron that is spliced by Ire1). A positive control using genomic DNA is fully digested by PstI.
Taking into account that the predicted Xbp1unspliced protein encoded by Excision101 does not have HR2 or CTR, we used two different assays to verify if Excision101 mRNA is spliced by Ire1. In a first assay, we were able to distinguish the mRNA of the Xbp1spliced form from the Xbp1unspliced form by digestion with the restriction enzyme PstI of a cDNA/PCR-amplified fragment containing the Ire1 splicing site. A PstI restriction site is present in the Xbp1 intron that is removed by Ire1, and consequently PstI only cuts the fragment corresponding to the Xbp1unspliced form. Heterozygous Excision 101/Cyo third instar larvae were dissected (cut in half) and treated with 5 mM DTT (dithiothreitol, in PBS or Schneider cells medium) for 5 hours, to activate Ire1. Total RNA was extracted and, after cDNA synthesis, PCR products were generated with primers that allow the specific amplification from Excision 101 or the CyO (balancer chromosome that serves as “wild-type” control) alleles (Figure 3A, D). The Excision101-specific fragment was mostly digested with PstI, although a faint PstI-resistant band, corresponding to the Xbp1spliced, was also observed. So most of the Excision101-specific mRNA is not cleaved by Ire1 and remains in the Xbp1unspliced form, even after 5 hours of DTT treatment. In the CyO-specific fragment, the PstI-resistant band (Xbp1spliced) is more prominent, indicating that more Xbp1 mRNA is spliced by Ire1 in this case. A positive control using genomic DNA was fully digested by PstI, indicating that the observed PstI-resistant fragments should not be due to incomplete digestion (Figure 3D).
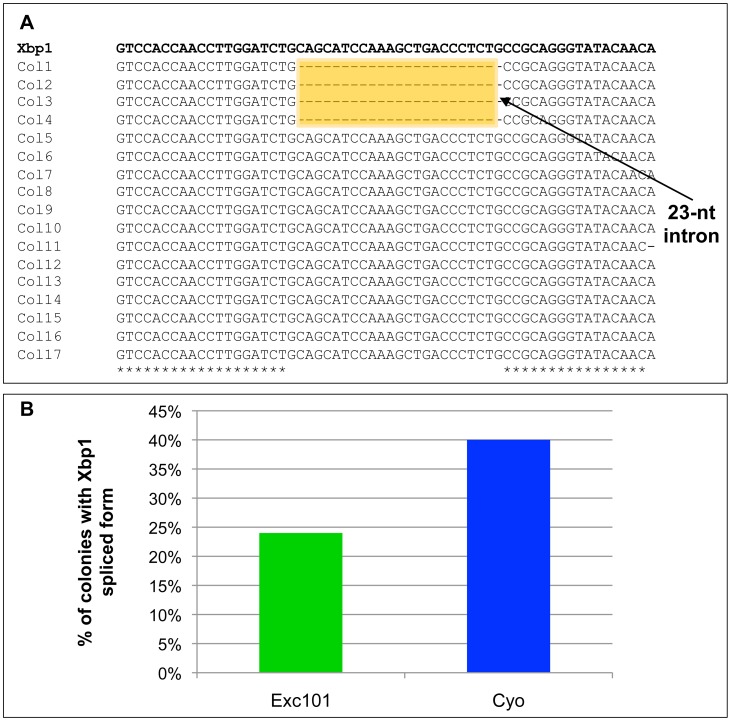
In a second approach, we sequenced plasmids from several single colonies that were obtained after cloning of the Excision101 or CyO specific PCR fragments, to confirm whether or not the intron was present. In the CyO control, approximately 40% of the colonies (15 out of 37) corresponded to the Xbp1spliced form (and 60% to the Xbp1unspliced form). In Excision101 approximately 23% of the colonies (9 out of 38) corresponded to the Xbp1spliced form (and 77% to the Xbp1unspliced form), which represents a reduction in the amount of Xbp1spliced form, in comparison with the CyO control (Figure 4). These results indicate that the elements deleted from the 3′region of Xbp1 in Excision101, such as HR2 and CTR, are important for Ire1 mediated splicing of Xbp1 mRNA. Nevertheless, around 23% of the colonies obtained from Excision101 amplification corresponded to the Xbp1spliced form, which demonstrates that Xbp1 splicing still occurs, even in the absence of HR2 and translational pausing. It is possible that the HR1 of Xbp1unspliced, which is still present in Excision101, is sufficient to promote the association of Xbp1 mRNA with the ER membrane.
Figure 4. cDNA sequencing demonstrates splicing in Excision101 specific mRNA.
Sequencing of single colonies obtained after cloning of Excision101 specific cDNA. The absence of the intron in colonies 1–4 demonstrates that Excision101 mRNA is spliced by Ire1. Percentage of colonies containing Xbp1spliced form in Excision101 or CyO control chromosome. The total number of colonies sequenced was 38 for Excision101 and 37 for the CyO control.
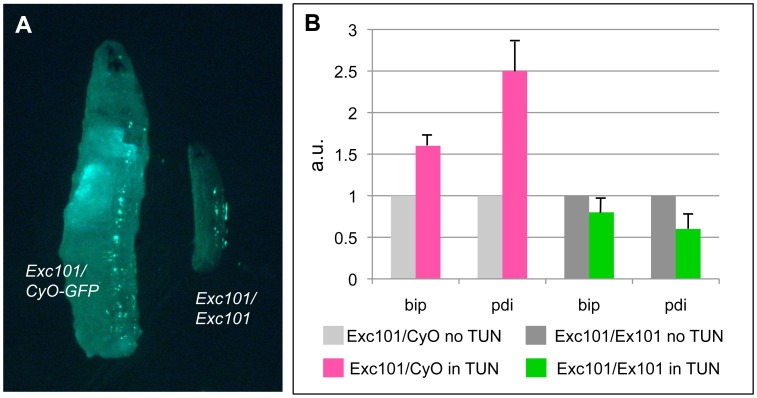
Finally, we tested the susceptibility of Excision101 homozygous larvae upon treatment with tunicamycin, an ER stress-inducing drug. Larvae homozygous for Excision101 arrest development during the first instar stage and die 2 to 3 days after egg laying, while similarly aged heterozygous Excision101/CyO-GFP sibling control larvae develop normally (Figure 5A). In fact, lethality of Excision101 homozygous larva occurs at the same stage than larva homozygous for Excision 250, a previously described total deletion of Xbp1 [24], suggesting that Xbp1spliced from Excision101 does retain little or no activity. This is expected, since the C-terminal half of Xbp1spliced is deleted in Excision101. We exposed larva to yeast paste food containing tunicamycin and assayed for induction of ER stress markers (BiP and Pdi) by real time RT-PCR (Figure 5B). As expected, the induction of ER stress markers is compromised in organisms homozygous for Excision 101.
Figure 5. Excision101 homozygous larvae have impaired UPR activation.
(A) Picture of 3-day old Excision101 homozygous larva and heterozygous Excision101/CyO-GFP sibling control. Excision101 homozygous larva arrest development during first instar stage. (B) Quantitative RT-PCR for BiP and Pdi mRNA levels in Excision101 homozygous or control heterozygous Excision101/CyO-GFP larvae treated with/without tunicamycin food. Induction of BiP and Pdi upon tunicamycin treatment is impaired in Excision101 homozygous larva. mRNA levels are indicated as a mean +/− standard deviation (a.u. - arbitrary units).
The features of C-terminal region in Xbp1unspliced protein, including the high hydrophobicity profile and critical amino acid residues to translational pausing, are shared between vertebrates and invertebrates, suggesting that the mechanism to target Xbp1 mRNA to the ER membrane described in humans is conserved across metazoans. This terminal hydrophobic region 2 is predicted to form an alpha helix [30], which is important for the association with lipid bilayers, dragging the mRNA-ribosome-nascent polypeptide complex to the ER membrane. Using an Xbp1 mutant lacking the C-terminal region, we investigated the mechanism underlying the targeting of Xbp1 mRNA to the ER membrane in Drosophila. We found the lack of the C-terminal region decreases the splicing efficiency of Xbp1 mRNA, but it does not abolish splicing completely. Our results suggest the existence of additional mechanisms for ER membrane targeting of Xbp1 mRNA that are independent of the C-terminal domain of Drosophila Xbp1unspliced.
Acknowledgments
We would like to thank the Bloomington Stock Center for fly stocks. We thank IGC for access to fly facilities. We would like to thank Hyung Don Ryoo and Colin Adrain for comments on our manuscript. This work was supported by Fundação para a Ciência e a Tecnologia, Portugal, through grants PEst-OE/EQB/LA0004/2011, PTDC/BIA-BCM/105217/2008, PTDC/SAU-OBD/104399/2008 and FCT-ANR/NEU-NMC/0006/2013, by a Marie Curie International Reintegration grant (PIRG03-GA-2008-230935). D.S.C was supported by a doctoral fellowship from the Gulbenkian Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Fundação para a Ciência e a Tecnologia, Portugal, through grants PEst-OE/EQB/LA0004/2011, PTDC/BIA-BCM/105217/2008, PTDC/SAU-OBD/104399/2008 and FCT-ANR/NEU-NMC/0006/2013, by a Marie Curie International Reintegration grant (PIRG03-GA-2008-230935). D. S. C. was supported by a doctoral fellowship from the Gulbenkian Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Moore KA, Hollien J (2012) The unfolded protein response in secretory cell function. Annu Rev Genet 46: 165–183. [DOI] [PubMed] [Google Scholar]
- 2. Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529. [DOI] [PubMed] [Google Scholar]
- 3. Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334: 1081–1086. [DOI] [PubMed] [Google Scholar]
- 4. Shamu CE, Walter P (1996) Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. Embo J 15: 3028–3039. [PMC free article] [PubMed] [Google Scholar]
- 5. Tirasophon W, Welihinda AA, Kaufman RJ (1998) A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev 12: 1812–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu CY, Wong HN, Schauerte JA, Kaufman RJ (2002) The protein kinase/endoribonuclease IRE1alpha that signals the unfolded protein response has a luminal N-terminal ligand-independent dimerization domain. J Biol Chem 277: 18346–18356. [DOI] [PubMed] [Google Scholar]
- 7. Credle JJ, Finer-Moore JS, Papa FR, Stroud RM, Walter P (2005) On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc Natl Acad Sci U S A 102: 18773–18784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gardner BM, Walter P (2011) Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 333: 1891–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cox JS, Walter P (1996) A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 87: 391–404. [DOI] [PubMed] [Google Scholar]
- 10. Sidrauski C, Walter P (1997) The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell 90: 1031–1039. [DOI] [PubMed] [Google Scholar]
- 11. Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107: 881–891. [DOI] [PubMed] [Google Scholar]
- 12. Shen X, Ellis RE, Lee K, Liu CY, Yang K, et al. (2001) Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell 107: 893–903. [DOI] [PubMed] [Google Scholar]
- 13. Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, et al. (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415: 92–96. [DOI] [PubMed] [Google Scholar]
- 14. Ryoo HD, Domingos PM, Kang MJ, Steller H (2007) Unfolded protein response in a Drosophila model for retinal degeneration. Embo J 26: 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Plongthongkum N, Kullawong N, Panyim S, Tirasophon W (2007) Ire1 regulated XBP1 mRNA splicing is essential for the unfolded protein response (UPR) in Drosophila melanogaster. Biochem Biophys Res Commun 354: 789–794. [DOI] [PubMed] [Google Scholar]
- 16. Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH (2003) Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc Natl Acad Sci U S A 100: 9946–9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee AH, Scapa EF, Cohen DE, Glimcher LH (2008) Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320: 1492–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamamoto K, Sato T, Matsui T, Sato M, Okada T, et al. (2007) Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell 13: 365–376. [DOI] [PubMed] [Google Scholar]
- 19. Yoshida H, Oku M, Suzuki M, Mori K (2006) pXBP1(U) encoded in XBP1 pre-mRNA negatively regulates unfolded protein response activator pXBP1(S) in mammalian ER stress response. J Cell Biol 172: 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hollien J, Weissman JS (2006) Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313: 104–107. [DOI] [PubMed] [Google Scholar]
- 21. Cross BC, Bond PJ, Sadowski PG, Jha BK, Zak J, et al. (2012) The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc Natl Acad Sci U S A 109: E869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kimmig P, Diaz M, Zheng J, Williams CC, Lang A, et al. (2012) The unfolded protein response in fission yeast modulates stability of select mRNAs to maintain protein homeostasis. Elife 1: e00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hollien J, Lin JH, Li H, Stevens N, Walter P, et al. (2009) Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol 186: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coelho DS, Domingos PM (2014) Physiological roles of regulated Ire1 dependent decay. Front Genet 5: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maurel M, Chevet E, Tavernier J, Gerlo S (2014) Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem Sci 39: 245–254. [DOI] [PubMed] [Google Scholar]
- 26. Gaddam D, Stevens N, Hollien J (2013) Comparison of mRNA localization and regulation during endoplasmic reticulum stress in Drosophila cells. Mol Biol Cell 24: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hollien J (2013) Evolution of the unfolded protein response. Biochim Biophys Acta 1833: 2458–2463. [DOI] [PubMed] [Google Scholar]
- 28. Moore KA, Plant JJ, Gaddam D, Craft J, Hollien J (2013) Regulation of sumo mRNA during endoplasmic reticulum stress. PLoS One 8: e75723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aragon T, van Anken E, Pincus D, Serafimova IM, Korennykh AV, et al. (2009) Messenger RNA targeting to endoplasmic reticulum stress signalling sites. Nature 457: 736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yanagitani K, Imagawa Y, Iwawaki T, Hosoda A, Saito M, et al. (2009) Cotranslational targeting of XBP1 protein to the membrane promotes cytoplasmic splicing of its own mRNA. Mol Cell 34: 191–200. [DOI] [PubMed] [Google Scholar]
- 31. Yanagitani K, Kimata Y, Kadokura H, Kohno K (2011) Translational pausing ensures membrane targeting and cytoplasmic splicing of XBP1u mRNA. Science 331: 586–589. [DOI] [PubMed] [Google Scholar]
- 32. Souid S, Lepesant JA, Yanicostas C (2007) The xbp-1 gene is essential for development in Drosophila. Dev Genes Evol 217: 159–167. [DOI] [PubMed] [Google Scholar]
- 33. Griciuc A, Aron L, Roux MJ, Klein R, Giangrande A, et al. (2010) Inactivation of VCP/ter94 suppresses retinal pathology caused by misfolded rhodopsin in Drosophila. PLoS Genet 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kang MJ, Ryoo HD (2009) Suppression of retinal degeneration in Drosophila by stimulation of ER-associated degradation. Proc Natl Acad Sci U S A 106: 17043–17048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mendes CS, Levet C, Chatelain G, Dourlen P, Fouillet A, et al. (2009) ER stress protects from retinal degeneration. Embo J 28: 1296–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coelho DS, Cairrao F, Zeng X, Pires E, Coelho AV, et al. (2013) Xbp1-independent Ire1 signaling is required for photoreceptor differentiation and rhabdomere morphogenesis in Drosophila. Cell Rep 5: 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- 38. Sone M, Zeng X, Larese J, Ryoo HD (2012) A modified UPR stress sensing system reveals a novel tissue distribution of IRE1/XBP1 activity during normal Drosophila development. Cell Stress Chaperones 18: 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Casas-Tinto S, Zhang Y, Sanchez-Garcia J, Gomez-Velazquez M, Rincon-Limas DE, et al. (2011) The ER stress factor XBP1s prevents amyloid-beta neurotoxicity. Hum Mol Genet 20: 2144–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ryoo HD, Steller H (2007) Unfolded protein response in Drosophila: why another model can make it fly. Cell Cycle 6: 830–835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.