Abstract
The External Quality Assurance Program Oversight Laboratory (EQAPOL) Flow Cytometry Program assesses the proficiency of NIH/NIAID/DAIDS-supported and potentially other interested research laboratories in performing Intracellular Cytokine Staining (ICS) assays. The goal of the EQAPOL Flow Cytometry External Quality Assurance Program (EQAP) is to provide proficiency testing and remediation for participating sites. The program is not punitive; rather, EQAPOL aims to help sites identify areas for improvement. EQAPOL utilizes a highly standardized ICS assay to minimize variability and readily identify those sites experiencing technical difficulties with their assays. Here, we report the results of External Proficiency 3 (EP3) where participating sites performed a 7-color ICS assay. On average, sites perform well in the Flow Cytometry EQAP (median score is “Good”). The most common technical issues identified by the program involve protocol adherence and data analysis; these areas have been the focus of site remediation. The EQAPOL Flow Cytometry team is now in the process of expanding the program to 8-color ICS assays. Evaluating polyfunctional ICS responses would align the program with assays currently being performed in support of HIV immune monitoring assays.
Keywords: ICS, Antigen specific, Proficiency testing, Flow cytometry, Site performance, Grading
1. Introduction
Over the last decade, the applications of polychromatic flow cytometry (PFC) have significantly broadened in scope as a result of technologic improvements in instrumentation, the development of new fluorescent probes, and the generation of novel statistical tools that greatly facilitate the analysis of large and highly complex data sets. Present day PFC platforms, for example, have the capacity to not only determine the frequencies of relatively rare, pheno-typically defined cellular subtypes within a mixed population, but also to simultaneously analyze qualitative features such as polyfunctionality by intracellular cytokine staining (ICS) within individual cells comprising such subpopulations. As the complexity of PFC analyses has increased, the need for more stringent quality assurance and comprehensive proficiency testing has become more critical. This manuscript describes the efforts of the NIH-supported EQAPOL Flow Cytometry EQAP to establish an external quality assurance program for PFC that provides both comprehensive proficiency testing and remediation for site laboratories performing polyfunctional ICS assays.
2. Material and methods
2.1. Participating sites
There are currently fourteen sites, across six different countries, participating in the Flow Cytometry ICS Quality Assurance Program. All sites previously agreed to share their data for publication. Most of the participating sites were involved with previous efforts to standardize the ICS assay as well as the previous DAIDS ICS Quality Assurance Program (Maecker et al., 2005; Jaimes et al., 2011).
2.2. Peptide pools
The Flow Cytometry EQAPOL Oversight Laboratory (EOL) purchased raw materials used to prepare stimulation lyoplates from JPT (Berlin, Germany). The two PepMixes routinely used are PepMix HCMVA (pp65), lyophilized aliquots containing a pool of 138 15-mer peptides overlapping by 11 amino acids derived from the 65 kDa phosphoprotein (pp65) of human cytomegalovirus (CMV), and PepMix CEF Pool (extended), lyophilized aliquots containing a pool of 32 Major Histocompatibility Complex (MHC) Class I-restricted immunodominant T cell epitopes for CMV, Epstein Barr virus (EBV), and Influenza (JPT). The CMVpp65 peptide mix is used to measure both MHC Class I (CD8+) and MHC Class II (CD4+) restricted responses. The CEF peptide mix, comprised of optimal 8- and 9-mers, is used to measure MHC Class I (CD8+) restricted responses.
2.3. Lyophilized plates
BD Biosciences Custom Technology Team (BD/CTT, San Diego, CA) provided lyophilized 96-well V-bottom polypropylene stimulation plates and staining plates as described by Jaimes et al. (2011). The antibody panel for staining includes gating markers for CD3, CD4, and CD8 as well as functional markers for interferon gamma (IFN-γ), interleukin 2 (IL-2), and tumor necrosis factor alpha. Lyophilized lyoplates are subjected to performance and stability testing by BD/CTT and shipped to the Flow Cytometry EOL along with a Certificate of Analysis (COA).
2.4. Instrument performance
Following procedures published in a technical bulletin by Meinelt et al. (2012), instrument performance data were used by BD/CTT to generate instrument-specific target channels. Target channel values were used by sites to establish instrument settings.
2.5. Intracellular cytokine staining kits
The Flow Cytometry EOL and EQAPOL Central Laboratory worked together to create a kit for each proficiency program that contained three blinded donor Peripheral Blood Mononuclear Cell (PBMC) samples with different reactivities, lyoplates for stimulation and staining, reagents for permeabilizing cells and instrument setup and performance assessment. Additionally, sites were provided with a detailed assay protocol, questionnaire, reporting templates, and instructions for how to analyze and report data. Methods used were described by Jaimes et al. (2011).
2.6. Samples
The Flow Cytometry EOL obtained cryopreserved PBMCs from the EQAPOL Repository to screen for ICS assay reactivity before selecting specific donors for use in flow cytometry proficiency panels Garcia et al. (in this issue). Donors were selected based on the following criteria:
Availability of at least 200 cryovials
Cryovials not reserved for another EQAPOL program
Viability >80%
Recovery >90%. Note, a higher recovery is used as part of the donor selection to ensure that sites that might obtain somewhat lower recoveries will have sufficient cells to perform the proficiency panel.
Repetitive values for %CD3, %CD4, and %CD8 within a range of 4% (maximum frequency minus minimum frequency), following criteria established by Gelman and Wilkening (2000) and currently being used by NIH/NIAID/DAIDS Immunology Quality Assessment Program for acceptable intra-laboratory ranges on blinded replicates. Repetitive values were used as a measure of the quality of sample staining and were calculated for a given marker, for example %CD3, by subtracting the lowest value from the highest value obtained across all stimulations and replicates for a specific donor.
Low background responses (<0.05%)
A mixture of cytokine responses for CD4+ and CD8+ subsets against CMVpp65 and CEF peptide mixes, such that among the three donors selected for a proficiency panel there are at least 4 response levels: no response, low response (0.05% to 0.50%), medium response (0.51% to 0.99%), and high response (> 1.00), where a positive response is defined as >0.05 and at least 2x background.
2.7. Bridging studies
After selecting donors for use in a proficiency panel, the Flow Cytometry EOL then performed a bridging study. Bridging studies were designed to evaluate the performance of the lyoplates to be used for proficiency panel. Donors selected using the donor screening data were used for the Bridging study to compare evaluation plates against donor screening plates. In the bridging study, results from the send-out plates were compared against results from the donor screening data. Acceptable performance for bridging studies was defined as follows:
%CD3, %CD4, and %CD8 (range within 4%)
Low cytokine backgrounds (≤0.05%)
Cytokine responses consistent with donor screening data (high, medium, low, and no response)
2.8. Data reporting and analysis
Data were returned to EQAPOL in the form of an Excel template containing a summary of results, a PowerPoint template showing gates and backgates for each sample, a completed assay questionnaire, and raw flow cytometry standard (FCS) files. All data were reported using a web-based application developed for the EQAPOL program. The EQAPOL Flow Cytometry Laboratory performed centralized analysis on all data files (EOL manual or EOLm). Backgating was used to maximize the positive responses and minimize the negative responses for each donor. Centralized analysis gates were independently assessed by a second operator for accurate placement. Assay questionnaires, power point documents, and instrument performance standards were used to compile site performance tables and comments. The EQAPOL Statistician calculated confidence boundaries using the reported data as described by Rountree et al. (in this issue). EQAPOL then used the performance summary tables and comments, together with statistical information, to compile and distribute the final site specific reports.
2.9. Grading criteria
At the recommendation of the EQAPOL Scientific Advisory Board, grading criteria were harmonized across all EQAPOL programs and shared the same performance criteria:
“Excellent” (scores between 91 to 100)
“Good” (scores between 75 and 90)
“Fair” (scores between 66 and 74)
“Poor” (scores between 0 and 65).
Grading criteria that were common across EQAPOL programs include Timeliness, PBMC Processing, and Protocol Adherence. Timeliness — on time completion of proficiency panel assays was critical to the success of our program. Therefore, Timeliness was weighted heavily across all EQAPOL programs. No site that reported late data was permitted to obtain an “Excellent” score. PBMC Processing — properly thawing and counting PBMC samples was essential to a site's ability to successfully perform an ICS assay. Jaimes et al. (2011) reported that poor viability resulted in suboptimal ICS responses and low recovery resulted in too few events being collected, leading to greater assay variability.
Protocol adherence included Instrument Setup (target channel values, data annotation) and Data Collection and Analysis (Number of events collected and Following gating strategy provided in the Protocol). Target channels were used to standardize instruments across laboratories and facilitate centralized analysis. Without instrument standardization cell populations would have fallen outside of the expected analysis region boundaries, negating the use of standardized gating. Data annotation — in the context of a standardized assay where all of the reagents used are identical across sites, poor annotation created data that were difficult to interpret. However, poorly annotated data obtained from an in-house assay, where the reagents are no longer standardized, would be nearly impossible to interpret. Improperly annotated data were not compatible with centralized analysis. Centralized analysis was used in our program to isolate data analysis errors from technical issues with the assay, helping us to provide constructive feedback to the sites during site remediation. Number of events acquired — collecting too few events impacts accuracy and precision and creates a unique challenge when analyzing polyfunctional responses Jaimes et al. (2011). Finally, Following the gating strategy included in the Protocol — following the gating strategy was necessary for the laboratories to complete the EQAPOL standardized ICS assay kit. Failing to follow the suggested gating strategy resulted in data that could not be meaningfully compared with other participating laboratories. Overall, failure to follow the protocol resulted in a substantial penalty, up to 20 points.
Specific grading criteria for the ICS Assay included Basic Subset Evaluation, Deviation of EOLm Analyzed Data from Consensus, and Deviation of EOLm and Site Analyzed Data. Intra-laboratory Performance for Basic Lymphocytes Subset Identification measured how well a site was able to reproducibly identify the basic lymphocyte subsets CD3, CD4, and CD8 based on repetitive values for each of these markers across stimulations. Reproducibility of CD3, CD4, and CD8 subsets was used during remediation to differentiate those samples with more global technical issues from those samples with specific problems measuring cytokine responses.
The majority of points were awarded in the two main categories: Deviation of EOLm Analyzed Data from Consensus and Deviation of EOLm from Site Analyzed Data. Deviation of EOLm Analyzed Data from the Consensus was used as a measure for overall performance based on EOLm-analyzed results. Sites that performed the assay well scored high in this category, irrespective of whether data were analyzed properly by the site. Deviation of EOLm and Site Analyzed Data was used as a measure for agreement between site analysis and EOLm. The criterion assessed how well a site analyzed their data, irrespective of how well a site performed their assay. The placement of gates has been previously shown to contribute to inter-assay variability to a degree that might preclude across laboratory studies using ICS or other rare event assays McNeil et al. (2013). Therefore, we devised these two grading categories that together enabled EQAPOL to isolate specific errors associated with the assay versus problems with gating and analysis.
Instrument performance will affect the ability of a given site to accurately measure positive responses. Instrument performance was not graded; however it was assessed for all sites following methods established by Wood and Hoffman (1998). Instrument performance parameters for background (Br) and detection efficiency (Qr) were calculated by BD/CTT based on the site-specific CS & T reports. Instrument performance assessed at the time of sample acquisition aided in site remediation by differentiating problems with the instrument from problems with the assay.
2.10. Site remediation
Sites that received a score of “Fair” or “Poor” were contacted for remediation. Remediation and training were offered as an option to help sites improve their performance in future proficiency panels. A teleconference was conducted with the site to review the potential areas for improvement. Together, the EQAPOL and the respective site identified what areas are most important and designed a plan for how to help the site improve their performance. All site remediation comments and suggestions were recorded in the EQAPOL web-based system.
2.11. EQAPOL Quality Assurance Unit
The Flow Cytometry EOL operates under compliance with Good Clinical Laboratory Practice guidelines. The EQAPOL Quality Assurance Unit helped the laboratory institute Good Clinical Laboratory Practice guidelines and was responsible for distributing Standardized Operating Procedures as well as auditing the Flow Cytometry EOL Todd et al. (in this issue).
2.12. EQAPOL Flow Cytometry Advisory Committee
We established an external advisory committee composed of experts in the field. They offered input and advice on all aspects of the Flow Cytometry Program. The EQAPOL Flow Cytometry Advisory Committee played a pivotal role in developing the Flow Cytometry EQAP. Their input was critical to the overall success of the program, as they addressed key issues such as panel design, grading criteria, positivity criteria, and program advancement.
3. Results
3.1. External proficiency 3 (EP3) results
EP3 was the most recent Flow Cytometry proficiency panel (Table 1). EP3 included both 4-color and 7-color ICS proficiency panels (see supplemental data for 4-color results). A total of eleven sites participated in EP3. The average score for the 7-color ICS panel was 80.85 (“Good”). Color coding was added to the table to illustrate the categories where sites score the lowest performance: 66–74%, equivalent to “Fair” performance in respective categories (yellow highlight); 0-65 points, equivalent to “Poor” performance (orange highlight), and red text identifies the two sites that performed “Fair” and “Poor,” triggering Remediation. The three main categories where sites lost the majority of points, collectively, were Protocol Adherence, Deviation of EOLm Analyzed Data from Consensus, and Deviation of EOLm from Site Analysis. Therefore, examples provided below are focused on these three main criteria.
Table 1.
EP3 grading results.
| Criteria | Sub-criteria | 001 | 003 | 004 | 006 | 007 | 008 | 010 | 011 | 013 | 031 | 044 | Maximum points | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Timeliness | 10 | 0 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| PBMC processing | Cell viability % | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 3 | 3 | |
| Cell recovery % | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 0 | 3 | 3 | 2 | 3 | ||
| Protocol adherence | Instrument setup | Scatter TC | 6 | 6 | 6 | 6 | 6 | 4 | 5.5 | 5.5 | 5.5 | 6 | 6 | 6 |
| Fluorescence TC | 4 | |||||||||||||
| Data collection and analysis | # events | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Gating | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | ||
| Basic subset | Intra-laboratory performance for basic lymphocytes subset identification | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 2.67 | 4 | 4 | |
| Deviation of EOLm analyzed data from consensus | 24 | 20 | 28.67 | 18.67 | 18 | 29.33 | 22 | 20.67 | 19.33 | 13.33 | 18.67 | 30 | ||
| Deviation of EOLm andsite analyzed data | 29.33 | 20 | 30 | 25.33 | 27.33 | 21.33 | 30 | 19.33 | 28.67 | 14 | 29.33 | 30 | ||
| Total | 93.33 | 66 | 94.67 | 80 | 81.33 | 84.67 | 91.5 | 72.5 | 83.5 | 60 | 83 | 100 | ||
| Grade | Excellent | Fair | Excellent | Good | Good | Good | Excellent | Fair | Good | Poor | Good | Excellent | ||
Key:
66 - 74% of category
0 - 65% of category
Overall score = fair/poor (site remediation)
3.2. Protocol adherence
3.2.1. Instrument setup
The majority of sites lost points for not setting target channels according to the protocol, although all of these sites reported having followed the protocol. The protocol for EP3 specified target channel ranges for each fluorescence parameter as well as scatter and the detection threshold. Our experience with earlier EPs demonstrated the importance of these parameters. The protocol for EP1 did not include instructions for sites to follow for establishing scatter target channels. As a result, the settings used for forward and side scatter varied greatly across sites (Fig. 1A). Given the high degree of variability in scatter settings across sites for EP1, the EOL was unable to use standardized scatter gates for centralized analysis. For EP2 sites were given target channels for both forward and side scatter parameters, and there was much less variability in scatter settings across sites (Fig. 1B). A standard gate has been placed on all plots to illustrate the variation in settings. Agreement across sites for scatter settings improved significantly since EP2, aiding in the use of standardized gating for EOLm.
Fig. 1.
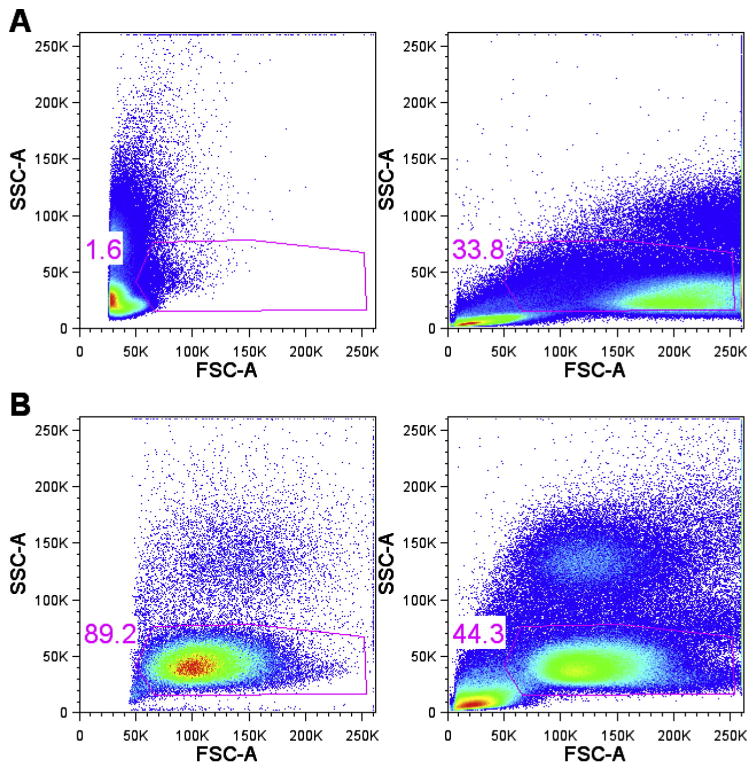
Scatter plot variance shown with and without target channels.
3.2.2. Data annotation
Data annotation was not scored in EP3; however, the majority of sites did not properly annotate their data files. Sites were provided with a protocol that specified how to annotate data. Two examples of poorly annotated data obtained from a sample known to be stained with CD4 FITC were “<Alexa Fluor 488-A>: CD4” and “<FITC-A>: CD4.” The instrument configuration in the examples was “<Alexa Fluor 488-A>” and “<FITC-A>”, respectively. However, the parameter annotation for both examples included only “CD4.” The correct parameter annotation was “CD4 FITC” after the configuration (for example “<Blue A-A>: CD4 FITC”).
3.3. Deviation of EOLm analyzed data from consensus
Most sites lost points in the category of Deviation of EOLm Analyzed Data from Consensus. The majority of points deducted for Deviation of EOLm Analyzed Data from Consensus were due to issues where replicate values were inconsistent. In the assay questionnaire only three sites reported having used a manifold to aspirate supernatant (sites 004, 008, and 044). Jaimes et al. (2011) reported that using a manifold would improve agreement across replicates in an ICS assay. Use of a manifold does seem to account for some variability; however, there are other assay components that contribute as well.
3.4. Deviation of EOLm and site analyzed data
3.4.1. Gate placement
In EP3, all sites followed the gating strategy suggested in the protocol. However, all but one site lost points for Deviation of EOLm and Site Analyzed Data, indicating that the actual placement of gates and analysis regions was a major issue across sites. According to McNeil et al. (2013), the most important aspects of gate placement is the inclusion of dim CD3+ and CD8+ cells. Previous reports have shown that both CD3 and CD8 are down regulated on stimulation with specific antigen, such as with an ICS assay (Valitutti et al., 1997; Lamoreaux et al., 2006). Therefore, excluding CD3+ and CD8+ dim cells selectively excludes antigen specific cells and lowers response rates. Fig. 2 includes an example of a site excluding antigen-specific CD3+ dim cells. For CMVpp65 stimulated cells, the site reported 0.596% for CD4+ IFN-γ+IL-2+, while the centralized analysis for the same FCS file was 0.85% (see supplemental data). Data were not collected from the sites in a manner that enabled us to backgate the cytokine positive response using site analysis. Therefore, we performed a mock site analysis that resembled the original site analysis as closely as possible (Fig. 2). The only difference between plots shown in 2A versus plots shown in 2B, centralized analysis, was the placement of the CD3 gate. In Fig. 2A the first three plots from the left are CD3 and CD4 gates and the cytokine analysis region, respectively. Gates for each plot are listed at the top. To backgate, the cytokine analysis region shown in the CD4+ gated CD3 versus IFN-γ+IL-2 plot (middle) was placed on the ungated population, shown in the ungated CD3 versus IFN-γ+IL-2 plot (second plot from the right). This identified all of the IFN-γ+IL-2+ events in the FSC file. The cytokine analysis region on the ungated plot was then used as a gate to create a second plot showing CD3 versus SSC (last plot on the right). Finally, the original CD3 gate (far left) was then copied to the ungated/cytokine gated CD3 versus IFN-γ+IL-2 plot (far right). Using this backgating method enabled us to observe the degree to which the original CD3 gate was selectively missing the IFN-γ+IL-2+ responding cells. Equivalent plots from the centralized analysis are shown in Fig. 2B, where the CD3 gate included the CD3 dim+ events that are IFN-γ+IL-2+ responding cells. Note that the use of CD3 versus cytokine helps to visualize the cytokine positive cell population as a clear cluster with the centralized analysis (2B middle plot). However, the cytokine positive cell population was truncated in the site analysis, where CD3 dim+ cells are missing (2A middle plot). Fig. 3 includes an example of a site excluding CD8+ dim cells. The only difference between mock site analysis plots shown in 3A versus centralized analysis plots shown in 3B was the placement of the CD8 gate. Original site analysis results for CEF stimulated CD8+ T-cells was 0.36%, and centralized analysis of the same file was 0.63% (see supplemental data). CD8 gate (far left plots) and cytokine analysis region (second plots) followed by the backgating of the cytokine positives (third and fourth plots) illustrate the degree of CD8 downregulation missed by the mock site analysis (3A). Fig. 3B plots were obtained using centralized analysis and illustrate proper placement of the CD8 gate, including cytokine responding cells that are CD8 dim positive.
Fig. 2.
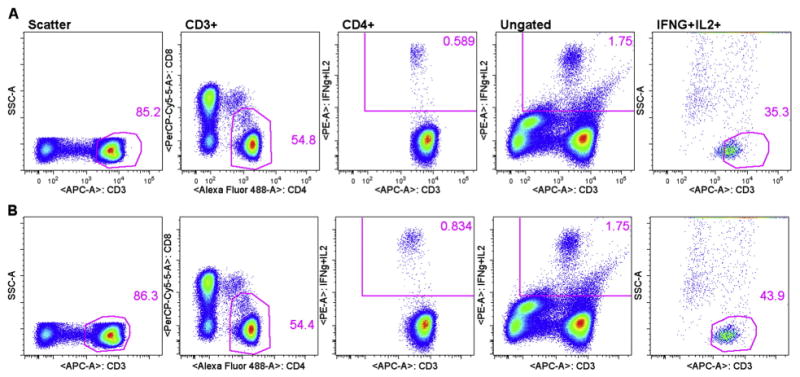
Backgating improves magnitude of functional response by including CD3 dim+ antigen-specific cells in the CD3 gate. Mock site analysis (2A) versus centralized analysis (2B).
Fig. 3.
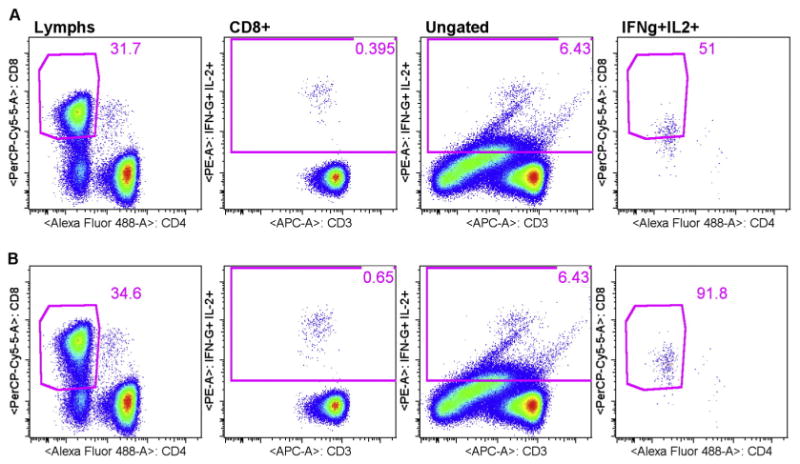
Backgating to include dim CD8+ cells improves magnitude of measured response. Mock site analysis (3A) versus centralized analysis (3B).
3.4.2. Compensation
For multicolor flow cytometry assays, electronic compensation for spectral overlap is necessary Roederer (2001). Some sites still experienced minor compensation errors, mostly due to a lack of verifying that compensation had been set appropriately by the software. Fig. 4 contains an example of a compensation error that resulted in a false positive, where the only difference between the mock site analysis in 4A and the centralized analysis in 4B was the compensation for PE - %FITC. For EP2, one site reported a false CD4 response to CEF (0.114%, see supplemental data). EQAPOL centralized analysis of the same file did not result in a CD4 response to CEF (0.0237%), consistent with results obtained from all other sites. The false positive site-reported CD4 CEF response for this donor was due to an error in site analysis. Upon visually comparing the backgate plots, provided by the site, we noticed that the false positive population appeared to be very dimly positive for CD4. The dim CD4 positive in the site analysis was not present in the centralized analysis, pointing to compensation as a possible cause for this error. We were unable to view the exact plots showing the compensation error based on the data provided by the site; however, we were able to recreate the error using mock site analysis by changing only the compensation between PE and FITC. Fig. 4A shows mock site analysis for the CD4 gate (CD4 vs CD8, second plot) and CD4+ and CD8+ cytokine analysis regions (CD3 vs IFN-γ+IL-2, third and fourth plots, respectively). The CD4+ cytokine analysis region was backgated (fifth and six plots) to show that the cytokine positive events were dim CD4+ and dim CD8+. The compensation error is noticeable by viewing the IFN-γ+IL-2 PE vs CD4 FITC, where the IFN-γ+IL-2 PE positive population was spilling over into the CD4 FITC population due to under-compensation (PE - %FITC = 8.589). For mock site analysis, compensation was calculated by the software with no manual adjustment. The result of the compensation error is that the mock site analysis resulted in responding cells for both CD4 (0.123%) and CD8 gates (0.226%). MHC Class II-restricted CD4+ cells should not be responding to the CEF peptide pool that consists of optimal 8- and 9-mers. In Fig. 4B the exact same series of plots are shown for centralized analysis, where the compensation was manually increased for PE - %FITC to 10.6. Centralized analysis results are negative for CD4 (0.0238%) and positive for CD8 (0.436%) gates.
Fig. 4.
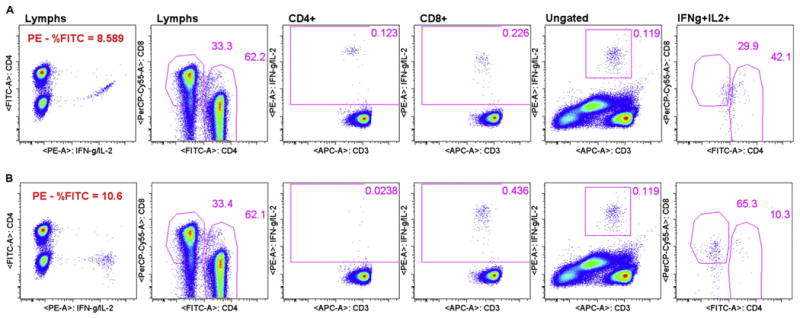
Minor compensation error leads to a false positive response (FP). Mock site analysis (4A) versus centralized analysis (4B).
4. Discussion
4.1. Approaches to improve site performance
The primary goals of the Flow Cytometry EQAP are to assess proficiency and provide support to help sites improve assay performance. The Flow Cytometry EQAP is able to readily identify specific components of the ICS assay that contribute to poor outcomes by using highly standardized reagents and protocols. Grading criteria have been developed to categorically address specific elements in the assay that are known to contribute the most variability. To date the categories where sites are penalized most include Protocol Adherence (instrument setup data annotation), Deviation of EOLm Analyzed Data from Consensus (use of manifold), Deviation of EOLm and Site Analyzed Data (compensation, placement of CD3 and CD8 gates to include dim positives).
The future addition of in-house assays will help sites identify potential technical issues with their own assays, by comparing results against the EQAPOL standardized assay.
4.2. 8-Color assay
Reports suggest that HIV-specific polyfunctional cells are associated with immunologic control of viremia among HIV elite controllers Betts et al. (2006), Makedonas and Betts (2006), and Hersperger et al. (2011) and possibly represent a correlate of protection for yellow fever and small pox vaccines (Makedonas and Betts (2006), Precopio et al. (2007), Gaucher et al. (2008). Therefore, introducing an 8-color ICS assay to evaluate polyfunctional ICS responses represents the next logical step for advancing the program. Evaluating sites on four functions simultaneously will add some unique challenges with regard to grading site performance. Both the EQAPOL Scientific Advisory Board and Flow Cytometry Advisory Committee will aid in determining the final grading criteria to be used for evaluating polyfunctional responses.
In future send-outs, an 8-color ICS assay will include a fourth functional measure, CD107a or lysosomal-associated membrane protein 1 that has been shown to correlate with cytotoxic activity Betts and Koup (2004). All current participating laboratories have instrumentation that is compatible with the fluorophores selected for use in the 8-color ICS panel (see supplemental data for 8-color panel). CD107a, a degranulation marker of cytotoxic T-Lymphocyte function, was added to the panel as a first choice. According to Betts et al. (2003) CD107a expression is a measure of cytotoxic potential of antigen-specific CD8 T-cells and in some patients a substantial population of C8+ T-cells will degranulate but not produce IFN-γ.
Initial grading for the 8-color ICS panel will be similar to the grading used in the EP3 7-color ICS panel. Sites will be asked to report single functions for each functional antibody as well as Boolean gate values, as a measure of polyfunctionality. The Flow Cytometry EQAP will then evaluate single function versus polyfunctionality to see whether there are any significant differences in grading.
4.3. Future directions
As the EQAPOL moves toward evaluating polyfunctional response, performing centralized analysis manually will rapidly become a rate-limiting step. Manual analysis is limited by conventional analysis tools that utilize sequential two dimensional gating and local operator expertise. To overcome this issue the Flow Cytometry EQAP includes the development and implementation of automated analysis tools Richards et al. (in this issue). The Flow Cytometry EQAP partnered with Flow Cytometry: Critical Assessment of Population Identification Methods (FlowCAP) to facilitate development and implementation of an optimized automated analysis approach for accurate rare event quantification. FlowCAP is designed to compare the performance of different computational methods for their ability to automatically identify cell populations in PFC data. Collaborating with FlowCAP ensures that automated analysis procedures used by EQAPOL will be compared against other automated computational methods. The long-term goal is to develop robust and user-friendly automated analysis software based on the lessons learned in Flow Cytometry EOL automated centralized analysis for sites to evaluate.
Supplementary Material
Acknowledgments
This project has been funded with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272201000045C. We thank the Flow Cytometry Advisory Committee members for their continued support, including Mario Roederer, Stephen DeRosa, and Charles Rinaldo.
The Flow Cytometry EOL receives support from the Duke University Center for AIDS Research (CFAR), an NIH funded program (5P30 AI064518).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jim.2014.05.021.
References
- Maecker HT, Reinfret A, D'Souza P, Darden J, Roig E, Landry C, Hayes P, Birungi J, Anzala O, Garcia M, Harari A, Frank I, Baydo R, Baker M, Holbrook J, Ottinger J, Lamoreaux L, Epling CL, Sinclair E, Suni MA, Punt K, Calarota S, El-Bahi S, Alter G, Maila H, Kuta E, Cox J, Gray C, Altfield M, Nougarede N, Boyer J, Tussey L, Tobery T, Bredt B, Roederer M, Koup R, Maino VC, Weinhold K, Pantaleo G, Gilmour J, Horton H, Sekaly RP. Standardization of cytokine flow cytometry assays. BMC Immunol. 2005;6:13. doi: 10.1186/1471-2172-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimes MC, Maecker HT, Yan M, Maino VC, Hanley MB, Greer A, Darden JM, D'Souza MP. Quality assurance of intracellular cytokine staining assays: analysis of multiple rounds of proficiency testing. J Immunol Methods. 2011;363(2):143. doi: 10.1016/j.jim.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinelt E, Reunanen M, Edinger M, Jaimes M, Stall A, Sasaki D, Trotter J. Standardizing application setup across multiple flow cytometers using BD FACSDiva version 6 software. BD Biosciences Technical Bulletin. 2012 Mar;:1–16. [Google Scholar]
- Garcia A, Keinonen S, Sanchez AM, Ferrari G, Denny TN, Moody MA. Leukopak PBMC sample processing for preparing quality control material to support proficiency testing programs. J Immunol Methods. 2014 doi: 10.1016/j.jim.2014.05.019. Submitted. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman R, Wilkening C. Analysis of quality assessment studies using CD45 for gating lymphocytes for CD3+CD4+% Cytometry. 2000;42:1. doi: 10.1002/(sici)1097-0320(20000215)42:1<1::aid-cyto1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Rountree W, Vandergrift N, Bainbridge J, Sanchez AM, Denny TN. Statistical methods for the assessment of EQAPOL proficiency testing: ELISpot, Luminex, and Flow Cytometry. J Immunol Methods. 2014 doi: 10.1016/j.jim.2014.01.007. 11789 (XXX:XXX-XXX) In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil LK, Price L, Britten CM, Jaimes M, Maecker H, Odunsi K, Matsuzaki J, Staats JS, Thorpe J, Yuan J, Janetzki S. A harmonized approach to intracellular cytokine staining gating: results from an international multiconsortia proficiency panel conducted by the Cancer Immunotherapy Consortium (CIC/CRI) Cytometry. 2013;83A:728. doi: 10.1002/cyto.a.22319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JCS, Hoffman RA. Evaluating fluorescence sensitivity on flow cytometers: an overview. Cytometry. 1998;33:256. [PubMed] [Google Scholar]
- Todd CA, Sanchez AM, Garcia A, Denny TN, Sarzotti-Kelsoe M. Implementation of Good Clinical Laboratory Practice (GCLP) guidelines within the External Quality Assurance Program Oversight Laboratory (EQAPOL) J Immunol Methods. 2014 doi: 10.1016/j.jim.2013.09.012. 11731 (XXX:XXX-XXX) in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valitutti S, Muller S, Salio M, Lanzavecchia A. J Exp Med. 1997;185:1859. doi: 10.1084/jem.185.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoreaux L, Roederer M, Koup R. Intracellular cytokine optimization and standard operating procedure. Nat Protoc. 2006;1:1507. doi: 10.1038/nprot.2006.268. [DOI] [PubMed] [Google Scholar]
- Roederer M. Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry. 2001;45:194. doi: 10.1002/1097-0320(20011101)45:3<194::aid-cyto1163>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Betts MR, Nason MC, West SM, DeRosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makedonas G, Betts MR. Polyfunctional analysis of human T cell responses: importance in vaccine immunogenicity and natural infection. Springer Semin Immunopathol. 2006;28:209. doi: 10.1007/s00281-006-0025-4. [DOI] [PubMed] [Google Scholar]
- Hersperger AR, Migueles SA, Betts MR, Connors M. Qualitative features of the HIV-specific T-cell response associated with immunologic control. Curr Opin HIV AIDS. 2011;6:169. doi: 10.1097/COH.0b013e3283454c39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE, Douek DC, Harari A, Pantaleo G, Baller R, Graham BS, Roederer M, Koup RA. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007;204:1405. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher D, Therrien R, Kettaf N, Asngermann BR, Buoocher G, Filali-Mouhim A, Moser JM, Mehta RS, Drake DR, III, Castro E, Akondy R, Rinfret A, Yassine-Diab B, Said EA, Chouikh Y, Cameron MJ, Clum R, Delvin D, Somogyi R, Greller LD, Balderas RS, Wilkinson P, Pantaleo G, Tartaglia J, Haddad EK, Sekaly R. Yellow fever vaccine induces integrated multilineage and polyfunctional immune repsonses. J Exp Med. 2008;205:3119. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MR, Koup RA. Detection of T-cell degranulation: CD107a and b. Methods Cell Biol. 2004;75:497. doi: 10.1016/s0091-679x(04)75020-7. [DOI] [PubMed] [Google Scholar]
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. J Immunol Methods. 2003;281:65. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Richards AJ, Staats J, Enzor J, McKinnon K, Frelinger J, Denny TN, Weinhold KJ, Chan C. Setting objective thresholds for rare event detection in flow cytometry. J Immunol Methods. 2014 doi: 10.1016/j.jim.2014.04.002. 11843 (XXX:XXX-XXX) in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


