Abstract
We report activation of the immediate-early gene Egr-1 in the lateral amygdala (LA), hippocampus (CA1), and medial prefrontal cortex (mPFC) 30-min following the training phase in the context pre-exposure facilitation effect (CPFE) and standard context fear conditioning (180 sec context exposure → shock). On day one of the CPFE paradigm, postnatal day (PD) 31 rats (±1) were pre-exposed to Context A (Pre) or Context B (Alt-Pre) for 5 min followed by five additional 1-minute exposures. A day later, Pre and Alt-Pre rats received a 2-sec, 1.5 mA footshock immediately upon placement in Context A. Animals included in in situ hybridization were then sacrificed 30 (±3) min later. On day three, the behaviorally-tested Pre rats showed significantly more fear-conditioned freezing in Context A than Alt-Pre rats. Standard context fear conditioning groups showed much greater freezing than the Pre group, as well as no shock and immediate-shock controls. Thirty minutes after immediate shock training, Pre rats showed increased Egr-1 mRNA in the prelimbic mPFC relative to Alt-Pre rats. Standard context conditioning selectively increased Egr-1 in CA1. In the LA and mPFC, Egr-1 increased to a similar extent in no shock, immediate shock, and standard context conditioning relative to homecage controls. The present study demonstrates that Egr-1 mRNA expression has a complex relationship to fear learning in different brain regions and variants of context conditioning.
Keywords: hippocampus, amygdala, prefrontal cortex, Egr-1, context fear conditioning, CPFE
1. Introduction
In Pavlovian fear conditioning, a conditioned stimulus (CS) is paired with an aversive unconditioned stimulus (US) such that future presentations of the CS alone elicit species-typical defensive responses (e.g., freezing). In standard context fear conditioning (sCFC), the training context, rather than a discrete CS, is paired with an aversive stimulus (Maren et al., 1997; O’Reilly and Rudy, 2001). sCFC requires a context exposure period in which a representation of the context is formed prior to learning the context-US association; if animals are shocked immediately upon being placed in a context for the first time, they fail to form the context-US association, a phenomenon termed the “immediate-shock deficit” (Fanselow, 1990). This deficit is used as a control procedure in a variant of sCFC, the context pre-exposure facilitation effect (CPFE), which permits the dissociation of context learning from context-shock learning by separating them into different procedural phases. This is important because it is not possible to distinguish between these different forms of learning in a standard context fear experiment. In the CPFE paradigm, animals pre-exposed to Context A (Pre) and given an immediate shock in Context A 24-hours later exhibit a freezing response when returned to Context A the following day, whereas animals pre-exposed to an alternative Context B on the first day (Alt-Pre) do not display freezing when tested in Context A. Our research group has recently employed this paradigm to study correlates of immediate-early gene expression associated with the context acquisition and the context-shock association phases of the CPFE (Asok et al., 2013b).
Our previous investigations have focused on the expression of the immediate-early gene early-growth response gene 1 (Egr-1, a.k.a. Krox 24, NGFI-1, Zif268, TIS8), an inducible transcription factor associated with neuronal plasticity and implicated in various forms of learning, such as fear conditioning and spatial learning (for reviews, see Alberini, 2009; Davis et al., 2003; Knapska and Kaczmarek, 2004; Rosen, 2004; Rosen and Donley, 2006; Veyrac et al., 2014). Egr-1 expression is increased in the lateral nucleus of the amygdala (LA) of adult animals that learn context fear in sCFC relative to those that display the immediate-shock deficit (Malkani and Rosen, 2000) and context fear conditioning is abolished following antisense knockdown of EGR-1 protein in the LA (Malkani et al., 2004). Recently, we have extended these findings to the CPFE in adolescent rats (Asok et al., 2013b) by examining patterns of activity in the dorsal hippocampus (dHPC) and LA, as well as in the prelimbic (PL) and infralimbic (IL) divisions of the medial prefrontal cortex (mPFC) following the preexposure and training phases of the CPFE procedure. Taken together, these studies suggest a unique role for Egr-1 during consolidation of context fear memories throughout different brain regions.
Interestingly, Asok et al. (2013b) obtained findings that indicate a novel role of Egr-1 in the mPFC during the context-shock association in the CPFE (Asok et al., 2013b). This adds to a recent growing literature on the role of prefrontal cortex in contextual fear conditioning: this region seems to be particularly involved in the retrieval of contextual representations (Asok et al., 2013b; Baeg et al., 2001; Frankland and Bontempi, 2005; Hyman et al., 2012; Raybuck and Gould, 2010; Rudy, Biedenkapp, & O’Reilly, 2005; Sotres-Bayon and Quirk, 2010), although the role of the mPFC in the acquisition of sCFC remains unclear (cf. Beeman et al., 2013; Gilmartin and Helmstetter, 2010; Morgan et al., 1993; Morgan and LeDoux, 1995; Quinn et al., 2008; Raybuck and Gould 2010; Zhao et al., 2005). Accordingly, the present study sought to compare, for the first time, patterns of Egr-1 expression during training on the CPFE vs. standard contextual fear conditioning in adolescent rats.
This comparison also addresses an alternate hypothesis of Asok et al. (2013b), namely that the prefrontal cortex is involved in weak forms of fear conditioning (Rudy et al., 2005). We used a multiple-exposure CPFE paradigm similar to Dokovna, Jablonski, & Stanton (2013) to increase the overall amount of learning in the CPFE and to reduce the potential confounds of Egr-1 expression owing to novelty or unpredictability (Hall, Thomas, & Everitt, 2000; Rosen and Donley, 2006) in favor of fear learning (Lee, 2010; Malkani and Rosen, 2000; Malkani et al., 2004). Thus, in addition to investigating mPFC Egr-1 expression in sCFC, we also sought to extend our findings of learning-related Egr-1 expression in the CPFE to a multiple-exposure paradigm that would, in theory, feature less of a “novelty” component.
The purpose of the present study was to extend Asok et al (2013b) in a manner that affords a comparison of Egr-1 expression in the CPFE and standard contextual conditioning. As in our previous study, we examined Egr-1 expression in the PL, IL, CA1 of the dHPC, and the LA in PD 31 (± 1) rats 30 min following training. The design of our sCFC study included experimental groups similar to those used by Malkani and Rosen (2000): a group that received equivalent handling without any exposure to context A or B which received no shock (No Shock) or an immediate shock upon placement in the training context (Imm Shock), as well as a group of animals that underwent sCFC (180-sec context exposure followed by footshock). This allowed us to test several hypotheses: (1) whether associations of a retrieved context representation with a shock drives gene expression similar to associations of a concurrently experienced context with shock (Pre and sCFC, respectively), (2) whether Egr-1 expression in the mPFC is driven by learning of standard contextual fear (No Shock/Imm Shock vs. sCFC), and (3) whether a multiple-exposure CPFE paradigm might reduce uncertainty-based Egr-1 responses in LA and CA1 that might “unmask” fear-based responses that were not evident in our previous study (Asok et al., 2013b).
2. Materials and Methods
2.1 Subjects
Subjects and animal husbandry were as described in our previous reports (e.g., Asok et al., 2013b; Schiffino et al., 2011). Subjects were 185 (101 males and 84 females) Long Evans rats derived from 36 time-bred dams in the University of Delaware breeding colony. Of these, 74 animals (40 males, 34 females) were assigned to the 6 experimental groups in the in situ hybridization assay, and 114 (63 males 51 females) were assigned to the 5 groups that underwent behavioral testing (see Table 1, design and procedure below). The date of birth (PD 0) was determined by checking for births during the light cycle on GD 21 and 22. On PD 3, litters were culled to 8 pups (usually 4 males and 4 females) and paw-marked by subcutaneous injections of non-toxic black ink for identification purposes. Pups were kept with the dam in a clear polypropylene cage (45 × 24 × 21 cm) until PD 21, after which they were weaned and housed with same-sex littermates in 45 × 24 × 17 cm cages. Two days prior to the start of the experiment (PD 29 ± 1), rats were individually-housed in opaque white cages (24 × 18 × 13 cm), where they lived for the remainder of the study. Same-sex littermates were assigned to different behavioral or assay conditions so that no more than one same-sex littermate was represented in a particular experimental condition. In three cases in which same-sex littermates were inadvertently assigned to the same experimental group in the behavioral experiment (2 males in group Pre, 1 female in group No Shock), the animals’ data were averaged together to yield a single data point. The final number of animals in the behavioral assay was 111 (61 males, 50 females). Animals had ad libidum access to food and water throughout the experiment. All subjects were treated in accordance with the Institutional Animal Care and Use Committee at the University of Delaware.
Table 1.
Subject assignment and experimental design.
| ------Experimental Condition------ | ----------------Experimental Phase--------------- | ||||
|---|---|---|---|---|---|
| Behavioral | Pre-exposure | Training | Sac | Testing | |
| In situ | Baseline | Context A/B | Home cage | x | |
| Pre | Context A | Context A | x | ||
| Alt-Pre | Context B | Context A | x | ||
| Imm Shock | Handle | Context A | x | ||
| No Shock | Handle | Context A | x | ||
| Standard Context Fear | Handle | Cxt A 180s | x | ||
| Behavior | Pre | Context A | Context A | Context A | |
| Alt-Pre | Context B | Context A | Context A | ||
| Imm Shock | Handle | Context A | Context A | ||
| No Shock | Handle | Context A | Context A | ||
| Standard Context Fear | Handle | Cxt A 180s | Context A | ||
2.2 Apparatus and Stimuli
The apparatus is the same one described previously (Asok et al., 2013b; Murawski and Stanton, 2010; 2011). There were two pre-exposure contexts, the training context (Context A) and an alternate context (Context B). Context A was a clear Plexiglas chamber measuring 16.5 × 21.1 × 21.6 cm with a floor consisting of 9 stainless steel bars (0.5 cm diameter placed 1.25 cm apart) connected to a shock scrambler that delivered a 2 sec 1.5 mA footshock (Med Associates, Georgia, VT ENV-414S). Four chambers (Context A) were placed on a Plexiglas stand (2 chambers per row and column) within a fume hood, which provided background light and ambient noise. The sides of each chamber were made opaque to prevent animals from viewing one another. Activity was recorded with a camera connected to a computer running FreezeFrame software (Actimetrics, Wilmette, IL). Freezing was defined as a bout of 0.75 seconds or longer without changes in pixel luminance. Context B was a modification of Context A to include a wire mesh insert covering the floor and protruding into the chamber to alter the spatial configuration of the context. Opaque paper was draped across three of the four outside walls of Context B; only the wall facing the camera remained visible. Chambers were cleaned with a 5% ammonium hydroxide solution immediately prior to use. Transport cages (11 × 11 × 18 cm) made of Lexan and surrounded with opaque paper on all four outside walls were used to move individual rats to and from their home cages in the colony room.
2.3 Design and Procedures
Design
There were eleven experimental conditions in this study with different littermates being assigned to each condition (Table 1). These eleven conditions were comprised of five core conditions: Handled No Shock (handled and exposed to the context without receiving a footshock; “No Shock”), Handled Immediate Shock (handled and shocked immediately upon placement in the training context; “Imm Shock”), Alt-Pre (pre-exposed to Context B, see Apparatus and Stimuli), Pre (pre-exposed to Context A, see Apparatus and Stimuli), and Standard Context Fear (handled on pre-exposure day, received 180 sec acclimation period followed by a shock on training day; “sCFC”). These 5 conditions were assigned to behavioral testing (Fig. 1) or were sacrificed on the training day for an in situ hybridization assay that included a sixth home-cage baseline group (Fig. 2, 3).
Figure 1.
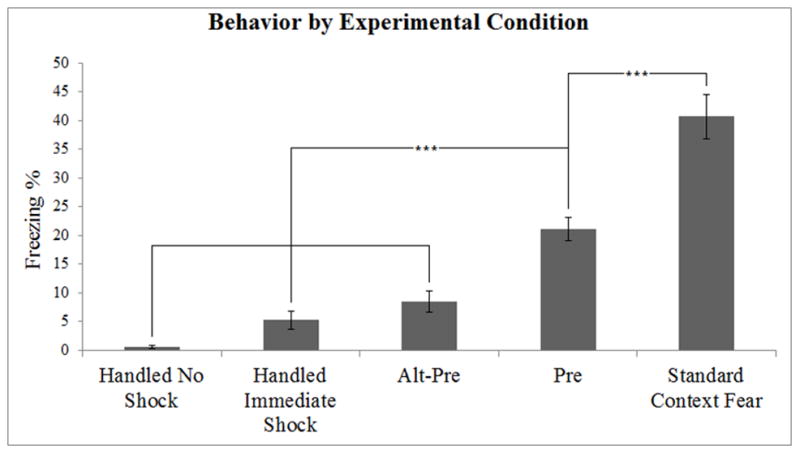
Behavioral data from the five experimental groups. Both Pre and Standard Context Fear animals showed higher levels of learning than Handled No Shock, Handled Immediate Shock, and Alt Pre animals. Standard Context Fear animals showed more learning than Pre animals. Error bars indicate standard error of the mean. ***p < 0.01.
Figure 2.
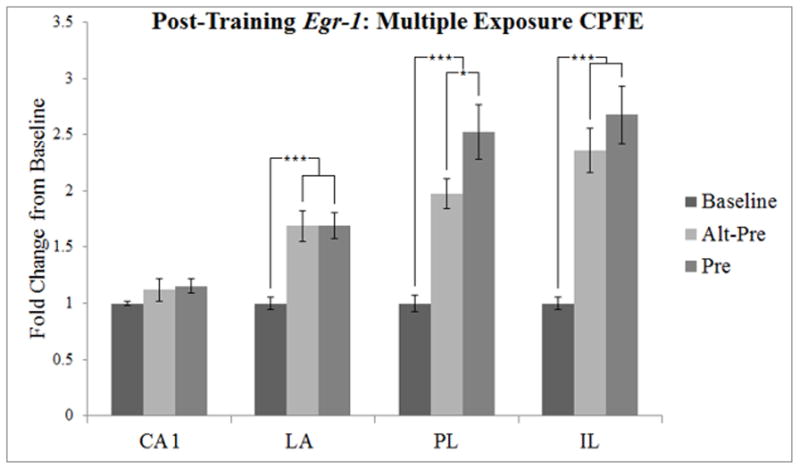
Post-training Egr-1 expression, depicted as a three-group design similar to Asok et al. (2013b). Error bars indicate standard error of the mean. *p < 0.05, *** p < 0.01.
Figure 3.
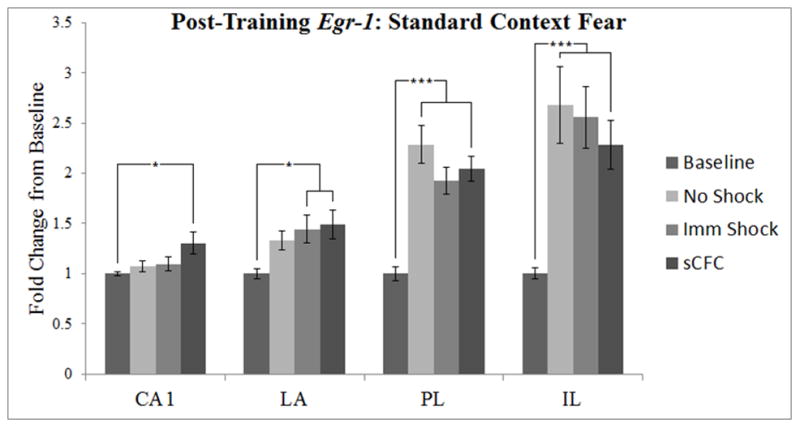
Post-training Egr-1 expression, depicted as a four-group design similar to Malkani and Rosen (2000). Error bars indicate standard error of the mean. *p < 0.05, ***p < 0.01.
Procedure
The general training procedure has been described previously (Dokovna et al., 2013). Contextual fear conditioning occurred over three days (Pre-exposure/Handling, Training, and Testing) starting on PD 31(±1). Pre-exposure consisted of a 5-minute exposure to one of two distinct contexts (Context A or Context B) followed by an additional five 1-minute exposures. Animals that did not receive Pre-exposure (Handled Immediate Shock, Handled No Shock, and Standard Context Conditioning) received equivalent handling in a hallway outside the behavior room roughly equivalent to the handling experiences of their pre-exposed counterparts. Animals assigned to any of the six experimental groups in the in situ hybridization assay were sacrificed following training; littermates of these animals that were not sacrificed during training were tested for behavioral expression of the CPFE.
2.3.1 Pre-exposure
Animals were weighed and transported to a place outside the conditioning room. Animals were loaded into individual contexts (A or B) and allowed to explore the context for a 5-min period, after which they were returned to their transport cages. Approximately one minute later, animals received an additional five sessions of 1-minute exposures to the pre-exposure context, separated by 1-minute periods in their transport cages. The total procedure consisted of six exposures: one 5-minute exposure followed by five 1-minute exposures. After the final 1-minute pre-exposure, animals were loaded into their transport cages and brought back to their home cages in the colony room.
While animals in the Pre and Alt-Pre conditions were being exposed to Context A or B, animals in the Imm Shock, No Shock, and sCFC groups received similar handling from an experimenter in a hallway outside of the behavior room (i.e. they were briefly picked up, and then replaced in their transport cages), but were not exposed to either Context A or Context B on the pre-exposure day.
2.3.2 Training
Twenty-four hours after pre-exposure, animals in the in situ hybridization condition (Table 1) were again weighed and transported four at a time to the behavior room (see Pre-exposure). Animals were then individually brought into the conditioning room and received a 2 sec 1.5 mA footshock immediately upon placement into Context A (except sCFC animals, which experienced a 180-sec acclimation period prior to shock, see Design). Animals in the No Shock condition were placed in chambers with inactivated shock grids, although all other elements of the Training procedure were identical to animals in the Pre, Alt-Pre, and Imm Shock groups. Animals were immediately removed, returned to their transport cages, brought back to their home cages in the colony room, and sacrificed 30 (± 3) min later (see below). HC (baseline control) animals remained undisturbed in their home cages and were sacrificed while their littermate counterparts were undergoing training. Care was taken to ensure that HC controls had the same experimental history as their counterparts. For the in situ hybridization condition, the HC control group was comprised approximately equally of rats that had received Pre or Alt-Pre exposures the previous day (Table 1).
2.3.3 Retention Testing
Twenty-four hours after training, the remaining littermates in the Behavior condition (Table 1) were weighed and transported identically as described in the Pre-exposure and Training phases. Animals were loaded into the same chambers where they received training (Context A), and were monitored for freezing behavior over a 5-min testing period.
2.4 Brain Collection
The procedure was the same as previously described (Asok et al., 2013b). Rats were sacrificed by rapid decapitation, and brains were removed and frozen in −45°C isopentane and stored at −80°C until sectioned. Sixteen micrometer coronal brain sections corresponding to the medial prefrontal cortex, lateral nucleus of the amygdala, and CA1 subfield of the dorsal hippocampus were sectioned on a cryostat (Leica Inc., Deerfield, IL) using the Paxinos and Watson stereotaxic brain atlas as a guide (Paxinos and Watson, 2007). Two brain sections were placed on each slide, and slides were stored at −80°C until they were processed for in situ hybridization.
2.5 In situ Hybridization
In situ hybridization was conducted as described previously (Asok et al., 2013a; Asok et al., 2013b). An antisense RNA probe (riboprobe) was transcribed from a plasmid containing a sense cDNA sequence coding for a 230 bp sequence of Egr-1 (gift from J. Milbrandt, Washington University, St. Louis, MO). The transcribed riboprobe incorporated a radioactively labeled 35S UTP (approximately 1×106 dpm) using a T7 RNA polymerase Maxiscript kit according to the manufacturer’s instructions (Life Technologies, Grand Island, NY). After hybridization and washing, the dry slides were exposed to Kodak Biomax MR Film for 2 days.
2.6 In Situ Hybridization Image and Statistical Analysis
As previously described (Asok et al., 2013b), autoradiograms were captured and digitized to 8-bit gray values via a Dage CCD video camera with ImageJ 1.45m program (Wayne Rasband, NIMH). ImageJ was used to subtract the background (2D-rolling ball radius of 50.0 pixels) and measure the mean density (mean gray value) within a specific area. The mean density of all mRNA labeling was analyzed for the prelimbic and infralimbic divisions of the medial prefrontal cortex (using Plate 11 of Paxinos and Watson as a guide; Paxinos and Watson, 2007), as well as the lateral nucleus of the amygdala and CA1 of the hippocampus (using Plate 57 of Paxinos and Watson as a guide; Paxinos and Watson, 2007).
The mean gray value of the left and right side of the brain was averaged within slices and then within slides. A 14C standard with known amounts of radioactivity was exposed and captured with the slides. The standard was used to generate a 3rd degree polynomial equation and calibrate the mean grey values from the slides against the radioactive standard (in nCi/g). The nCi/g value was then normalized against the average nCi/g of all home-cage animals in that region to obtain a proportionate score (the average proportionate score of the home cage group equaled 1). For each assay, when nCi/g scores differed by ± 1.96 standard deviations from the nCi/g group mean for a particular region, that score was defined as an outlier and was excluded from the calculation of proportionate scores and further analysis.
In situ hybridization data (proportionate scores) were analyzed by separate one-way ANOVAs on each brain region and type of experiment (CPFE, Fig. 2; sCFC, Fig. 3; see below). Post-hoc analyses included Dunnett’s tests to contrast each group with home-cage baseline and Newman-Keuls tests to contrast the remaining groups with each other (statistically significant differences, p < 0.05).
2.7 Behavioral Statistical Analysis
Freezing behavior was scored using FreezeFrame software by an observer blind to the experimental condition of the animals as described previously (Schiffino et al., 2011). Activity thresholds were adjusted on an individual basis to exclude small movements from being calculated as part of an animal’s total freezing. Data were analyzed via one-way ANOVA, as well as Newman-Keuls and Dunnett’s post-hoc tests (Fig. 1).
3. Results
3.1 Behavior
After excluding outliers (N = 1 female, Standard Context Fear; N = 2 males, Alt-Pre; N = 1 female, 1 male, Pre; N = 1 female, 1 male, Handled Immediate Shock; N = 2 males, Handled No Shock), freezing behavior was scored for the remaining 102 animals across the five training conditions.
A 5 (Condition) × 2 (Sex) factorial ANOVA revealed a main effect of Condition only [F(4, 92) = 41.77, p < 0.01] (see Figure 1). There was no main effect of Sex, or Sex × Condition interaction (p’s > 0.06), indicating no sex differences in freezing behavior. Newman-Keuls analysis conducted on the main effect of Condition showed that freezing in the Standard Context Fear condition (40.66 ± 3.87 %) was significantly higher than all other conditions. Animals in the Pre condition (21.10 ± 2.00%) froze significantly more than those in all remaining control conditions, which did not differ among themselves: Alt-Pre (8.54 ± 1.84 %), Handled Immediate Shock (5.22 ± 1.60 %), or Handled No Shock (0.53 ± 0.23 %) conditions. Importantly, animals in the Pre condition froze significantly more than animals in the Alt-Pre condition (p < 0.001), confirming the CPFE in this study.
In summary, our behavioral data replicate and extend our previous findings with the multiple-exposure CPFE paradigm (Dokovna et al., 2013). Standard Context Fear conditioning produced higher levels of freezing than any group, including the Pre group, indicating that this is a stronger form of fear conditioning than the CPFE. Lastly, animals in the Alt-Pre condition do not show significantly different amounts of freezing from animals that do not learn either because of the immediate shock deficit (Handled Immediate Shock) or because of the lack of an aversive stimulus (Handled No Shock).
3.2 Gene Expression
An omnibus ANOVA simultaneously comparing all of the experimental groups in this study is underpowered by the large number of groups relative to effect sizes and also includes many irrelevant contrasts (e.g. Alt-Pre vs. sCFC). We therefore analyzed CPFE and sCFC data separately, in a comparable fashion to previous studies (Asok et al., 2013b; Malkani and Rosen, 2000).
3.2.1. CPFE Analysis
To examine our data under similar conditions as Asok et al. (2013b), we analyzed all brain regions across three groups that define the CPFE: Baseline, Alt-Pre, and Pre. Initially, analyses were conducted in a factorial (condition × sex) ANOVA. Because there were no significant main effects or interactions involving sex (p’s > 0.22), we collapsed across this variable and all further analyses were conducted with a one-way ANOVA. The data from this analysis are depicted in Figure 2.
In CA1, there was no significant main effect of Condition (p > 0.3). Thus, the multiple-exposure procedure used in this study may have reduced the “novelty response” in CA1 that appeared on the training day with a single exposure procedure in our previous study (Asok et al., 2013b).
In the LA, there was a significant main effect of Condition [F(2, 26) = 12.61, p < 0.001]. Dunnett’s test showed that both Pre and Alt-Pre groups differed significantly from Baseline (p’s < 0.001). Newman-Keuls analysis showed that gene expression in Pre (1.69 ± 0.12) and Alt-Pre (1.69 ± 0.14) groups did not differ from one another (p > 0.98). Thus, greater fear conditioning in Group Pre relative to Alt-Pre (Figure 1) did not differentially elevate Egr-1 in the LA.
In the PL, there was a significant main effect of Condition [F(2, 30) = 17.43, p < 0.001]. Dunnett’s test showed that both Pre and Alt-Pre groups differed significantly from Baseline (p’s < 0.01). Newman-Keuls analysis showed that gene expression in the Pre group was significantly higher than in the Alt-Pre group (p < 0.04). This replicates and extends our previous finding with the single-exposure CPFE procedure (Asok et al., 2013b).
In the IL, there was a significant main effect of Condition [F(2, 29) = 17.45, p < 0.001]. Dunnett’s test showed that both Pre and Alt-Pre groups differed significantly from Baseline (p’s < 0.001). Newman-Keuls analysis showed that gene expression in Pre (2.68 ± 0.26) and Alt-Pre (2.36 ± 0.20) groups did not differ from one another (p > 0.27). This outcome contrasts with our previous study (see General Discussion).
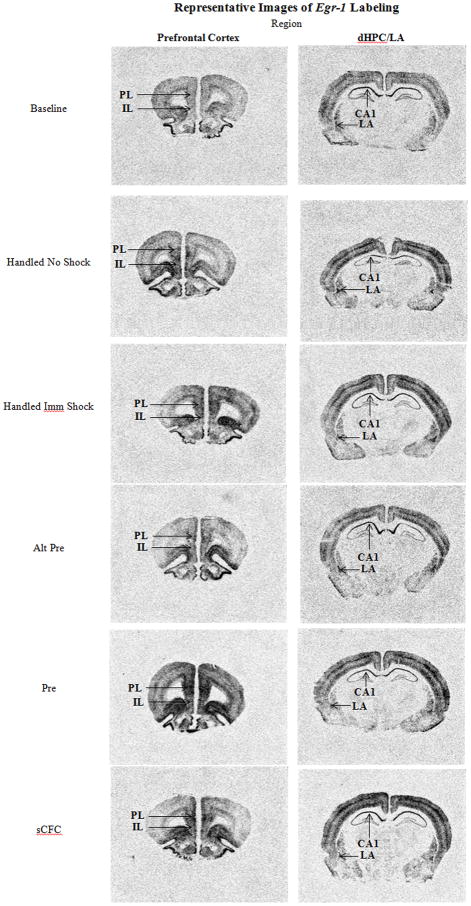
In summary, following context-shock training in the CPFE paradigm there was a significant fear-conditioning related difference between Pre and Alt-Pre groups in the PL wherein animals in the Pre condition showed elevated levels of Egr-1 above animals in the Alt-Pre condition. This differential effect was not present in any of the other brain regions, although there were increases over baseline in the LA, PL, and IL in Pre and Alt-Pre conditions (see Fig. 5 for representative digitized enhanced contrast images).
Figure 5.
Digitized enhanced contrast images of animals across all experimental conditions in the prelimbic (PL) and infralimbic (IL) of the prefrontal cortex (PFC). CA1 region of the dorsal hippocampus (dHPC) and lateral nucleus of the amygdala (LA).
3.2.2. Standard Contextual Fear Conditioning (Baseline, No Shock, Imm Shock, sCFC)
To examine our data under similar conditions to Malkani and Rosen (2000), we analyzed all brain regions while contrasting four conditions: Baseline, No Shock, Imm Shock, and sCFC (Figure 3). There were no significant main effects or interactions of sex (p’s > 0.20) in an initial 2 × 4 ANOVA, so we collapsed across this variable and all further analyses were conducted with a one-way ANOVA.
In CA1, there was a significant main effect of Condition [F(3, 36) = 3.10, p < 0.04]. Dunnett’s test showed that gene expression in the sCFC group was significantly higher than Baseline (p < 0.02). Newman-Keuls analysis also showed that Egr-1 expression did not differ among the No Shock, Imm Shock, or sCFC groups (p’s > 0.05).
In the LA, there was a significant main effect of Condition [F(3, 38) = 3.18, p < 0.04]. Dunnett’s test showed that gene expression in the Imm Shock and sCFC groups were elevated above baseline (p’s < 0.05), but not the No Shock group (p > 0.15). Newman-Keuls showed that Egr-1 expression did not differ among the No Shock, Imm Shock, or sCFC groups (p’s > 0.49).
In the PL, there was a significant main effect of Condition [F(3, 34) = 16.33, p < 0.001]. Dunnett’s test showed that gene expression in the No Shock, Imm Shock, and sCFC groups were elevated above baseline (p’s < 0.001). Newman-Keuls analysis showed Egr-1 expression in these groups was not significantly different from one another (p’s > 0.16).
In the IL, there was a significant main effect of Condition [F(3, 35) = 7.36, p < 0.001]. Dunnett’s test showed that gene expression in the No Shock, Imm Shock, and sCFC groups were elevated above baseline (p’s < 0.01). Newman-Keuls analysis showed Egr-1 expression in these groups did not significantly differ from one another (p’s > 0.48).
In summary, in the sCFC paradigm there was a significant elevation of Egr-1 expression above baseline in the PL and IL in all groups, but these elevations did not differ between the No Shock, Imm Shock, and sCFC groups. The sCFC group showed an increase in Egr-1 expression in both CA1 and the LA, and the Imm Shock group showed an increase over Baseline in the LA (see Fig. 5). The lack of a significant difference between the sCFC group and either No Shock or Imm Shock groups suggests that the LA effect reported by Malkani and Rosen (2000) might not extend to the conditions of the present study (see General Discussion).
4. General Discussion
The present study extends previous research from our laboratory on amygdala Egr-1 expression in context fear conditioning (Malkani and Rosen, 2000; Malkani et al., 2004), as well as amygdala, hippocampal, and prefrontal egr-1 expression in the CPFE (Asok et al., 2013b). We examined if the post-training pattern of Egr-1 expression observed by Asok et al. (2013b) in the medial prefrontal cortex extended to context-shock learning in the multiple pre-exposure CPFE paradigm and to standard contextual fear conditioning. Additionally, we used a multiple pre-exposure procedure in an attempt to reduce the additive effect of environmental novelty on fear-learning-related increases of Egr-1 mRNA in the amygdala and hippocampus (Hall et al., 2000; Malkani and Rosen, 2000).
The present study extends previous reports by comparing how sCFC and CPFE training procedures activate Egr-1 in prefrontal cortical regions. The CPFE differs from sCFC in some important respects. First, the CPFE depends on an association between a retrieved context representation and shock (Rudy, Huff & Matus-Amat, 2004). The retrieved representation is arguably not as salient as the context itself, which can be directly associated with shock in sCFC. Second, the CPFE is a weaker form of conditioning, as reflected in levels of freezing that are typically half of what is observed in sCFC. The levels of fear conditioning in our study (Fig. 1) show strong agreement both with our previous studies of the CPFE (Asok et al., 2013b; Schiffino et al., 2011) and with Malkani and Rosen’s (2000) study of sCFC. Makani and Rosen (2000) found an increase in LA Egr-1 in a standard context fear conditioning group over an immediate-shock control group (Malkani and Rosen, 2000; Rosen et al., 1998). The present experiment did not obtain evidence of this effect in the LA and failed to replicate or extend it to any of the brain regions analyzed. Various methodological differences could contribute to these findings, such as handling procedures, post-shock behavior testing, or foot shock salience (length and intensity; Rosen and Donley, 2006). Additionally, Malkani and Rosen (2000) used adult male Sprague-Dawley rats, whereas the present study used adolescent Long-Evans rats of both sexes (although there were no significant sex differences in any of our reports). Fear learning during development differs from adulthood, especially with respect to the anatomical and psychological changes that characterize the adolescent developmental period (Kim et al., 2011; Markham et al., 2007; Pattwell et al., 2011; Richardson and Hunt. 2010; Rubinow and Juraska, 2009; Spear, 2000; Stanton, 2000). However, recent findings from our research group suggest that adult rats show similar patterns in Egr-1 expression as adolescents when trained with the same general procedures used here (Chakraborty et al., 2013), suggesting that ontogenetic differences between adolescents and adults cannot explain the different outcomes of the present study versus Malkani & Rosen (2000).
The functional significance of Egr-1 expression patterns in CA1, the LA, the PL, and the IL is discussed in Asok et al. (2013b). It is possible that during CPFE training, animals that were exposed to an alternative context (Context B; Alt-Pre) form a context-shock association with the previously acquired context (Context B) during the immediate shock in Context A (Rudy and O’Reilly, 2001; Rudy et al., 2004). Our data do not support this theory, specifically because of the lack of significant difference in freezing between animals exposed to the alternate context and animals shocked without preexposure to either context (Alt-Pre vs. Imm Shock). It is also worth noting that the learning-dependent expression of Egr-1 mRNA in the prelimbic mPFC reported by Asok et al. (2013b) has since been replicated and reported twice outside of the current report (Chakraborty et al., 2013; Jablonski et al., submitted). The prelimbic mPFC is implicated in memory storage, retrieval, and context conditioning (Baeg et al., 2001; Frankland et al., 2004; Frankland and Bontempi, 2005; Hall et al., 2001; Rudy et al., 2005; as cited in Asok et al., 2013b). Egr-1 is increased in the prelimbic cortex of Pre above Alt-Pre animals in the CPFE; however, no difference is observed between sCFC animals over Imm Shock animals, suggesting that the prelimbic cortex is involved in context-sensitive fear conditioning under certain conditions (Maren et al., 2013; Zelikowsky et al., 2013). Specifically, activity in the prelimbic cortex during the CPFE might aide in promoting successful retrieval of a recently acquired weak context memory and the subsequent consolidation of a new context-shock association (Rudy et al., 2005).
Although we failed to observe a statistically significant difference between the Pre and Alt-Pre groups in the infralimbic cortex, our data exhibit the same trend in which Egr-1 mRNA expression in the Pre group is higher than the Alt-Pre group as in our previous report (Asok et al., 2013b). We have subsequently replicated Asok et al. (2013b)’s infralimbic cortex effect with adult rats (Chakraborty et al., 2013), and found similar effects in another study of juvenile rats (Jablonski et al., submitted). That the increased Egr-1 in infralimbic cortex of the Pre vs. the Alt-Pre groups was negative statistically in the present study seems to have limited generality and should thus be interpreted with caution.
It remains to be determined whether Egr-1 expression serves exclusively as a molecular marker of learning in the CPFE. In Lee (2010), an analogue to our Pre group (including multiple pre-exposure) showed an increase in EGR-1 protein in the dHPC following training over all their experimental groups (including one similar to our Imm Shock group). The CPFE was prevented when Egr-1 antisense was infused into the dHPC 90-min before training, leading Lee (2010) to conclude that EGR-1 protein in the hippocampus was specifically involved in updating contextual memories to include footshock. In our experiment, the only significant increase in hippocampal Egr-1 expression above baseline was observed in the sCFC group (Figs. 2 & 3), although this could be owing to a difference between the use of mRNA vs. protein expression in measuring Egr-1 responses (Maier et al., 2009). It is also known that antisense knockdown of EGR-1 in the LA (Maddox et al., 2011; Malkani et al., 2004) causes deficits in fear learning, suggesting that Egr-1 is causally and specifically related to fear conditioning. However, the broader literature on Egr-1 expands its purview beyond “fear learning” (e.g. Pre vs. Alt-Pre) to include “novelty/uncertainty responses” (e.g. Imm Shock vs. sCFC; see Davis et al., 2003; Hall et al., 2000; Knapska and Kaczmarek, 2004; Rosen and Donley, 2006; Veyrac et al., 2014). Our data suggest that elevated Egr-1 mRNA expression in various regions during fear conditioning may be driven by multiple processes, including novelty and threat detection appraisals of uncertain and novel situations.
In summary, the present study expands upon previous research indicating that the CPFE causes Egr-1 expression in the PL in which Egr-1 mRNA is higher in group Pre than in group Alt-Pre, corresponding to behavioral expression of acquired fear. This increase was not seen during standard contextual fear conditioning, suggesting that the mPFC becomes more critical for contextual fear conditioning when context retrieval is important or when context cues are weak. Our findings also suggest that regional Egr-1 expression may not be a “marker” of fear learning per se, but rather likely has a broader role as a regulator of neuronal plasticity and as a molecular priming agent that responds to novelty, uncertainty, and other factors which are necessary for learning to occur.
Highlights.
During training, the CPFE increases Egr-1 mRNA in prelimbic cortex.
Egr-1 mRNA in sCFC may reflect processes other than specific context fear learning.
PL, IL, and LA Egr-1 mRNA expression may reflect responding to unpredictability.
Acknowledgments
This research was supported by 1-R21-HD070662-01 (to MES and JBR), 1-F31-AA021317-01 (to SAJ), and by The University of Delaware
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89:121–45. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asok A, Ayers LW, Awoyemi B, Schulkin J, Rosen JB. Immediate early gene and neuropeptide expression following exposure to the predator odor 2,5-dihydro-2,4,5-trimethylthiazoline (TMT) Behav Brain Res. 2013a;248:85–93. doi: 10.1016/j.bbr.2013.03.047. [DOI] [PubMed] [Google Scholar]
- Asok A, Schreiber WB, Jablonski SA, Rosen JB, Stanton ME. Egr-1 increases in the prefrontal cortex following training in the context preexposure facilitation effect (CPFE) paradigm. Neurobiol Learn Mem. 2013b;106C:145–153. doi: 10.1016/j.nlm.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb Cortex. 2001;11:441–51. doi: 10.1093/cercor/11.5.441. [DOI] [PubMed] [Google Scholar]
- Beeman CL, Bauer PS, Pierson JL, Quinn JJ. Hippocampus and medial prefrontal cortex contributions to trace and contextual fear memory expression over time. Learn Mem. 2013;20:336–343. doi: 10.1101/lm.031161.113. [DOI] [PubMed] [Google Scholar]
- Chakaborty T, Asok A, Jablonski S, Schreiber W, Stanton M, Rosen J. Egr-1 gene expression in the prefrontal cortex, hippocampus, and amygdala in the CPFE fear conditioning paradigm. Poster presented at the annual meeting of the Society of Neuroscience; San Diego, CA. 2013. Nov, [Google Scholar]
- Davis S, Bozon B, Laroche S. How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behav Brain Res. 2003;142:17–30. doi: 10.1016/s0166-4328(02)00421-7. [DOI] [PubMed] [Google Scholar]
- Dokovna LB, Jablonski SA, Stanton ME. Neonatal alcohol exposure impairs contextual fear conditioning in juvenile rats by disrupting cholinergic function. Behav Brain Res. 2013;248:114–20. doi: 10.1016/j.bbr.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Factors governing one-trial contextual conditioning. Anim Learn Behav. 1990;18:264–270. [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–3. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–30. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, Helmstetter FJ. Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learn Mem. 2010;17:289–296. doi: 10.1101/lm.1597410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–5. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci. 2001;21:2186–93. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Ma L, Balabuer-Ballester E, Durstewitzm D, Seamans JK. Contextual encoding by ensembles of medial prefrontal cortex neurons. Proc Natl Acad Sci USA. 2012;109:5086–5091. doi: 10.1073/pnas.1114415109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski SA, Schiffino FL, Stanton ME. Role of age, post-training consolidation, and conjunctive associations in the ontogeny of the context preexposure facilitation effect. Dev Psychobiol. 2012;54:714–22. doi: 10.1002/dev.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski SA, Schrieber WB, Asok A, Rosen JB, Stanton ME. Impairment of the Context Pre-Exposure Facilitation Effect in Juvenile Rats by Neonatal Alcohol Exposure is Associated with Decreased Egr-1 mRNA Expression in the Prefrontal Cortex. Behav Neurosci. doi: 10.1037/bne0000272. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Li S, Richardson R. Immunohistochemical Analyses of Long-Term Extinction of Conditioned Fear in Adolescent Rats. Cereb Cortex. 2011;21:530–538. doi: 10.1093/cercor/bhq116. [DOI] [PubMed] [Google Scholar]
- Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Lee JL. Memory reconsolidation mediates the updating of hippocampal memory content. Front Behav Neurosci. 2010;4:168. doi: 10.3389/fnbeh.2010.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox SA, Monsey MS, Schafe GE. Early growth response gene 1 (Egr-1) is required for new and reactivated fear memories in the lateral amygdala. Learn Mem. 2011;18:24–38. doi: 10.1101/lm.1980211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Maier T, Guell M, Serrano L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009;583:3966–73. doi: 10.1016/j.febslet.2009.10.036. [DOI] [PubMed] [Google Scholar]
- Malkani S, Rosen JB. Specific induction of early growth response gene 1 in the lateral nucleus of the amygdala following contextual fear conditioning in rats. Neuroscience. 2000;97:693–702. doi: 10.1016/s0306-4522(00)00058-0. [DOI] [PubMed] [Google Scholar]
- Malkani S, Wallace KJ, Donley MP, Rosen JB. An egr-1 (zif268) antisense oligodeoxynucleotide infused into the amygdala disrupts fear conditioning. Learn Mem. 2004;11:617–24. doi: 10.1101/lm.73104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–74. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Murawski NJ, Stanton ME. Variants of contextual fear conditioning are differentially impaired in the juvenile rat by binge ethanol exposure on postnatal days 4–9. Behav Brain Res. 2010;212:133–42. doi: 10.1016/j.bbr.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski NJ, Stanton ME. Effects of dose and period of neonatal alcohol exposure on the context preexposure facilitation effect. Alcohol Clin Exp Res. 2011;35:1160–70. doi: 10.1111/j.1530-0277.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly RC, Rudy JW. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychol Rev. 2001;108:311–45. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Pattwell SS, Bath KG, Casey BJ, Ninan I, Lee FS. Selective early-acquired fear memories undergo temporary suppression during adolescence. P Natl Acad Sci. 2011;108:1182–1187. doi: 10.1073/pnas.1012975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; Boston: 2007. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Ma QD, Tinsley MR, Koch C, Fanselow MS. Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learn Mem. 2008;15:368–372. doi: 10.1101/lm.813608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. The role of nicotinic acetylcholine receptors in the medial prefrontal cortex and hippocampus in trace fear conditioning. Neurobiol Learn Mem. 2010;94:353–363. doi: 10.1016/j.nlm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson R, Hunt P. Ontogeny of Fear Conditioning. In: Blumberg M, Freeman J, Robinson S, editors. Oxford Handbook of Developmental Behavioral Neuroscience. New York: Oxford University Press; 2010. pp. 527–545. [Google Scholar]
- Rosen JB, Fanselow MS, Young SL, Sitcoske M, Maren S. Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning. Brain Res. 1998;796:132–42. doi: 10.1016/s0006-8993(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Rosen JB. The neurobiology of conditioned and unconditioned fear: a neurobehavioral system analysis of the amygdala. Behav Cogn Neurosci Rev. 2004;3:23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Donley MP. Animal studies of amygdala function in fear and uncertainty: relevance to human research. Biol Psychol. 2006;73:49–60. doi: 10.1016/j.biopsycho.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Biedenkapp JC, O’Reilly RC. Prefrontal cortex and the organization of recent and remote memories: an alternative view. Learn Mem. 2005;12:445–6. doi: 10.1101/lm.97905. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neuroscience & Biobehavioral Reviews. 2004;28(7):675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cognitive, Affective, & Behavioral Neuroscience. 2001;1(1):66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Juraska JM. Neuron and glia numbers in the basolateral nucleus of the amygdala from preweaning through old age in male and female rats: A stereological study. J Comp Neurol. 2009;512:717–725. doi: 10.1002/cne.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffino FL, Murawski NJ, Rosen JB, Stanton ME. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiol Learn Mem. 2011;95:190–8. doi: 10.1016/j.nlm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–5. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience Biobehav R. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stanton ME. Multiple memory systems, development and conditioning. Behavioural Brain Res. 2000;110:25–37. doi: 10.1016/s0166-4328(99)00182-5. [DOI] [PubMed] [Google Scholar]
- Veyrac A, Besnard A, Caboche J, Davis S, Laroche S. The transcription factor Zif268/Egr1, brain plasticity, and memory. Prog Mol Biol Transl Sci. 2014;122:89–129. doi: 10.1016/B978-0-12-420170-5.00004-0. [DOI] [PubMed] [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–92. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Hast TA, Bennett RZ, Merjanian M, Nocera NA, Ponnusamy R, Fanselow MS. Cholinergic blockade frees fear extinction from its contextual dependency. Biol Psychiatry. 2013;73:345–352. doi: 10.1016/j.biopsych.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, et al. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47:859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]