Abstract
Aims
To determine if the presence of diabetes autoantibodies predicts the development of diabetes among participants in the Diabetes Prevention Program.
Methods
A total of 3050 participants were randomized into three treatment groups: intensive lifestyle intervention, metformin and placebo. Glutamic acid decarboxylase (GAD) 65 autoantibodies and insulinoma-associated-2 autoantibodies were measured at baseline and participants were followed for 3.2 years for the development of diabetes.
Results
The overall prevalence of GAD autoantibodies was 4.0%, and it varied across racial/ethnic groups from 2.4% among Asian-Pacific Islanders to 7.0% among non-Hispanic black people. There were no significant differences in BMI or metabolic variables (glucose, insulin, HbA1c, estimated insulin resistance, corrected insulin response) stratified by baseline GAD antibody status. GAD autoantibody positivity did not predict diabetes overall (adjusted hazard ratio 0.98; 95% CI 0.56–1.73) or in any of the three treatment groups. Insulinoma-associated-2 autoantibodies were positive in only one participant (0.033%).
Conclusions
These data suggest that ‘diabetes autoimmunity’, as reflected by GAD antibodies and insulinoma-associated-2 autoantibodies, in middle-aged individuals at risk for diabetes is not a clinically relevant risk factor for progression to diabetes.
Introduction
The prevention or delay of diabetes in adults is possible based on a number of randomized controlled trials [1-7]. Most studies have been conducted in individuals at high risk with impaired glucose tolerance and/or impaired fasting glucose. Diabetes was diagnosed using an oral glucose tolerance test and cases were presumed to have Type 2 diabetes. In addition to this common form of diabetes among adults, there have been consistent findings of cases of autoimmune diabetes occurring in adults over the age of 30–40 years, often termed latent autoimmune diabetes of adulthood [8], with a more rapid requirement for insulin therapy [9].
The Diabetes Prevention Program was designed to investigate the prevention of diabetes in adults at high risk. During the design phase it was not clear what proportion of subjects would have diabetes autoantibodies. Based on previous studies [10-13], we expected that the Diabetes Prevention Program population would have a prevalence of diabetes autoantibodies of <5%; therefore, it was decided that diabetes autoantibodies would not be an exclusion criterion but, instead, the presence of diabetes autoantibodies would be characterized after the study began. The aim of the present study was to explore whether the presence of diabetes autoantibodies at baseline influenced diabetes risk, overall or by treatment group.
Participants and Methods
The Diabetes Prevention Program methods have been published previously [1]. Participants had available data on elevated fasting and post-load glucose levels and were randomized to either intensive lifestyle therapy, metformin or placebo. Diabetes was diagnosed using an annual oral glucose tolerance test and semi-annual fasting glucose levels, with confirmation of positive tests. The ClinicalTrials.gov registration number was NCT00004992 (www.clinicaltrials.gov). All participants provided written informed consent through their local institutional review boards.
Diabetes autoantibody measurements were performed at the Northwest Lipid Research Laboratory, University of Washington, Seattle, WA, USA. Glutamic acid decarboxylase (GAD) 65 autoantibodies and insulinoma-associated-2 autoantibodies were measured at baseline for all randomized participants according to assays used at the time. Of the 3234 randomized participants, 94.3% had samples for diabetes autoantibody analysis at randomization. The clinical and metabolic characteristics of these particpants did not differ from participants without samples. After completion of these assays, an international laboratory harmonization project was undertaken [14] to allow better comparison across laboratories, including the Diabetes Prevention Program laboratory.When the harmonized assay became available, we repeated the tests on all samples previously positive for either the GAD antibody or insulinoma-associated-2 autoantibody assays (n=367) and a race/ethnicity-stratified random sample of participants (n=500) previously negative on both tests. Insulin autoantibody testing was not conducted since it was not standardized at the time of the first assay and was not included in the subsequent harmonization effort.
Samples were analysed for GAD and insulinoma-associated-2 autoantibodies using the harmonized protocol [14]. A radio-labelled human recombinant GAD65 was produced (Promega Inc., Madison, WI, USA) using a pThGAD65 clone. The concentration of GAD autoantibodies was determined using the standard curve and was expressed in GAD65 harmonized DK units. A DK value of ≥33, equivalent to the 99th percentile in 500 Diabetes Autoantibodies Standardization Program control subjects, was considered positive, with 88% sensitivity and 94.9% specificity compared with Diabetes Autoantibodies Standardization Program 2010 workshop standards. The insulinoma-associated-2 autoantibody assay was identical to the GAD65 autoantibody assay except that 35S-labelled insulinoma-associated-2 was used as a tracer. The assay used a pSP64-PolyA-IA-2ic clone (amino acids 601–979) in the in vitro insulinoma-associated-2 IC transcription and translation system to produce 35S-IA2 trace. Using the standard curve a sample with DK value ≥ 5 was positive (99th percentile in Diabetes Autoantibodies Standardization Program control subjects) for a 62% sensitivity and 100% specificity, similar across laboratories [14].
Statistical analysis
The prevalence of diabetes autoantibody positivity and baseline characteristics was estimated for all 3050 participants using a stratified sampling method with inverse probability weighting [28]. The diabetes incidence rate by baseline diabetes autoantibody status was calculated as number of new events per 100 person-years of follow-up. Cox’s proportional hazard modelling [15] assessed the association between diabetes autoantibody status and diabetes risk, without or with adjustment for covariates. Treatment groups by antibody interactions were assessed as in previous Diabetes Prevention Program analyses [1]. An a priori aim was to assess the effect of baseline diabetes autoantibody positivity on diabetes incidence in each treatment group, with the hypothesis that any effect would be apparent among untreated individuals (placebo), but treatment with metformin or intensive lifestyle intervention would modify this association. Although interaction terms were not statistically significant, we stratified the diabetes incidence analyses by treatment group as an exploratory analysis.
Results
Among the 3050 participants, only one participant was insulinoma-associated-2 autoantibody-positive, an estimated prevalence of 0.033%. All subsequent analyses focused only on GAD autoantibody status at baseline. Table 1 shows that there were no significant differences in age, BMI or metabolic variables according to baseline GAD autoantibody status. Overall, the estimated prevalence of GAD autoantibodies was 4.0% and varied by race/ethnicity, from 2.4% among Asian Pacific Islanders, 2.5% in Hispanic participants, 3.3% in non-Hispanic white participants, 5.4% in American-Indian participants, to 7.0% in non-Hispanic black participants.
Table 1.
Baseline characteristics of Diabetes Prevention Program participants, by glutamic acid decarboxylase autoantibody positivity
| GAD autoantibodies* | |||
|---|---|---|---|
|
| |||
| Characteristics | Negative | Positive | P† |
| No. of particpants, n | 2928 | 122 | |
| Median (IQR) age at randomization, years | 50.7 (45.0–58.4) | 49.5 (44.8–58.5) | 0.5855 |
| Sex, % | |||
| Male | 96.0 | 4.0 | 0.9652 |
| Female | 96.0 | 4.0 | |
| Diabetes Prevention Program intervention % | |||
| Placebo | 96.8 | 3.2% | 0.5921 |
| Metformin | 95.1 | 4.9% | |
| Lifestyle | 96.0 | 4.0% | |
| Median (IQR) BMI, kg/m2 | 32.2 (28.8–37.2) | 30.7 (27.1–37.8) | 0.5506 |
| Median (IQR) fasting glucose, mmol/l | 5.8 (5.5–6.1) | 5.8 (5.6–6.1) | 0.9539 |
| Median (IQR) 2-h glucose, mmol/l | 9.1 (8.3–10.0) | 9.3 (8.5–9.9) | 0.2891 |
| Median (IQR) HbA1c | |||
| mmol/mol | 40 (38–44) | 40 (38–44) | 0.4641 |
| % | 5.8 (5.6–6.2) | 5.8 (5.6–6.2) | |
| Median (IQR) fasting insulin, pmol/l | 142.8 (99.6–192.6) | 149.4 (106.2–207.6) | 0.3048 |
| Median (IQR) insulin resistance, HOMA‡ | 6.3 (4.4–8.6) | 6.5 (4.5–9.0) | 0.3280 |
| Median (IQR) insulin secretion, CIR§ | 0.6 (0.3–0.8) | 0.6 (0.3–0.9) | 0.1648 |
GAD, glutamic acid decarboxylase; IQR, interquartile range; HOMA, homeostatic model assessment; CIR, corrected insulin response.
Values are estimates for all 3050 participants using a stratified sampling method with inverse probability weighting [28].
Pearson’s chi-squared test for quantitative variables, and Wilcoxon’s rank sum tests for qualitative variables.
Fasting insulin (IU/mL) × fasting glucose (mmol/L) / 22.5 [29]: (100 × 30-min insulin) / (30-min glucose × 30-min glucose-70 mg/dl) [30].
CIR was expressed using glucose and insulin measured in conventional units, as a formula using SI units has not yet been developed).
Next, we explored whether baseline GAD autoantibody status predicted diabetes incidence. In none of the sequentially adjusted models tested was there a significant association of GAD autoantibody positivity with incident diabetes (unadjusted hazard ratio: 0.77, 95% CI: 0.42–1.41; hazard ratio adjusted for age, sex, race/ethnicity and treatment assignment: 0.74, 95% CI: 0.40–1.37; hazard ratio additionally adjusted for fasting and 2-h glucose and insulin, HbA1c, homeostatic model assessment of insulin resistance, corrected insulin response and BMI: 0.98, 95% CI: 0.56–1.73). Interactions between treatment group and GAD autoantibody positivity were not significant (P<0.05). Figure 1 shows that diabetes incidence did not differ by GAD autoantibody status in any treatment group. GAD autoantibodies were not significantly predictive using different thresholds for positivity or when titres were analysed as a continuous variable (not shown).
FIGURE 1.
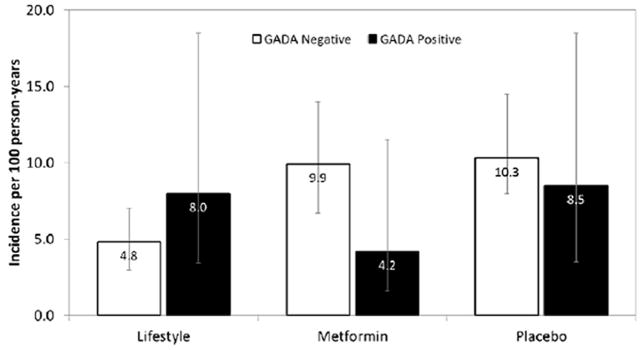
Incidence of diabetes over 3.2 years (per 100 person-years with 95% CIs), by treatment group and glutamic acid decarboxylase (GAD) status at baseline in the Diabetes Prevention Program.
Discussion
The present study provides evidence that positivity for GAD autoantibodies at baseline in participants at high risk in the Diabetes Prevention Program did not predict the incidence of diabetes over 3 years of follow-up. With the harmonized insulinoma-associated-2 autoantibody assay only one participant was positive, which was not informative.
Comparisons of our results with other studies should be performed with persons at risk of diabetes and not with those with diagnosed diabetes. GAD autoantibody prevalence has been reported to range from 0% in Japanese people with impaired glucose tolerance [16] to 3.6% in the Finnish Botnia Study [17], with intermediate prevalence rates in other reports [13,18-20]. The prevalence of insulinoma-associated-2 autoantibodies among adults at risk for diabetes has been less well studied, but was found to be 0% in Northern Italy [18] and 1.2% in Sweden [20].
Our results for GAD autoantibodies, although not those for insulinoma-associated-2 autoantibodies, in a multiethnic population, are somewhat higher than these estimates. Studies of autoantibodies in minority populations are limited. Among individuals at low risk and aged ≥40 years in the third National Health and Nutrition Examination Survey, 2.0% of non-Hispanic white people, 1.3% of non-Hispanic black people, and 2.6% of Hispanic people were GAD autoantibody-positive [21]. Among young Pima Indian people with diabetes, 5.6% were GAD antibody-positive, though at low titres [22], and none had typical Type 1 diabetes. Among Filipino people with Type 2 diabetes and control subjects, no insulinoma-associated-2 autoantibodies were detected [23]. These results are more consistent with our estimates.
Our finding that GAD autoantibody positivity did not predict diabetes incidence is consistent with some [18,24], but not all studies [12,25,26]. The Atherosclerosis Risk in Communities Study found no excess risk (hazard ratio: 1.04, 95% CI: 0.55–1.96) for GAD autoantibody-positive participants [24]. In the Cremona Italy study, none of the participants with normal glucose tolerance who were GAD autoantibody- and/or insulinoma-associated-2 autoantibody-positive progressed to diabetes over an 8-year follow-up [18]. By contrast, the Multiple Risk Factor Intervention Trial found an adjusted odds ratio for the association between GAD autoantibodies and diabetes risk of 3.6 (0.7–18.8) [12] In the Botnia Study, GAD autoantibody positivity was associated with higher diabetes risk 8 years later (14.2 vs 5.3%, P<0.00001) [26]; however, they reported that baseline GAD autoantibody status did not predict diabetes in a subset of individuals with no family history of diabetes, and low to medium levels of GAD autoantibodies, results similar to those of the present study. In the follow-up of the Västerbotten County Project [25], 28% of the GAD autoantibody-positive participants developed diabetes (relative risk: 7.2, 3.7–13.9). The authors also found an overall increase in GAD autoantibody epitopes detected, a different epitope pattern than was detected in school children with pre-diabetes developing Type 1 diabetes [27]. It is possible that the epitopes detected by our harmonized GAD autoantibody assay differed from those in studies that did observe a higher risk of diabetes development among GAD autoantibody-positive individuals. Interestingly, in the Diabetes Prevention Program, the number of diabetes autoantibody-positive participants was lower when using the harmonized GAD and insulinoma-associated-2 autoantibody assays than with the ones in use at the start of the study (122 vs 367).
The present study has limitations and strengths. We only measured diabetes autoantibodies in a single specimen at baseline; measurements were not repeated at diabetes onset, so their persistence was not assessed. The sample size, despite being the largest study of individuals with impaired glucose tolerance reporting diabetes autoantibodies testing, may have been too small to detect a low excess risk associated with diabetes autoantibody positivity. Importantly, the present study is one of only few studies to include a high proportion of participants of minority race/ethnicity, with over 3 years of careful prospective follow-up, high retention rates and standardized testing for diabetes outcomes.
We showed that GAD autoantibody positivity at baseline was not associated with higher diabetes risk and the overall profile of other risk factors was similar according to baseline GAD autoantibody status. These data suggest that ‘diabetes autoimmunity’ as reflected by GAD and insulinoma-associated-2 autoantibody positivity in middle-aged individuals at high risk for diabetes is not a clinically relevant risk factor for progression to diabetes.
Supplementary Material
What’s new?
It is unclear whether diabetes autoantibodies predict Type 2 diabetes among people with pre-diabetes (impaired fasting glucose and impaired glucose intolerance), especially in minority populations.
Diabetes autoantibodies (such as glutamic acid decarboxylase autoantibodies and insulinoma-associated-2 autoantibodies) were measured at baseline in the Diabetes Prevention Program and participants were followed for 3 years
Diabetes autoantibodies did not predict the development of diabetes in any treatment or race/ethnic group.
Measuring diabetes autoantibodies in middle-aged adults at high risk is not clinically useful for diabetes prediction.
Acknowledgments
The Investigators gratefully acknowledge the commitment and dedication of the participants of the Diabetes Prevention Program. Members of the Diabetes Prevention Program Research Group are shown in Appendix S1.
Funding sources
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centres and the coordinating centre for the design and conduct of the study, and collection, management, analysis and interpretation of the data (U01 DK048489). The Southwestern American Indian Centers were supported directly by the NIDDK and by the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources supported data collection at many of the clinical centres. Funding for data collection and participant support was also provided by the Office of Research on Minority Health, the National Institute of Child Health and Human Development, the National Institute on Aging, the Centers for Disease Control and Prevention, the Office of Research on Women’s Health, the Department of Veterans Affairs, and the American Diabetes Association. Bristol-Myers Squibb and Parke-Davis provided medication. This research was also supported, in part, by the intramural research program of the NIDDK. Lipha (Merck-Sante) provided medicines, and LifeScan Inc., Health O Meter, Hoechst Marion Roussel, Inc., Merck-Medco Managed Care, Inc., Merck and Co., Nike Sports Marketing, Slim Fast Foods Co., and Quaker Oats Co. donated materials, equipment or medicines for concomitant conditions. The opinions expressed are those of the investigators and do not necessarily reflect the views of the funding agencies. A complete list of centres, investigators, and staff can be found in Appendix S1.
Footnotes
Competing interests
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article: Appendix S1 Diabetes Prevention Program research group investigators.
References
- 1.The Diabetes Prevention Program Research Group. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 3.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar A, Vijay V, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49:289–297. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 4.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 5.The Diabetes Prevention Program Research Group. Prevention of Type 2 Diabetes With Troglitazone in the Diabetes Prevention Program. Diabetes. 2005;54:1150–1156. doi: 10.2337/diabetes.54.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo RA, Tripathi R, Schwenke DC, Banerji MA, Bray GA, Buchanan TA, et al. Pioglitazone for Diabetes Prevention in Impaired Glucose Tolerance. New Engl J Med. 2011;364:1104–1115. doi: 10.1056/NEJMoa1010949. [DOI] [PubMed] [Google Scholar]
- 8.Pozzilli P, di Mario U. Autoimmune diabetes not requiring insulin at diagnosis (latent autoimmune diabetes of the adult): definition, characterization, and potential prevention. Diabetes Care. 2001;24:1460–1467. doi: 10.2337/diacare.24.8.1460. [DOI] [PubMed] [Google Scholar]
- 9.Leslie RD, Williams R, Pozzilli P. Type 1 diabetes and latent autoimmune diabetes in adults (LADA): one end of the rainbow. J Clin Endocrinol Metab. 2006;91:1654–1659. doi: 10.1210/jc.2005-1623. [DOI] [PubMed] [Google Scholar]
- 10.Turner R, Stratton I, Horton V, Manley S, Zimmet P, Mackay IR, et al. UKPDS 25: Autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet. 1997;350:1288–1293. doi: 10.1016/s0140-6736(97)03062-6. [DOI] [PubMed] [Google Scholar]
- 11.Pietropaolo M, Barina-Mitchell E, Pietropaolo SL, Kuller LH, Trucco M. Evidence of islet cell autoimmunity in elderly patients with type 2 diabetes. Diabetes. 2000;49:32–38. doi: 10.2337/diabetes.49.1.32. [DOI] [PubMed] [Google Scholar]
- 12.Zimmet PZ, Shaten BJ, Kuller LH, Rowley MJ, Knowles WJ, Mackay IR. Antibodies to glutamic acid decarboxylase and diabetes mellitus in the Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1994;140:683–690. doi: 10.1093/oxfordjournals.aje.a117316. [DOI] [PubMed] [Google Scholar]
- 13.Ruige JB, Batstra MR, Aanstoot H-K, Bouter LM, Bruining GJ, deNeeling JND, et al. Low prevalence of antibodies to GAD65 in a 50- to 74-year old general Dutch population. The Hoorn Study. Diabetes Care. 1997;20:1108–1110. doi: 10.2337/diacare.20.7.1108. [DOI] [PubMed] [Google Scholar]
- 14.Bonifacio E, Yu L, Williams AK, Eisenbarth GS, Bingley PJ, Marcovina SM, et al. Harmonization of Glutamic Acid Decarboxylase and Islet Antigen-2 Autoantibody Assays for National Institute of Diabetes and Digestive and Kidney Diseases Consortia. J Clin Endocrinol Metab. 2010;95:3360–3367. doi: 10.1210/jc.2010-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox DR. Regression Models and Life-Tables. JRSS (B) 1972;74:187–220. [Google Scholar]
- 16.Tsuruoka A, Matsuba I, Toyota T, Isshiki G, Nagataki S, Ikeda Y. Antibodies to GAD in Japanese diabetic patients: a multicenter study. Diabetes Res Clin Pract. 1995;28:191–199. doi: 10.1016/0168-8227(95)01101-i. [DOI] [PubMed] [Google Scholar]
- 17.Tuomi T, Carlsson A, Li H, Isomaa B, Miettinen A, Nilsson A, et al. Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes. 1999;48:150–157. doi: 10.2337/diabetes.48.1.150. [DOI] [PubMed] [Google Scholar]
- 18.Bosi EP, Garancini MP, Poggiali F, Bonifacio E, Gallus G. Low prevalence of islet autoimmunity in adult diabetes and low predictive value of islet autoantibodies in the general adult population of northern Italy. Diabetologia. 1999;42:840–844. doi: 10.1007/s001250051235. [DOI] [PubMed] [Google Scholar]
- 19.Breidert M, Temelkova-Kurktschiev T, Hanefeld M, Leonhardt W, Schmoeckel A, Seissler J. Prevalence of diabetes-specific autoantibodies in patients at risk for adult onset diabetes mellitus. Exp Clin Endocrinol Diabetes. 1998;106:113–116. doi: 10.1055/s-0029-1211961. [DOI] [PubMed] [Google Scholar]
- 20.Rolandsson O, Hagg E, Hampe C, Sullivan EP, Jr, Nilsson M, Jansson G, et al. Glutamate decarboxylase (GAD65) and tyrosine phosphatase-like protein (IA-2) autoantibodies index in a regional population is related to glucose intolerance and body mass index. Diabetologia. 1999;42:555–559. doi: 10.1007/s001250051194. [DOI] [PubMed] [Google Scholar]
- 21.Barinas-Mitchell E, Pietropaolo S, Zhang YJ, Henderson T, Trucco M, Kuller LH, et al. Islet cell autoimmunity in a triethnic adult population of the Third National Health and Nutrition Examination Survey. Diabetes. 2004;53:1293–1302. doi: 10.2337/diabetes.53.5.1293. [DOI] [PubMed] [Google Scholar]
- 22.Dabelea D, Palmer JP, Bennett PH, Pettitt DJ, Knowler WC. Absence of glutamic acid decarboxylase antibodies in Pima Indian children with diabetes mellitus [letter] Diabetologia. 1999;42:1265–1266. doi: 10.1007/s001250051303. [DOI] [PubMed] [Google Scholar]
- 23.Medici F, Hawa MI, Giorgini A, Panelo A, Solfelix CM, Leslie RD, et al. Antibodies to GAD65 and a tyrosine phosphatase-like molecule IA-2ic in Filipino type 1 diabetic patients. Diabetes Care. 1999;22:1458–1461. doi: 10.2337/diacare.22.9.1458. [DOI] [PubMed] [Google Scholar]
- 24.Vigo A, Duncan BB, Schmidt MI, Couper D, Heiss G, Pankow JS, et al. Glutamic acid decarboxylase antibodies are indicators of the course, but not of the onset, of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities Study. Braz J Med and Biol Res. 2007;40:933–941. doi: 10.1590/s0100-879x2006005000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hampe CS, Hall TR, Agren A, Olandsson O. Longitudinal changes in epitope recognition of autoantibodies against glutamate decarboxylase 65 (GAD65Ab) in prediabetic adults developing diabetes. Clin Exp Immunol. 2007;148:72–78. doi: 10.1111/j.1365-2249.2007.03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundgren VM, Isomaa B, Lyssenko V, Laurila E, Korhonen P, Groop LC, et al. GAD Antibody Positivity Predicts Type 2 Diabetes in an Adult Population. Diabetes. 2010;59:416–422. doi: 10.2337/db09-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlosser M, Banga JP, Madec AM, Binder KA, Strebelow M, Rjasanowski I, et al. Dynamic changes of GAD65 autoantibody epitope specificities in individuals at risk of developing type 1 diabetes. Diabetologia. 2005;48:922–930. doi: 10.1007/s00125-005-1719-1. [DOI] [PubMed] [Google Scholar]
- 28.Lohr SL. Sampling: Design and Analysis. 2. Boston, MA: BROOKS/COLE Cengage Learning; 2010. [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and B-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 30.Hanson RL, Pratley RE, Bogardus C, Venkat Narayan KM, Roumain JM, Imperatore G, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol. 2000;151:190–198. doi: 10.1093/oxfordjournals.aje.a010187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


