Abstract
Objective
To determine the 12-month cost-effectiveness of a collaborative care (CC) program for treating depression following coronary artery bypass graft (CABG) surgery versus physicians’ usual care (UC).
Methods
We obtained 12 continuous months of Medicare and private medical insurance claims data on 189 patients who screened positive for depression following CABG surgery, met criteria for depression when reassessed by telephone two-weeks following hospitalization (9-item Patient Health Questionnaire ≥10), and were randomized to either an 8-month centralized, nurse-provided, and telephone-delivered collaborative care (CC) intervention for depression or to their physicians’ usual care (UC).
Results
At 12-months following randomization, CC patients had $2,068 lower but statistically similar estimated median costs compared to UC (P=0.30) and a variety of sensitivity analyses produced no significant changes. The incremental cost effectiveness ratio of CC was −$9,889 (−$11,940 to −$7,838) per additional quality-adjusted life-year (QALY), and there was 90% probability it would be cost-effective at the willingness to pay threshold of $20,000 per additional QALY. A bootstrapped cost-effectiveness plane also demonstrated a 68% probability of CC “dominating” UC (more QALYs at lower cost).
Conclusions
Centralized, nurse-provided, and telephone-delivered CC for post-CABG depression is a quality-improving and cost-effective treatment that meets generally accepted criteria for high-value care.
Keywords: Coronary artery bypass grafting, depression, cost-benefit analysis
Approximately 1 in 4 patients with cardiac disease report significant elevations in mood symptoms and strong evidence links depression to reduced health-related quality of life (HRQoL),1–3 increased morbidity and mortality,4–8 and higher treatment costs independent of cardiac disease severity.9 Randomized trials clearly demonstrate that effective depression treatment can reduce mood symptoms and improve HRQoL in patients with cardiac disease,2,10–13 and despite conflicting opinions,2,10,14,15 an American Heart Association (AHA) Science Advisory recommends that screening and treatment programs for depression be deployed into routine cardiac care.16
Numerous trials have supported the effectiveness17 and cost-effectiveness18–21 of collaborative care (CC) strategies for treating depression in primary care settings. Based on Wagner’s Chronic Care Model,22 it involves care managers who follow an evidence-based treatment protocol under the supervision of a primary care physician (PCP), and systematically contact patients to monitor their mood symptoms and recommend adjustments in treatment accordingly. Yet, collaborative care has not been widely adopted in primary care let alone cardiac care settings. Given the concern about rising health care costs and resource limitations, payers and health systems are likely to require rigorous economic analyses before investing in new programs. Still, just one group has reported the cost-effectiveness of a depression treatment program for patients with cardiac disease.23,24
We conducted the Bypassing the Blues (BtB) Trial, the first study to apply a CC approach for treating depression following an acute cardiac event,25 and reported that an 8-month nurse-led and telephone-delivered CC intervention for treating depression following coronary artery bypass graft (CABG) surgery could produce higher levels of HRQoL and lower levels of mood symptoms12 and pain26 than patients randomized to their doctors’ usual care. We now report the 12-month cost-effectiveness of the BtB intervention strategy.
METHODS
BtB compared the impact of telephone-delivered CC for treating post-CABG depression versus doctors’ usual care on HRQoL (primary outcome), mood symptoms, physical functioning, health services utilization, and health care costs. All study procedures were approved by the institutional review boards of the (removed to preserve blind) and our study hospitals, and by an independent data and safety monitoring board appointed by the National Heart Lung and Blood Institute. Published details of the BtB protocol,25 recruitment patterns, and main clinical outcomes12,25 are briefly summarized herein.
Setting and Participants
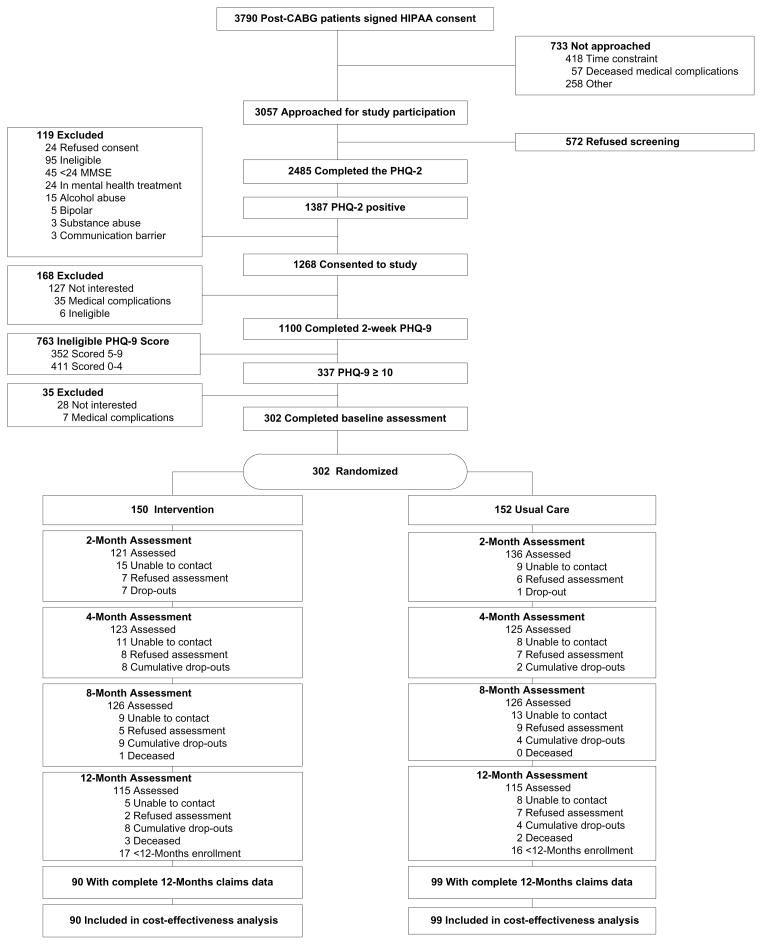
From 3/2004 to 9/2007 when our randomization target was achieved (N=300),12 study nurse-recruiters identified 2,485 hospitalized patients who had just undergone CABG surgery at one of seven (removed to preserve blind) area hospitals and provided their signed informed consent to undergo our depression screening procedure with the Patient Health Questionnaire (PHQ-2).27 Of these, 1,387 (56%) screened positive, and 1,268 (91%) met all preliminary eligibility criteria and consented to enroll into our trial and allow us to obtain claims data from their insurer should they remain protocol-eligible following our two-week telephone follow-up assessment. Later, 1,100 (87%) completed the PHQ-928 following hospital discharge and 337 (31%) scored ≥ 10 signifying at least a moderate level of depressive symptoms. Of these, 302 (90%) met all other eligibility criteria and were randomized to either their physicians’ “usual care” (UC) (n=152) or our CC intervention (n=150).
Collaborative Care Intervention
Following randomization a nurse care manager telephoned intervention patients to: review their psychiatric history; provide basic psychoeducation about depression; and describe various treatment options that included: (1) a workbook we mailed patients to enhance their ability to self-care for depression;29 (2) initiation or adjustment of a course of antidepressant pharmacotherapy prescribed under their PCPs’ direction; (3) assistance with referral to a local mental health specialist in the event of poor treatment response, severe psychopathology, or complex psychosocial problems; or (4) “watchful waiting” for mildly elevated mood symptoms.
The care manager subsequently presented the patient’s information to the study psychiatrist and internist at a weekly case-review session. Treatment recommendations were then formulated and conveyed by the nurse to the patient via telephone, and to his/her PCP for consideration via fax, telephone, or mail depending upon the urgency of response. However, we required that PCPs approve and prescribe all of their patients’ pharmacotherapy and we never dispensed any medications.
During the “acute phase” of treatment, the care manager telephoned patients bi-weekly to administer the PHQ-9 and assess treatment response; monitor antidepressant pharmacotherapy; review workbook lesson plans; and inform them of new treatment recommendations generated at our case-review sessions. Depending upon a patient’s treatment choice(s), symptoms, and motivation, these telephone contacts lasted 15–45 minutes and continued for two to four months. The patient subsequently transitioned to the “continuation phase” of care during which the care manager initiated contacts every 1–2 months until completion of our 8-month intervention.
Patient-Level Outcomes
Nurse-recruiters collected information on patients’ sociodemographic characteristics and conducted a detailed chart review of co-morbid medical conditions, and medication use prior to hospital discharge. Telephone assessors blinded to the patient’s treatment assignment administered the SF-3630 to determine generic mental (MCS) and physical (PCS) HRQoL, and the 17-item Hamilton Rating Scale for Depression (HRS-D)31 at baseline and at 2-, 4-, 8-, and 12-months following randomization. We attempted to complete 8-month telephone assessments on all patients (8-months was our Trial’s “main outcomes” time-point12), and 12-month telephone assessments on all who were enrolled for at least 12 months at the time we concluded our final 8-month telephone assessment.
We used the approach developed by Lave, et al.32 to calculate the effectiveness of our intervention in terms of added quality adjusted life years (QALYs) and depression-free days (DFDs). To estimate QALYs, we converted the SF-36 scores collected at each time point into a preference-based utility between 0 (death) and 1 (perfect health) summed over a 12-month period.33 Similarly, to calculate DFDs, we categorized the HRS-D score at each time point as either a “full” DFD (HRS-D ≤ 7), partial DFD (HRS-D >7 ≤ 21 weighted proportionately), or a “zero” DFD (HRS-D >21) that we summed over the total 12-month period.
Cost Analyses
We conducted our cost analyses from the perspective of the health care payer. In an a priori economic power calculation submitted to our funding agency (2002), we estimated that 150 subjects per trial arm would provide 90% power to detect log-transformed differences of $2,400 between-groups assuming: an intent-to-treat analytic plan; 2-tailed alpha ≤ 0.05; ≤5% missing claims rate; and 12-month UC medical costs of $3,400 following CABG surgery.
We sought all available medical claims and enrollment data from Medicare and the two largest private insurers in western Pennsylvania who covered the majority of BtB participants to 12/31/2008 so as to ensure that the last randomized patients had 12 months of follow-up claims. We included trial patients who were continuously enrolled with these three insurers for a 12-month period following the date of randomization including those who switched from one of these plans to another, and those with Medicare plus a supplemental Medigap policy through one of the two private insurers. Using outpatient and inpatient insurance claims data, we then constructed measures of total 12-month health care spending. Outpatient costs included physician visits to PCPs and specialists, laboratory testing, imaging, emergency department use, facility fees, and all other outpatient health care. Inpatient costs included all acute inpatient medical or surgical admissions but excluded the initial admission for CABG surgery or any other care prior to randomization. Although self-reported rates of antidepressant pharmacotherapy use differed slightly at 8-month follow-up (44% CC vs. 31% UC; P=0.00812), we did not include prescription drug spending because Medicare lacked a drug benefit until 2006, and increasing numbers of patients over the course of the trial were utilizing pharmacies that offered a month’s supply of a generic antidepressant for $4 without generating an insurance claim.34
To account for differences in reimbursement rates across our three payers, we assigned standard Medicare costs to the claims-based utilization data. We obtained the mean cost per discharge by diagnosis-related group from the 2007 Healthcare Costs and Utilization Project National Inpatient Sample and merged it with the inpatient data (http://www.hcup-us.ahrq.gov). We determined the mean cost per physician visit/procedure using the Medicare Part B Extract Summary System by CPT code and assigned a cost to all physician claims (http://www.cms.hhs.gov/NonIdentifiableDataFiles). Because of limited power, skewed cost data, and to avoid making multiple comparisons with small sample sizes, we restricted our analyses to those of total outpatient, inpatient, and overall total costs.
We estimated the mean 8-month cost of our intervention at $460 per patient. We calculated this value using: (1) the median number of 10 telephone contacts made by our nurse care managers to each patient over the course of his or her participation in our 8-month intervention (range: 0–28)12; (2) trial documentation that each call took approximately one hour of time (25 minutes call + 35 minutes recordkeeping and other outreach efforts); and (3) the actual salary and fringe benefit rate paid to our study nurses ($31/hour × 10 = $310). Then, per Katon et al.,20 we added a fixed $100 for each patient to cover the costs of physician supervision and our care manager’s tracking registry,20,25 plus an additional $50 to cover the cost of our workbook, mailings, and other miscellaneous support.
Statistical Analyses
We compared baseline sociodemographic, clinical, and functional status measures by both availability of complete 12-month claims data and randomization status using t-tests for continuous data and chi-square analyses for categorical data. We calculated the incremental cost-effectiveness ratio (ICER) of our intervention using both actual and estimated measures of effectiveness and costs. To estimate QALYs, we first estimated the preference-based utilities at each time point using a mixed model that included treatment, time point, age, gender, and race as independent variables and each subject as a random intercept to account for unobserved heterogeneity among individuals. We then added two- and three-way interaction terms between treatment, time point and gender to the model to account for observed gender differences in treatment response,12 with time treated as a categorical variable. We used the iterative Markov chain Monte Carlo method to impute missing values of our effectiveness measure, and calculated the mean utilities from the two adjacent time points and summed the product of the mean utility and the duration of time between those points.
To estimate total 12-month costs, we used Generalized Linear Models (GLM) with Gamma distribution to correct for skewness in our cost data given the large differences between the actual mean and median costs. The model included a log-link function with treatment group, age, gender and race as independent variables, and an interaction term of treatment X gender. We then calculated the incremental cost per QALY and DFD using the mean cost difference between the CC and UC groups divided by the difference in mean QALYs and DFSs between the groups, respectively. We used the bootstrapping procedure35 with 1,000 replications of the same size as our study sample to calculate the ICER with 95% confidence intervals. We then generated a cost-effectiveness acceptability curve to display the probability that the intervention is cost-effective at or below a given willingness to pay. We created a scatterplot of the estimated incremental cost versus incremental QALYs and reported the probability that CC would “dominate” UC (i.e., greater improvement in QALYs at lower cost).
We conducted sensitivity analyses to determine how robust our estimate of cost-effectiveness was to changes in how costs were determined. First, we calculated the ICER using raw data on costs and effectiveness. Second, as the mean costs of our small sample were heavily influenced by a single high-cost CC outlier whose total 12-month cost of care was $258,801, we removed this patient from both our actual and estimated numbers (the next highest cost patient, also randomized to CC, had 12-month costs of $109,668). Third, we doubled the estimated cost of our care manager intervention to $920 to account for any costs we may have overlooked in our base case (e.g., pharmacotherapy costs) and repeated our calculations. We used SAS 9.2 (SAS Institute Inc, Cary, North Carolina) for all analyses.
RESULTS
We obtained completed SF-36 questionnaires on 82–85% of our cohort at our 2-, 4-, and 8-months assessments points (N=302); on 87% of our cohort who remained eligible for a 12-month assessment (230/269);12-month vital status on 100% (302/302), and complete 12-months insurance claims data on 63% (189/302) (Figure 1). Just 4 subjects (1%) died (3 CC and 1 UC), and we were able to obtain 12-months claims on the two who died in months 9 and 12 after randomization (both CC). They and the other subjects included in our cost-effectiveness analyses were more likely to be working at baseline than the 113 depressed subjects for whom we lacked complete data (70% vs. 48%; P <0.001), but were otherwise similar on their sociodemographic and clinical criteria (Table 1). As with our full trial cohort (N=302),12 the 189 depressed subjects included in our cost-effectiveness analyses were balanced by randomization status (Table 1), and CC patents reported greater improvements on the SF-36 and HRS-D at various follow-up times, and more QALYs than UC patients (0.70 QALYs vs. 0.65 QALYs, p=0.004; eTable 1). However, these differences were not uniformly statistically significant as described earlier12 perhaps due to the smaller sample size.
Figure 1.
CONSORT Flowchart. CABG indicates coronary artery bypass graft; HIPAA, Health Insurance Portability and Accountability Act; MDD, major depressive disorder; MMSE, Mini-Mental State Examination; PHQ, Patient Health Questionnaire.
Table 1.
Baseline sociodemographic, clinical, and mental health characteristics among depressed post-CABG patients by inclusion in our cost effectiveness analyses (CEA) and randomization status
| Included in Cost Effectiveness Analysis | Treatment Assignment (within CEA) | |||||
|---|---|---|---|---|---|---|
| No (N = 113) | Yes (N = 189) | P | Usual Care (N = 99) | Intervention (N = 90) | P | |
| Age, mean (SD) | 58.8 (10.1) | 67.0 (10.4) | 0.74 | 67.1 (11.5) | 66.9 (9.0) | 0.88 |
| Male | 54% | 61% | 0.21 | 59% | 64% | 0.41 |
| Caucasian | 88% | 93% | 0.15 | 93% | 92% | 0.85 |
| > High School education | 57% | 54% | 0.61 | 49% | 60% | 0.15 |
| Married | 68% | 68% | 0.94 | 67% | 69% | 0.74 |
| Working, full, part-time | 48% | 70% | <0.0001 | 75% | 66% | 0.17 |
| SF-36v2 MCS, mean (SD) | 42.8 (11.5) | 43.1 (11.5) | 0.86 | 42.5 (11.6) | 43.7 (11.4) | 0.48 |
| SF-36v2 PCS, mean (SD) | 30.3 (6.2) | 31.0 (7.5) | 0.40 | 30.2 (7.5) | 31.8 (7.5) | 0.15 |
| PHQ-9 score, mean (SD) | 13.6 (3.4) | 13.5 (3.4) | 0.91 | 13.7 (3.7) | 13.3 (3.0) | 0.40 |
| Hamilton Rating Scale for Depression, mean (SD) | 16.5 (7.3) | 16.0 (6.9) | 0.54 | 15.7 (6.8) | 16.4 (6.9) | 0.46 |
| Diabetes | 43.4% | 42% | 0.79 | 44% | 39% | 0.44 |
| Chronic obstructive pulmonary disease | 19% | 24% | 0.38 | 23% | 24% | 0.85 |
| Myocardial infarction | 44% | 48% | 0.57 | 44% | 51% | 0.36 |
| Ejection fraction, mean (SD) | 51 (12.2) | 50 (12.6) | 0.73 | 51 (12.4) | 49 (12.7) | 0.41 |
| Medicare only | 28% (52) | -- | 31% (31) | 23% (21) | 0.50 | |
| Private health plan 1 only | 36% (68) | 34% (34) | 38% (34) | |||
| Private health plan 2 only | 21% (39) | 17% (17) | 24% (22) | |||
| Medicare plus private health plan | 16% (30) | 17% (17) | 15% (13) | |||
Table 2 displays the total 12-month costs by randomization status. Using actual claims data and including the $460 estimated cost of our intervention, CC patients had a non-significantly $779 higher total median per capita medical costs than UC patients at 12-months following randomization ($8,359 vs. $7,580; P= 0.60), and neither excluding the one high-cost outlier from our intervention group ($258,801) nor categorizing total costs into outpatient and inpatient costs (eTable 2) affected these results. Estimating costs using the GLM procedure, CC patients had a $2,068 lower but still non-statistically significant difference in median 12-month costs than UC ($16,126 vs. $18,194; P=0.30) that was robust to either excluding the one high-cost outlier or doubling the estimated per capita cost of our intervention.
Table 2.
12-Month Total cost comparisons between collaborative care and usual care for post-CABG depression.
| Usual Care | Collaborative Care | Cost Difference Usual Care − Collaborative Care |
|||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | Mean (SD) | Median | Mean | Median | Pb | |
| Actual, All observations Including intervention costs ($460) |
$17,522 ($21,072) | $7,580 | $19,279 ($31,369) | $8,359 | −$1,757 | −$779 | 0.60 |
| Actual, Excluding 1 high-cost outlier in Intervention group Including intervention costs ($460)c |
$17,522 ($21,072) | $7,580 | $16,552 ($18,050) | $8,292 | $971 | −$712 | 0.69 |
| Actual, All observations Double intervention costs ($920) |
$17,522 ($21,072) | $7,580 | $19,739 ($31,369) | $8,819 | −$2,217 | −$1,239 | 0.34 |
| Estimated, All observations Including intervention costs ($460) |
$18,622 ($6,292) | $18,194 | $18,173 ($8,316) | $16,126 | $449 | $2,068 | 0.30 |
| Estimated, Excluding 1 high-cost outlier in Intervention group c Including intervention costs ($460) |
$18,622 ($6,292) | $18,194 | $17,986 ($8,174) | $15,931 | $636 | $2,263 | 0.24 |
| Estimated, All observations Double intervention costs ($920) |
$18,558 ($6,148) | $18,159 | $18,660 ($8,332) | $16,652 | −$102 | $1,507 | 0.30 |
Estimated costs were based on generalized linear models with gamma distribution and log link function, including gender, age, race, treatment group, and interaction term between gender and treatment group.
Due to the skewed distribution of the data, Wilcoxon non-parametric tests were used to test the differences between the medians of the groups.
The single extreme high-cost outlier in the CC group generated $258,801 in total 12-month costs.
Table 3 displays the 12-month incremental costs per QALY and DFD produced by CC. In our estimated models, CC “strongly dominated” UC (ICER = −$9,889; 95% CI: −$11,940 to −$7,838), and removing the extreme cost outlier from our analyses resulted in even greater dominance (−$14,539; −$16,125 to −$12,952). CC remained cost-effective even when taking the most conservative approach using actual costs and including all observations ($54,605; $44,593 to $64,617), and dominated UC again when we removed the single high-cost outlier (−$21,591; −$26,064 to −$17,118). Furthermore, the estimated base case model demonstrated a cost savings of −$48 per DFD (−$64 to −$31) that remained significant even after we doubled the estimated cost of our intervention (−$13; −$26 to −$1).
Table 3.
Incremental mean cost per quality adjusted life year (QALY) and per depression free day (DFD).
| Incremental Cost per QALY, $b (95% CI) | Incremental Cost per DFD, $b (95% CI) | |
|---|---|---|
| Actual All observations |
54,605 (44,593 to 64,617) | −4 (−391 to 384) |
| Actual, Excluding 1 high-cost outlier in CC groupc | −21,591 (−26,064 to −17,118) | −59 (−142 to 25) |
| Actual All observations Intervention cost doubled to $920 |
65,943 (55,234 to 76,653) | 44 (−320 to 409) |
| Estimateda, All observations | −9,889 (−11,940 to −7,838) | −48 (−64 to −31) |
| Estimateda, Excluding 1 high-cost outlier in CC groupc | −14,539 (−16,125 to −12,952) | −49 (−54 to −44) |
| Estimateda, All observations Intervention cost doubled to $920 |
3,743 (−163 to 7,649) | −13 (−26 to −1) |
QALYs and DFDs were estimated using a mixed model that included treatment, time point, age, gender, and race, and two- and three-way interaction terms between treatment, time point and gender and bootstrapped to calculate the ICER with 95% confidence intervals.
Costs were estimated using GLM model that included treatment group, age, gender, race and interaction term between treatment and gender. Assume $460 mean per capita cost for intervention unless otherwise noted.
The single extreme high-cost outlier in the CC group generated $258,801 in total 12-month costs.
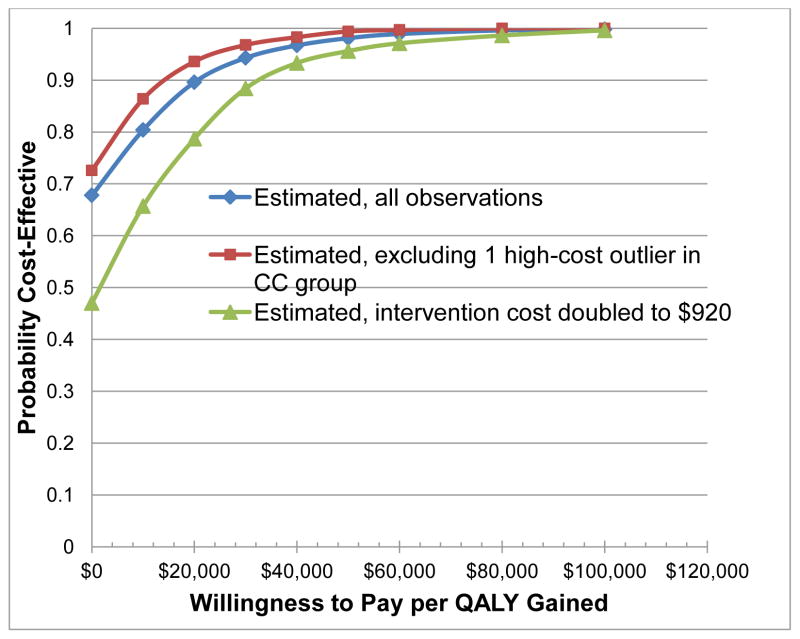
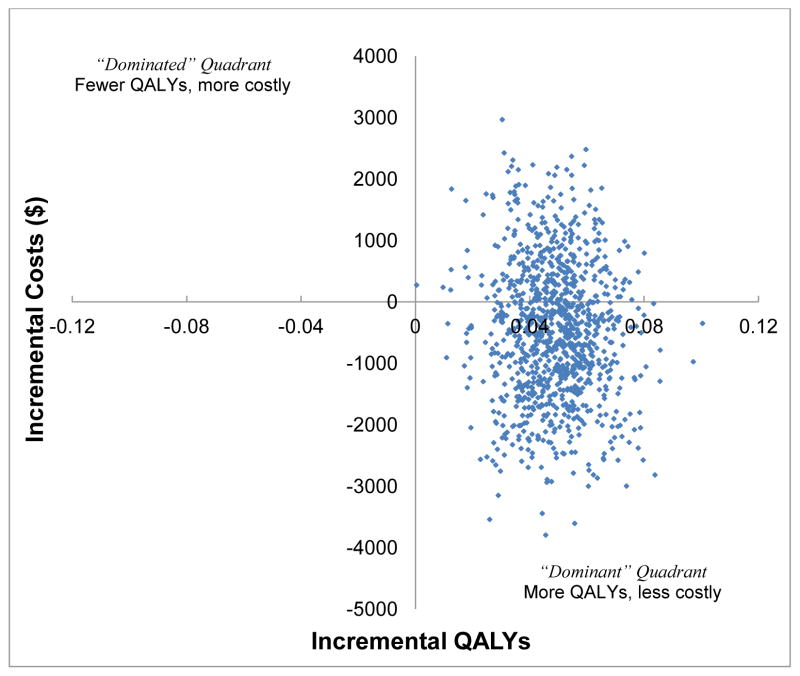
Figure 2 illustrates the estimated cost-effectiveness acceptability curves for our three scenarios. We see the base case analysis of the BtB intervention as having a 98% probability of being cost-effective at a societal willingness to pay up to $50,000 per additional QALY, and a 90% probability of being cost-effective at the $20,000 “high value care” threshold recommended for rapid dissemination.36 Moreover, these probabilities were little affected by excluding the high-cost outlier, doubling the cost of our intervention (96% and 79% probability cost-effective at the $20,000 threshold, respectively), or removing the three-way treatment, time point, and gender interaction term. A cost-effectiveness scatterplot involving 1,000 bootstrapped replication of the Bypassing the Blues trial population found CC as likely to be both more effective and cost less than UC as 68% of replications fell in the “dominant” right lower quadrant (Figure 3). Moreover, removing the single high-cost outlier or doubling the cost of the BtB intervention had only modest affects on the probability that CC would continue to “dominate” UC (73% and 47%, respectively; not shown).
Figure 2.
Cost-effectiveness acceptability curves of collaborative care (CC) versus usual care for post-CABG depression.
Figure 3.
Estimated cost-effectiveness plane of incremental cost versus incremental quality adjusted life years (QALYs) based on 1,000 bootstrapped replication of the Bypassing the Blues trial population and all observations. Notably, 68% of replications are in the “dominant” quadrant.
DISCUSSION
To the best of our knowledge, this is the first economic evaluation of a CC strategy for treating depression following an acute cardiac event. Just one other group has reported the cost-effectiveness of a systematic depression treatment program for patients with coronary disease.23,24 They employed mental health professionals who delivered their interventions either face-to-face13,23 or in combination with telephone and video contacts among patients with stable cardiac disease who were recruited from outpatient settings13,24 and also reported non-significant estimated total cost reductions favoring their intervention (e.g., −$325 lower mean costs; 95% CI: −$2,639 to $1,98924). Yet while they used estimated rather than actual Medicare claims data, our findings are consistent as they also reported their program was likely to be cost-effective (98% probability cost-effective at a willingness to pay of $30,000 per additional QALY23).
This is also the first report to describe a statistically significant negative ICER for a CC strategy for treating depression in any patient population. Gilbody et al.’s 2006 systematic review of 11 randomized economic evaluations of collaborative care interventions delivered in primary care to younger and mixed-age populations concluded that these programs improve outcomes, but at a greater cost than usual care.18 Although they were unable to calculate a summary measure of incremental cost per QALY, they did summarize the cost per added DFD at $24 (−$105 to $148) versus the −$48 cost per added DFD we report (−$64 to −$31; Table 3).
Not included in Gilbody et al.’s review are the cost-effectiveness analyses from the Improving Mood Promoting Access to Collaborative Treatment (IMPACT)19 and TEAMcare Trials.20 IMAPCT involved 1,801 primary care patients aged 60 and older who were randomized to either a CC intervention provided by a nurse- or psychologist-care manager or to their PCPs’ UC.37 At 2-years follow-up, intervention patients had $691 higher total health care costs ($16,175 vs. $15,484) and 107 more DFDs which translated to an incremental outpatient cost per QALY of $2,519 (−$4,517 to $9,554) and $2.76 cost per additional DFD (−$4.95 to $10.47).19 TEAMcare randomized 214 depressed adults with poorly controlled diabetes or coronary heart disease to either 12 months of a nurse-led “blended” CC strategy for treating depression and diabetes, or to their doctors’ UC.38 At two-years follow-up, their intervention produced favorable but non-significant cost-savings versus UC ($594 lower estimated mean outpatient costs; ICER: −$1,773 estimated outpatient costs per added QALY (−$2,878 to $2,878)), at an outpatient cost of −$5.26 per DFD (−$29,76 to $19.17).20 However, as in IMPACT, most cost savings occurred between the first and second year of follow-up, stressing the continued need for adequately powered and longer-term trials that examine the effects of depression treatment on costs. Given the increasingly negative (favorable) cost per DFD found when CC is applied to more medically ill populations, we conclude that the cost-effectiveness of CC programs are likely to be greater when applied to patients who tend to utilize high levels of medical services (e.g., the elderly, those with cardiovascular disease) than to undifferentiated groups of primary care patients whose baseline ambulatory care-sensitive use of non-mental health specialists, emergency rooms, and hospitalizations, and medical spending on pharmaceuticals and other services are relatively lower.
Confidence in our findings is further strengthened as we used the actual numbers of nurse contacts,12 incorporated physician supervision time and other expenses to calculate the $460 cost to provide our 8-month intervention, and bootstrapped our estimate of the ICER rather than use a raw number coming from a single sample. Thus it is of great interest that the estimated ICER of our intervention was negative and remained so even after we doubled the cost of our intervention to $920 and well above the $53523 to $68724 estimated 6-month costs to provide mental health care to depressed cardiac patients, the $515 1-year costs to deliver collaborative care for depression to predominantly low-income Hispanics,39 or the $591 1-year cost to provide the IMPACT version of CC to depressed older primary care patients.19
Despite our encouraging findings, uptake of the BtB intervention and others similar to it will depend in large part on whether its key components are adequately reimbursed by payers – either as individual services, bundled payments, or as incentives for achieving predefined goals (e.g., reduced use of emergency rooms and hospitalizations) - to allow payment for depression screening, telephone outreach, nurse care management and coordination, and psychiatrist supervision.40 Because payers make coverage and reimbursement decisions based on information about an intervention’s value at a population-level, implementation of CC is thus reliant on high-quality evidence demonstrating that it is both effective and cost-effective. Although there is no official U.S. standard, medical treatments that have an incremental cost per QALY of $50,000 or less are considered reasonable values, and those that cost under $20,000 are often recognized as “high-value” care recommended for rapid dissemination.36 Therefore, if we estimate 400,000 CABG surgeries are performed annually in the U.S.,41 and assume a conservative 20% incident rate of post-CABG depression,3,12 and a median 12-month savings of $2,068 per treated patient (Table 2), then telephone-delivered CC for post-CABG depression has the potential to save $165 million in the first year following surgery as well as generate significant improvements in HRQoL and speed recovery.12,26
The results of our analyses should be interpreted in light of several limitations. First, BtB was powered to detect differences in HRQoL between study arms12 rather than economic outcomes. Still, analyses of our trial data provides an unbiased view of the comparative outcome of clinical strategies.42 Second, although we noted no differences in baseline characteristics other than employment status, we were only able to obtain complete 12-month claims on 62% of trial participants and lacked complete information on prescription drug spending. Third, because of our relatively small sample size and non-significant overall cost differences, we choose not to categorize cost data into multiple cost categories. However, we did split total medical costs into its inpatient and outpatient cost components and found no significant differences by treatment assignment (eTable 2). Finally, while our findings are consistent with other trials suggesting CC is cost-effective, BtB was a single trial conducted in one region of the country with relatively short follow-up, and thus our findings should be confirmed in a larger multisite trial with longer follow-up.
In summary, centralized, nurse-provided, and telephone-delivered collaborative care for post-CABG depression is a quality-improving, cost-effective, and high-value treatment that meets generally accepted criteria for rapid dissemination. Moreover, our findings support the AHA’s Science Advisory advocating the increased awareness, and routine screening and treatment of all patients with cardiovascular disease for depression, and have important implications for health policy makers and providers considering integrated delivery of mental health and medical services.
Acknowledgments
This work was supported by NIH grants R01 HL70000 (Rollman) and P30 MH71944 and P30 MH90333 (Reynolds), and by a grant from The Fine Foundation (Donohue).
Footnotes
Clinical Trial Registration: Clinicaltrials.gov Identifier: NCT00091962 (http://clinicaltrials.gov/ct2/show/NCT00091962?term=rollman+cabg&rank=1)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kendel F, Gelbrich G, Wirtz M, et al. Predictive relationship between depression and physical functioning after coronary surgery. Arch Intern Med. 2010;170:1717–21. doi: 10.1001/archinternmed.2010.368. [DOI] [PubMed] [Google Scholar]
- 2.Thombs BD, de Jonge P, Coyne JC, et al. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA. 2008;300:2161–71. doi: 10.1001/jama.2008.667. [DOI] [PubMed] [Google Scholar]
- 3.Pignay-Demaria V, Lesperance F, Demaria RG, Frasure-Smith N, Perrault LP. Depression and anxiety and outcomes of coronary artery bypass surgery. Ann Thorac Surg. 2003;75:314–21. doi: 10.1016/s0003-4975(02)04391-6. [DOI] [PubMed] [Google Scholar]
- 4.Tully PJ, Winefield HR, Baker RA, Turnbull DA, de Jonge P. Confirmatory factor analysis of the Beck Depression Inventory-II and the association with cardiac morbidity and mortality after coronary revascularization. J Health Psychol. 2011;16:584–95. doi: 10.1177/1359105310383604. [DOI] [PubMed] [Google Scholar]
- 5.Connerney I, Sloan R, Shapiro P, Bagiella E, Seckman C. Depression is associated with increased mortality 10 years after coronary artery bypass surgery. Psychosom Med. 2010;72:874–81. doi: 10.1097/PSY.0b013e3181f65fc1. [DOI] [PubMed] [Google Scholar]
- 6.Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379–88. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumenthal JA, Lett HS, Babyak MA, et al. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet. 2003;362:604–9. doi: 10.1016/S0140-6736(03)14190-6. [DOI] [PubMed] [Google Scholar]
- 8.Oxlad M, Stubberfield J, Stuklis R, Edwards J, Wade TD. Psychological risk factors for cardiac-related hospital readmission within 6 months of coronary artery bypass graft surgery. J Psychosom Res. 2006;61:775–81. doi: 10.1016/j.jpsychores.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Frasure-Smith N, Lesperance F, Gravel G, et al. Depression and health-care costs during the first year following myocardial infarction. J Psychosom Res. 2000;48:471–8. doi: 10.1016/s0022-3999(99)00088-4. [DOI] [PubMed] [Google Scholar]
- 10.Rutledge T, Redwine LS, Linke SE, Mills PJ. A meta-analysis of mental health treatments and cardiac rehabilitation for improving clinical outcomes and depression among patients with coronary heart disease. Psychosom Med. 2013;75:335–49. doi: 10.1097/PSY.0b013e318291d798. [DOI] [PubMed] [Google Scholar]
- 11.Freedland KE, Skala JA, Carney RM, et al. Treatment of Depression After Coronary Artery Bypass Surgery: A Randomized Controlled Trial. Arch Gen Psychiatry. 2009;66:387–96. doi: 10.1001/archgenpsychiatry.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(ref removed to preserve blind).
- 13.Davidson KW, Rieckmann N, Clemow L, et al. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: coronary psychosocial evaluation studies randomized controlled trial. Arch Intern Med. 2010;170:600–8. doi: 10.1001/archinternmed.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whooley MA. To screen or not to screen? Depression in patients with cardiovascular disease. J Am Coll Cardiol. 2009;54:891–3. doi: 10.1016/j.jacc.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 15.Ziegelstein RC, Thombs BD, Coyne JC, et al. Routine screening for depression in patients with coronary heart disease never mind. J Am Coll Cardiol. 2009;54:886–90. doi: 10.1016/j.jacc.2009.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lichtman JH, Bigger JT, Jr, Blumenthal JA, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768–75. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 17.Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. 2012;10(4):CD004274. CD006525. doi: 10.1002/14651858.CD006525.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbody S, Bower P, Whitty P. Costs and consequences of enhanced primary care for depression: systematic review of randomised economic evaluations. Br J Psychiatry. 2006;189:297–308. doi: 10.1192/bjp.bp.105.016006. [DOI] [PubMed] [Google Scholar]
- 19.Katon WJ, Schoenbaum M, Fan MY, et al. Cost-effectiveness of improving primary care treatment of late-life depression. Arch Gen Psych. 2005;62:1313–20. doi: 10.1001/archpsyc.62.12.1313. [DOI] [PubMed] [Google Scholar]
- 20.Katon W, Russo J, Lin EH, et al. Cost-effectiveness of a Multicondition Collaborative Care Intervention: A Randomized Controlled Trial. Arch Gen Psychiatry. 2012;69:506–14. doi: 10.1001/archgenpsychiatry.2011.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katon WJ, Russo JE, Von Korff M, et al. Long-term effects on medical costs of improving depression outcomes in patients with depression and diabetes. Diabetes Care. 2008;31:1155–9. doi: 10.2337/dc08-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Quarterly. 1996;74:511–44. [PubMed] [Google Scholar]
- 23.Ladapo J, Shaffer JA, Fang Y, Ye S, Davidson KW. Cost-effectiveness of enhanced depression care after acute coronary syndrome: Results from the coronary psychosocial evaluation studies randomized controlled trial. Arch Intern Med. 2012;172:1682–4. doi: 10.1001/archinternmed.2012.4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson KW, Bigger JT, Burg MM, et al. Centralized, stepped, patient preference–based treatment for patients with post–acute coronary syndrome depression: CODIACS vanguard randomized controlled trial. JAMA Internal Medicine. 2013;173:997–1004. doi: 10.1001/jamainternmed.2013.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.(ref removed to preserve blind).
- 26.Morone NE, Weiner DK, Belnap BH, et al. The impact of pain and depression on recovery after coronary artery bypass grafting. Psychosomatic Med. 2010;72:620–5. doi: 10.1097/PSY.0b013e3181e6df90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–92. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 28.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katon W, Ludman E, Simon G. The Depression Helpbook. Boulder, CO: Bull Publishing; 2002. [Google Scholar]
- 30.Ware J, Kosinski M, Keller S. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. 2. Boston: New England Medical Center; 1994. [Google Scholar]
- 31.Freedland KE, Skala JA, Carney RM, et al. The Depression Interview and Structured Hamilton (DISH): Rationale, Development, Characteristics, and Clinical Validity. Psychosomatic Med. 2002;64:897–905. doi: 10.1097/01.psy.0000028826.64279.29. [DOI] [PubMed] [Google Scholar]
- 32.Lave JR, Frank RG, Schulberg HC, Kamlet MS. Cost-effectiveness of treatments for major depression in primary care practice. Arch Gen Psychiatry. 1998;55:645–51. doi: 10.1001/archpsyc.55.7.645. [DOI] [PubMed] [Google Scholar]
- 33.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21:271–92. doi: 10.1016/s0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 34.Choudhry NK, Shrank WH. Four-dollar generics--increased accessibility, impaired quality assurance. N Engl J Med. 2010;363:1885–7. doi: 10.1056/NEJMp1006189. [DOI] [PubMed] [Google Scholar]
- 35.Campbell MK, Torgerson DJ. Bootstrapping: estimating confidence intervals for cost-effectiveness ratios. QJ Med. 1999;92:177–82. doi: 10.1093/qjmed/92.3.177. [DOI] [PubMed] [Google Scholar]
- 36.Gold M. Cost Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 37.Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: A randomized controlled trial. JAMA. 2002;288:2836–45. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 38.Katon WJ, Lin EHB, Von Korff M, et al. Collaborative Care for Patients with Depression and Chronic Illnesses. N Eng J Med. 2010;363:2611–20. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hay JW, Katon WJ, Ell K, Lee P-J, Guterman JJ. Cost-effectiveness analysis of collaborative care management of major depression among low-income, predominantly Hispanics with diabetes. Value in Health. 2012;15:249–54. doi: 10.1016/j.jval.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katon WJ, Unützer J. Health reform and the Affordable Care Act: The importance of mental health treatment to achieving the triple aim. J Psychosom Res. 2013;74:533–7. doi: 10.1016/j.jpsychores.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Go AS, Mozaffarian D, Roger VL, et al. Heart Disease and Stroke Statistics—2013 Update: A Report From the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hlatky MA, Boothroyd DB, Johnstone IM. Economic evaluation in long-term clinical trials. Stat Med. 2002;21:2879–88. doi: 10.1002/sim.1292. [DOI] [PubMed] [Google Scholar]