Abstract
Aim
Advances in cardiopulmonary resuscitation (CPR) have focused on the generation and maintenance of adequate myocardial blood flow to optimize the return of spontaneous circulation and survival. Much of the morbidity associated with cardiac arrest survivors can be attributed to global brain hypoxic ischemic injury. The objective of this study was to compare cerebral physiological variables using a hemodynamic directed resuscitation strategy versus an absolute depth-guided approach in a porcine model of ventricular fibrillation (VF) cardiac arrest.
Methods
Intracranial pressure and brain tissue oxygen tension probes were placed in the frontal cortex prior to induction of VF in 21 female 3month old swine. After 7 minutes of VF, animalswere randomized to receive one of three resuscitation strategies: 1) Hemodynamic Directed Care (CPP-20): chest compressions (CCs) with depth titrated to a target systolic blood pressure of 100 mmHg and titration of vasopressors to maintain coronary perfusion pressure (CPP)> 20 mmHg; 2) Depth 33mm(D33): target CC depth of 33mm with standard American Heart Association (AHA) epinephrine dosing; or 3) Depth 51mm(D51): target CC depth of 51mm with standard AHA epinephrine dosing.
Results
Cerebral perfusion pressures (CerePP)were significantly higher in the CPP-20 group compared to both D33 (p<0.01) and D51 (P=0.046), and higher in survivors compared to non-survivors irrespective of treatment group (P<0.01).Brain tissue oxygen tension was also higher in the CPP-20 group compared to both D33 (P<0.01) and D51 (P=0.013), and higher in survivors compared to non-survivors irrespective of treatment group (P<0.01).Subjects with a CPP > 20 mm Hg were 2.7 times more likely to have a CerePP > 30 mm Hg (P< 0.001).
Conclusions
Hemodynamic directed resuscitation strategy targeting coronary perfusion pressure > 20 mmHg following VF arrest was associated with higher cerebral perfusion pressures and brain tissue oxygen tensions during CPR.
Keywords: cardiac arrest, cardiopulmonary resuscitation, coronary perfusion pressure, cerebral perfusion pressure, intracranial pressure, brain tissue oxygen tension, ventricular fibrillation, swine
Introduction
Advances in cardiopulmonary resuscitation (CPR) have focused on the generation and maintenance of adequate myocardial blood flow to optimize the return of spontaneous circulation (ROSC) and survival [1-4]. However, much of the morbidity associated with cardiac arrest survivors can be attributed to global brain hypoxic ischemic injury.
It is unclear whether CPR strategies to optimize myocardial blood flow will improve or compromise cerebrovascular hemodynamics and brain tissue oxygenation. During CPR, coronary perfusion pressure (CPP) is the primary determinant of myocardial blood flow [1, 5, 6], whereas cerebral perfusion pressure (CerePP), the aortic pressure minus the intracranial pressure, influences cerebral blow flow (CBF). However, cerebrovascular autoregulation, the mechanism by which the brain is protected from injury during hypotension and hypertension, complicates the relationship between CerePP and CBF. Previous investigation in swine models of cardiac arrest have demonstrated the negative influence of incomplete chest wall decompression and positive influence of active decompression and augmentation of negative intrathoracic pressure on cerebral perfusion pressure during CPR [7, 8].
Previously we have demonstrated that a therapeutic strategy to titrate compression depth and vasopressor dosing to optimize physiological conditions for myocardial blood flow improvedshort term survival following ventricular fibrillation (VF) cardiac arrest in a swine model[9]. In this study we compare CerePP with a hemodynamic directed resuscitation strategy intended to attain CPPs > 20 mmHg (CPP-20) versus absolute depth-guided CPR from data obtained in the previous randomized swine investigation of VF cardiac arrest. Depth-guided CPR was further divided into two groups: one with CC depth targeted to previously documented “usual care” of 33 mm (D33) and one with CCs targeted to the American Heart Association (AHA) 2010 guideline recommended depth of 51 mm (D51). We hypothesized that a myocardial focused hemodynamic directed strategy would improve CerePP and brain tissue oxygen tension (PBtO2) compared to standard depth-guided CPR.
Materials and Methods
Animal Preparation
The experimental protocol was approved by The University of Pennsylvania Institutional Animal Care and Use Committee. Twenty-one healthy 3-month old female domestic swine(32.4 ± 2.0 kg) were anesthetized and mechanically ventilated using a Datex Ohmeda anesthesia machine (Modulus SE) on a mixture of room air and titrated isoflurane (∼1.0% to 2.5%) with a tidal volume of 12mL / kg, PEEP 6 cm H2O, rate of 12 breaths / minute, and titration of rate to maintain end-tidal carbon dioxide (ETCO2) at 38 – 42 mmHg (NICO, Novametrix Medical Systems Inc.).
For intracranial monitoring, a burr hole was drilled 10 mm to the left of the sagittal suture, and 10 mm posterior to the coronal suture. A double lumen bolt was placed and a brain tissue oxygen tension probe (Licox system, Integra Lifesciences, Plainsboro, NJ) and fiberoptic parenchymal intracranial pressure monitor (Integra Lifesciences, Plainsboro, NJ) were introduced.
High fidelity, solid-state, micromanometer-tipped catheters (MPC-500, Millar Instruments) were advanced through the right femoral artery and external jugular vein into thoracic locations to measure continuous aortic and right atrial pressures respectively. A Swan-Ganz Thermodilution catheter (Edwards Lifesciences) was advanced into the pulmonary artery, and a bipolar pacing catheter (Edwards Lifesciences) was advanced into the right ventricle. All catheter placements were confirmed with fluoroscopy. Unfractionated heparin 200 U/kg was provided to prevent catheter clotting. Prior to obtaining any baseline measurements, animals received normal saline intravenously according to baseline pulmonary capillary wedge pressure as follows: 20 mL/kg if < 14 mm Hg, 10 mL/kg if < 16 mm Hg, and no fluid if > 16 mm Hg.
Measurements
Thermodilution cardiac outputs (ICU monitor: model HP66, Hewlett Packard) were obtained at baseline. Arterial blood gas specimens were obtained from the thoracic aorta at baseline (before VF), at 2 minutes, 4 minutes, and 6 1/2 minutes of VF, and then 2 1/2 minutes and 6 minutes after the initiation of CPR. Coronary perfusion pressure (CPP) was calculated by subtracting the mid-diastolic right atrial pressure from the mid-diastolic aortic pressure. Cerebral perfusion pressure (CerePP) was calculated by subtracting the intracranial pressure (ICP) from the mean arterial pressure.
To guide and record manual CPR quality, the Philips Heart Start MRx defibrillator with QCPR option was deployed during the experimental protocol. Using force transducer / accelerometer technology, the defibrillator records CPR quality and provides audiovisual feedback to the chest compression (CC) provider for rate (CC/min), depth (mm), and incomplete chest wall recoil [residual leaning force (grams)][10-13].
Experimental Protocol
The protocol described in this experiment was designed to addressCPR goals for VF cardiac arrest[9]. VF was induced by electrical pacing. No changes in mechanical ventilation or oxygenation were made during the initial seven minutes of VF. Following seven minutes of untreated VF, animals were randomized to one of three different CPR and advanced life support strategies for aten-minute duration before attempts at defibrillation. Fourteen of the 21 animals were part of an initial cohort examining 45-minute survival[9]. Intracranial monitoring during active CPR is known to be technically difficult and successful acquisition of intracranial data did not occur for all animals. Therefore, additional animals were added and individually randomized to one of the three groups after surgical preparation until there were at least 6 subjects in each arm of the study with full intracranial variable datasets. Any animals that did not have intracranial data were kept in the overall cohort nonetheless which resulted in overall unequal treatment groups for the survival analysis. All data for each variable represents data from the same cohort of 21 animals.
In all treatment arms, metronome-guided CCs were provided with a target rate of 100 CC/min and ventilations at 6 breaths per minute with 100% oxygen. Brief interruptions in CPR every two minutes mimicked pulse checks/rhythm analysis. Animals randomly received one of three resuscitation strategies as previously described [9, 14]: 1) Hemodynamic Directed Care (CPP-20): CCs with depth titrated to a target systolic blood pressure of 100 mmHg and titration of vasopressors to maintain CPP > 20 mmHg; 2) Depth 33mm (D33)or usual depth as reported in the literature[10, 15]: target CC depth of 33mm [10-13, 15, 16] with standard AHA epinephrine dosing interval; or 3) Depth 51mm (D51): target CC depth of 51mm (2010 AHA guidelines) with standard AHA epinephrine dosing interval[17]. After 10 minutes of CPR (minute 17 of the protocol), the initial 200J biphasic waveform defibrillation attempt was provided. Resuscitation according to treatment strategy continued until sustained return of spontaneous circulation (ROSC) was attained or at minute 27 of the protocol (after an additional ten minutes of resuscitation post-initial defibrillation attempt). If ROSC was attained, the animals were supported for 45 minutes in a simulated intensive care setting. After ROSC, mechanical ventilation was provided with 100% oxygen and adjusted to maintain an ETCO2 of 38 to 42 mmHg and pulse oximetry saturations 92% to 96%. Isoflurane was administered as necessary. At 45 minutes, the animals were euthanized with pentobarbital and potassium chloride.
Data Analysis / Outcomes
The primary outcome of the study was cerebral perfusion pressure. Secondary outcomes included PBtO2 and 45-minute survival. Normality of continuous variables was assessed using the Skewness-Kurtosis test. Normally distributed continuous variables were summarized as mean ± SEM and compared across groups by ANOVA. Continuous variables that were not normally distributed were summarized as median (25%, 75%) and compared by the Kruskal-Wallis test. Comparisons of dichotomous variables, such as 45-minute ICU survival and rate of ROSC were evaluated by Fisher's exact test. Differences in mean CerePP and PBtO2 over time and between treatment groups and between survivors / non-survivors were assessed using generalized estimating equations (GEE) with an identity link[18].A robust variance estimator with an exchangeable correlation structure was used to account for longitudinal correlation, which arose from collecting observations on the same study animals over time. To estimate the correlation between CPP and CerePP, the repeated measures for each animal were reduced to a mean over all times[19]. For assessment of the correlation between CPP > 20 mm Hg and CerePP > 30 mm Hg, a GEE with a logistic link was used for the binary outcome of CerePP > 30 mm Hg, with the primary exposure of CPP > 20 mm Hg and adjusted for time. Statistical analyses were completed using Stata-IC 12.0 (StataCorp, College Station, TX) or R 3.0.1 (R Development Core Team, Vienna, Austria).
Results
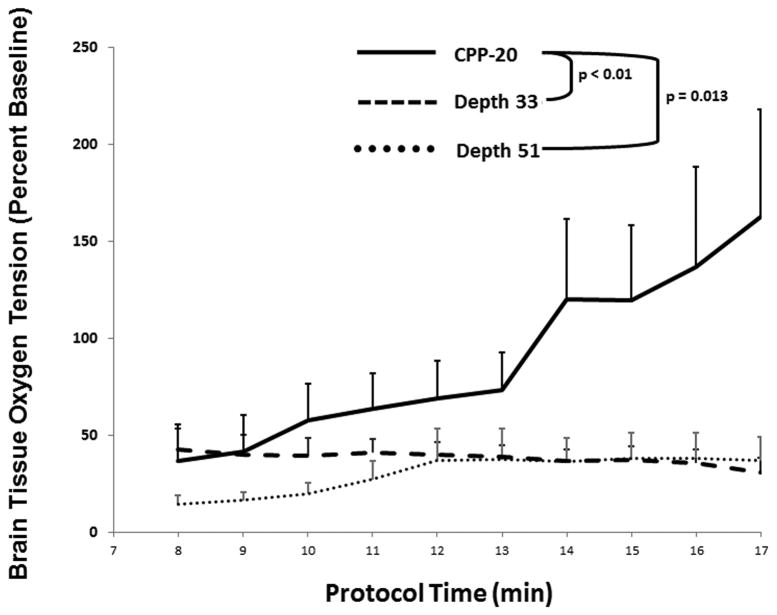
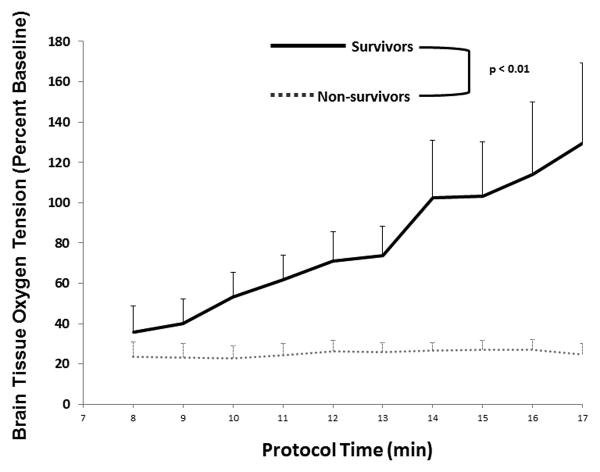
The primary outcome of cerebral perfusion pressure was significantly higher in the CPP-20 group compared to both D33 (P <0.01) and D51 (P =0.046) (Figure 1), and higher in survivors compared to non-survivors irrespective of treatment group (P <0.01) (Figure 2).The secondary outcome of PBtO2 was also higher in the CPP-20 group compared to both D33 (P < 0.01) and D51 (P=0.013) (Figure 3), and higher in survivors compared to non-survivors irrespective of treatment group (P <0.01) (Figure 4). Of note, 45-minute ICU survival was significantly higher in the CPP-20 group (7/8), compared to D51 (3/6) and D33 (1/7) (p<0.022).
Figure 1.
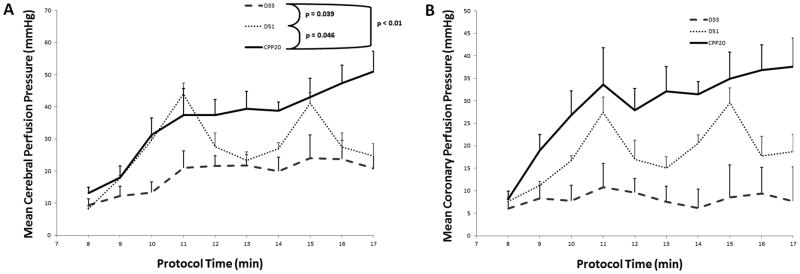
A) Mean cerebral perfusion pressure (CerePP) during each minute of cardiopulmonary resuscitation (CPR) across treatment groups. B) Mean coronary perfusion pressure (CPP) during each minute of cardiopulmonary resuscitation (CPR) across treatment groups. Error bars represent SEM. Depth 33 and Depth 51 refer to depth-guided CPR at 33 mm and 51 mm, respectively. CPP-20 = CPR directed to attain coronary perfusion pressure > 20 mm Hg.
Figure 2.
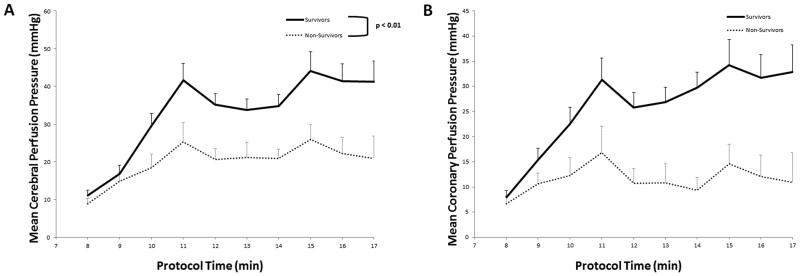
A) Mean cerebral perfusion pressure (CerePP) during each minute of cardiopulmonary resuscitation (CPR) between survivors and non-survivors. B) Mean coronary perfusion pressure (CPP) during each minute of cardiopulmonary resuscitation (CPR) between survivors and non-survivors. Error bars represent SEM.
Figure 3.
Mean brain tissue oxygenation as a percentage of baseline during each minute of cardiopulmonary resuscitation (CPR) across treatment groups. Error bars represent SEM. Depth 33 and Depth 51 refer to depth-guided CPR at 33 mm and 51 mm, respectively. CPP-20 = CPR directed to attain coronary perfusion pressure > 20 mm Hg.
Figure 4.
Mean brain tissue oxygenation as a percentage of baseline during each minute of cardiopulmonary resuscitation (CPR) between survivors and non-survivors. Error bars represent SEM.
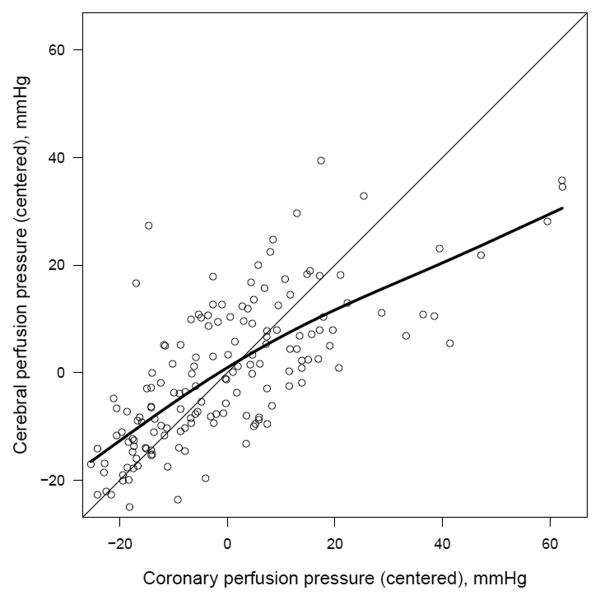
CPP and CerePP were well correlated across treatment groups and survival status, r = 0.76 (P< 0.001) (Figure 5). Subjects with a CPP > 20 mm Hg were 2.7 times more likely to achieve a CerePP > 30 mm Hg (P< 0.001).
Figure 5.
Scatterplot of coronary perfusion pressure (CPP) and cerebral perfusion pressure (CerePP) residuals calculated utilizing a linear regression model for each variable adjusted for time; r = 0.76, P<0.001. Smoothing spline (bold line) demonstrates strong positive correlation between CPP and CerePP.
Resuscitation Variables
Chest compression depth was significantly different among groups: D33 = 34 ± 1.0mm; D51 = 49 ± 0.5mm; CPP-20 = 46 ± 0.6mm (P <0.01). Other CPR quality variables were not different (rate = 101 ± 0.2 CC/min; CPR fraction = 96.7 ± 0.3%; and no delivered compressions had leaning exceeding 2.5kg). Total number of vasopressors doses administered was not different (D33 = 5 (5, 5); D51 =4 (2, 5); CPP-20 = 3 (3, 5), P =0.31).Interestingly, the variability of vasopressor need to attain that goal was great (including the use of no vasopressor during the entire CPR period when CPP-20 was maintained by CPR only).Similarly, total number of epinephrine doses was not different (D33 = 5 (5, 5); D51 =5 (4, 5); CPP-20 = 2 (2, 4), P =0.09). By protocol design, CPP-20 received first vasopressor dose as early as 1 minute after initiation of CPR, compared to 2 minutes in the D33 and D55 groups. Overall number of defibrillation attempts was not different among groups (D33 = 3 (1, 5); D51 = 2 (1, 5); CPP-20 = 1 (1, 2), P =0.13).
Hemodynamics and Arterial Blood Gases
Hemodynamic variables were not different at pre-arrest baseline or at the end of untreated VF period (Table 1). During the last minute of the resuscitation period (minute 16 – 17), there were significant differences across treatment groups for aortic systolic and diastolic pressure, CerePP, PbtO2 as a percentage of baseline, and end tidal CO2, but not intracranial pressure. Higher CerePP in the CPP-20 group compared to D33 and D55 was the result of higher aortic diastolic pressures, and in turn mean arterial pressures, in the CPP-20 group. There was higher end tidal CO2 in the D51 and CPP-20 groups compared to D33. There were no differences in arterial blood gases obtained at baseline (Table 2). At the end of the resuscitation, in general, the D33 group was characterized by a higher arterial pH, lower PCO2, and higher PaO2 compared to either D51 or CPP-20.
Table 1.
Pressures and PbtO2 in mmHg.AoS indicates aortic systolic pressure; AoD, aortic diastolic pressure; RAD, right atrial diastolic pressure; ICP, intracranial pressure; CerePP, cerebral perfusion pressure; PbtO2, brain tissue oxygen tension; ET CO2, end tidal carbon dioxide. Depth 33 (D33) and Depth 51 (D51) refer to depth-guided CPR at 33mm and 51 mm, respectively. CPP-20 refers to CPR directed to attain coronary perfusion pressure >20 mmHg. Data presented as mean (SEM).
| Depth 33 (n=7) | Depth 51 (n=6) | CPP-20 (n=8) | p | |
|---|---|---|---|---|
| Baseline | ||||
| Weight (kg) | 32 (0.5) | 33 (0.2) | 33 (1) | 0.77 |
| CO (L/min) | 3.3 (0.3) | 3.0 (0.3) | 2.8 (0.3) | 0.49 |
| AoS | 113(5) | 99(6) | 99(4) | 0.09 |
| AoD | 81(4) | 71(7) | 73(3) | 0.29 |
| CPP | 68 (5) | 66 (6) | 67 (4) | 0.93 |
| ICP | 16 (1) | 20 (3) | 18 (2) | 0.48 |
| CerePP | 76(4) | 64(8) | 65(6) | 0.34 |
| PbtO2 | 22 (4) | 29 (6) | 17 (7) | 0.43 |
| End of Untreated VF* | ||||
| AoS | 26(1) | 25(3) | 23(1) | 0.37 |
| AoD | 20(1) | 15(3) | 20(1) | 0.22 |
| CPP | 2 (1) | 1 (1) | 2 (1) | 0.74 |
| ICP | 15 (1) | 16 (3) | 16 (1) | 0.80 |
| CerePP | 7(2) | 3(4) | 7(2) | 0.77 |
| PbtO2 | 3 (1) | 15 (5) | 4 (3) | 0.08 |
| PbtO2 (% baseline) | 13(4) | 46 (11) | 22(16) | 0.14 |
| End of Resuscitation Period† | ||||
| AoS | 62(11)‡ | 87(9)§ | 110(5) | <0.01 |
| AoD | 23(3)‖ | 31(6)¶ | 47(5) | <0.01 |
| CPP | 8 (2)** | 19 (6) | 38 (7) | <0.01 |
| ICP | 15 (1) | 21 (5) | 22 (2) | 0.18 |
| CerePP | 21 (4)** | 25(8) †† | 51(6) | <0.01 |
| PbtO2 | 10 (5) | 8 (2) | 20 (8) | 0.23 |
| PbtO2 (% baseline) | 37 (12)§§ | 31 (7)‡‡ | 163(55) | 0.019 |
| ET CO2 | 20(3)¶¶ | 35(5)‖‖ | 29(2) | 0.02 |
Last epoch during untreated VF period (minute 6 – 7)
Last epoch during protocol resuscitation period CPR (minute 16 – 17)
CPP-20 vs. D33: p<0.01;
CPP-20 vs. D33: p<0.01;
CPP-20 vs. D33: p<0.01;
p=0.015;
p=0.02.
D51 vs. D33: p=0.025.
CPP-20 vs. D51: p=0.03;
p=0.052;
p=0.024;
p=0.018.
Table 2.
Depth 33 and Depth 51 refer to depth-guided CPR at 33mm and 51 mm, respectively. CPP-20 refers to CPR directed to attain coronary perfusion pressure >20 mmHg.
| Depth 33(n=6) | Depth 51 (n=6) | CPP-20 (n=8) | p | |
|---|---|---|---|---|
| Baseline | ||||
| pH | 7.52(0.01) | 7.52(0.01) | 7.52(0.01) | 0.97 |
| PCO2 (mmHg) | 43(3) | 46(2) | 43(3) | 0.62 |
| PaO2 (mmHg) | 135(5) | 114(8) | 133(7) | 0.12 |
| End of Untreated VF* | ||||
| pH | 7.78(0.01)‡ | 7.75(0.07) | 7.60(0.05) | 0.03 |
| PCO2 (mmHg) | 16(2) | 22(6) | 27(7) | 0.43 |
| PaO2 (mmHg) | 91(12) | 118(16) | 106(21) | 0.47 |
| After 6 Minutes of CPR† | ||||
| pH | 7.48(0.04)‖ | 7.33(0.03)§ | 7.30(0.02) | < 0.01 |
| PCO2 (mmHg) | 33(4)** | 47(4)¶ | 61(9) | 0.022 |
| PaO2 (mmHg) | 369(68)†† | 223(48) | 101(40) | <0.01 |
Sample drawn at 6min 30s during VF period and
Sample drawn at 6 minutes during protocol resuscitation period.
CPP-20 vs. D33: p<0.01;
CPP-20 vs. D33: p<0.01;
CPP-20 vs. D33: p<0.01;
p=0.02.
D51 vs. D33: p<0.01;
p=0.048.
p=0.048.
Discussion
In the present study, cerebral perfusion pressure after VF cardiac arrest was improved with hemodynamic directed CPR to maintain coronary perfusion pressure > 20 mmHg (CPP-20) compared to a rescuer-centric resuscitation strategy with depth of compressions guided to 33mm or 51mm and standard AHA vasopressor dosing. Our hemodynamic directed resuscitation strategy included titration of chest compression depth to systolic blood pressure and titration of timing and frequency of vasopressor administration to coronary perfusion pressure. Brain tissue oxygen tension (PBtO2) was higher in the CPP-20 group compared to D33 and D51. Irrespective of treatment group, cerebral perfusion pressures and PBtO2 were higher in survivors compared to non-survivors, similar to our previous observations demonstrating higher coronary perfusion pressures in survivors compared to non-survivors (9).
We have previously demonstrated in our swine model of VF cardiac arrest, that a treatment algorithm focused on hemodynamic directed titration of chest compressions and vasopressor administration improved rates of ROSC and short term survival[9]. Hemodynamic directed resuscitation targeting CPP >20 mm Hg significantly improved cerebral perfusion pressure and brain tissue oxygen tension as well and did not compromise cerebrovascular hemodynamics. The ratio of intensive care unit versus ward pediatric CPR events has increased over the past several years [20]. With many of these patients already having invasive monitoring in place, there is an opportunity to utilize a hemodynamic directed resuscitation strategy.
Previous studies in immature swine have observed the lower limit of autoregulation to be a cerebral perfusion pressure of 30 mmHg, below which CBF is assumed to not be maintained[21]. Only animals in the CPP-20 group were able to consistently maintain a CerePP of 30 mmHg. CerePP in the D51 group was transiently above 30 mmHg, coinciding with administration of epinephrine, but was not consistently above the lower limit of autoregulation, demonstrating that titration of vasopressors to hemodynamic goals may have a positive effect on cerebrovascular hemodynamics. CerePP and CoPP correlated well across resuscitation strategies (Figure 5). This is not surprising since the main determinants of CerePP and CoPP are MAP and aortic diastolic pressure respectively. Animals achieving a CPP > 20 mm Hg were 2.7 times more likely to achieve CerePP > 30 mm Hg. Titration of vasopressors to hemodynamic goals (CPP > 20 mm Hg) may have a positive effect on cerebrovascular hemodynamics by generating a CerePP above the lower inflexion point of cerebral autoregulation and maintain adequate cerebral blood flow during CPR. These data suggest that a hemodynamic directed myocardial perfusion focused resuscitation strategy may also be a neuroprotective resuscitation strategy.
Epinephrine's alpha1-agonist activity has been previously demonstrated to have adverse effects on cerebral microvascular blood flow[22, 23]. In our studies, a hemodynamic directed resuscitation strategy which included early administration of epinephrine produced significant increases in PBtO2. Using PBtO2 as a surrogate marker of CBF, early administration of epinephrine during CPR after VF cardiac arrest in our model may improve regional CBF.
There was a non-significant increase in ICP in the D51 and CPP-20 groups at the end of the resuscitation (Table 1), perhaps related to the increased chest compression depth observed in the D51 and CPP-20 groups compared to D33. In the CPP-20 group, the greater rise in MAP in relation to ICP resulted in marked improvement in CerePP compared to the D51 group. Our findings are consistent with previous observations of improved cerebral perfusion pressures with other advanced CPR techniques such as active compression-decompression with an impedance threshold device[7].
There are several limitations to our experimental design that must be considered when translating our results to the care of the cardiac arrest patient. First, brain tissue oxygen tension was measured regionally in the subcortical white matter of the right frontal cortex. Measurement of brain tissue oxygen tension in regions known to be highly susceptible to hypoxic-ischemic injury such as the hippocampus were not technically possible, but subcortical measurements of brain tissue oxygen tension in swine models of traumatic brain injury have been demonstrated to be associated with changes incerebral blood flow[24]. Second, we did not assess real-time changes in cerebral blood flow during the three different resuscitation strategies. However, techniques to measure CBF, such as microspheres, do not allow for the same temporal resolution during CPR that we obtained with ICP and brain tissue oxygen tension measurements.Third, all the animals in this study were young had no known coronary artery disease which may limit translatability to an older adult population. Furthermore, ventricular fibrillation as the initial rhythm occurs in only 25% of in-hospital pediatric cardiac arrests[25]. Animals in this study also did not experience asphyxia prior to inducing VF and were observed to have a respiratory alkalosis at end of the untreated VF period. Future investigations studying cerebral hemodynamics after asphyxia arrest are planned which will control for the alkalosis observed in our current study. Fourth, there are differences in chest wall appearance between swine and humans which may limit the translatability of the hemodynamic differences observed across different depth directed compression strategies. Fifth, there were differences in pH between the groups at end of untreated VF and during CPR which may have influenced cerebral blood flow. Finally, although animals were randomized to treatment group, the study was not blinded. Those participating in the resuscitations were aware of the treatment strategy. However, aside from protocolized differences in chest compression depth, other CPR quality variables were similar, minimizing concerns of bias.
Conclusion
Hemodynamic directed resuscitation targeting CPPs > 20 mmHg during 10 minutes of CPR following VF arrest improves cerebral perfusion pressures and brain tissue oxygen tension when compared to resuscitation with depth of compressions guided to 33mm or 51mm and standard AHA vasopressor dosing. A resuscitation protocol individualized to goal coronary perfusion pressure is associated with improvements in cerebral perfusion and oxygenation and may be neuroprotective.
Acknowledgments
Financial Disclosure: This study was funded by, the National Institute of Neurological Disorders and Stroke (SHF K08), the National Institute of Child Health and Human Development (RMS K23), the Laerdal Foundation for Acute Care Medicine, and The Russell Raphaely Endowed Chair Funds at The Children's Hospital of Philadelphia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halperin HR, Lee K, Zviman M, Illindala U, Lardo A, Kolandaivelu A, et al. Outcomes from low versus high-flow cardiopulmonary resuscitation in a swine model of cardiac arrest. The American journal of emergency medicine. 2010;28:195–202. doi: 10.1016/j.ajem.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Kern KB, Ewy GA, Voorhees WD, Babbs CF, Tacker WA. Myocardial perfusion pressure: a predictor of 24-hour survival during prolonged cardiac arrest in dogs. Resuscitation. 1988;16:241–50. doi: 10.1016/0300-9572(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 3.Paradis NA, Martin GB, Rivers EP, Goetting MG, Appleton TJ, Feingold M, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA : the journal of the American Medical Association. 1990;263:1106–13. [PubMed] [Google Scholar]
- 4.Ralston SH, Voorhees WD, Babbs CF. Intrapulmonary epinephrine during prolonged cardiopulmonary resuscitation: improved regional blood flow and resuscitation in dogs. Annals of emergency medicine. 1984;13:79–86. doi: 10.1016/s0196-0644(84)80566-1. [DOI] [PubMed] [Google Scholar]
- 5.Berg RA, Sanders AB, Kern KB, Hilwig RW, Heidenreich JW, Porter ME, et al. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104:2465–70. doi: 10.1161/hc4501.098926. [DOI] [PubMed] [Google Scholar]
- 6.Kern KB, Lancaster L, Goldman S, Ewy GA. The effect of coronary artery lesions on the relationship between coronary perfusion pressure and myocardial blood flow during cardiopulmonary resuscitation in pigs. American heart journal. 1990;120:324–33. doi: 10.1016/0002-8703(90)90076-a. [DOI] [PubMed] [Google Scholar]
- 7.Metzger AK, Herman M, McKnite S, Tang W, Yannopoulos D. Improved cerebral perfusion pressures and 24-hr neurological survival in a porcine model of cardiac arrest with active compression-decompression cardiopulmonary resuscitation and augmentation of negative intrathoracic pressure. Critical care medicine. 2012;40:1851–6. doi: 10.1097/CCM.0b013e318246b9ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuercher M, Hilwig RW, Ranger-Moore J, Nysaether J, Nadkarni VM, Berg MD, et al. Leaning during chest compressions impairs cardiac output and left ventricular myocardial blood flow in piglet cardiac arrest. Critical care medicine. 2010;38:1141–6. doi: 10.1097/CCM.0b013e3181ce1fe2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friess SH, Sutton RM, Bhalala U, Maltese MR, Naim MY, Bratinov G, et al. Hemodynamic Directed Cardiopulmonary Resuscitation Improves Short-Term Survival From Ventricular Fibrillation Cardiac Arrest. Critical care medicine. 2013;41:2698–704. doi: 10.1097/CCM.0b013e318298ad6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abella BS, Alvarado JP, Myklebust H, Edelson DP, Barry A, O'Hearn N, et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA : the journal of the American Medical Association. 2005;293:305–10. doi: 10.1001/jama.293.3.305. [DOI] [PubMed] [Google Scholar]
- 11.Abella BS, Edelson DP, Kim S, Retzer E, Myklebust H, Barry AM, et al. CPR quality improvement during in-hospital cardiac arrest using a real-time audiovisual feedback system. Resuscitation. 2007;73:54–61. doi: 10.1016/j.resuscitation.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 12.Edelson DP, Litzinger B, Arora V, Walsh D, Kim S, Lauderdale DS, et al. Improving in-hospital cardiac arrest process and outcomes with performance debriefing. Archives of internal medicine. 2008;168:1063–9. doi: 10.1001/archinte.168.10.1063. [DOI] [PubMed] [Google Scholar]
- 13.Sutton RM, Niles D, Nysaether J, Abella BS, Arbogast KB, Nishisaki A, et al. Quantitative analysis of CPR quality during in-hospital resuscitation of older children and adolescents. Pediatrics. 2009;124:494–9. doi: 10.1542/peds.2008-1930. [DOI] [PubMed] [Google Scholar]
- 14.Sutton RM, Friess SH, Bhalala U, Maltese MR, Naim MY, Bratinov G, et al. Hemodynamic directed CPR improves short-term survival from asphyxia-associated cardiac arrest. Resuscitation. 2013;84:696–701. doi: 10.1016/j.resuscitation.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edelson DP, Abella BS, Kramer-Johansen J, Wik L, Myklebust H, Barry AM, et al. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation. 2006;71:137–45. doi: 10.1016/j.resuscitation.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Nishisaki A, Nysaether J, Sutton R, Maltese M, Niles D, Donoghue A, et al. Effect of mattress deflection on CPR quality assessment for older children and adolescents. Resuscitation. 2009;80:540–5. doi: 10.1016/j.resuscitation.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Travers AH, Rea TD, Bobrow BJ, Edelson DP, Berg RA, Sayre MR, et al. Part 4: CPR overview: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S676–84. doi: 10.1161/CIRCULATIONAHA.110.970913. [DOI] [PubMed] [Google Scholar]
- 18.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 19.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 2--Correlation between subjects. Bmj. 1995;310:633. doi: 10.1136/bmj.310.6980.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg RA, Sutton RM, Holubkov R, Nicholson CE, Dean JM, Harrison R, et al. Ratio of PICU versus ward cardiopulmonary resuscitation events is increasing. Critical care medicine. 2013;41:2292–7. doi: 10.1097/CCM.0b013e31828cf0c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brady KM, Lee JK, Kibler KK, Easley RB, Koehler RC, Czosnyka M, et al. The lower limit of cerebral blood flow autoregulation is increased with elevated intracranial pressure. Anesth Analg. 2009;108:1278–83. doi: 10.1213/ane.0b013e3181964848. [DOI] [PubMed] [Google Scholar]
- 22.Ristagno G, Sun S, Tang W, Castillo C, Weil MH. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Critical care medicine. 2007;35:2145–9. doi: 10.1097/01.ccm.0000280427.76175.d2. [DOI] [PubMed] [Google Scholar]
- 23.Ristagno G, Tang W, Huang L, Fymat A, Chang YT, Sun S, et al. Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Critical care medicine. 2009;37:1408–15. doi: 10.1097/CCM.0b013e31819cedc9. [DOI] [PubMed] [Google Scholar]
- 24.Friess SH, Smith C, Kilbaugh TJ, Frangos SG, Ralston J, Helfaer MA, et al. Early cerebral perfusion pressure augmentation with phenylephrine after traumatic brain injury may be neuroprotective in a pediatric swine model. Critical care medicine. 2012;40:2400–6. doi: 10.1097/CCM.0b013e31825333e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samson RA, Nadkarni VM, Meaney PA, Carey SM, Berg MD, Berg RA, et al. Outcomes of in-hospital ventricular fibrillation in children. The New England journal of medicine. 2006;354:2328–39. doi: 10.1056/NEJMoa052917. [DOI] [PubMed] [Google Scholar]