Abstract
External proficiency testing programs designed to evaluate the performance of end-point laboratories involved in vaccine and therapeutic clinical trials form an important part of clinical trial quality assurance. Good Clinical Laboratory Practice (GCLP) guidelines recommend both assay validation and proficiency testing for assays being used in clinical trials, and such testing is facilitated by the availability of large numbers of well-characterized test samples. These samples can be distributed to laboratories participating in these programs and allow monitoring of laboratory performance over time and among participating sites when results are obtained with samples derived from a large master set. The leukapheresis procedure provides an ideal way to collect samples from participants that can meet the required number of cells to support these activities. The collection and processing of leukapheresis samples requires tight coordination between the clinical and laboratory teams to collect, process, and cryopreserve large number of samples within the established ideal time of ≤8 hours. Here, we describe our experience with a leukapheresis cryopreseration program that has been able to preserve the functionality of cellular subsets and that provides the sample numbers necessary to run an external proficiency testing program.
1. Introduction
Apheresis is a procedure during which the blood of a donor is passed through an apparatus that separates out one particular blood constituent and returns the remaining blood components to the circulation (Ganzel et al., 2012). Leukapheresis is a specialized modification of apheresis specifically designed to remove leukocytes and return the remaining cells (i.e., granulocytes, platelets, erythrocytes) and plasma to the participant. The leukapheresis procedure can be performed using a low extracorporeal volume, such that the apheresis circuit does not have to be primed with blood products prior to the procedure. Thus, it is an ideal method for obtaining blood components from a participant without exposing them or their blood to material from another human donor.
In order to enrich for a cellular component of interest, centrifugation is used to separate blood components during the apheresis procedure based on density. Typically, Continuous Flow Centrifugation (CFC) is used (Graw et al., 1971), where blood is collected, spun, and returned continuously while the participant is connected to the apheresis circuit. The main advantage of this system is the low extracorporeal volume (i.e., blood in the circuit not present in the participant's circulation) required by the procedure; this makes the procedure more readily tolerated by participants who would be more sensitive to blood loss, such as the elderly and children. During this procedure, the needed component is collected, and the “unused” blood components are returned to the donor. In general, fluid replacement is not needed during this procedure; however, the procedure does require the placement of two separate venous catheters to provide the needed access.
In order to obtain a large number of leukocytes for use in quality assurance programs, we have utilized a leukapheresis procedure that preferentially excludes polymorphonuclear leukocytes (PMNs), basophils, and eosinophils to obtain a leukopheresis product (LP) that is rich in mononuclear leukocytes (i.e., lymphocytes and monocytes). The LP processed by the laboratory results in a large number of sample vials of cryopreserved Peripheral Blood Mononuclear Cells (PBMCs) prepared from a single participant sample that form a reliable test material for quality assurance.
2. Materials and Methods
2.1 Inclusion and Exclusion Criteria
All participants were recruited into protocols approved by the Duke Medical Center Institutional Review Board (IRB); all donors provided written informed consent prior to participation. LPs were obtained from either asymptomatic HIV-1 seropositive or healthy HIV-1 seronegative donors who had been pre-screened to meet the inclusion criteria (Table 1); these criteria were established to conform to guidelines from the American Association of Blood Banking and the American Society for Apheresis to maximize participant safety (AABB, Standards Program Committee, 2012). Participants were assessed during a pre-screen visit to ensure all clinical safety indicators were met, and donors who met the screening criteria were scheduled to undergo leukapheresis in the Apheresis Unit at Duke University Medical Center. In order to ensure participant safety, pre-screen visits and leukapheresis visits occurred within 48-72 hours of each other.
Table 1.
Donor criteria for participation in the leukapheresis protocol.
| Criterion | Acceptable Value |
|---|---|
| Participant Assessment / Interview | |
| Ability to provide informed consent | Yes |
| Willing to receive HIV test results (if tested as part of entry into the study) | Yes |
| Ability and willingness to undergo the leukapheresis procedure for approximately 3 hours | Yes |
| Participant age | ≥18 years (and ≤60 years if HIV-1 infected) |
| Participant weight | ≥110 lbs (50 kg) |
| Appropriate venous access possible in both arms | Yes |
| Symptomatic of infection (including opportunistic infections if HIV-1 infected) | No |
| History of AIDS diagnosis | No |
| History of CD4 count <200 cell/μL in previous 6 months | |
| History of autoimmune disease or other chronic disease (other than HIV-1 infection) for which leukapheresis would be contraindicated | No |
| Pregnancy | No |
| Ongoing bleeding disorder / hemorrhage or current anticoagulant therapy | No |
| History of malignancy (except squamous cell carcinoma) in previous 5 years | No |
| On an investigational drug | No |
| Pulse, blood pressure, and temperature at the time of screening and at the procedure | Within accepted normal limits for age / gender |
| Currently on angiotensin converting enzyme (ACE) inhibitor therapy | No |
| Participant Screening Laboratory Values | |
| White blood count | >3.8 × 109 cells/L |
| CD4 count | ≥300 cells/μL if HIV-1 infected, no criterion if HIV-1 negative |
| Hematocrit | ≥35% |
| Platelet count | 150 × 109 platelet/L |
| Albumin | ≥3.5 g/dL |
| Ionized calcium | ≥1.12 mmol/L |
2.2. Clinical procedure
The leukapheresis procedure was performed using the COBE Spectra Apheresis System (Figure 1A; Terumo BCT, Lakewood, CO). Each procedure took 3-4 hours and was performed under careful supervision by the apheresis clinical nursing staff.
Figure 1.
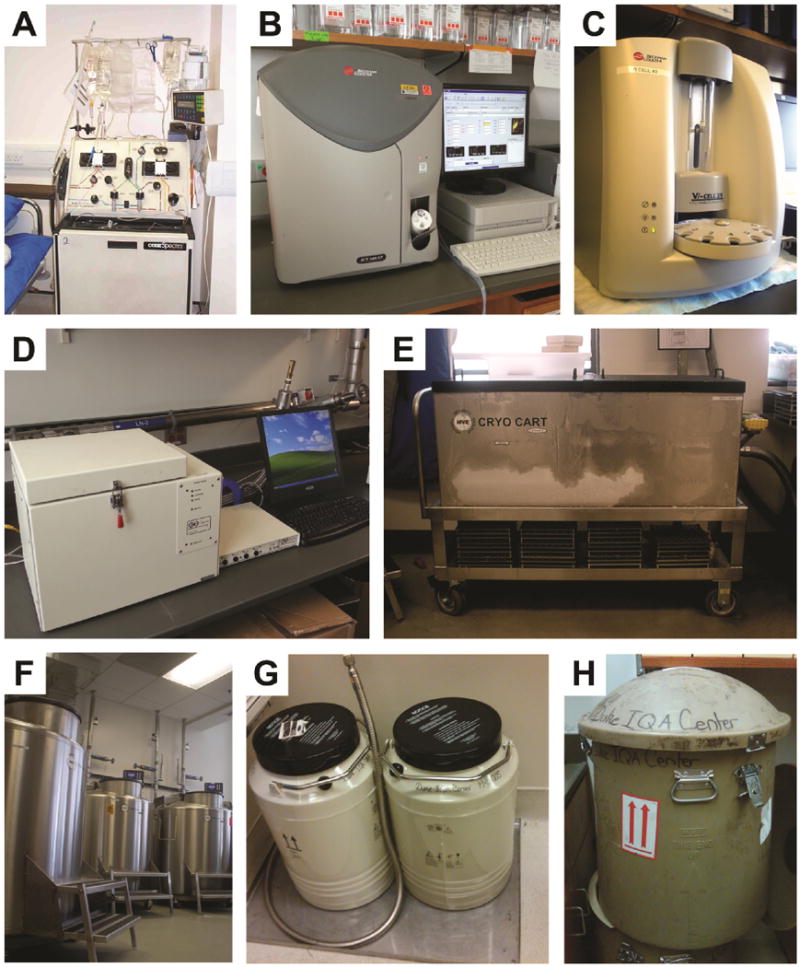
Equipment used for the preparation, storage, and shipment of PBMC.
A. Leukapheresis machine (Terumo BCT COBE Spectra Apheresis System).
B. Hematology counter (Beckman Coulter Ac*T 5 Diff CP).
C. Cell / viability counter (Beckman Coulter Vi-Cell XR).
D. Controlled rate freezer (Gordinier Model #9010).
E. Cryogenic cart (MVE CHART CryoCart™).
F. Liquid nitrogen vapor phase storage tanks.
G. Dry shipper container (MVE CHART); Dewar is inserted into this container.
H. External shipping cover for dry shipper.
2.3. Isolation of the PBMC
Once the leukapheresis procedure was completed, the LP was labeled with a unique participant identification number (PID#) and was delivered at room temperature to the laboratory within the hour. On average, the LP volume was 167mL. The PBMC isolation process started within two hours of the LP delivery using the processing plan summarized in Figure 2.
Figure 2.
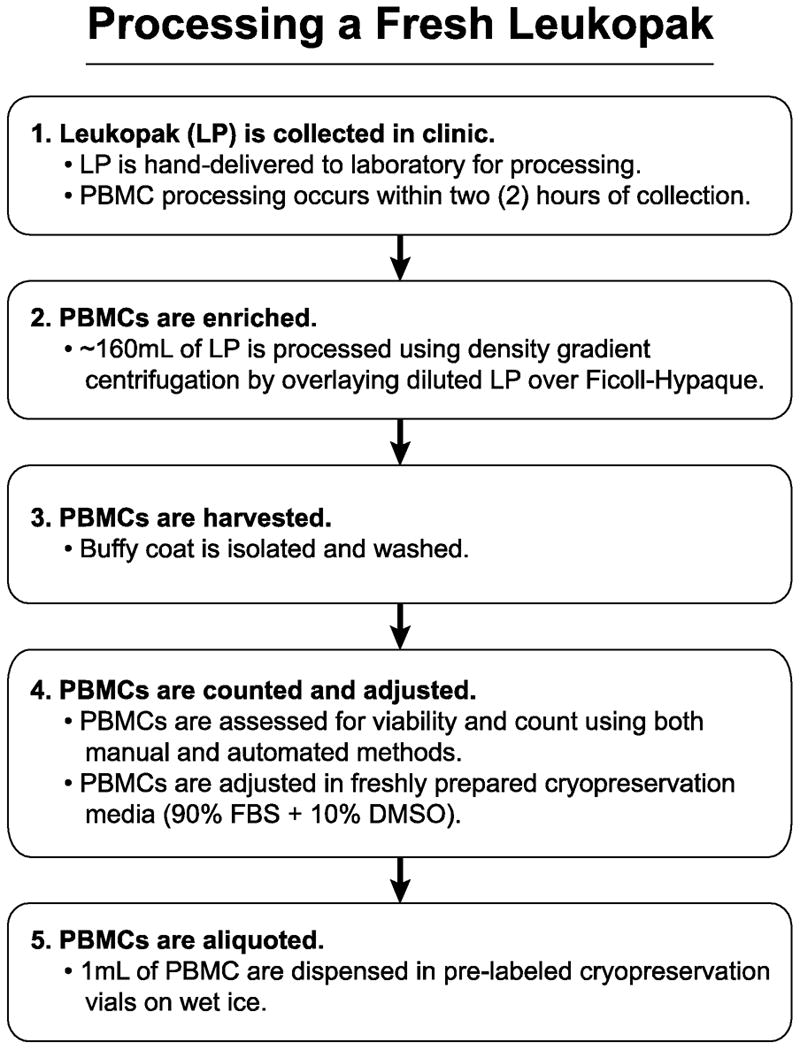
Leukopak processing. The steps required for correct and timely processing of a leukapheresis product are shown in chronological order.
The PBMC isolation procedure was adapted from density gradient procedures used for whole blood. The initial testing resulted in acceptable yields; the protocol was then standardized to the present procedure. The processing laboratory performed these procedures following GCLP guidelines; all personnel were trained, certified, and followed standard operating procedures (SOPs). The laboratory performing the work was audited semiannually by the Quality Assurance Unit (Duke University, Durham, NC) to assess and confirm GCLP compliance.
Processing was started by performing an initial white blood cell count (WBC) on the LP. The WBC was obtained using an automated hematology counter Ac*T 5 Diff CP (Figure 1B; Beckman Coulter, Miami, FL). Each LP was divided into four equal volumes to distribute the work and to improve the processing efficiency. Each part was diluted to a total volume of 140mL using Dulbecco's phosphate buffered saline (D-PBS; Gibco / Life Technologies, Grand Island, NY) without added calcium or magnesium. The diluted LP was then overlaid onto Ficoll-Paque™ Premium media (GE Healthcare, Pittsburg, PA), and the material was centrifuged for 30 min at 800×g / 25°C with no brake. After this density gradient separation, the PBMCs were harvested and washed twice with D-PBS followed by centrifugation at 300×g for 10 min at 25°C with brake. Cells were then pooled and mixed to produce a homogeneous product from which 3 PBMC samples were taken to assess cell count and viability. Each sample was counted on three platforms in triplicate, for a total of nine cell count determinations. Multiple assessments were performed due to the large number of cells being processed (mean of 12.7×109 cells per LP). Cells were counted and assessed for viability using a Vi-Cell XR automated cell counter (Figure 1C; Beckman Coulter, Miami, FL) and an Ac*T 5Diff CP automated analyzer (Figure 1B; Beckman Coulter, Miami FL). A manual cell count and viability assessment was also performed using a hemacytometer to visualize trypan blue dye exclusion. For each sample's count / viability measurement type, means were calculated and compared within and across the three platforms (manual, ViCell, and Ac*T 5Diff CP) to ensure that all values fell within 20% of each platform mean value. If outliers occurred for any measurement type, they were excluded from the final calculation for the overall cell adjustment.
The PMBCs were then suspended in freshly-made cryopreservation media (90% fetal bovine serum [FBS; Gemini Bio-Products, West Sacramento, CA]+ 10% dimethylsulfoxide [DMSO; Sigma-Aldrich, St. Louis, MO]) to a cell concentration of 20×106/mL. Cells were then aliquoted into pre-labeled cryopreservation vials placed in steel racks sitting over wet ice. For each PBMC harvesting process, a minimum of 4 technicians worked in order to ensure that PBMCs could be cryopreserved within 8 hours of collection.
2.4. Cryopreservation of PBMC
Once cells were counted, diluted, and aliquoted, the cryopreservation vials were treated using the cryopreservation freezing process illustrated in Figure 3. PBMC vials were frozen using an automated programmed controlled-rate freezer unit, Model #9010 (Figure 1D; Gordinier, Roseville, MI) that decreased the temperature of the vials in the racks by -1°C per minute. This controlled freezing process continued until the chamber cooled from 4°C to −90°C, at which point the steel racks were quickly transferred to a MVE CryoCart™ (Figure 1E; CHART, Garfield Heights, OH). The CryoCart™ served both as a workbench and provided a temperature-controlled environment (consistently less than −90°C) for the frozen samples. Vials were transferred from the steel racks to cardboard storage boxes, and the boxes were placed in designated racks in two different vapor phase liquid nitrogen (LN2) freezers (Figure 1F) maintained at −190°C. PBMC vials were split between LN2 freezer units for safety and redundancy. All temperatures from the LN2 freezer units were continuously electronically monitored and logged using a computerized system (Rees Scientific, Trenton, NJ).
Figure 3.
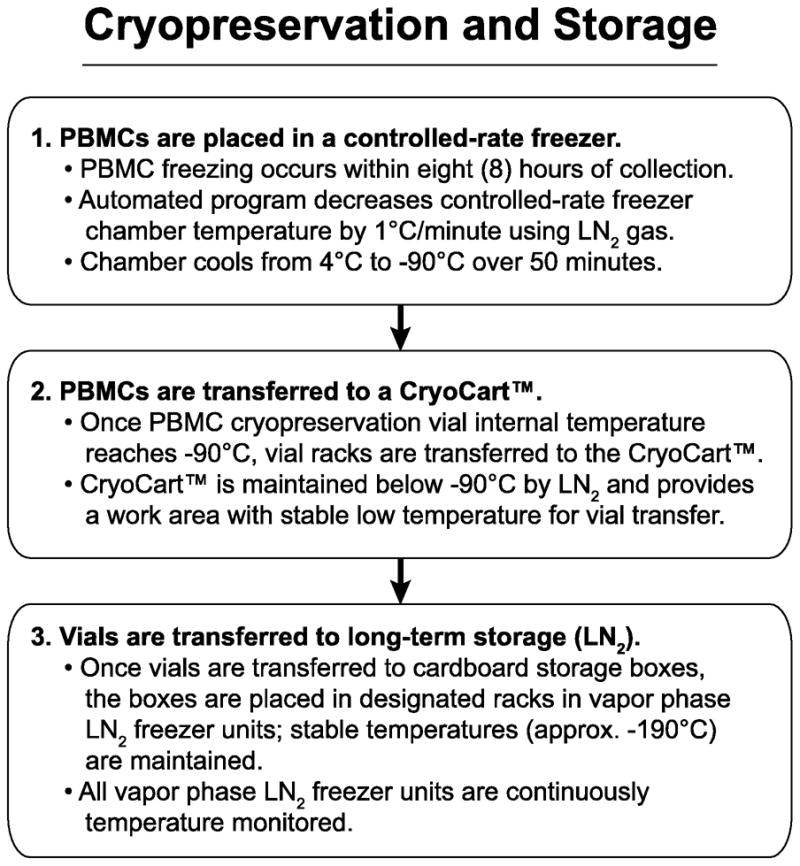
Cryopreservation and storage of PBMC samples. A description of the requirements for long-term storage of the samples from time of collection is provided.
The total time from the end of the LP collection to PBMCs being cryopreserved was ≤8 hours. This strict timeline was created to meet previously reported requirements for the preservation of functionality of cellular subsets in PBMC (Bull et al., 2007; Weinberg et al., 2010).
2.5. Storage and shipping of the PBMC samples
The PBMC storage inventory was tracked using Laboratory Data Management System (LDMS, Frontier Science and Technology Research Foundation, Inc., Amherst, NY). Upon sponsor approval, confirmation of an executed Material Transfer Agreement (MTA), and following verification that human subjects regulations (i.e., IRB compliance) were satisfied, PBMCs were shipped to laboratories worldwide using the scheme shown in Figure 4. First, samples were identified based on the requirements of the particular shipment. Then using a request system that queried the LDMS inventory system (Figure 5), specific PBMC vials were identified and assembled into a panel. Individual PBMC vials were retrieved from the freezer and transferred to a labeled box kept in a CryoCart™. Boxes containing PBMC panels were then transferred from the CryoCart™ into a pre-chilled external container (commonly called a Dewar). The Dewar was then placed into an LN2 dry shipper (Figures 1G & 1H) that was closed and fitted with a temperature data logger within the lid of the shipper. Finally, the data logger was activated and left running in order to record the transit temperature. The data loggers were deactivated once reaching the receiving laboratory. All shipments were performed in compliance with International Air Transport Association (IATA) regulations (3.6.2 Infectious Substance, 3.6.2.2.2. Category B: Biological Substance; https://www.iata.org/whatwedo/cargo/dgr/Documents/DGR52_InfectiousSubstances(DG R362).pdf).
Figure 4.
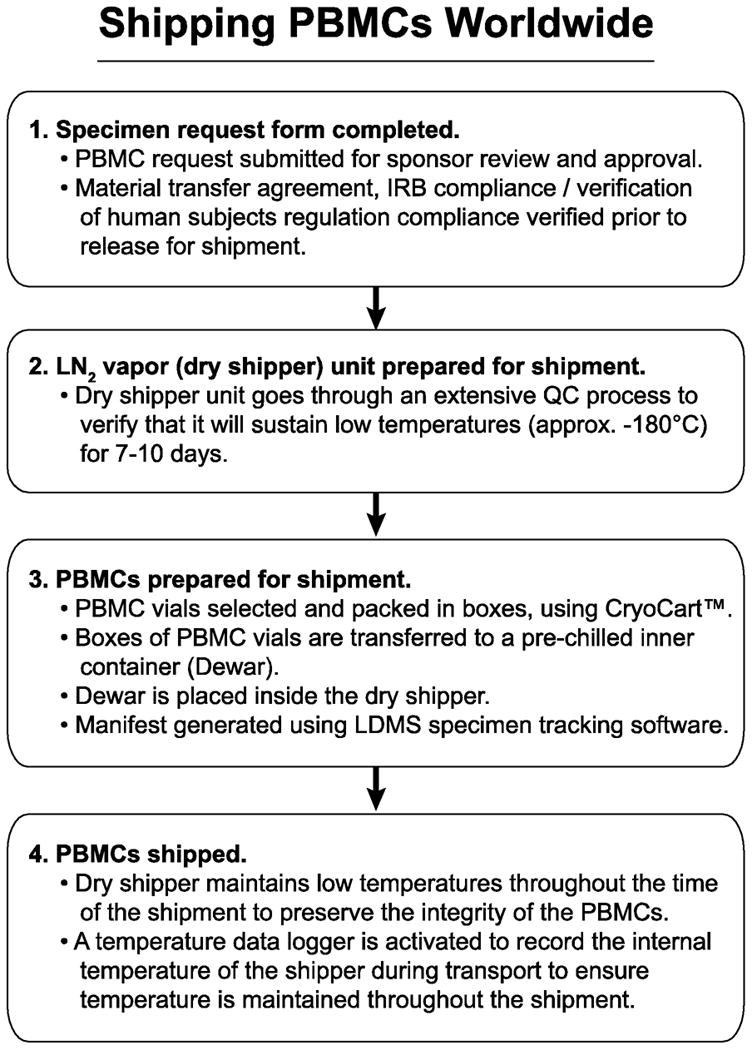
Shipping of cryopreserved samples. The steps required for proper distribution of the sample within country and internationally are provided. These steps have insured consistent quality (viability, recovery, and functionality) of the cryopreserved samples received by an end-point laboratory.
Figure 5.
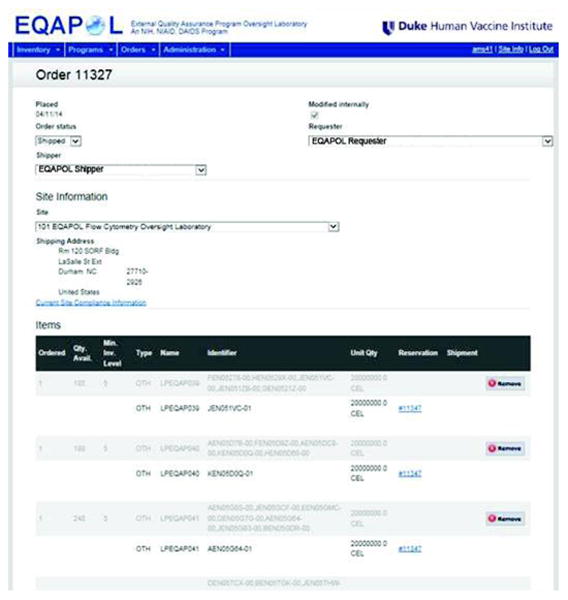
Sample selection screenshot. Sample selection is performed using a web-based interface that queries the LDMS record system. In the system, multiple samples can be selected and assembled into a shipping request. Prior to shipment, MTA and regulatory compliance is confirmed.
The use of LN2 dry shippers (MVE CHART Garfield Heights, OH) for PBMC transport allowed for shipping in a manner that preserved PBMC integrity. In order to ensure that the PBMC shipment process would preserve cell function, the LN2 dry shippers underwent a quality control (QC) process to verify that the shipper could sustain low temperatures (about −180°C) for 7-10 days. Once the LN2 dry shipper was returned to the processing/shipper lab, the data from the data logger were downloaded and analyzed to ensure that temperature excursions did not occur and that proper temperatures less than −135°C were maintained throughout the shipment.
2.6. Use of PBMC samples outside of the quality assurance program
Highly characterized PBMC are a valuable resource for human research. The primary use of the PBMC described here is for quality assurance. PBMC can be shared if they are deemed to be “in excess” of what is needed for the program. To share PBMC outside of the program network or for research not associated with quality assurance, permission must be obtained both from the sponsoring organization (e.g., Division of AIDS, NIAID, NIH) and from the Duke IRB. Sharing of PBMC requires an executed MTA between both institutions. In addition, approval for the release must be obtained from the Duke IRB; this requires that the researchers receiving specimens obtain IRB approval at their institution.
We invite inquiries about our PBMC collection, but note that samples can only be shared as a research collaboration. The goals of any proposed research must align with those of our sponsors and our own research programs.
3. Results
Over the last 7 years (Aug 2007- Sept 2013), we have used these procedures to collect and process LP samples from HIV-1 seronegative and seropositive donors. We have collected over 280 LPs, yielding over 89,000 total vials of PBMCs at 20×106 PBMC/mL.
A summary of yields of the apheresis procedure and processing are shown in Table 2. The average volume (± standard deviation) of LP samples collected from donors was 167 (± 15 mL). These LPs yielded an average of 6.71×109 PBMC (± 2.10×109) per LP, and on average this resulted in 325 vials of PBMCs at 20×106 PBMC/mL per LP. The average viability of these samples, after PBMC isolation, was 96.2% (±1.4%).
Table 2.
Leukapheresis protocol yields.
| Parameter | Average yield (range) |
|---|---|
| Leukapheresis product (LP) | |
| Volume of leukapheresis | 167 mL (35-198) |
| Total leukocytes in LP (based on total count) | 13.7×109 (4.7-29.7×109) |
| Total leukocytes in LP (based on lymphocytes + monocytes) | 12.7×109 (4.3-25.4×109) |
| Fraction of neutrophils in LP | 5% (0-37%) |
| Fraction of lymphocytes in LP | 77% (2-90%) |
| Fraction of monocytes in LP | 17% (7-73%) |
| After processing | |
| Total PBMC | 6.7×109 (1.4-14.4×109) |
| Leukocyte recovery (based on total count) | 50% (17-87%) |
| Leukocyte recovery (based on lymphocytes + monocytes) | 52% (6-91%) |
| Viability of recovered PBMC | 96% (91-99%) |
| PBMC vials obtained at 20×106 per vial | 327 (61-701) |
The average time from leukopak collection to the cryopreservation of PMBCs 4.15 hours (± 0.53; range 2.50-7.08 hrs) and was within the established limit of eight hours that has been shown to preserve the functionality of the cellular subsets of interest (Bull et al., 2007; Weinberg et al., 2010). Data regarding the viability, recovery, and functionality over time of these collected samples is described in this special issue [Sambor et al. in this issue].
To date, 98,300 vials of PBMC have been prepared using these methods and 31,712 have been requested and transferred out of the inventory. Of the PBMC samples stored, all specimens have viability and count information available. Additional assay data is available for subsets of the samples, including ∼90% with associated interferon-γ ELISPOT data, ∼50% with CD4/CD8 count data, and ∼50% with intracellular cytokine staining data.
4. Conclusions
In order to meet the needs of quality assurance programs and to comply with GCLP guidelines, we implemented a protocol of leukapheresis that allowed us to collect PBMC samples from healthy donors and HIV-1 seropositive donors. We followed GCLP guidelines for all procedures (processing, storing, shipping of samples) including the training and certification of our personnel, and the operational maintenance of our instrumentation. The procedure allowed us to store large numbers of vials of PBMCs derived from specimens obtained from individual participants, permitting validation of in vitro assays and functional evaluation of B, T, and NK cell subsets circulating in human peripheral blood [Sambor, et al. in this issue]. Moreover, these samples have been used for longitudinal external proficiency testing as described in this issue [see Sarzotti-Kelsoe, et al.; Sanchez, et al.; and Staats, et al.; all in this issue]. These samples have been used to validate in vitro assays and to evaluate the functionality different cell subsets circulating in human peripheral blood. Key features of the program included implementing a clinical protocol that allowed us to recruit the relevant participants, rapid processing of the samples in a manner that preserved cell functionality, a storage and retrieval system that permitted accurate location of cryopreserved cells and linking to the donor from whom they were derived, and a shipping process that preserved cell functionality for end-user laboratories.
A harmonized set of principles for GCLP compliance has been proposed (Sarzotti-Kelsoe et al., 2009), two of which are assay validation and proficiency testing. Use of both of these components can improve confidence in the results of critical endpoint assessments of large clinical trials. During the validation process, large quantities of PBMC samples such as those described herein are useful for establishing assay precision by allowing an assay to be performed repeatedly on samples that should give the same results. Furthermore, by providing samples to multiple laboratories performing the same assay, inter-laboratory accuracy can be assessed.
Proficiency testing programs provide an external assessment mechanism for endpoint laboratories. As part of a proficiency testing program, there is a need for standardized specimens that can be provided along with a reagent set (Sarzotti-Kelsoe et al., 2009). For cell-based assays like ELISpot and flow cytometry with or without intracellular cytokine staining, well-characterized replicate PBMC samples allow for many laboratories to be assessed using the same material.
Using the leukapheresis procedure to obtain PBMC requires specialized equipment and may not be feasible for all laboratories. As noted, the average yield from our LP samples was 325 vials at 20×106 PBMC/mL; in contrast, from a single 500 mL blood draw the expected number of equivalent vials would be 10-43 [based on the yields reported in (Crosley et al., 2009)]. Based on this, the leukapheresis procedure allows for a larger number of equivalent PBMC vials to be stored per participant, permitting more comparisons across laboratories or across assay types. Tests that use whole blood (e.g., CD4 count proficiency testing as is done by the Immunology Quality Assessment Center) can be provided for by means other than leukapheresis.
Other alternatives to leukapheresis-derived specimens include stabilized cells and stabilization media. Multiple products are available from commercial vendors (e.g., Streck, Omaha, NE); stabilized cells can be useful for some assays but are not useful for those assays requiring viable cells (i.e., ELISpot, intracellular cytokine staining following antigen stimulation). Thus, the leukapheresis-derived PBMC product described here provides a useful resource for proficiency testing and assay validation of GCLP-compliant protocols, especially those typically used to assess HIV-1 vaccine trials.
Because of the specialized equipment and procedures required, it may be impractical to implement this set of procedures in areas where some clinical trials are being performed. It is possible that programs using this procedure may not have a complete representation of the demographic, ethnic, and genetic diversity present in a population where a trial is being performed. For this reason, ongoing efforts to increase the diversity of well-characterized specimens should continue. However, the use of standard specimens across labs is still useful, as labs in different regions performing the same assay should obtain similar results, regardless of the local population. Such comparisons provide a baseline standard that can be enhanced as more diverse specimens become available.
The cryopreservation of PBMC from leukapheresis in a manner that preserves the integrity of the cells requires careful coordination and processes that keep the cells in a stable low temperature environment until they are selected for use. Through our work on this protocol, we have found that the procedures require close interaction between the clinical and laboratory personnel to minimize the time elapsed between collection and cryopreservation of the samples. We believe that the detailed description of these procedures and the steps taken by our group will assist others in implementing such protocols. For those laboratories that plan to establish leukapheresis protocols, we strongly recommend the use of similar procedures in order to ensure the viability and functionality of the cryopreserved cells.
Finally, shipping of leukapheresis-derived PBMC to end-user laboratories must also be a carefully coordinated process. Maintaining cell integrity, viability, and functionality requires an intact cold chain and specialized equipment (for both transport and maintenance at the receiving site); for this reason, we use digital recording thermometers to document the cold chain during transport. We also provide instructions for the removal of the Dewar, turning off the recording thermometer, and instructions on how to return the shipping equipment, usually with a pre-prepared shipping waybill or invoice. In addition, there may be restrictions on what samples may be imported to a particular site. For example, cells preserved in FBS-containing media may require documentation that the cows from which the serum was derived were free of bovine spongiform encephalopathy (i.e., “mad cow” disease); additional requirements may vary by locality. It is, therefore, critical to have close communication between the central laboratory and the receiving sites to ensure that samples are not compromised due to delays at the receiving end and that all paperwork is properly prepared before the shipment departs.
Acknowledgments
This work was supported by a CTVIMC grant (DENN11VIMC2) to T.N Denny and G. Ferrari from R.A. Koup at the Foundation for the NIH; by the Immunology Quality Assurance contract to T.N. Denny from NIH NIAID (N01-AI-95356); by the External Quality Assurance Program Oversight Laboratory (EQAPOL) contract to T.N. Denny from NIH NIAID (HHSN272201000045C); and by the Center for HIV/AIDS Vaccine Immunology (CHAVI) grant to B.F. Haynes from NIH NIAID (AI067854).
Footnotes
5. Additional Information: SOPs that were developed to support this work are available upon request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AABB, Standards Program Committee. Standards for blood banks and transfusion services. 28. American Association of Blood Banks; Bethesda, MD: 2012. [Google Scholar]
- Bull M, Lee D, Stucky J, Chiu YL, Rubin A, Horton H, McElrath MJ. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J Immunol Meth. 2007;322:57–69. doi: 10.1016/j.jim.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosley LK, Duthie SJ, Polley AC, Bouwman FG, Heim C, Mulholland F, Horgan G, Johnson IT, Mariman EC, Elliott RM, Daniel H, de Roos B. Variation in protein levels obtained from human blood cells and biofluids for platelet, peripheral blood mononuclear cell, plasma, urine and saliva proteomics. Genes Nutr. 2009;4:95–102. doi: 10.1007/s12263-009-0121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzel C, Becker J, Mintz PD, Lazarus HM, Rowe JM. Hyperleukocytosis, leukostasis and leukapheresis: Practice management. Blood Reviews. 2012;26:117–122. doi: 10.1016/j.blre.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Graw RG, Herzig GP, Eisel RJ, Perry S. Leukocyte and platelet collection from normal donors with the continuous flow blood cell separator. Transfusion. 1971;11:94–101. doi: 10.1111/j.1537-2995.1971.tb04383.x. [DOI] [PubMed] [Google Scholar]
- Sarzotti-Kelsoe M, Cox J, Cleland N, Denny T, Hural J, Needham L, Ozaki D, Rodriguez-Chavez IR, Stevens G, Stiles T, Tarragona-Fiol T, Simkins A. Evaluation and recommendations on good clinical laboratory practice guidelines for phase I-III clinical trials. Plos Med. 2009;6:e1000067. doi: 10.1371/journal.pmed.1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Song LY, Wilkening CL, Fenton T, Hural J, Louzao R, Ferrari G, Etter PE, Berrong M, Canniff JD, Carter D, Defawe OD, Garcia A, Garrelts TL, Gelman R, Lambrecht LK, Pahwa S, Pilakka-Kanthikeel S, Shugarts DL, Tustin NB. Optimization of storage and shipment of cryopreserved peripheral blood mononuclear cells from HIV-infected and uninfected individuals for ELISPOT assays. J Immunol Meth. 2010;363:42–50. doi: 10.1016/j.jim.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]


