Summary
Aims
Diabetes mellitus increases the risk of stroke, but the mechanisms are unclear. The present study tested the hypothesis that diabetes mellitus disturbs the brain microcirculation and increases the susceptibility to cerebral damage in a middle cerebral artery occlusion (MCAO) model of ischemia.
Methods
Diabetes was induced by streptozocin in mice expressing green fluorescent protein in endothelial cells (Tie2‐GFP mice). Four weeks later, they were subjected to transient (20 min) MCAO. In vivo blood flow was measured by two‐photon laser‐scanning microscopy (TPLSM) in cerebral arteries, veins, and capillaries.
Results
There was a significant decrease in red blood cell (RBC) velocity in capillaries in diabetic mice as assessed by TPLSM, yet the regional cerebral blood flow, as assessed by laser Doppler flowmetry, was maintained. Brain capillary flow developed turbulence after MCAO only in diabetic mice. These mice sustained increased neurological deficits after MCAO which were accompanied by an exaggerated degradation of tight junction proteins and blunted CaMKII phosphorylation in cerebral tissues indicating disruption of the blood–brain barrier and disturbed cognitive potential.
Conclusion
Diabetic mice are more susceptible to disturbances of cerebral capillary blood flow which may predispose them to neurovascular defects following ischemia.
Keywords: Capillary, Cerebral blood flow, Diabetes, Hyperglycemia, Ischemia, Two‐photon laser‐scanning microscopy
Introduction
Diabetes mellitus (DM) is a common condition that leads over time to the development of macro‐ or microvascular complications manifested in the brain as ischemic stroke or cognitive defects 1, 2, 3.
Recent studies have reported that chronic hyperglycemia per se is directly detrimental to the brain function 4. Hyperglycemia may cause the accumulation of toxic reactive oxygen species (ROS) and advanced glycation end‐products (AGEs) accumulating in the brain during hyperglycemia and disturbing cerebral function 5, 6, 7. However, present techniques have not been adequate to explore whether hyperglycemia increases the susceptibility to cerebral disturbances caused by defective regulation of the microcirculation. Spatially averaged cerebral blood flow can be assessed by laser Doppler flowmetry, but this is inadequate to evaluate the microcirculation. Therefore, we have adopted intravital two‐photon laser‐scanning microscopy (TPLSM) to study cerebral blood flow in individual microvessels and capillaries 8 which can be at depths of 500–1000 μm into the brain tissue 9. This has permitted the dynamic measurements of cerebral microvascular function in living mice.
Diabetes mellitus not only damages microvascular function but also induces blood–brain barrier (BBB) dysfunction perhaps by generation of ROS 10. Therefore, we investigated the effects of DM on cerebral microcirculation over ischemia using intravital TPLSM and correlated with this functional outcome to validate this method for the study of dynamic changes of brain capillary blood flow. The expression of degraded tight junction proteins and CaMKII indexed damage to the blood–brain barrier and to cognitive function, respectively. These results demonstrated that disturbed cerebral capillary blood flow and disrupted BBB worsened the outcome of cerebral ischemia in diabetic mice.
Materials and methods
Reagents
All chemicals were purchased from Sigma‐Aldrich Chemical Co. (St. Louis, MO, USA) unless otherwise noted.
Mouse Strains and Induction of Diabetes
Tie2‐GFP (STOCK Tg [TIE2GFP] 287Sato/J, Bar Harbor, ME, USA) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) to provide ready identification of cerebral microvessels. Adult male C57BL/6J wild‐type (WT) mice were also used in this study for Western blot studying. All mice were housed under 12‐h light/dark cycle and had access to food and water ad libitum. All animal procedures were performed in accordance with the “Guide for the Care and Use of Laboratory Animals” (NIH publication 86‐23, 1996). The appropriate guidelines for the care and use of laboratory animals were also approved by the Committee for Animal Experiments of Zhejiang University in China. Diabetes was induced by a single injection of freshly prepared streptozocin (STZ, 150 mg/kg; intraperitoneal; Sigma‐Aldrich), whereas control mice were injected only with vehicle (citrate buffer). Before diabetes induction, the animals were weighed and fasted for 12 h. The concentration of blood glucose was repeatedly monitored by test reagent strips (Accu‐Chek Performa, Roche®, Indianapolis, IN, USA) every week after STZ injection. Only mice with glucose levels >11.1 mmol/L were considered diabetic.
Transient Middle Cerebral Artery Occlusion in Mice
Preparation for the transient ischemia/reperfusion middle cerebral artery occlusion (MCAO) model in mice was carried out as previously described 11. For this model, 20 min of ischemia was followed by reperfusion. Mice were randomly assigned to four groups: control, diabetes, MCAO, and diabetes + MCAO. Tie2‐GFP mice were used for TPLSM imaging to identify blood vessels. The rectal temperature was monitored throughout the surgery and was maintained at 37 ± 0.5°C with a heating blanket. Neurological scores were determined 24 h after MCAO as described 11.
Preparation for In Vivo Imaging
After anesthetizing the mice with an intraperitoneal injection of chloral hydrate (400 mg/kg body weight), cerebral blood vessels were imaged through a craniotomy window centered at stereotactic coordinates 2.5 mm caudal to bregma and 2.5 mm lateral to midline 12. The area to be observed is periinfarcted zone in MCAO mice. The dura was removed and a metal frame of diameter 10.0 mm with a removable cover glass lid (diameter 6.0 mm) was glued to the skull to cover the craniotomy window. The space between the exposed brain surface and the cover glass was filled with 1.5% (w/v) low melting point agarose in an artificial cerebrospinal fluid (ACSF) 13. A bolus of 5 mg/kg Texas Red dextran (70 kDa, Molecular Probes, Invitrogen, Carlsbad, California) in 0.9% NaCl was injected into the tail vein before TPLSM imaging to outline the blood vessels.
Laser Doppler Flowmetry
Measurement of regional cerebral blood flow (rCBF) was performed with a laser Doppler flowmeter (Periflux System 5010; Perimed, Jarfalla, Sweden) 14.The laser was transmitted through a flexible fiber‐optic probe affixed to the skull at about 2.5 mm caudal to bregma and 2.5 mm lateral to midline (proximally the center of the craniotomy window). The cerebral regional blood flow was equated with the absolute RBC flux.
Vessel Imaging and Analysis
We use an Olympus Fluoview1000 two‐photon microscope (BX61W1‐FV1000, Olympus, Ltd., Tokyo, Japan) with an excitation source of a Spectra‐Physics MaiTai HP DeepSee femtosecond Ti:Sa laser. To acquire images (either stacks or single focal planes) from GFP‐positive vessels, a long working distance (2 mm) water‐immersion objective (×25, NA 1.05) was used for line scan measurements. The images were taken at 12‐bit depth with resolution of 1024 × 1024 pixels. We chose a scanning rate of 10 μs/pixel and 2000 lines in total to follow the velocity of RBCs. The average RBC velocity speed has been reported 8, 15. In vivo vessel diameters were measured manually using ImageJ 16. RBC velocity and flux were calculated with an automated image‐processing algorithm using MATLAB software (The MathWorks Inc., Natick, Massachusetts) 8, 15.
Western blot Analysis
Brain cortex of penumbra region was used in Western blot, which matched the region under two‐photon observation. Samples containing equivalent amounts of extracted protein were loaded onto 10% gel for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS‐PAGE) 11. Membranes were blotted using the following primary antibodies: spectrin, phospho‐GluR1 (Ser831; Millipore, Billerica, MA, USA), ZO‐1 (Invitrogen, San Diego, CA, USA), phospho‐Thr286‐CaMKII, phospho‐Ser603‐synapsin I (ThermoScientific, Waltham, MA, USA), and β‐actin (Sigma‐Aldrich).
Statistical Analysis
The data are presented as mean ± SEM. Statistical significance was determined using one‐way analysis of variance (ANOVA) followed by Tukey's test for multigroup comparisons. And unpaired t‐test was performed when determining the neurological deficit. P < 0.05 indicated statistically significant differences.
Results
Diabetes Increases Susceptibility to Disturbances of Blood Flow After Brain Ischemia
Four weeks after STZ injection, the blood glucose concentrations of diabetic mice were significantly higher (22.07 ± 0.88 mmol/L; n = 34) than in the controls (5.21 ± 0.26 mmol/L; n = 26). The ischemia caused by the MCAO was similar in control and diabetic mice using a laser Doppler flowmetry (Figure 1A). We measured the rCBF in the ipsilateral brain hemisphere in each group of mice. No differences in the basal rCBF were observed between control and diabetic mice. However, diabetic mice exhibited an approximately 50% greater reduction in rCBF following MCAO (Figure 1A).
Figure 1.
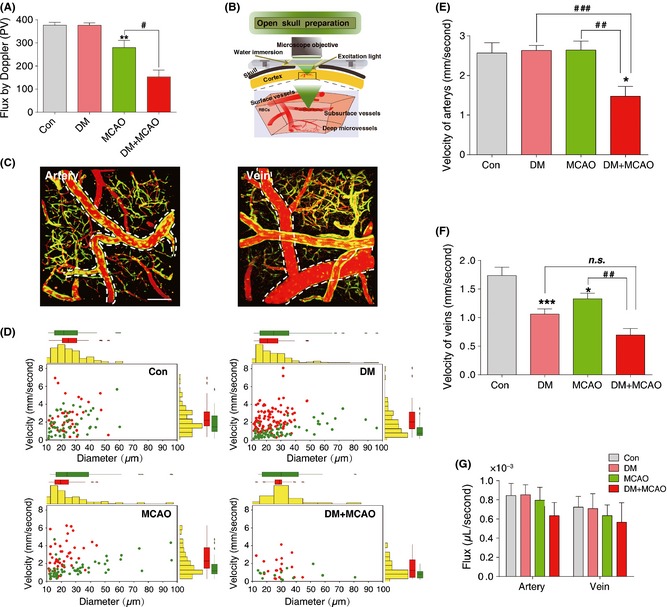
Diabetes increases susceptibility to ischemic insults. (A) Changes in regional cerebral blood flow (rCBF) in control (Con) and diabetic (DM) mice after middle cerebral arterial occlusion (MCAO) using a laser Doppler flowmeter for measurements. The values read from the flowmeter directly and expressed as mean ± SEM (n = 6‐8), **P < 0.01; versus control mice; # P < 0.05 versus diabetes + MCAO mice. (B) In vivo imaging of blood flow by two‐photon laser‐scanning microscopy (TPLSM) through a cranial window. (C) TPLSM in conjunction with labeling of the blood plasma with Texas Red dextran is used for mapping the angioarchitecture as well as quantify the transport of individual red blood cell (RBC) in arteries and veins. Bar = 100 μm. (D) The changes in velocity of RBC plotted against diameter in artery (red) and vein (green) in control, diabetic, MCAO, and diabetic + MCAO mice were detected using TPLSM. Bar graphs display velocity of artery (E) and vein (F) in control (Con), diabetic (DM), MCAO, and diabetic + MCAO (DM + MCAO) mice. The data are expressed as the mean ± SEM (n = 6–8), *P < 0.05; ***P < 0.001 versus control mice; ## P < 0.01; ### P < 0.001 versus DM+MCAO mice. (G) Changes in volume flux (μL/second) in artery and vein in control, diabetic, MCAO, and diabetic + MCAO mice.
Two‐photon laser‐scanning microscopy in conjunction with labeling of the blood plasma with Texas Red dextran was used to map the angioarchitecture 8, 15, 17, 18 and to quantify the transit of individual RBCs (Figure 1B) by TPLSM as previously described (Figure S1, Movie S1) 19. The expression of GFP by endothelial cells enabled the visualization of the blood vessel walls. We categorized cortical vessels located within a depth of 100–200 μm from the surface by their topology, vessel wall morphology, diameter, and RBC flow direction (Figure 1B,C; Figures S1 and S2) to identify distinct blood vessel (Figure 1C, Movie S1). To quantify center line RBC velocity and the direction of blood flow in individual vessel, a line scan method was established by scanning single vessels and extracting the average speed over this period (Figure S2–S4). We also measured the cerebrovascular blood flow in veins and arteries by this line scan method. Bar graphs display marginal means, and scatter plots display the different patterns of distribution for vessels characterized by their diameters and average RBC velocities in different experimental groups (Figure 1D). Unexpectedly, diabetic mice had no significant changes in RBC velocity in arteries (Figure 1E), but had a decreased blood velocity in veins (Figure 1F), implying that the veins were dilated or arteries were constricted. A decline in blood velocity in both arteries and veins was observed in diabetic mice after MCAO (Figure 1E, F; Movie S2–S5). The oxygen and nutrient carrying capacities of a blood vessel are proportional to their RBC and fluid flux 20, but no difference in nutrient carrying capacity was detected as the RBC volume flux (μL/second) was similar in arteries and veins in all groups (Figure 1G).
Diabetes Increases Susceptibility to Cerebral Capillary RBCs Velocity Disturbances in Brain Ischemia
The cerebral microcirculation is closely coupled to normal brain metabolism and function. Although hyperglycemia can injure brain capillaries 1, 3, it is unclear whether brain capillary flow is sufficient to meet the energy demands of the parenchyma during diabetes. Thus, RBC velocities were measured by intravital TPLSM after MCAO to examine whether induced ischemia altered cerebral capillary blood flow. Figure 2A and B provides representative tracings of vessel wall morphology and RBC velocity in brain capillaries. Bar graphs of marginal means with individual scatter plots indicated that the RBC velocity in capillaries of diabetic mice was decreased (0.93 ± 0.11 mm/second, Figure 2C, D) and decreased further in the diabetic mice after MCAO (0.62 ± 0.07 mm/second, Figure 2C, D). Data on RBC velocity and vessel diameter were used to calculate the RBC flux in a given vessel to provide an index of the vascular oxygen and nutrient carrying capacity of the blood vessel 21. Whereas MCAO did not change volume flux of RBCs (in μL/s) in arteries or veins, there was a decrease in capillary RBC volume flux and this was exacerbated by diabetes (Figure 2E). We did not detect a change in the shape or diameter of RBCs to explain these findings (Figure S5).
Figure 2.
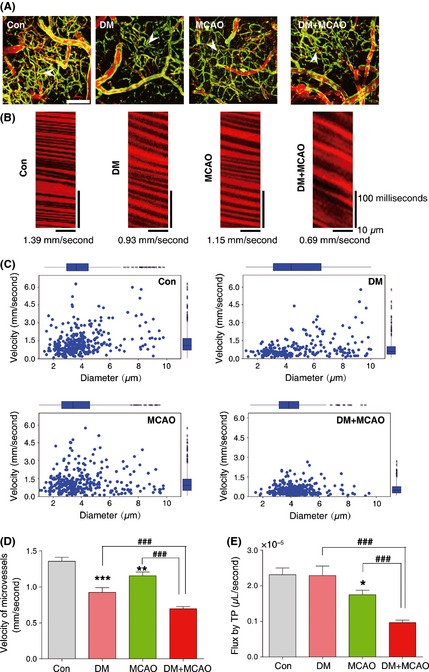
Diabetes increases disturbances in cerebral capillary red blood cells (RBCs) velocity in brain ischemia. (A) Two‐photon laser‐scanning microscopy (TPLSM) in conjunction with labeling of the blood plasma with Texas Red dextran used for mapping the angioarchitecture of capillaries in control, diabetic, middle cerebral artery occlusion (MCAO), and diabetic + MCAO mice. Bar = 100 μm. (B) Line scan data of the blood flow pattern in capillaries of control, diabetic, MCAO, and diabetic + MCAO mice. (C) The changes in RBC velocity versus diameter in capillary in control, diabetic, MCAO, and diabetic + MCAO mice using TPLSM. (D) Bar graphs display RBC velocity in capillaries in diabetic, MCAO, and diabetic + MCAO mice. (E) Volume flux changes in capillaries in control, diabetic, MCAO, and diabetic + MCAO mice. The data are expressed as mean ± SEM (n = 6–8), *P < 0.05; **P < 0.01; ***P < 0.001 versus control mice; ### P < 0.001 versus diabetic + MCAO mice.
Two‐Photon Imaging of Cortical Surface Microvessels Reveals Turbulent Brain Capillary Flow in Diabetic Mice
Blood flow turbulence increases flow resistance and slows blood flow in the circulation and can predispose the vessel wall to remodeling. The RBCs patterns in vessels from control and diabetic mice maintained a unidirectional flow (Figure 3A‐a, A‐b; B‐a, B‐b). Unexpectedly, the pattern of blood flow in MCAO developed a sinuous character (Figure 3C‐a, C‐b), with turbulence detected in brain capillary flow in diabetic mice after MCAO (Figure 3D‐a, D‐b). The direction of blood flow after MCAO in control mice changed in an “in‐line” pattern, but in diabetic mice, it became sinusoidal. The “back‐and‐forth” pattern of flow after MCAO in normal mice should provide a reasonable supply of oxygen and nutrients. In contrast, the more extreme sinuous patterns after MCAO in diabetic mice led to episodes of blood flow standstill. Such a pattern of severely disturbed flow after the combination of ischemia and hyperglycemia may be detrimental to cerebral function or survival. The capillary blood flow becomes very severely disrupted with turbulence developing only in this diabetic group of mice after MCAO.
Figure 3.
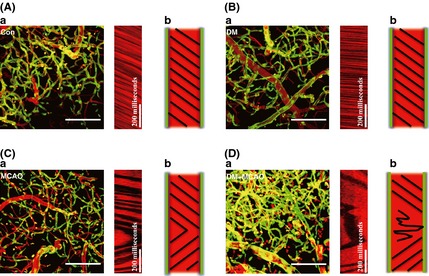
Two‐photon imaging of cortical surface microvessels reveals turbulent brain capillary flow in diabetic mice. (A) In control mice, the RBCs keep moving in one direction and no reverse are observed. The dark streaks running from upper left to lower right reflect the normal direction of brain capillary flow in control mice. (B) The pattern of RBCs moving was recorded in diabetic mice. (C) The reversed direction of brain capillary flow in middle cerebral artery occlusion (MCAO) mice. It demonstrated that the direction of blood flow changes in a “straightforward” pattern that the reverse flow occurs paroxysmally. (D) Turbulent brain capillary flow was observed in diabetic + MCAO mice. Different with MCAO mice, a “sinuous” pattern was observed in diabetic + MCAO group, which seems to be more severe and leads to cessation of blood flow supply in a certain time period. Bar = 100 μm.
Diabetes Potentiates Neurovascular Damage in Brain Ischemia
Neurological degeneration is associated with disturbed microcirculation in the brain, which may be potentiated by diabetes 22. Therefore, neurological scores were determined after MCAO to quantify the neurological functions 24 h after induction of ischemia (Figure 4A). More severe neurological deficits were observed after MCAO in diabetic mice. Neurological damage was further quantified by immunoblotting for the expression of a 150‐kDa spectrin breakdown product. A significant increase in spectrin breakdown was observed after MCAO only in diabetic mice (Figure 4B). Preservation of an intact BBB depends on normal function of tight junction proteins 7. There was extensive degradation of zona occludens (ZO‐1) and occludin after MCAO in diabetic mice (Figure 4B).
Figure 4.
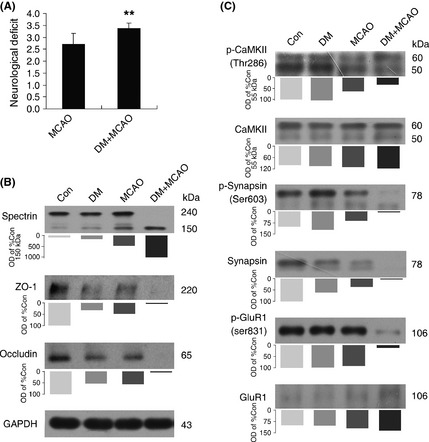
Diabetes increases susceptibility to neurovascular damage in brain ischemia. (A) Neurological scores were determined in middle cerebral artery occlusion (MCAO) and diabetic + MCAO mice. The data are expressed as mean ± SEM (n = 6–8), **P < 0.01 versus MCAO mice. (B) Immunoblot analysis of spectrin, ZO‐1, and occludin in brain cortex of control, diabetic, MCAO, and diabetic + MCAO mice. The bar graphs indicate the ratio of OD for indicated proteins. (C) Immunoblot analysis of the phosphorylation of CaMKII (Thr286), synapsin I (Ser603), and GluR1 (Ser831) in brain cortex of control, diabetic, MCAO, and diabetic + MCAO mice. The bar graphs indicate the ratio of OD for indicated proteins.
Multifunctional CaMKII has been used to assess cognition 23. It phosphorylates a variety of substrates such as synapsin I and GluRI 23. Although CaMKII (Thr 286), synapsin I (Ser603), and GluR1 (Ser831) phosphorylation were preserved after MCAO in control mice, there was a significant decrease in phospho‐CaMKII and its substrates after MCAO in diabetic mice (Figure 4C).
Discussion
This study has demonstrated that the improved spatial and temporal resolution of quantifying blood flow using intravital TPLSM in mice was clearly more sensitive than laser Doppler flowmetry. It permitted the quantification of RBC velocity in capillary blood flow and demonstrated that the disturbed pattern of cerebral capillary blood flow in diabetes was associated with a worsening of neurovascular damage after brain ischemia.
The use of TPLSM in Tie2‐GFP mice enabled the individual assessment of cerebral blood flow in arteries, veins, and capillaries. We confirm the results of Knudsen that, overall, cerebral blood flow is maintained in diabetes 24. However, despite unchanged RBC volume flux in arteries or veins after MCAO, diabetic mice had markedly abnormal capillary blood flow following MCAO. The turbulent capillary blood flow detected after MCAO in diabetic mice is potentially damaging to the endothelium 20, 21 and may worsen cerebral microvascular perfusion after ischemia.
Disturbances in BBB function are seen in diabetic animals 7. Diabetes increased the degradation of tight junction proteins after MCAO indicating impaired cerebral endothelial function. This may have contributed to the defects in BBB function and to the worsened cognitive outcomes. We confirm the reports 5, 6, 10 that hyperglycemia worsened loss of cerebral function after ischemia, as assessed from a decrease in phospho‐CaMKII (Thr286) and its substrates.
A transient 20 min MCAO 4 weeks after induction of diabetes was selected to ensure survival of the mice 7, 25. Simultaneous measurements of capillary blood flow, calcium imaging, and blood gas analysis should throw further light on neurovascular dynamics in chronic hyperglycemia. However, this study has identified disturbed capillary blood flow as a therapeutic target to lessen stroke and cognitive decline in diabetes.
In summary, the present study using intravital TPLSM has demonstrated that disturbances of cerebral capillary blood flow in diabetes are associated with exaggerated neurovascular and probably cognitive defects after ischemia.
Sources of Funding
This study was funded in part by National Natural Science Foundations of China (81120108023, 81202533) and NIH Grants (DK‐49870; DK‐36079; HL‐68686).
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Figure S1. The 3D view of architecture of cerebral vessels by TPLSM.
Figure S2. Choosing scanning rate.
Figure S3. Line scan method to quantify the flow speed of RBCs.
Figure S4. Determination of blood flow direction.
Figure S5. Representative images of RBCs morphology by Wright‐Giemsa staining.
Movie S1. Movie to show the 3‐dimensional reconstruction of arteries, veins and capillaries.
Movie S2. Time‐lapse of blood flow in control mice.
Movie S3. Time‐lapse of blood flow in diabetic mice.
Movie S4. Time‐lapse of blood flow in MCAO mice.
Movie S5. Time‐lapse of blood flow in diabetci+MCAO mice.
The first two authors contributed equally to this work
References
- 1. Brismar T, Maurex L, Cooray G, et al. Predictors of cognitive impairment in type 1 diabetes. Psychoneuroendocrino 2007;32:1041–1051. [DOI] [PubMed] [Google Scholar]
- 2. Jones DT. Neural networks, cognition, and diabetes: What is the connection? Diabetes 2012;61:1653–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ryan CM, Geckle MO, Orchard TJ. Cognitive efficiency declines over time in adults with Type 1 diabetes: Effects of micro‐ and macrovascular complications. Diabetologia 2003;46:940–948. [DOI] [PubMed] [Google Scholar]
- 4. Northam EA, Rankins D, Cameron FJ. Therapy insight: The impact of type 1 diabetes on brain development and function. Nat Clin Pract Neurol 2006;2:78–86. [DOI] [PubMed] [Google Scholar]
- 5. Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006;114:597–605. [DOI] [PubMed] [Google Scholar]
- 6. Jakobsen J, Nedergaard M, Aarslew‐Jensen M, Diemer NH. Regional brain glucose metabolism and blood flow in streptozocin‐induced diabetic rats. Diabetes 1990;39:437–440. [DOI] [PubMed] [Google Scholar]
- 7. Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase‐9 activation after focal cerebral ischemia/reperfusion in rats: Relation to blood‐brain barrier dysfunction. Stroke 2007;38:1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schaffer CB, Friedman B, Nishimura N, et al. Two‐photon imaging of cortical surface microvessels reveals a robust redistribution in blood flow after vascular occlusion. PLoS Biol 2006;4:e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Helmchen F, Denk W. Deep tissue two‐photon microscopy. Nat Methods 2005;2:932–940. [DOI] [PubMed] [Google Scholar]
- 10. Liu C, Wu J, Zou MH. Activation of AMP‐activated protein kinase alleviates high‐glucose‐induced dysfunction of brain microvascular endothelial cell tight‐junction dynamics. Free Radic Biol Med 2012;53:1213–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shioda N, Ishigami T, Han F, et al. Activation of phosphatidylinositol 3‐kinase/protein kinase B pathway by a vanadyl compound mediates its neuroprotective effect in mouse brain ischemia. Neuroscience 2007;148:221–229. [DOI] [PubMed] [Google Scholar]
- 12. Itoh Y, Toriumi H, Yamada S, Hoshino H, Suzuki N. Resident endothelial cells surrounding damaged arterial endothelium reendothelialize the lesion. Arterioscler Thromb Vasc Biol 2010;30:1725–1732. [DOI] [PubMed] [Google Scholar]
- 13. Moriguchi S, Oomura Y, Shioda N, et al. Ca2 + /calmodulin‐dependent protein kinase II and protein kinase C activities mediate extracellular glucose‐regulated hippocampal synaptic efficacy. Mol Cell Neurosci 2011;46:101–107. [DOI] [PubMed] [Google Scholar]
- 14. Fan YY, Hu WW, Dai HB, et al. Activation of the central histaminergic system is involved in hypoxia‐induced stroke tolerance in adult mice. J Cereb Blood Flow Metab 2011;31:305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kleinfeld D, Mitra PP, Helmchen F, Denk W. Fluctuations and stimulus‐induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc Natl Acad Sci U S A 1998;95:15741–15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fischer MJ, Uchida S, Messlinger K. Measurement of meningeal blood vessel diameter in vivo with a plug‐in for ImageJ. Microvasc Res 2010;80:258–266. [DOI] [PubMed] [Google Scholar]
- 17. Hirase H, Creso J, Buzsaki G. Capillary level imaging of local cerebral blood flow in bicuculline‐induced epileptic foci. Neuroscience 2004;128:209–216. [DOI] [PubMed] [Google Scholar]
- 18. Zhang S, Murphy TH. Imaging the impact of cortical microcirculation on synaptic structure and sensory‐evoked hemodynamic responses in vivo . PLoS Biol 2007;5:e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nishimura N, Rosidi NL, Iadecola C, Schaffer CB. Limitations of collateral flow after occlusion of a single cortical penetrating arteriole. J Cereb Blood Flow Metab 2010;30:1914–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shih AY, Friedman B, Drew PJ, Tsai PS, Lyden PD, Kleinfeld D. Active dilation of penetrating arterioles restores red blood cell flux to penumbral neocortex after focal stroke. J Cereb Blood Flow Metab 2009;29:738–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Long DS, Smith ML, Pries AR, Ley K, Damiano ER. Microviscometry reveals reduced blood viscosity and altered shear rate and shear stress profiles in microvessels after hemodilution. Proc Natl Acad Sci U S A 2004;101:10060–10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huber JD, VanGilder RL, Houser KA. Streptozotocin‐induced diabetes progressively increases blood‐brain barrier permeability in specific brain regions in rats. Am J Physiol Heart Circ Physiol 2006;291:H2660–H2668. [DOI] [PubMed] [Google Scholar]
- 23. Fukunaga K, Miyamoto E. A working model of CaM kinase II activity in hippocampal long‐term potentiation and memory. Neurosci Res 2000;38:3–17. [DOI] [PubMed] [Google Scholar]
- 24. Knudsen GM, Gobel U, Paulson OB, Kuschinsky W. Regional density of perfused capillaries and cerebral blood flow in untreated short‐term and long‐term streptozotocin diabetes. J Cereb Blood Flow Metab 1991;11:361–365. [DOI] [PubMed] [Google Scholar]
- 25. Luitse MJ, Biessels GJ, Rutten GE, Kappelle LJ. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol 2012;11:261–271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The 3D view of architecture of cerebral vessels by TPLSM.
Figure S2. Choosing scanning rate.
Figure S3. Line scan method to quantify the flow speed of RBCs.
Figure S4. Determination of blood flow direction.
Figure S5. Representative images of RBCs morphology by Wright‐Giemsa staining.
Movie S1. Movie to show the 3‐dimensional reconstruction of arteries, veins and capillaries.
Movie S2. Time‐lapse of blood flow in control mice.
Movie S3. Time‐lapse of blood flow in diabetic mice.
Movie S4. Time‐lapse of blood flow in MCAO mice.
Movie S5. Time‐lapse of blood flow in diabetci+MCAO mice.


